Abstract
Two conserved epitopes, located in the membrane-proximal external region (MPER) of the human immunodeficiency virus type 1 (HIV-1) gp41, are recognized by two HIV-1 broadly neutralizing antibodies 2F5 and 4E10, and are promising targets for vaccine design in efforts to elicit anti-HIV-1 broadly neutralizing antibodies. Since most HIV-1 infections initiate at mucosal surfaces, induction of mucosal neutralizing antibodies is necessary and of utmost importance to counteract HIV-1 infection. Here, we utilized a mucosal vaccine vector, bovine papillomavirus (BPV) virus-like particles (VLPs), as a platform to present HIV-1 neutralizing epitopes by inserting the extended 2F5 or 4E10 epitope or the MPER domain into D-E loop of BPV L1 respectively. The chimeric VLPs presenting MPER domain resembled the HIV-1 natural epitopes better than the chimeric VLPs presenting single epitopes. Oral immunization of mice with the chimeric VLPs displaying the 2F5 epitope or MPER domain elicited epitope-specific serum IgGs and mucosal secretory IgAs. The induced antibodies specifically recognized the native conformation of MPER in the context of HIV-1 envelope protein. The antibodies induced by chimeric VLPs presenting MPER domain are able to partially neutralize HIV-1 viruses from clade B and clade C.
Keywords: HIV-1, membrane-proximal external region, neutralizing antibody, mucosal, bovine papillomavirus-like particle, vaccine
1. Introduction
Although anti-HIV-1 (human immunodeficiency virus type 1) drugs are effective in controlling development of AIDS, HIV-1 infection still remains an enormous problem worldwide. An effective HIV-1 vaccine is urgently needed but its development is hindered by the huge variability of the virus. A recent HIV-1 vaccine phase III clinical trial, the RV144 trial conducted in Thailand, for the first time showed modest efficacy against acquisition of infection, which raises hope for developing an effective HIV-1 vaccine [1, 2].
A number of groups have isolated several monoclonal antibodies (mAbs) that can neutralize a variety of HIV-1 isolates [3-12]. Among those mAbs, 2F5, 4E10, Z13 and the recently reported 10E8 are shown to target conserved epitopes within the membrane-proximal external region (MPER) of gp41 [12-21]; The isolation of these and other broadly neutralizing antibodies (b12, 2G12, VRC01, CH01 to CH04, PG9, PG16) is a milestone in the field. The epitopes located at the MPER of gp41 recognized by broadly neutralizing mAbs 2F5 and 4E10 are promising targets for vaccine design. Unfortunately, many attempts at eliciting broadly neutralizing antibodies by targeting neutralizing epitopes of MPER failed [22-27]. Two recent studies reported that presenting the ELDKWA epitope by human rhinovirus [28] or a whole MPER domain with mutations (T569A and I675V) by NCM antigen [29] successfully induced anti-HIV-1 cross-clade neutralizing antibodies.
HIV-1 is transmitted both venereally and hematogeneously and vaginal and rectal mucosa serve as major sites for viral entry and initial infection [30, 31]. Thus, an effective HIV-1 vaccine must elicit both mucosal immunity to prevent entry of HIV-1 into the mucosa, and systemic immunity to restrict viral transmission via blood stream [32]. Bovine papillomavirus 1 (BPV) virus-like particle (VLP) is an ideal mucosal delivery vector that not only carries peptide/protein/DNA to protect them from degradation by the gastric and intestinal environment but also serves as a strong immune activating adjuvant eliciting both mucosal and systemic immune responses via the oral immunization route [27, 33-37]. Additionally, since BPV is not a natural pathogen for humans, the likelihood of preexisting “vector neutralization” that would presumably attenuate vaccine efficacy is minimized.
In this study, we used BPV VLPs to present HIV-1 gp41 MPER or extended 2F5 epitope and determined if the epitopes are presented in their proper conformation. Induction of systemic and mucosal antibody responses was measured after oral immunization of the chimerical VLPs in mice. We used HIV-1 infected cells and an in vitro neutralization test to assess the binding and neutralizing activities of the induced HIV-1-specific antibodies.
2. Materials and methods
2.1. Cell lines
Sf9 (Spodoptera frugiperda) cells were obtained from the American Type Culture Collection and grown at 28°C in HyGro Sf-PFM insect medium (JR Scientific, Woodland, CA). Human T-lymphoblastic cell lines H9 and CEM-NKR-CCR5 were obtained from the AIDS Research and Reference Reagent Program, NIH [38, 39]. T cells were cultured in L-glutamine containing DMEM medium supplemented with 10% fetal bovine serum, 100 U of penicillin and 100 μg of streptomycin per ml (HyClone, Logan, UT).
2.2. Construction and generation of CVLPs
The C-terminal truncated BPV1 L1 gene [40] was used as a scaffold to insert the extended 2F5, 4E10 epitope and membrane proximal external region of HIV-1 gp41 (Fig. 1A). To generate chimeric genes, the 2F5 (ELLELDKWASLWN), 4E10 epitope (NWFDITNWLWYIK) or MPER domain (ELLELDKWASLWNWFDITNWLWYIK) were inserted into D-E loop to replace sequence encoding L1 amino acids 130 -136 by overlap PCRs (Fig. 1A). The recombinant baculoviruses containing the chimeric genes coding for L1- 2F5 (2-1), L1-4E10 (4-1) and L1-MPER were generated using Bac-to-Bac baculovirus expression system according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Chimeric proteins were expressed in sf9 cells in form of chimeric virus-like particles (CVLPs) and purified as described previously [34].
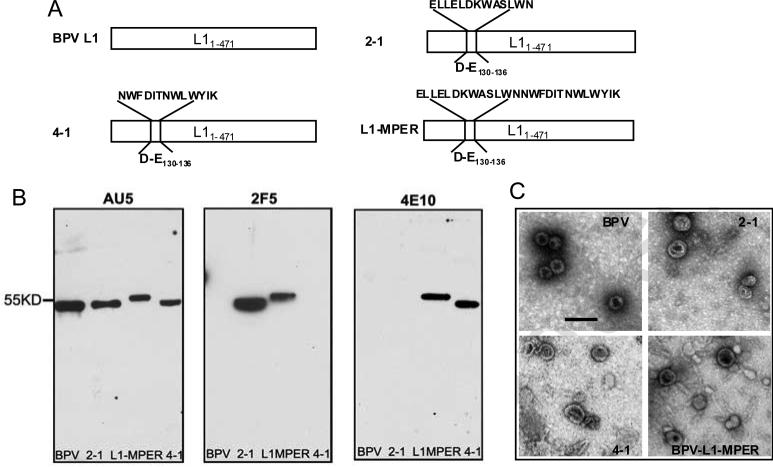
Fig. 1.
Construction and analysis of BPV-gp41 CVLPs. (A) BPV1 L1 was truncated at the C-terminal, and the D-E130-136 loop of L1 was replaced with HIV-1 clade B gp41 conserved epitopes (2-1: ELLELDKWASLWN, 4-1: NWFDITNWLWYIK) or MPER domain (L1-MPER: ELLELDKWASLWNWFDITNWLWYIK) respectively using overlap PCR. (B) The purified BPV and BPV-gp41 particles (2-1, 4-1, L1-MPER) were analyzed by western-blotting, using anti-BPV L1 mAb AU5 (left), anti-HIV-1 mAb 2F5 (middle) and mAb 4E10 (right). (C) Electron micrographs of BPV VLPs and CVLPs. The chimeric proteins-generated CVLPs had sizes similar to those of wild type BPV-L1 VLPs. Bar=100 nm, magnification 40,000×.
2.3. Western blotting and electron microscopy
The extracted fractions from infected sf9 cells were separated by SDS-10% PAGE and transferred to nitrocellulose membrane. Western blots were performed using monoclonal antibody AU5 against BPV1 L1 (Covance, Denver, PA) or mAb 2F5 and 4E10 (NIH AIDS Reagent Program) [41-43], and HRP-conjugated anti-mouse or anti-human secondary antibody. The size and shape of CVLPs and VLPs were examined by Zeiss EM900 electron microscopy as described previously [34].
2.4. Mice and immunizations
Six to eight weeks old female Balb/C mice were purchased from The Jackson Laboratory. The study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committees of Loyola University Chicago (permit# 2008088). Mice were immunized by oral gavage with 10μg dialyzed CVLPs or VLPs in 200 μl PBS without adjuvant and boosted twice at two-week intervals.
2.5. Sample collection
Two weeks after the final boost, blood samples were collected from the heart of mice and sera were recovered by centrifugation and stored at −20°C. Fecal samples collected from individual mice were extracted by making a 1: 6 suspension (wt/vol) in 0.05 mg/ml soybean trypsin inhibitor (Sigma-Aldrich, Carlsbad, CA) containing PBST. The suspension was vortexed and centrifuged for 10min at 12,000g. The supernatant was collected and stored at −20°C. Vaginal wash samples were collect from individual mouse and stored at −20°C until test.
2.6. Enzyme-linked immunosorbent assays
Maxisorp 96-well plates (Corning Inc., Corning, NY) were coated with CVLPs or peptide 2F5, Z13, 4E10, MPER or OVA323-339 in coating buffer (pH9.6) overnight at 4°C. Plates were blocked with blocking buffer (5% nonfat dry milk in PBST) at 37°C for 1 hour. After one wash with PBST, mAb 2F5 or 4E10 with or without serial diluted 2F5 peptide, 4E10 peptide or OVA323-339 peptide (BioSynthesis, Lewisville, TX) was added to CVLPs coated plates (for HIV-1 neutralizing antibody binding ELISAs and competition ELISAs), or serial dilutions of serum or fecal extracts were added into peptide coated wells (for peptide ELISAs) and incubated at 37°C for 1 hour. After 3 washes, HRP-conjugated anti-human IgG ɣ-chain-specific, anti-mouse IgG ɣ-chain-specific or IgA α-chain-specific antibodies (Sigma-Aldrich) were added at a dilution of 1:8,000 in blocking buffer and incubated for 1 hour at 37°C. Following 4 additional washes, the plates were developed with 3,3’5,5’-tetramethylbenzidine as substrate. The reaction was stopped by adding 1M H2SO4 and the optical density (OD) of each well was measured at a wavelength of 450 nm. In competition ELISA, the calculation of the percent of binding in each dilution was: (OD-peptide dilution – OD-negative)/(OD-nonpeptide – OD negative) × 100. (OD: mean of triplicate wells in each dilution). Cut-off value = 3 X OD450 of PBS control well.
2.7. Flow cytometry
One million HIV-1MN-infected H9 cells were washed twice with PBS and resuspended in 0.2 ml FACS buffer. Cells were incubated with 0.25 μg of mAb 2F5 or 4E10, or 10 μl of serum or 20 μl of fecal extracts at 4°C for 30 min. In other tubes, cells were incubated with serum or fecal extracts plus 2 μg of 2F5 peptide or MPER peptide (in samples from 2-1 or L1-MPER immunized mice respectively) at 4°C for 30 min. This was followed by 2 washes, and 0.5 μg of FITC-conjugated goat anti-human IgG or goat anti-mouse IgA or IgG (Sigma-Aldrich) was incubated with cells for 30 min at 4°C. After 2 additional washes, the stained cells were fixed with 4% paraformaldehyde, and then analyzed on a FACS Canto flow cytometer (BD Biosciences, CA). FACS data were analyzed using FlowJo software (Treestar).
2.8. Virus neutralization assay
HIV-1 viruses (obtained from the AIDS Research and Reference Reagent Program[44]) were used for testing the neutralizing activity. 200 pg p24 of HIV-1 viruses (clade A: HIV-1UG273, clade B: HIV-1MN, HIV-1Bal and HIV-1US4, clade C: HIV-1SE364, clade D: HIV-1UG274) were mixed with diluted samples (sera or fecal extracts) and incubated at 37°C for 4 hours. Then, 105 CEM-NKR-CCR5 cells were added to each mixture and incubated overnight/24 hours at 37°C with 5%CO2. The cells were washed 3 times and then further cultured with fresh medium. The serum samples were diluted 1:20 or 1:40, and fecal extractions were diluted 1:2.5 or 1:5. On day 9, HIV-1 virus production in culture supernatant was assayed by p24 ELISA as described previously [45].
3. Results
3.1. Design and generation of CVLPs
We used BPV1 L1 VLPs to present HIV gp41 neutralizing epitopes to develop an HIV-1 vaccine. We used an extended 13aa 2F5 epitope, 13aa 4E10 epitope or an MPER peptide containing both epitopes in the vaccine constructs. Three chimeric L1 VLPs were made: 2-1 (presenting ELLELDKWASLWN), 4-1 (presenting NWFDITNWLWYIK) and L1-MPER (presenting ELLELDKWASLWNWFDITNWLWYIK) (Fig. 1A). Purified CVLPs were identified by western-blotting using anti-L1 mAb AU5 (Fig. 1B). Western-blot using 2F5 or 4E10 mAb showed positive bands in the CVLPs 2-1 and L1-MPER or 4-1 and L1-MPER respectively (Fig. 1B), which confirmed that the epitope sequences were present in the particles. VLP assembly was confirmed by electron microscopy. Samples from gradient centrifugation showed the presence of virus-like particles that have morphology and sizes similar to those of wild-type BPV1 L1 VLPs (Fig. 1C). The morphology of CVLPs is a little bit more heterogeneous than that of wild type BPV VLPs, but all CVLPs and VLPs showed similar stability after a long-term (3 to 7 months) storage in −20°C (Fig. S1).
3.2. Presentation of the epitopes
To determine whether the HIV-1 epitopes presented on the surface of CVLPs could be recognized by the broadly neutralizing antibodies in vitro, we initially performed binding ELISA to characterize the specificity of the chimeric particles. As shown in Figure 2A, 2F5 and 4E10 mAbs recognized 2-1 and 4-1 respectively, and both mAbs were able to bind L1-MPER, but not BPV VLPs. Moreover, at lower concentrations of CVLPs (from 0.156 to 10μg/ml), mAb (2F5 or 4E10) binding to L1-MPER showed much higher OD450 than their binding to 2-1 or 4-1 (Fig. 2A). The mAb 4E10 binding to 4-1 or L1-MPER showed a lower OD than the binding of mAb 2F5 to 2-1 or L1-MPER (OD450 at 20 μg/ml: 0.73, 0.83 vs. 3.12, 2.94 respectively), which is likely due to the lack of a lipid bilayer in the binding assay since previous studies have suggested that efficient 4E10 binding requires lipid interaction [18, 25].
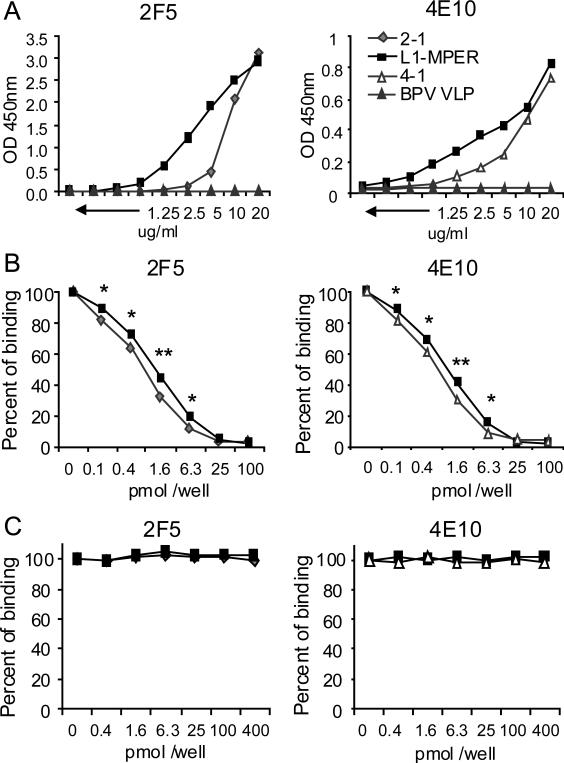
Fig. 2.
Binding of mAb 2F5 or 4E10 to BPV-gp41 CVLPs. (A) The direct binding of 2F5/4E10 mAb to CVLPs. Serially diluted CVLPs were coated onto microtiter plate wells and indirect ELISAs were performed to detect 2F5/4E10 antibody binding to the presented 2F5/4E10 epitope by CVLPs. (B) Competitive binding of 2F5/4E10 mAb to BPV-gp41 CVLPs in the presence of peptide ELLELDKWASLWN (left panel) or NWFDITNWLWYIK (right panel) respectively. 5μg/ml of CVLPs were coated onto plate well. After blocking, 1pmol of 2F5 or 4E10 mAbs were mixed with serial diluted (0-100 pmol/well) epitope peptides and then added in plate wells. MAb-CVLP binding was detected by incubating with anti-human-IgG-HRP antibody and then followed with 3,3’5,5’-tetramethylbenzidine substrate for ELISA development. The percent of MAb-CVLP binding was shown. (C) Competitive binding of 2F5/4E10 mAbs to BPV-gp41 CVLPs in the presence of control peptide OVA323-339 ISQAVHAAHAEINEAGR at 0.4-400 pmol/well. The percent of MAb-CVLP binding was shown. The percent of binding = (OD-peptide dilution – OD-negative)/ (OD-no peptide – OD negative) × 100. Data are representative of three independent experiments. OD: optical density. * p < 0.05 ,* * p < 0.01.
To confirm that the strong binding between the CVLPs and mAbs was specific to the CVLP-presented epitopes, we performed competition ELISA by mixing 2F5 or 4E10 mAb with their epitope peptide respectively. As shown in Figure 2B-C, the CVLP-antibody binding was reduced with increasing concentrations of epitope peptide but not control peptide. In the presence of 0.1-6.3 pmol/well of epitope peptides, MPER-presenting CVLPs bound better to the antibodies than single epitope presenting CVLPs 2-1 or 4-1 (Fig. 2B). Together, these results confirmed that the CVLPs-antibody binding was epitope-specific, and indicated that 2F5 and 4E10 epitopes presented by MPER domain are better recognized by the broadly neutralizing antibodies.
3.3. Immunogenicity
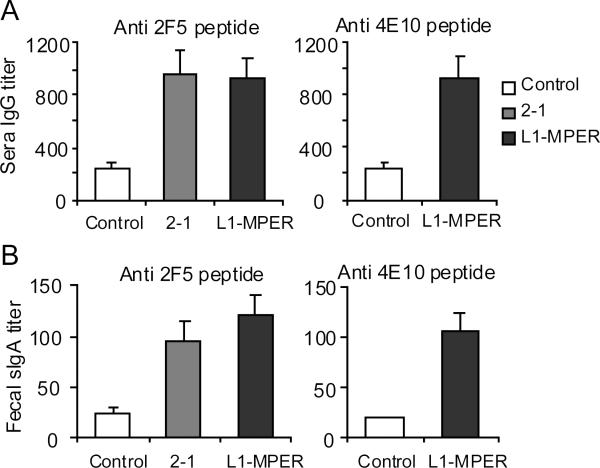
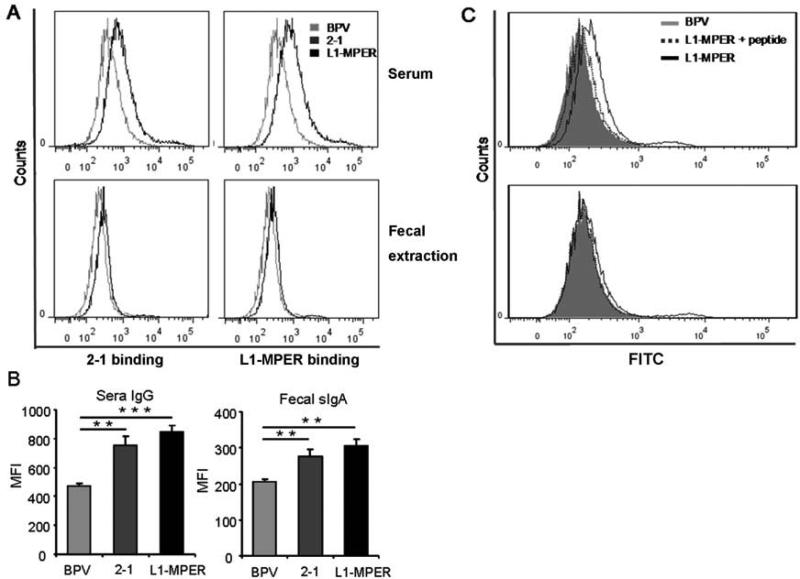
Inducing intestinal mucosal neutralizing antibody is pivotal for preventing HIV-1 infection through a rectal mucosa route. As BPV VLP is able to deliver antigens to intestinal mucosal lymphoid tissues through the oral route, we tested whether oral immunization of mice with 2-1 or L1-MPER CVLPs induces mucosal and systemic specific antibodies. Balb/C mice were orally administered with a 10 μg/dose of CVLPs or BPV VLPs for three times and antibodies against the 2F5 and 4E10 epitopes in sera and fecal extracts were measured by ELISAs. In mice immunized with 2-1 or L1-MPER, 2F5 peptide-specific IgG from sera and secretory IgA (sIgA) from fecal extracts were detected (Fig. 3). To test whether vaginal mucosal specific sIgA was induced, 2F5-specific peptide ELISA was performed using 1:4 diluted vaginal washing samples. Only one from 2-1-immunized mice and two from L1-MPER-immunized mice showed specific reaction (data not shown). All mice generated anti-vector (BPV VLP) vaginal IgGs with titers of 50-100 (data not shown). The results suggest that the oral immunization induced weak vaginal HIV-specific sIgA responses in mice.
Fig. 3.
Epitope-specific IgG and sIgA antibody responses in mice after oral immunization with BPV-gp41 CVLPs. Mice (3-5 per group) were orally immunized 3 times at two-week intervals with 10ug of 2-1 or L1-MPER CVLPs. PBS or BPV VLPs orally immunized mice were control groups. Two weeks after the final boost, sera and fecal extracts were collected. Peptide ELISAs were performed to analyze 2F5 and 4E10 peptide reactivity of sera and fecal extracts. (A) 2F5 or 4E10 epitope-specific IgG titer in serum. (B) 2F5 or 4E10 epitope-specific secretory IgA (sIgA) titer in fecal extracts. Data are shown as geometrical mean ± s.e.m and are representative of two independent experiments.
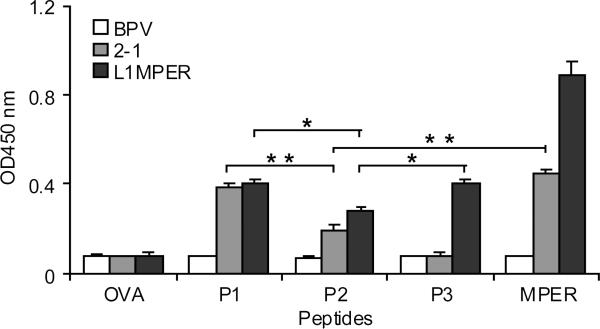
MPER domain also contains an additional neutralizing epitope (between 2F5 and 4E10 epitopes) recognized by the Z13 mAb which is capable of neutralizing some HIV-1 viruses from diverse subtypes [15]. To analyze the epitope recognition profiles of the induced antibodies, we used 4 peptides: 2F5, Z13, 4E10 and MPER to determine the binding specificity of the serum IgG antibodies. As shown in Fig. 4, 2-1 induced antibodies bound to 2F5, MPER and partially bound to Z13, whereas L1-MPER induced antibodies bound to 2F5, Z13, 4E10 and MPER. The binding capacity of L1-MPER-induced antibodies to Z13 epitope was not as high as that to 2F5 or 4E10 epitope, while they strongly reacted with MPER peptide (Fig. 4). These data suggest that CVLPs presenting MPER domain were able to induce multiple HIV-1 epitopes specific antibodies and the dominant antibodies are 2F5 and 4E10 epitopes-specific.
Fig. 4.
Binding specificity of IgG antibodies induced by BPV-gp41 CVLPs. P1: ELLELDKWASLWN; P2: LDKWASLWNWFDIT; P3: NWFDITNWLWYIK; MPER: ELLELDKWASLWNWFDITNWLWYIK; OVA323-339 ISQAVHAAHAEINEAGR is a control peptide. 5 ug/ml of peptide were coated on plate wells and a 1:100 dilution of serum was used to analyze the antibody-peptide binding in ELISA. Data are shown as mean ± S.D. and are representative of three independent experiments. OD: optical density. * p < 0.05 ,* * p < 0.01.
3.4. The antibodies recognize HIV-1-infected cells
To further characterize 2F5 and 4E10 epitopes-specific IgG and sIgA antibodies, we tested whether they were able to recognize MPER epitopes presented by HIV-1 virus. Flow cytometric analysis was performed using HIV-1MN-infected H9 cells for binding of antibody to gp41 presented on cell surface. The results showed that both serum IgG and mucosal sIgA from CVLPs-immunized mice bound to the surface of HIV-1MN-infected H9 cells (Fig. 5A, B). As a comparison, mAbs 2F5 and 4E10 are able to bind strongly to the HIV-1MN-infected H9 cells (Fig. S2). To confirm that the antibody-HIV-1 binding is specific for the epitopes, we analyzed the antibody-HIV-1MN-infected H9 cell binding in the presence of competitive peptide. The presence of competitive peptide greatly abrogated the binding of the IgG or sIgA antibodies to HIV-1MN-infected H9 cells (Fig. 5C and data not shown). These results indicate that BPV-gp41 CVLPs-induced antibodies could specifically recognize HIV-1 MPER epitopes presented in their native conformation.
Fig. 5.
Flow cytometry analysis of HIV-1-infected H9 cells incubated with the CVLP-induced antibodies. (A) 1 million of HIV-1MN-infected H9 cells were incubated with sera (top panel) or fecal extracts (bottom panel) from mice orally immunized with 2-1, L1-MPER CVLPs or BPV VLPs. FITC-conjugated goat anti-mouse IgA or IgG was used as secondary antibody, and the stained cells were fixed and analyzed by FACS. (B) Calculation of geometric mean florescent intensities from each group (n=3-5). (C) HIV-1MN-infected H9 cells were incubated with sera and MPER peptide (top panel) or fecal extracts and MPER peptide (bottom panel) from L1-MPER CVLPs or BPV VLPs immunized mice. Then the following procedures of FACS were performed as described before. Data are representative of two independent experiments. MFI: mean fluorescence intensity, * * p < 0.01, * * * p < 0.001.
3.5. Neutralizing activities
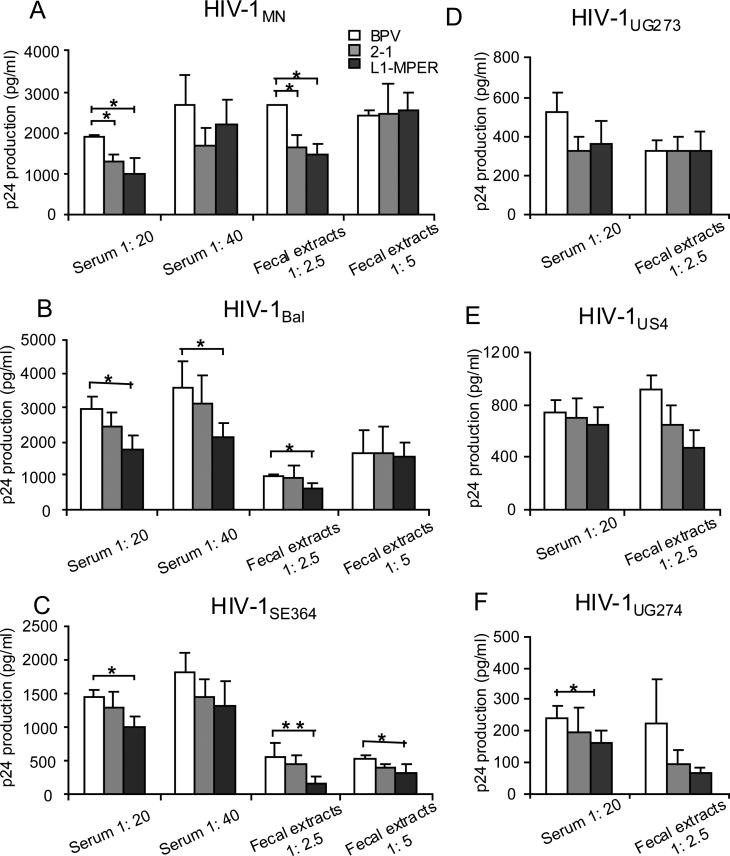
To determine the neutralizing activity of the epitope-specific antibodies, the sera and fecal extracts from CVLPs or control VLP-immunized mice were diluted and tested in a neutralizing assay against the clade A virus HIV-1UG273, clade B viruses HIV-1MN, HIV-1Bal and HIV-1US4, clade C virus HIV-1SE364 and clade D virus HIV-1UG274. Preincubation of HIV-1MN with 1:20 diluted sera and 1:2.5 diluted fecal extracts from either 2-1 or L1-MPER-immunized mice resulted in less than 50%, but significant reduction of HIV-1 p24 production (Fig. 6A). Notably, the same dilutions of sera and fecal extracts from L1-MPER-immunized mice also showed significant neutralization against HIV-1Bal and HIV-1SE364 (Fig. 6B, C). In addition, the 1:40 diluted sera also significantly reduced HIV-1Bal p24 production (Fig 6B) and 1:5 diluted fecal extracts reduced HIV-1SE364 p24 (Fig. 6C). In HIV-1 UG273, HIV-1US4 and HIV-1UG274, only the sera from L1-MPER-immunized mice significantly reduced the production of p24 in clade D virus HIV-1UG274 at 1:20 dilution (Fig. 6D-F). These results indicate that sera IgG and mucosal sIgA induced by 2-1 are able to partially neutralize clade B HIV-1MN, while the sera IgG and mucosal sIgA induced by L1-MPER, are able to partially neutralize HIV-1 from both clade B and clade C.
Fig. 6.
HIV neutralization assay for sera or fecal extracts from mice orally immunized with BPV-gp41 CVLPs. (A, B and C) 200pg of HIV-1 viruses (clade B: HIV-1 MN, Bal, clade C: HIV-1 SE364) were preincubated with 1: 20, 1: 40 diluted sera or 1: 2.5, 1: 5 diluted fecal extracts from mice orally immunized with CVLPs (2-1, L1-MPER) or BPV VLPs. (D, E and F) 200pg of HIV-1 viruses (clade A: HIV-1 UG273, clade B: HIV-1 US4, clade D: HIV-1 UG274) were preincubated with 1: 20 diluted sera or 1: 2.5 diluted fecal extracts from mice orally immunized with CVLPs (2-1, L1-MPER) or BPV VLPs. The viruses was added to the CEM-NKR-CCR5 cells and virus replication was assessed by ELISA on day 9 by detecting p24 antigen in culture supernatants. Values are shown as mean ± S.D. (3-5 mice/group). * p < 0.05, ** p < 0.01. Data are representative of two independent experiments.
4. Discussion
In this study, we developed chimeric BPV1-gp41 to elicit mucosal and systemic anti-HIV-1 broadly neutralizing antibodies. We inserted the extended 2F5 and 4E10 epitopes as well as MPER domain containing three neutralizing epitopes, into the D-E loop of BPV L1 to broaden the presentation of the HIV-1 2F5/Z13/4E10 epitope on the BPV CVLPs as well as the protection against HIV-1. Our results demonstrated that the MPER-displaying CVLPs induced HIV-specific mucosal and systemic cross-clade neutralizing antibodies, although modest, with improved breadth of HIV-1 neutralization in comparison with the previous ELDKWA-displaying CVLPs [27].
BPV VLP is capable of delivering target antigen via mucosal route to induce both mucosal and systemic immune responses [27, 36]. Notably, BPV VLPs possess strong immunogenicity and it has been shown by Bryce Chackerian et al. that immunization of BPV L1-CCR5 CVLPs, which had a self-antigen peptide from mouse or macaque chemokine receptor CCR5 inserted into D-E loop, even broke self-tolerance and induced autoantibodies against CCR5 [37, 46]. Since 2F5/4E10-recognizing epitopes share similarities with conserved self antigens such as human kynureninase, splicing factor 3b subunit 3 and cardiolipin [47, 48], it is plausible to reason that BPV-VLP would be suitable for presenting such autoantigenic determinants.
Targeting neutralizing epitopes of MPER has been extensively studied [22-29, 49]. However, most of attempts failed to induce 2F5 or 4E10-like broadly neutralizing antibodies. Recent studies revealed that both 2F5 and 4E10 epitopes have specific conformational structure when interacting with their neutralizing mAbs [16-19]. In the current study, we chose the extended 13 aa sequences ELLELDKWASLWN and NWFDITNWLWYIK as HIV-1 2F5 and 4E10 epitopes to develop subunit vaccine. These epitopes may contain entire residues for the epitope-neutralizing antibody (2F5/4E10) interaction, and thus may elicit antibodies mimicking the properties of 2F5/4E10 broadly neutralizing antibodies if the natural conformation of these epitopes could be presented.
We found that all BPV-gp41 CVLPs specifically bound to neutralizing antibodies 2F5 and/or 4E10 in vitro (Fig. 2). Compared with single epitopes, the MPER presenting CVLPs exhibited better antibody-epitope interaction, suggesting enhancement of epitope presentation. Mucosal sIgA is essential for containing HIV-1 infection at the frontline of virus entry sites [50, 51]. Our ultimate goal is to develop HIV-1 mucosal vaccine to stimulate both mucosal and systemic immunity. In line with our previous findings[27], oral immunization of those BPV-gp41 CVLPs induced HIV-specific mucosal and systemic immune responses, supporting the suitability of BVP VLP as an ideal mucosal vector for HIV-1 vaccine. However, the oral immunization does not induce high titer of antibodies (either anti-vector or anti-epitope) at vaginal mucosa. As intranasal and sublingual immunization with HPV VLPs are able to induce antibody responses at vaginal mucosa [33, 52, 53], the combination of different immunization routes may help improve the antibody responses at both intestinal and vaginal mucosal in the HIV-1vaccine development.
The goal of MPER epitope-based HIV-1 vaccine is to induce high titer antibodies with broadly neutralizing activity. We found that the CVLP presenting MPER domain improved the breadth of HIV-1 neutralization, resulting in a partial neutralization of viruses from clade B and clade C (fig. 6). The CVLP-presented MPER domain contains multiple epitopes (2F5, 4E10 and Z13) responsible for inducing neutralizing antibodies against different clades [13-15]. The 2F5 epitope-specific antibodies do not neutralize clade C virus HIV-1SE364 as there are mutations in the 2F5 core epitope [54]. The mAb Z13 has less neutralizing potency than 2F5 or 4E10 mAbs, and also does not neutralize NWFSIT (D to S mutation) containing HIV-1 while mAb 4E10 does [15]. This indicates that Z13-epitope specific antibodies may not contribute to the neutralization of HIV-1SE364 since it contains the NWFSIT mutation. Therefore, the 4E10 epitope-specific antibodies induced by L1-MPER are responsible for the neutralization of clade C HIV-1SE364. Also, the antibodies induced by L1-MPER neutralized two clade B viruses whereas those induced by 2-1 neutralized only HIV-1MN laboratory stain. This data suggests that the better presentation of the epitope by the VLPs may generate antibodies that better bind to HIV-1, thus, enhancing its neutralization. However, larger neutralization panels would be needed to verify this hypothesis. Although we successfully broaden the neutralization spectrum, the antibodies levels and neutralizing capacity induced by the CVLPs are still modest. Further investigations will be done to increase the titer and neutralizing abilities of these antibodies.
Supplementary Material
Chimeric BPV VLP vaccines were constructed to present HIV-1 MPER epitopes.
The induction of systemic and mucosal antibody responses was assessed in mice.
The induced IgG and sIgA recognized the MPER epitopes presented by HIV-1.
IgG and sIgA induced by L1-MPER are able to neutralize clade B and clade C HIV-1.
Acknowledgements
The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 gp41 mAbs 2F5 and 4E10 from Dr. Hermann Katinger; H9 cells, HIV-1MN and HIV-1Bal from Dr. Robert Gallo; HIV-1SE364, UG273, UG274, US4 from Dr. Nelson Michael. This work was supported by a grant from the National Institutes of Health (NIH) (grant # DE019075 to L.Q.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors have declared that no financial conflict of interest exists.
References
- 1.Fuchs JD, Sobieszczyk ME, Hammer SM, Buchbinder SP. Lessons drawn from recent HIV vaccine efficacy trials. J Acquir Immune Defic Syndr. 2010;55(Suppl 2):S128–31. doi: 10.1097/QAI.0b013e3181fbca02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266(5187):1024–7. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 4.Kessler JA, 2nd, McKenna PM, Emini EA, Chan CP, Patel MD, Gupta SK, et al. Recombinant human monoclonal antibody IgG1b12 neutralizes diverse human immunodeficiency virus type 1 primary isolates. AIDS Res Hum Retroviruses. 1997;13(7):575–82. doi: 10.1089/aid.1997.13.575. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, et al. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007;13(9):1032–4. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilgrim AK, Pantaleo G, Cohen OJ, Fink LM, Zhou JY, Zhou JT, et al. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997;176(4):924–32. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–61. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–9. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333(6049):1633–7. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85(19):9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333(6049):1593–602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491(7424):406–12. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conley AJ, Kessler JA, 2nd, Boots LJ, Tung JS, Arnold BA, Keller PM, et al. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc Natl Acad Sci U S A. 1994;91(8):3348–52. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67(11):6642–7. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75(22):10892–905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso RM, Brunel FM, Ferguson S, Zwick M, Burton DR, Dawson PE, et al. Structural basis of enhanced binding of extended and helically constrained peptide epitopes of the broadly neutralizing HIV-1 antibody 4E10. J Mol Biol. 2007;365(5):1533–44. doi: 10.1016/j.jmb.2006.10.088. [DOI] [PubMed] [Google Scholar]
- 17.Julien JP, Bryson S, Nieva JL, Pai EF. Structural details of HIV-1 recognition by the broadly neutralizing monoclonal antibody 2F5: epitope conformation, antigen-recognition loop mobility, and anion-binding site. J Mol Biol. 2008;384(2):377–92. doi: 10.1016/j.jmb.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, et al. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78(19):10724–37. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun ZY, Oh KJ, Kim M, Yu J, Brusic V, Song L, et al. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity. 2008;28(1):52–63. doi: 10.1016/j.immuni.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Brunel FM, Zwick MB, Cardoso RM, Nelson JD, Wilson IA, Burton DR, et al. Structure-function analysis of the epitope for 4E10, a broadly neutralizing human immunodeficiency virus type 1 antibody. J Virol. 2006;80(4):1680–7. doi: 10.1128/JVI.80.4.1680-1687.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, et al. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22(2):163–73. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Coeffier E, Clement JM, Cussac V, Khodaei-Boorane N, Jehanno M, Rojas M, et al. Antigenicity and immunogenicity of the HIV-1 gp41 epitope ELDKWA inserted into permissive sites of the MalE protein. Vaccine. 2000;19(7-8):684–93. doi: 10.1016/s0264-410x(00)00267-x. [DOI] [PubMed] [Google Scholar]
- 23.Eckhart L, Raffelsberger W, Ferko B, Klima A, Purtscher M, Katinger H, et al. Immunogenic presentation of a conserved gp41 epitope of human immunodeficiency virus type 1 on recombinant surface antigen of hepatitis B virus. J Gen Virol. 1996;77(Pt 9):2001–8. doi: 10.1099/0022-1317-77-9-2001. [DOI] [PubMed] [Google Scholar]
- 24.Ho J, MacDonald KS, Barber BH. Construction of recombinant targeting immunogens incorporating an HIV-1 neutralizing epitope into sites of differing conformational constraint. Vaccine. 2002;20(7-8):1169–80. doi: 10.1016/s0264-410x(01)00441-8. [DOI] [PubMed] [Google Scholar]
- 25.Phogat S, Svehla K, Tang M, Spadaccini A, Muller J, Mascola J, et al. Analysis of the human immunodeficiency virus type 1 gp41 membrane proximal external region arrayed on hepatitis B surface antigen particles. Virology. 2008;373(1):72–84. doi: 10.1016/j.virol.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Liu Z, Cheng X, Chen YH. The recombinant immunogen with high-density epitopes of ELDKWA and ELDEWA induced antibodies recognizing both epitopes on HIV-1 gp41. Microbiol Immunol. 2005;49(8):703–9. doi: 10.1111/j.1348-0421.2005.tb03657.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Huang Y, Fayad R, Spear GT, Qiao L. Induction of mucosal and systemic neutralizing antibodies against human immunodeficiency virus type 1 (HIV-1) by oral immunization with bovine Papillomavirus-HIV-1 gp41 chimeric virus-like particles. J Virol. 2004;78(15):8342–8. doi: 10.1128/JVI.78.15.8342-8348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold GF, Velasco PK, Holmes AK, Wrin T, Geisler SC, Phung P, et al. Broad neutralization of human immunodeficiency virus type 1 (HIV-1) elicited from human rhinoviruses that display the HIV-1 gp41 ELDKWA epitope. J Virol. 2009;83(10):5087–100. doi: 10.1128/JVI.00184-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Tong P, Lu L, Zhou L, Xu L, Jiang S, et al. HIV-1 gp41 core with exposed membrane-proximal external region inducing broad HIV-1 neutralizing antibodies. PLoS One. 2011;6(3):e18233. doi: 10.1371/journal.pone.0018233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambrose Z, Larsen K, Thompson J, Stevens Y, Finn E, Hu SL, et al. Evidence for early local viral replication and local production of antiviral immunity upon mucosal simian-human immunodeficiency virus SHIV(89.6) infection in Macaca nemestrina. J Virol. 2001;75(18):8589–96. doi: 10.1128/JVI.75.18.8589-8596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantis NJ, Kozlowski PA, Mielcarz DW, Weissenhorn W, Neutra MR. Immunization of mice with recombinant gp41 in a systemic prime/mucosal boost protocol induces HIV-1-specific serum IgG and secretory IgA antibodies. Vaccine. 2001;19(28-29):3990–4001. doi: 10.1016/s0264-410x(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 32.Letvin NL. Strategies for an HIV vaccine. J Clin Invest. 2002;110(1):15–20. doi: 10.1172/JCI15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu XS, Abdul-Jabbar I, Qi YM, Frazer IH, Zhou J. Mucosal immunisation with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology. 1998;252(1):39–45. doi: 10.1006/viro.1998.9442. [DOI] [PubMed] [Google Scholar]
- 34.Shi W, Liu J, Huang Y, Qiao L. Papillomavirus pseudovirus: a novel vaccine to induce mucosal and systemic cytotoxic T-lymphocyte responses. J Virol. 2001;75(21):10139–48. doi: 10.1128/JVI.75.21.10139-10148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Fayad R, Wang X, Quinn D, Qiao L. Human immunodeficiency virus type 1 gag-specific mucosal immunity after oral immunization with papillomavirus pseudoviruses encoding gag. J Virol. 2004;78(19):10249–57. doi: 10.1128/JVI.78.19.10249-10257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Fayad R, Smock A, Ullrich AM, Qiao L. Induction of mucosal and systemic immune responses against human carcinoembryonic antigen by an oral vaccine. Cancer Res. 2005;65(15):6990–9. doi: 10.1158/0008-5472.CAN-04-3669. [DOI] [PubMed] [Google Scholar]
- 37.Chackerian B, Lowy DR, Schiller JT. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proc Natl Acad Sci U S A. 1999;96(5):2373–8. doi: 10.1073/pnas.96.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann DL, O'Brien SJ, Gilbert DA, Reid Y, Popovic M, Read-Connole E, et al. Origin of the HIV-susceptible human CD4+ cell line H9. AIDS Res Hum Retroviruses. 1989;5(3):253–5. doi: 10.1089/aid.1989.5.253. [DOI] [PubMed] [Google Scholar]
- 39.Popovic M, Read-Connole E, Gallo RC. T4 positive human neoplastic cell lines susceptible to and permissive for HTLV-III. Lancet. 1984;2(8417-8418):1472–3. doi: 10.1016/s0140-6736(84)91666-0. [DOI] [PubMed] [Google Scholar]
- 40.Paintsil J, Muller M, Picken M, Gissmann L, Zhou J. Carboxyl terminus of bovine papillomavirus type-1 L1 protein is not required for capsid formation. Virology. 1996;223(1):238–44. doi: 10.1006/viro.1996.0473. [DOI] [PubMed] [Google Scholar]
- 41.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10(4):359–69. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 42.Purtscher M, Trkola A, Grassauer A, Schulz PM, Klima A, Dopper S, et al. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. Aids. 1996;10(6):587–93. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Purtscher M, Trkola A, Gruber G, Buchacher A, Predl R, Steindl F, et al. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1994;10(12):1651–8. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 44.Brown BK, Darden JM, Tovanabutra S, Oblander T, Frost J, Sanders-Buell E, et al. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J Virol. 2005;79(10):6089–101. doi: 10.1128/JVI.79.10.6089-6101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hart ML, Saifuddin M, Spear GT. Glycosylation inhibitors and neuraminidase enhance human immunodeficiency virus type 1 binding and neutralization by mannose-binding lectin. J Gen Virol. 2003;84(Pt 2):353–60. doi: 10.1099/vir.0.18734-0. [DOI] [PubMed] [Google Scholar]
- 46.Chackerian B, Briglio L, Albert PS, Lowy DR, Schiller JT. Induction of autoantibodies to CCR5 in macaques and subsequent effects upon challenge with an R5-tropic simian/human immunodeficiency virus. J Virol. 2004;78(8):4037–47. doi: 10.1128/JVI.78.8.4037-4047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308(5730):1906–8. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 48.Yang G, Holl TM, Liu Y, Li Y, Lu X, Nicely NI, et al. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med. 2013;210(2):241–56. doi: 10.1084/jem.20121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pastori C, Tudor D, Diomede L, Drillet AS, Jegerlehner A, Rohn TA, et al. Virus like particle based strategy to elicit HIV-protective antibodies to the alpha-helic regions of gp41. Virology. 2012;431(1-2):1–11. doi: 10.1016/j.virol.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Devito C, Broliden K, Kaul R, Svensson L, Johansen K, Kiama P, et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol. 2000;165(9):5170–6. doi: 10.4049/jimmunol.165.9.5170. [DOI] [PubMed] [Google Scholar]
- 51.Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, et al. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;2(5):412–26. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- 52.Revaz V, Zurbriggen R, Moser C, Schiller JT, Ponci F, Bobst M, et al. Humoral and cellular immune responses to airway immunization of mice with human papillomavirus type 16 virus-like particles and mucosal adjuvants. Antiviral Res. 2007;76(1):75–85. doi: 10.1016/j.antiviral.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Cho HJ, Kim JY, Lee Y, Kim JM, Kim YB, Chun T, et al. Enhanced humoral and cellular immune responses after sublingual immunization against human papillomavirus 16 L1 protein with adjuvants. Vaccine. 2010;28(14):2598–606. doi: 10.1016/j.vaccine.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78(23):13232–52. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.