Abstract
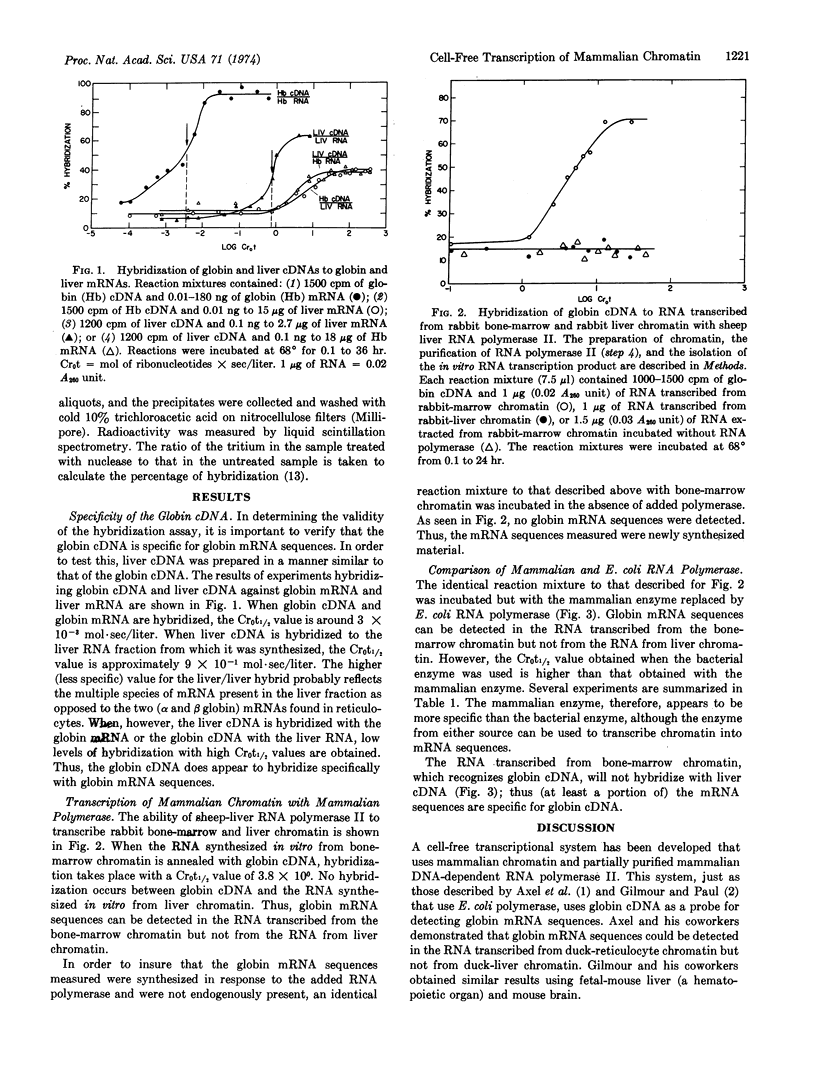
A mammalian cell-free transcriptional system was developed in which mammalian RNA polymerase synthesizes globin messenger RNA sequences from bone-marrow chromatin. The messenger RNA sequences are detected by measurement of the ability of the transcribed RNA to hybridize with globin complementary DNA. The globin complementary DNA is synthesized by the enzyme from avian myeloblastosis virus, RNA-directed DNA polymerase, with purified globin messenger RNA as template. The specificity of the globin complementary DNA in annealing reactions was verified by preparing DNA complementary to liver messenger RNA and showing that the globin and liver complementary DNAs are specific for their own messenger RNAs. Both DNA-dependent RNA polymerase II from sheep liver and RNA polymerase from Escherichia coli can transcribe globin messenger RNA sequences from rabbit bone-marrow chromatin; however, the mammalian enzyme appears to be more specific in that globin gene sequences represent a higher proportion of the RNA synthesized. Neither polymerase can transcribe globin messenger RNA sequences from rabbit-liver chromatin. This cell-free assay system should be useful in searching for mammalian transcriptional regulatory factors.
Keywords: complementary DNA, DNA·RNA hybridization, RNA synthesis
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank A., Terada M., Metafora S., Dow L., Marks P. A. In vitro synthesis of DNA components of human genes for globins. Nat New Biol. 1972 Feb 9;235(58):167–169. doi: 10.1038/newbio235167a0. [DOI] [PubMed] [Google Scholar]
- Benz E. J., Jr, Forget B. G. Defect in messenger RNA for human hemoglobin synthesis in beta thalassemia. J Clin Invest. 1971 Dec;50(12):2755–2760. doi: 10.1172/JCI106778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Nienhuis A. W., Elson N. A., Anderson W. F. Initiation of globin synthesis. Preparation and use of reticulocyte ribosomes retaining initiation region messenger ribonucleic acid fragments. J Biol Chem. 1972 Sep 10;247(17):5357–5368. [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Gilbert J. M., Anderson W. F. Cell-free hemoglobin synthesis. II. Characteristics of the transfer ribonucleic acid-dependent assay system. J Biol Chem. 1970 May 10;245(9):2342–2349. [PubMed] [Google Scholar]
- Gilbert J. M., Thornton A. G., Nienhuis A. W., Anderson W. F. Cell-free hemoglobin synthesis in beta-thalassemia. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1854–1861. doi: 10.1073/pnas.67.4.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman D., Forget B. G., Skoultchi A., Benz E. J., Jr Quantitative deficiency of chain-specific globin messenger ribonucleic acids in the thalassemia syndromes. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1809–1813. doi: 10.1073/pnas.70.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Gambino R., Dow L. W., Grossbard E., Natta C., Ramirez F., Spiegelman S., Marks P. A., Bank A. Decreased globin messenger RNA in thalassemia detected by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1886–1890. doi: 10.1073/pnas.70.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Kedinger C., Gissinger F., Gniazdowski M., Mandel J. L., Chambon P. Animal DNA-dependent RNA polymerases. 1. Large-scale solubilization and separation of A and B calf-thymus RNA-polymerase activities. Eur J Biochem. 1972 Jul 13;28(2):269–276. doi: 10.1111/j.1432-1033.1972.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Losick R. In vitro transcription. Annu Rev Biochem. 1972;41:409–446. doi: 10.1146/annurev.bi.41.070172.002205. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Anderson W. F. Isolation and translation of hemoglobin messenger RNA from thalassemia, sickle cell anemia, and normal human reticulocytes. J Clin Invest. 1971 Nov;50(11):2458–2460. doi: 10.1172/JCI106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Laycock D. G., Anderson W. F. Translation of rabbit haemoglobin messenger RNA by thalassaemic and non-thalassaemic ribosomes. Nat New Biol. 1971 Jun 16;231(24):205–208. doi: 10.1038/newbio231205a0. [DOI] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Isolation and partial characterization of reticulocyte factors M1 and M2. J Biol Chem. 1970 Nov 10;245(21):5553–5559. [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. I. RNA synthesis in vitro. Biochim Biophys Acta. 1971 Jan 1;228(1):202–211. doi: 10.1016/0005-2787(71)90560-0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]


