Abstract
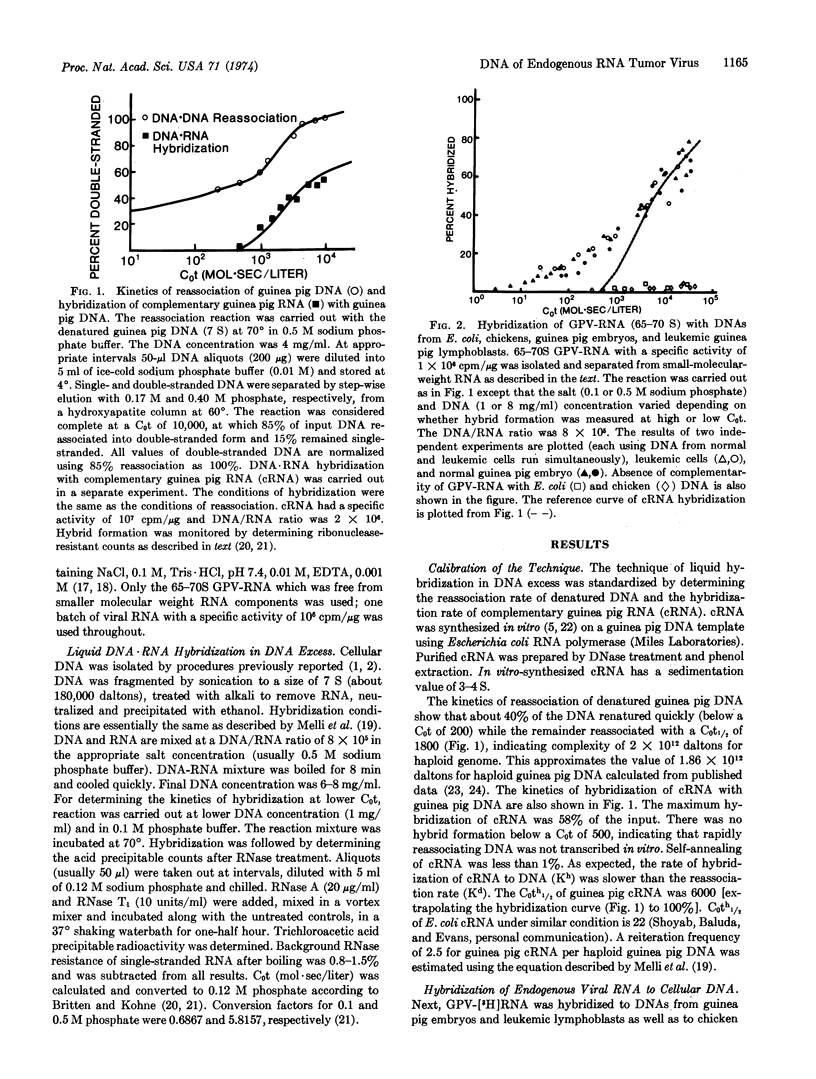
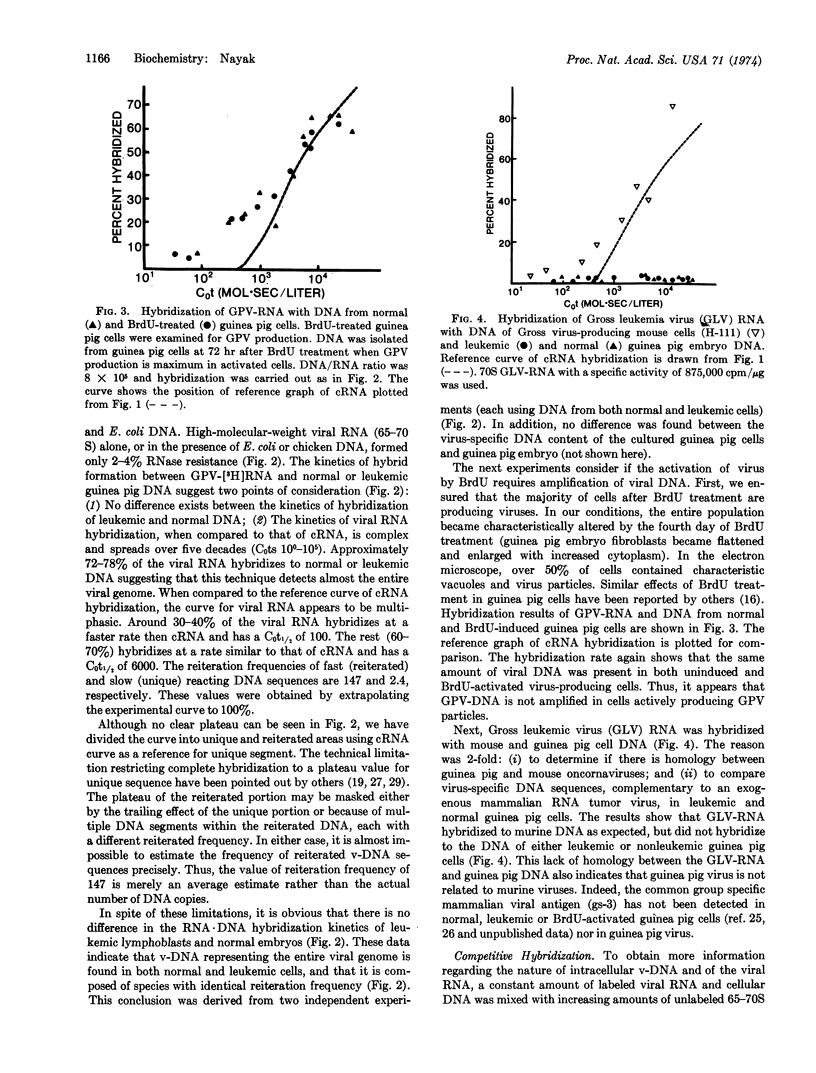
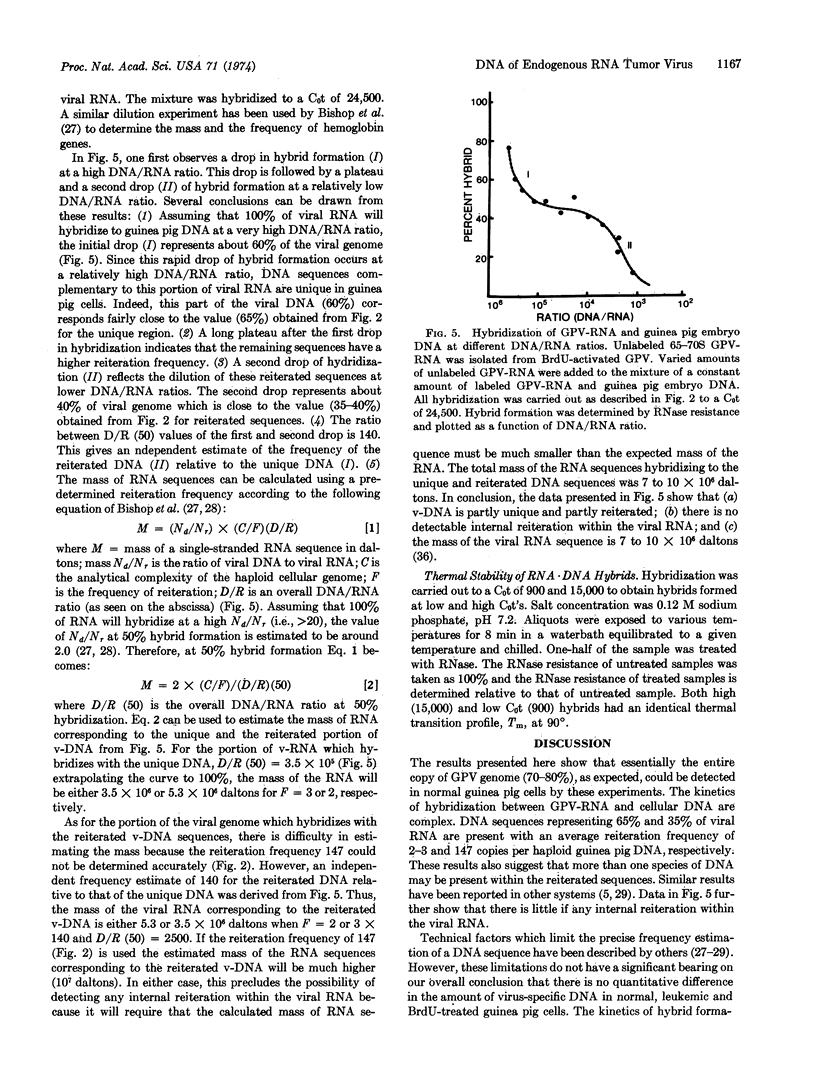
The kinetics of hybrid formation between the RNA of BrdU-activated endogenous guinea pig virus and the DNA of leukemic, normal, or BrdU-activated guinea pig cells were measured by the technique of RNA·DNA hybridization in DNA excess. The results suggest that virus-specific sequences representing some 60-70% of the viral genome are unique (2-3 copies per haploid cell genome), while the remainder (30-40%) are reiterated (147 copies), and that the reiterated virus-specific DNA may be composed of more than one species, each having a different reiteration frequency. No difference was found in the quantity of viral DNA sequences contained in normal, leukemic, or bromodeoxyuridine-activated guinea pig cells. These data are considerably different from those reported for exogenous (infectious) oncornaviruses, where cells infected or transformed by exogenous RNA tumor viruses have been shown to contain increased amounts of virus-specific DNA. The data reported here are consistent with the contention that preexisting viral genes are activated by bromodeoxyuridine treatment. Results of hybridization experiments done at different DNA/RNA ratios suggest that although the virus-specific DNA is partly unique and partly reiterated, the viral RNA does not contain any detectable internal reiteration. Total mass of the viral RNA sequences is around 0.7 to 1 × 107 daltons.
Keywords: DNA·RNA hybridization, BrdU activation, oncornavirus, reiteration
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. DNA complementary to viral RNA in leukemic cells induced by avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):329–336. doi: 10.1073/pnas.66.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Roy-Burman P. Partial characterization of RD114 virus by DNA-RNA hybridization studies. Nat New Biol. 1973 Jul 11;244(132):59–62. doi: 10.1038/newbio244059a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt W. G., Spiegelman S. Nuclear DNA sequences present in human leukemic cells and absent in normal leukocytes. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3737–3741. doi: 10.1073/pnas.69.12.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Pemberton R., Baglioni C. Reiteration frequency of haemoglobin genes in the duck. Nat New Biol. 1972 Feb 23;235(60):231–234. doi: 10.1038/newbio235231a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Q Rev Biol. 1971 Jun;46(2):111–138. doi: 10.1086/406830. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Corneo G., Ginelli E., Polli E. Different satellite deoxyribonucleic acids of guinea pig and ox. Biochemistry. 1970 Mar 31;9(7):1565–1571. doi: 10.1021/bi00809a014. [DOI] [PubMed] [Google Scholar]
- Feldman D. G., Gross L. Electron microscopic study of the guinea pig leukemia virus. Cancer Res. 1970 Nov;30(11):2702–2711. [PubMed] [Google Scholar]
- Gelb L. D., Milstien J. B., Martin M. A., Aaronson S. A. Characterization of murine leukaemia virus-specific DNA present in normal mouse cells. Nat New Biol. 1973 Jul 18;244(133):76–79. doi: 10.1038/newbio244076a0. [DOI] [PubMed] [Google Scholar]
- Gross P. A., Fong C. K., Hsiung C. D. Characterization of guinea pig C-type virus. Proc Soc Exp Biol Med. 1973 Jun;143(2):367–370. doi: 10.3181/00379727-143-37322. [DOI] [PubMed] [Google Scholar]
- Harel L., Harel J., Lacour F., Huppert J. Homologie entre génome du virus de la myéloblastose aviare (AMV) et génome cellulaire. C R Acad Sci Hebd Seances Acad Sci D. 1966 Aug 8;263(6):616–619. [PubMed] [Google Scholar]
- Hsiung G. D. Activation of guinea pig C-type virus in cultured spleen cells by 5-bromo-2'-deoxyuridine. J Natl Cancer Inst. 1972 Aug;49(2):567–570. [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Martelo O. J., Woo S. L., Reimann E. M., Davie E. W. Effect of protein kinase on ribonucleic acid polymerase. Biochemistry. 1970 Nov 24;9(24):4807–4813. doi: 10.1021/bi00826a027. [DOI] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- Nayak D. P. Activation of guinea pig herpesvirus antigen in leukemic lymphoblasts of guinea pig. J Virol. 1972 Nov;10(5):933–936. doi: 10.1128/jvi.10.5.933-936.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P. Isolation and characterization of a herpesvirus from leukemic guinea pigs. J Virol. 1971 Oct;8(4):579–588. doi: 10.1128/jvi.8.4.579-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Murray P. R. Induction of type C viruses in cultured guinea pig cells. J Virol. 1973 Jul;12(1):177–187. doi: 10.1128/jvi.12.1.177-187.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Rous sarcoma virus nucleotide sequences in cellular DNA: measurement by RNA-DNA hybridization. Science. 1972 Nov 17;178(4062):750–753. doi: 10.1126/science.178.4062.750. [DOI] [PubMed] [Google Scholar]
- Opler S. R. New oncogenic virus producing acute lymphatic leukemia in guinea pigs. Bibl Haematol. 1968;30:81–88. doi: 10.1159/000391228. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Baluda M. A. The nucleic acid from avian myeloblastosis virus compared with the RNA from the Bryan strain of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1686–1692. doi: 10.1073/pnas.54.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P. N., Robinson H. L., Robinson W. S., Hanafusa T., Hanafusa H. DNA in uninfected and virus-infected cells complementary to avian tumor virus RNA. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2336–2340. doi: 10.1073/pnas.68.10.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Friis R. R., Katz E., Vogt P. K. Induction of avian tumor viruses in normal cells by physical and chemical carcinogens. Virology. 1971 Dec;46(3):920–938. doi: 10.1016/0042-6822(71)90091-2. [DOI] [PubMed] [Google Scholar]
- Yoshikawa-Fukada M., Ebert J. D. Hybridization of RNA from Rous sarcoma virus with cellular and viral DNA's. Proc Natl Acad Sci U S A. 1969 Nov;64(3):870–877. doi: 10.1073/pnas.64.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]


