Abstract
Axonal injury and degeneration, whether primary or secondary, contribute to the morbidity and mortality seen in many acquired and inherited central nervous system (CNS) and peripheral nervous system (PNS) disorders, such as traumatic brain injury, spinal cord injury, cerebral ischemia, neurodegenerative diseases, and peripheral neuropathies. The calpain family of proteases has been mechanistically linked to the dysfunction and degeneration of axons. While the direct mechanisms by which transection, mechanical strain, ischemia, or complement activation trigger intra-axonal calpain activity are likely different, the downstream effects of unregulated calpain activity may be similar in seemingly disparate diseases. In this review, a brief examination of axonal structure is followed by a focused overview of the calpain family. Finally, the mechanisms by which calpains may disrupt the axonal cytoskeleton, transport, and specialized domains (axon initial segment, nodes, and terminals) are discussed.
Keywords: axon, calcium, calpain, ischemia, neurofilament, node, protease, synapse, trauma, Wallerian degeneration
I. Introduction
A growing body of work over the past 20 years has causally linked the calpain family of Ca2+ dependent cysteine proteases to axonal dysfunction and degeneration. Axons, which may reach over 1 meter in length in humans, are elegantly designed for anterograde and retrograde transport, conduction of electrical impulses, and release of neurotransmitters at synapses. To assist in these critical functions, axons have specialized domains and a unique subaxolemmal and central cytoskeleton, which are disrupted following injury in various experimental models. Strategies to injure axons, such as transection, stretch, ischemia, and complement activation, are known to activate intra-axonal calpains. In this review, I describe the findings from disparate lines of investigations and present a more unified narrative of the pathologic roles that calpains play within the mammalian axon.
II. Axonal dysfunction and degeneration underlie common neurologic diseases
A. Traumatic brain injury
Worldwide, traumatic brain injury (TBI) leading to hospitalization or death is estimated to afflict over 10 million people annually and is predicted to surpass many diseases as the major cause of death and disability by the year 2020 (Hyder et al., 2007). The high incidence does not include most patients with mild TBI, which comprises the majority of all brain injuries, as these patients typically do not seek medical treatment. There are no currently approved therapies that specifically target the molecular cascades acutely triggered by TBI. A significant contribution to the morbidity and mortality is from traumatic axonal injury (TAI; Gennarelli et al., 1982; Povlishock, 1992; Smith and Meaney, 2000). In blunt TBI, rotational and shearing forces disproportionately affect long white matter tracts. Except in the most severe cases, where axons tear at the moment of trauma (primary axotomy), injured axons initially show no overt disconnection. However, these injured axons may swell, which is likely due to a disruption of axonal transport, and/or subsequently lose continuity and degenerate (secondary axotomy; Jafari et al., 1997; Maxwell and Graham, 1997; Povlishock and Katz, 2005; Saatman et al., 2003). Better understanding of the molecular cascades that lead to axonal injury and secondary axotomy may allow for targeted therapies for TBI.
B. Spinal cord injury
In the United States, there are over 250,000 people living with spinal cord injury (SCI; Ray et al., 2011). Like TBI, the victims of SCI are mostly young adults. The only approved therapy for SCI is the steroid methylprednisolone, but its efficacy is highly controversial (Ray et al., 2011). The neurological deficits are predominantly caused by the disruption of ascending and descending tracts (Medana and Esiri, 2003). Immediately after SCI in humans, a proportion of injured axons are not transected (Kakulas, 1999). These axons are swollen and immunoreactive for β-amyloid precursor protein (β-APP), which indicates a disruption of fast anterograde transport. Interestingly, these findings are also commonly found after TBI and TAI.
C. Traumatic injury to peripheral nerves
Traumatic injury to peripheral nerves results in considerable worldwide disability (Robinson, 2000). Even though traumatic nerve injuries are commonly classified into three types (neuropraxia, axonotmesis, and neurotmesis), injury is probably often mixed; some axons are transected, while others suffer conduction block, which is likely due to focal ischemia and/or demyelination (Robinson, 2000). Similar to axons in the brain and spinal cord, a subset of peripheral nervous system (PNS) axons may not be physically disrupted when subjected to mechanical forces. While some axons may undergo repair, there may be molecular triggers or cascades that push some to subsequently degenerate. Some of these molecules may be intrinsic to axons and be shared by the PNS and central nervous system (CNS).
D. Cerebral ischemia
The worldwide incidence and burden of strokes and cardiac arrests, which cause global cerebral ischemia, are high (Berdowski et al., 2010; Bramlett and Dietrich, 2004; Gustavsson et al., 2011; Kim and Johnston, 2011). About 50% of the volume of the human brain is white matter, which is almost always injured to some degree in cardiac arrest and stroke (Goldberg and Ransom, 2003). Axons are sensitive to ischemia and hypoxia (Pantoni et al., 1996; Underhill and Goldberg, 2007) even when the ischemic injury does not involve their parental cell bodies (Medana and Esiri, 2003). Ischemia triggers multiple deleterious pathways in axons, some of which are also prominent after mechanical injury to axons. Therefore, therapeutic interventions to mitigate disruption of central conducting pathways may positively impact morbidity and mortality after both ischemia and trauma.
E. Other diseases
Axonal dysfunction and degeneration are likely important contributors to the morbidity and mortality caused by many other human diseases such as multiple sclerosis, amyotrophic lateral sclerosis, infections (e.g., human immunodeficiency syndrome and cerebral malaria), and peripheral neuropathies (Coleman, 2005; Coleman and Perry, 2002; Glass, 2004; Medana and Esiri, 2003; Shy et al., 2002). A good example is multiple sclerosis, which was initially characterized by multifocal demyelination with relative preservation of axons (Medana and Esiri, 2003). Later studies demonstrated that axon loss occurs earlier than would be expected for a primarily demyelinating disease, and that axon loss, but not demyelination, strongly correlates with disability (Das et al., 2008; Medana and Esiri, 2003). If axon loss is independent of demyelination, strategies to delay demyelination or encourage remyelination may have limited efficacy (Medana and Esiri, 2003). Thus, understanding axon physiology and pathophysiology is critically important to the development of therapeutic strategies for a broad spectrum of diseases and disorders.
III. Composition of specialized membrane domains and axonal cytoskeleton
A. Overview of specialized domains and subaxolemmal cytoskeleton
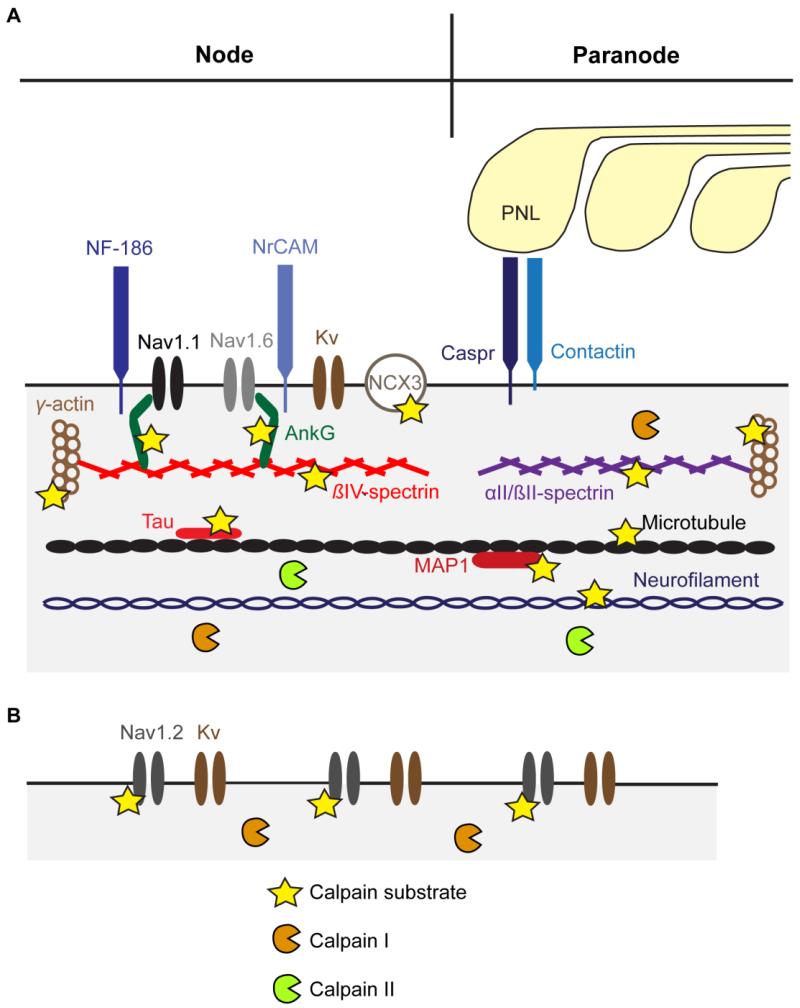
Broadly, there are two types of axons—myelinated and unmyelinated (Fig. 1). In myelinated axons, the clustering of ion channels and various proteins at the axon initial segment (AIS), nodes, and terminals are critical for action potential initiation, saltatory conduction, and neurotransmission. Both the mature AIS and node have high densities of voltage-gated Na+ channels (Nav). Nav1.1, Nav1.2 and Nav1.6 are found in the mature AIS, while Nav1.1 and Nav1.6 are found in the mature node (Debanne et al., 2011; Duflocq et al., 2008; Rasband, 2011). Developing nodes, however, also contain Nav1.2 (Rasband, 2011). Voltage-gated K+ channels (Kv) and/or cell adhesion molecules (CAMs) are also enriched in the AIS, nodes, and paranodes. The two main CAMs at the AIS and nodes are neurofascin-186 and NrCAM (neuronal cell adhesion molecule), while contactin and Caspr (contactin-associated protein) are present at the paranodes (Peles and Salzer, 2000; Rasband, 2011; Sherman et al., 2005). In unmyelinated axons, which are generally smaller and lack nodal domains, action potentials are thought to be propagated by a homogenous distribution of Nav1.2 (Debanne et al., 2011). Nav1.2 is also distributed throughout the length of axons of cultured cortical neurons (Iwata et al., 2004). In chemical synapses, axonal propagation culminates with the opening of presynaptic voltage-gated Ca2+ channels (Cav) and release of neurotransmitter into the synaptic cleft.
Fig. 1.
Selected calpain substrates and localization of calpain I and II in axons. Schematic of a representative myelinated (A) and unmyelinated axon (B). The nodal and paranodal regions of the myelinated axons are shown. The axon initial segment, which also contains Nav1.2, shares a similar molecular composition to the node. Calpain proteolysis of substrates in the axolemma and cytoskeleton (subaxolemmal and central) may contribute to the structural and functional disruption seen in injured axons. Whether or not calpain II is present in unmyelinated axons has not been reported. Represented locations of proteins are not exhaustive nor do they necessarily reflect their distribution in axons. AnkG, ankyrinG; Caspr, contactin-associated protein; Kv, voltage-gated K+ channel; MAP1, microtubule-associated protein 1; Nav, voltage-gated Na+ channel; NCX3, Na+-Ca2+ exchanger-3; neurofascin-186, NF-186; NrCAM, neuronal cell adhesion molecule; PNL, paranodal cytoplasmic loop
The subaxolemmal cytoskeleton consists of a lattice-like organization of various proteins, which contributes to the formation and stability of specialized electrical domains (Ogawa et al., 2006; Schafer et al., 2009). Of particular interest are spectrin, actin, and ankyrin, which are known calpain substrates (Roberts-Lewis et al., 1994; Schafer et al., 2009; Yoshida et al., 1984). One important function of spectrin is to link the cytoskeletal filament system, particularly actin filaments, to transmembrane proteins (Löfvenberg and Backman, 1999). Spectrins are comprised of α- and β-subunits, which associate laterally to form antiparallel heterodimers, and the heterodimers, in turn, are assembled head-to-head to form heterotetramers (Bennett and Baines, 2001). In humans, there are two α-subunits (αI and αII), four β-subunits (βI, βII, βIII, and βIV), and a β-H subunit (also referred to as βV). αII and βII-spectrins are located diffusely along the length of rodent optic nerve axons except for their exclusion from nodes and enrichment at paranodes (Ogawa et al., 2006). Similarly, in sciatic nerve axons, they are not detected at nodes but are enriched at paranodes. αII/βII-spectrin is also present in presynaptic terminals and may play a key role in synaptic transmission (Goodman, 1999). On the other hand, βIV-spectrin is restricted to the AIS and nodes (Berghs et al., 2000; Yang et al., 2004). Of the subunits, αII, βII, and βIV-spectrin are calpain substrates (Glantz et al., 2007; Harris and Morrow, 1990; Roberts-Lewis et al., 1994; Schafer et al., 2009).
The mammalian genome contains 6 distinct genes that encode different actin isoforms (Cheever and Ervasti, 2013). The 3 α-actin and γ–smooth isoforms are predominantly expressed in muscle. While α-actin is probably not in neurons, β-cytoplasmic and γ-cytoplasmic actins (or simply β- and γ-actins) are ubiquitous (Cheever and Ervasti, 2013). In large axons in adult animals, γ-actin is likely the predominant isoform, while β-actin is either absent or present at relatively low levels. β- and γ-actins differ at only 4 out of 375 amino acids. Under proper buffer conditions, actin monomers (G-actin) spontaneously assemble into filaments (F-actin) (Letourneau, 2009). F-actin forms ring-like structures that wrap around the circumference of axons and are uniformly spaced along the axon (Xu et al., 2013). In mature axons, polymerized actin contributes to the stability of axolemma and presynaptic membrane and serves as “tracks” for myosin motor proteins and their cargos (Letourneau, 2009).
The ankyrin family of proteins has a general role as an adapter between a variety of integral membrane proteins—particularly ion channels and CAMs—and the spectrin skeleton (Bennett and Baines, 2001). Of particular interest is ankyrinG (ankG). The 480-kD and/or 270-kDa isoforms of ankG are localized to the AIS and nodes (Kordeli et al., 1995), and, together with βIV-spectrin, are required for Na+ channel clustering (Komada and Soriano, 2002; Zhou et al., 1998).
Of the proteins discussed in this section, Nav1.2, αII-spectrin, βII-spectrin, βIV-spectrin, actin, and ankG are calpain substrates (Glantz et al., 2007; Harris and Morrow, 1990; Roberts-Lewis et al., 1994; Schafer et al., 2009; von Reyn et al., 2009; Yoshida et al., 1984). Calpains are also mechanistically linked to the disruption of the domain localization of two other key nodal or paranodal proteins—Nav1.6 and Caspr (McGonigal et al., 2010). The mislocalization of these transmembrane proteins may be due to proteolysis of the underlying subaxolemmal cytoskeleton or the proteins themselves.
B. Overview of axonal central cytoskeleton
Cytoskeletal disruption contributes to much of the axonal dysfunction and pathology observed after mechanical deformation or transection of axons. The axonal cytoskeleton is principally composed of neurofilaments and microtubules, which are well known calpain substrates (Billger et al., 1988; Kamakura et al., 1985, 1983; Malik et al., 1983; Schlaepfer et al., 1985).
Neurofilaments are the most abundant cytoskeletal protein in large myelinated axons and are longitudinally oriented and regularly spaced (see review by Perrot et al., 2008). Neurofilaments are ~10 nm in diameter and consist of homopolymers and heteropolymers of subunits with predicted molecular weights of 61.5, 102.5, and 112.5 kDa (in humans), known respectively as neurofilament light (NFL), medium (NFM), and heavy (NFH) subunits (Perrot et al., 2008). Each subunit has a ~46 nm-long central α-helical core or rod domain, flanked by a lobular amino-terminal head and non α-helical carboxy-terminal sidearm domain. The two larger isoforms are heavily phosphorylated, mainly in the sidearm domain. Phosphorylation is topographically regulated, as neurofilaments in axons are intensely phosphorylated (Perrot et al., 2008). Phosphorylation of the sidearms may influence inter-neurofilament distance and axonal caliber, an important determinant of conduction velocity. Dephosphorylation or proteolysis of the sidearms is a marker of TAI (Povlishock et al., 1997).
Microtubules are critically important for anterograde and retrograde transport in axons. Microtubules are hollow cylindrical structures with outer diameters of ~25 nm typically formed by 13 parallel protofilaments, each composed of alternating α- and β-tubulin molecules (Amos and Schlieper, 2005; Maccioni and Cambiazo, 1995; Stanton et al., 2011). Microtubule assembly, stability, and elongation are promoted by microtubule-associated proteins (MAPs; Amos and Schlieper, 2005; Maccioni and Cambiazo, 1995). The high molecular weight MAPs, particularly MAP1A and MAP1B, have widespread neuronal distribution and are present in both axons and dendrites (Bloom et al., 1984; Maccioni and Cambiazo, 1995; Noble et al., 1989). Tau, a low molecular weight MAP, is highly enriched in axons, while MAP2 is found only in dendrites and somata in the mature nervous system (Maccioni and Cambiazo, 1995). The disruption of anterograde and retrograde axonal transport commonly observed after TBI has been attributed to depolymerization or loss of microtubules (Maxwell, 1996; Maxwell and Graham, 1997; McCracken et al., 1999; Saatman et al., 2003).
IV. The Calpain/Calpastatin System
A. Overview
Calpains are a family of Ca2+-dependent non-lysosomal cysteine proteases, whose substrates are involved in cytoskeletal remodeling, signal transduction, cell differentiation, embryonic development, vesicular trafficking, apoptosis, and necrosis (Zatz and Starling, 2005). Since the discovery of the first calpain in 1964 (Guroff, 1964), it is now recognized that the human genome contains 15 genes encoding calpain-like proteases (Sorimachi et al., 2010). The ubiquitously expressed μ- and m-calpain are a focus of investigation in the diseased nervous system (Bevers et al., 2010; Geddes and Saatman, 2010; Yu et al., 2013). μ- and m-calpain are heterodimers that consist of 80 kDa catalytic subunits sharing 55-65% sequence homology (calpain 1 and calpain 2, respectively) and a common 28 kDa regulatory subunit, known as calpain 4 or common small subunit 1 (CSS1; Goll et al., 2003). CSS1 is likely important for the activity of both μ- and m-calpain. In 2002, a novel small subunit, named CSS2, was identified (Schád et al., 2002). Sharing significant sequence similarity with CSS1, CSS2 could substitute for CSS1 for m-calpain activity, at least in vitro. According to current scientific nomenclature, μ- and m-calpain refer to the heterodimers, while calpain 1 and 2 refer to the individual catalytic subunits.
μ- and m-calpains cleave their substrates in a limited manner, often modifying, rather than terminating, substrate functions (Sorimachi et al., 2010; Tompa et al., 2004). Even though some preferences for calpain cleavage site sequences have been identified, the complete rules governing substrate specificity remain unclear (Ray, 2006; Sorimachi et al., 2010). Despite sharing nearly identical substrate specificity (Goll et al., 2003), μ- and m-calpain serve unique physiological functions. Constitutive knockout of calpain 2 or CSS1 is embryonically lethal (Arthur et al., 2000; Dutt et al., 2006), while calpain 1-knockout mice are viable and fertile (Azam et al., 2001). A major biochemical difference between μ- and m-calpain is the in vitro Ca2+ concentration required for half-maximal activity, 3-50 μM and 400-800 μM, respectively (Goll et al., 2003). In the various injury models described in this review, axoplasmic [Ca2+] is elevated, thereby activating μ-calpains, m-calpains, or both.
In addition to calpain 1 and 2, several other calpain catalytic subunit isoforms (3, 5, and 10) have been identified in brain (Bevers and Neumar, 2008), but much less is known about them. Mutations in the gene for calpain 3, once thought to be muscle-specific, cause limb-girdle muscular dystrophy type 2A (Zatz and Starling, 2005). Even though present in neuron-like PC12 cells (Marcilhac et al., 2006), calpain 3 has been detected in astrocytes, but not neurons, in the rodent and primate brain (König et al., 2003). Its autoproteolytic activity appears to be Ca2+-independent (Kinbara et al., 1998), whereas in vitro proteolysis of the abundantly expressed muscle protein PDLIM1 is Ca2+-dependent (Bertipaglia et al., 2009). Calpain 5 mRNA has been identified in rat and human brain (Waghray et al., 2004), and the protein has been detected in photoreceptors, but not bipolar or retinal ganglion cells (Mahajan et al., 2012). Mutations in the calpain 5 gene result in an autosomal dominant neovascular inflammatory vitreoretinopathy, which can progress to complete blindness (Mahajan et al., 2012). Calpain 10 protein has been detected in rat brain homogenates (Ma et al., 2001) and is partially targeted to mitochondria, where it likely plays a pathologic role in mitochondrial dysfunction via cleavage of Complex 1 subunits and activation of mitochondrial permeability transition (Arrington et al., 2006). Evidence suggests that calpain 10 activity is regulated by Ca2+ (Arrington et al., 2006). In the various studies described in this review, it is not clear what pathologic roles, if any, these other calpain isoforms may concurrently play within the axon.
Calpastatin is the endogenous inhibitor of both μ- and m-calpain, and it is not known to inhibit proteases of any other family (Goll et al., 2003; Pietsch et al., 2010). Although there is a single calpastatin gene in humans, pigs, and cattle, alternative splicing and different promoters give rise to isoforms ranging in molecular mass from 17.5 to 84 kDa (Goll et al., 2003). Binding of calpastatin to both the catalytic and regulatory subunits is required for effective inhibition of μ- and m-calpains (Goll et al., 2003). Details about calpastatin inhibition of the other calpain isoforms found in brain are scarce. There is evidence to suggest that the autoproteolytic activity of calpain 3 is not inhibited by calpastatin (Kinbara et al., 1998). Calpains 3, 5, and 10 are not believed to associate with the small regulatory subunit (Sorimachi et al., 1995; Suzuki et al., 2004; Waghray et al., 2004), but the regulatory subunit is not absolutely required for inhibition by calpastatin (Hata et al., 2007). Because calpastatin has absolute specificity for calpains, its overexpression in neuronal populations is used to provide a mechanistic link between calpains and axonal dysfunction and degeneration (Ma et al., 2013, 2012b).
Currently, there are no pharmacologic inhibitors completely specific for calpains or individual calpain isoforms (Bevers et al., 2009; Goll et al., 2003). However, calpain specificity of protease inhibitors has been improved recently. A synthetic 27-residue peptide based on subdomain B of domain I of human calpastatin (calpastatin-derived peptide 1B or CP1B) inhibits μ- and m-calpain with IC50 values in the nanomolar range and weakly inhibits cathepsin L (Ki = 6 μM), but not trypsin, cathepsin B, 20S proteasome, caspase 3, or papain (Pietsch et al., 2010). Most of the studies linking calpains to axonal pathology, described herein, rely on ALLN (calpain inhibitor I; N-Ac-Leu-Leu-norleucinal), ALLM (calpain inhibitor II; N-Ac-Leu-Leu-methioninal), MDL-28170 (calpain inhibitor III; carbobenzylzoxy-Val-Phe-H), or AK295 (Z-Leu-aminobutyric acid-CONH(CH2)3-morpholine). The membrane permeable ALLN and ALLM also inhibit cathepsin B, cathepsin L, and proteasome (Goll et al., 2003). MDL-28170, one of the best studied and most utilized calpain inhibitors, readily crosses the blood-brain barrier and is somewhat more selective for calpains than cathepsin B (Ki = 25 and 10 nM for cathepsin B and calpain, respectively) (Markgraf et al., 1998). AK295 is membrane permeable, and significantly more selective for calpain (Ki = 27-42 nM) over other cysteine proteases, such as cathepsin B (Ki = 24 μM) (Saatman et al., 1996).
B. Localization of μ- and m-calpains in axons
Calpains 1 and 2 have been identified in axons. In the CNS, calpain 1 immunoreactivity is detectable in myelinated and unmyelinated axons and rarely in presynaptic terminals (Fig. 1; Perlmutter et al., 1990, 1988; Siman et al., 1985). Some axons (e.g., corpus callosum) though are either lightly or not detectably immunoreactive (Perlmutter et al., 1988). Hamakubo et al. (1986) localized calpain 2, but not calpain 1, immunoreactivity to myelinated axons in the cerebellum using light microscopy. Axonal localization of the calpain antibodies in other brain regions was not reported. Using electron microscopy, calpain 1 immunoreactivity is observed in cerebellar axons (Perlmutter et al., 1988). This discordance of calpain 1 immunoreactivity in cerebellar axons could be attributed to increased sensitivity of immunoelectron microscopy over standard immunohistochemistry. It is also not clear if the examined axons originated from the same neuronal population. In the PNS, calpain 2 immunoreactivity is observed in myelinated sciatic nerve axons (Mata et al., 1991).
In the rat cortex and hippocampus, immunoreactivity for CSS1 is readily detectable in neuronal somata and/or dendrites, while CSS2 staining is detectable mainly in the axon fibers and terminals (Friedrich et al., 2004). Their axonal localization in CNS white matter tracts or PNS has not been reported. Nonetheless, calpain activity has been identified in axons and synaptic terminals (Ma et al., 2013; O’Hanlon et al., 2003; Saatman et al., 2003). Identification of calpains and their activity in axons suggest that these proteases may play an important role in axonal dysfunction and degeneration.
V. Pathologic role of calpains in axons
The remainder of this review focuses on intra-axonal effects of pathologic calpain activity and the efficacy of calpain inhibitory strategies on preserving axonal function and morphology. Due to space constraints, our discussion of substrates of μ- and m-calpains will be limited to those that are proteolyzed under pathologic conditions and are either preferentially localized to distinct axonal domains or are critical to unique axonal functions. The role of calpains in specific organelles, like the endoplasmic reticulum and mitochondria, are reviewed elsewhere (Bevers and Neumar, 2008; Kar et al., 2010). Complex injury models (e.g. spinal cord crush and multiple sclerosis; Das et al., 2008; Ray and Banik, 2003), in which pathologic calpain activity in extra-axonal neuronal compartments, glia, or inflammatory immune cells may obfuscate the role of intra-axonal calpains, are deemphasized. Section V will serve to clarify the role of intra-axonal calpains in dysfunction and degeneration, highlight important gaps in our knowledge, and delineate the potential benefits and limitations of therapeutic calpain inhibition.
A. Degeneration of the axonal cytoskeleton in Wallerian degeneration
A.1. Overview of Wallerian degeneration
In 1850, Augustus Waller observed that nerve transection results in progressive degeneration of the distal portion of the nerve (Waller, 1850). After an axon is transected in vivo, there is a latency period where the axon remains structurally quiescent and electrically excitable (Wang et al., 2012). This is followed by a rapid, irreversible process, termed granular disintegration of the axonal cytoskeleton (GDC), in which neurofilaments, microtubules, and other cytoskeletal components disintegrate or disassemble (Vargas and Barres, 2007). Wallerian degeneration has attracted much scientific interest as the underlying molecular mechanisms are thought to be shared, at least in part, by many human diseases, such as TBI, cerebral ischemia, Alzheimer’s disease, Parkinson’s disease, and toxic and other neuropathies (Coleman, 2005; Glass, 2004; Saxena and Caroni, 2007; Wang et al., 2000; Wang et al., 2012).
It was once believed that when axons were separated from their somata, the cessation of anterogradely-delivered trophic factors was responsible for their passive degeneration (Vargas and Barres, 2007). However, with the discovery of the Wallerian degeneration slow mouse (Wlds), axonal degeneration is now established as an active process. The autosomal dominant Wlds mutation is spontaneously occurring. Located on mouse chromosome 4, the Wlds gene mutation is an 85-kb tandem triplication containing the full coding region of nicotinamide mononucleotide adenyl transferase 1 (Nmat-1), the coding region for the amino-terminal 70 amino acids of the ubiquitination factor E4B (Ube4b), and a unique 18 amino acid linker. The precise mechanism by which Wlds mediates axonal protection has not been clearly established, but the bulk of existing evidence favors a mitochondrial role of Nmnat-1 in preserving axons (Coleman and Freeman, 2010; Wang and Barres, 2012).
Additional compelling evidence that axon degeneration is a regulated and active process comes from the recent identification of the Drosophila Toll receptor adaptor dSarm (sterile α/Armadillo/Toll-Interleukin receptor homology domain protein) (Osterloh et al., 2012). Knockout of the mouse ortholog of dSarm, Sarm1, results in significant axonal and synaptic preservation after transection of sciatic nerves. These results provide convincing evidence that injured axons actively promote their own destruction and identify dSarm/Sarm1 as a member of this signaling pathway (Osterloh et al., 2012). Better understanding of the signals and mediators of Wallerian degeneration may reveal opportunities for therapeutic intervention in human disease.
A.2. Cytoskeletal components are calpain substrates
Early investigators implicated calpains as causative agents of GDC (Kamakura et al., 1985, 1983; Schlaepfer et al., 1984). All three neurofilament isoforms—NFL, NFM, and NFH—are calpain substrates (Fig. 1) (Kamakura et al., 1985, 1983; Malik et al., 1983; Schlaepfer et al., 1985). Of the three isoforms, NFM appears to be most susceptible to proteolysis (Kamakura et al., 1985). Except for two sites (after Lys467 and Lys516) identified in bovine NFM (Shaw et al., 2004), the calpain cleavage sites for the mammalian neurofilament isoforms have not been established. Even though the properties of individual neurofilament fragments have not been systematically examined, evidence suggests that calpain proteolysis of the neurofilament cytoskeleton results in loss of filamentous structure (Ma et al., 2013).
Similar to neurofilaments, microtubules are critical components of the axonal cytoskeleton and disintegrate during GDC (Donat and Wiśniewski, 1973; Schlaepfer, 1974). The early disintegration or loss of microtubules may be due to depolymerization, proteolysis of microtubule components, or a combination of the two. Most studies implicate tubulin as a calpain substrate (Fig. 1; Billger et al., 1988; Malik et al., 1987, 1983). The lack of tubulin proteolysis observed in the study by Sandoval and Weber (1978) is likely due to the relatively low concentration or activity of calpains in crude brain extract that was used as a source of the protease. It has been reported that in vitro calpain proteolysis of tubulin results in a ~50 kDa fragment (Billger et al., 1988), even though the fragment would expectedly be difficult to discriminate from intact tubulin on one-dimensional electrophoresis. Purified tubulin subjected to calpain proteolysis forms large aggregates without any obvious ultrastructure (Billger et al., 1988). Microtubule depolymerization may also be due to proteolysis of MAPs (Sandoval and Weber, 1978). MAP1 and tau are calpain substrates, and their proteolysis generates multiple fragments (Fischer et al., 1991; Sato et al., 1986; Yang and Ksiezak-Reding, 1995). When a purified mixture of microtubules, MAP1 and MAP2, is incubated with calpain, both MAPs are degraded and no longer bind to microtubules (Billger et al., 1988). Adding further complexity, microtubule depolymerization may result directly from elevated Ca2+ or by Ca2+ activation of calmodulin (Gaskin et al., 1975; Job et al., 1981; Keith et al., 1983; O’Brien et al., 1997). Therefore, in injured axons in vivo, it is difficult to tease apart the role of calpains from the other effects of elevated Ca2+. The mechanism of microtubule loss in GDC may be multifactorial.
A.3. Calpains and GDC in Wallerian degeneration
Evidence for the role of calpains in Wallerian degeneration initially came from in vitro studies (Table 1; Fig. 2). Reducing extracellular Ca2+ or application of pharmacologic calpain inhibitors decrease degeneration of transected neurites in dorsal root ganglia (DRG) and sympathetic superior ganglia (SCG) explant cultures, as assessed by phase contrast microscopy, MAP1B immunostaining, neurofilament immunolabeling, and neurofilament western blotting (Finn et al., 2000; George et al., 1995; Wang et al., 2000; Zhai et al., 2003). However, ALLN does not reduce axolemmal degeneration (Finn et al., 2000) or microtubule fragmentation, which refers to gaps in tubulin immunostaining of neurites (Zhai et al., 2003). Exactly what microtubule fragmentation represents on the ultrastructural level is unclear, but it may represent focal depolymerization of microtubules. In contrast, the Ca2+ chelator EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid) and pharmacologic inhibitors to the ubiquitin-proteasome system (UPS) prevent both neurofilament degradation and microtubule fragmentation. Another study did not find statistically significant protection from degeneration of βIII-tubulin-labeled transected axons (MAP2-negative neurites) with ALLN (Kilinc et al., 2011), but the large variability in the treatment group may have obscured any drug effects. These two in vitro studies, together with a third one that strongly links E3 ubiquitin ligase ZNRF1 (zinc and ring finger 1) to in vivo Wallerian degeneration (Wakatsuki et al., 2011), suggest that certain features of Wallerian degeneration, such as microtubule fragmentation, may be mediated by the UPS, and not by calpains. Nonetheless, the evidence in total supports that calpains are important for key aspects of neurite degeneration, particularly the loss of neurofilament integrity.
Table 1. Effect of calpain inhibitors in neurite/axon transection models.
| Model | Inhibitor(s) | Effect of Calpain Inhibitor(s) | Reference |
|---|---|---|---|
| Neurite transection; DRG explant culture |
|
Reduces neurite degeneration (phase contrast microscopy) 96 h post-transection. IC50 for all 3 = ~10-20 μM. |
George et al., 1995 |
| Neurite transection; DRG explant culture |
AK295 (50 μM) | Reduces neurite degeneration (MAP1B immunolabeling) 72 h post-transection. |
Wang et al., 2000 |
| Neurite transection; DRG explant culture |
ALLN (200 μM) | Preserves NFH immunolabeling of neurites 24 h post-transection. No effect on axolemmal degeneration (DIC microscopy or immunolabeling for cytoplasmic enzyme PGP 9.5 or cell-surface antigen Thy-1.1). |
Finn et al., 2000 |
| Neurite transection; SCG explant culture |
ALLN (50 μM) | Delays neurite degeneration (phase contrast microscopy). Decreases loss of intact NFL and NFM (western blot). No effect on “fragmentation” of microtubules (βIII-tubulin immunolabeling). |
Zhai et al., 2003 |
| Neurite transection (saponin); dissociated cortical neuronal culture |
ALLN (10 μM) | No effect on axon fragmentation (βIII-tubulin immunolabeling) 4, 8 and 24 h post- transection. |
Kilinc et al., 2011 |
| Optic nerve transection in vivo |
Calpastatin overexpression |
Reduces NFL proteolysis (western blot) 5 days post-transection. No ultrastructural protection of axonal cytoskeleton (electron microscopy). |
Ma et al., 2013 |
| Optic nerve crush in vivo |
Mu-F-hF-FMK (topical) |
Reduces axonal degeneration, myelin vacuolization and delamination, and reactive astrogliosis (electron microscopy) 4 days post-crush. |
Couto et al., 2004 |
| Sciatic nerve transection in vivo |
Calpastatin overexpression |
Reduces NFL proteolysis and NFH loss (western blot) 48 h post-transection. Protects axonal cytoskeleton (electron microscopy) 2 and 5 days post-transection. Preserves NMJ (neurofilament and synaptic vesicle immunolabeling) 18 h post-transection. No effect on CMAP amplitudes 14 h post- transection. |
Ma et al., 2013 |
| Sciatic nerve transection in vivo |
Leupeptin IP | No effect on axonal degeneration (light microscopy) or loss of intact NFL (western blot) 3 days post-transection. |
Glass et al., 2002 |
| Transection of GFP-labeled DRG axons in spinal cord in vivo |
Mixture of EST and MDL- 28170 |
Completely prevents acute axonal degeneration (fragmentation of GFP) between 5 and 30 min post-transection. |
Kerschensteiner et al., 2005 |
CMAP, compound muscle action potential; DIC, differential interference contrast; DRG, dorsal root ganglia; EST, (2S,3S)-trans-epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester; GFP, green fluorescent protein; IP, intraperitoneal; MAP1B, microtubule-associated protein 1B, NFH, neurofilament heavy; NFL, neurofilament light; NFM, neurofilament medium; NMJ, neuromuscular junction; SCG, sympathetic superior ganglia
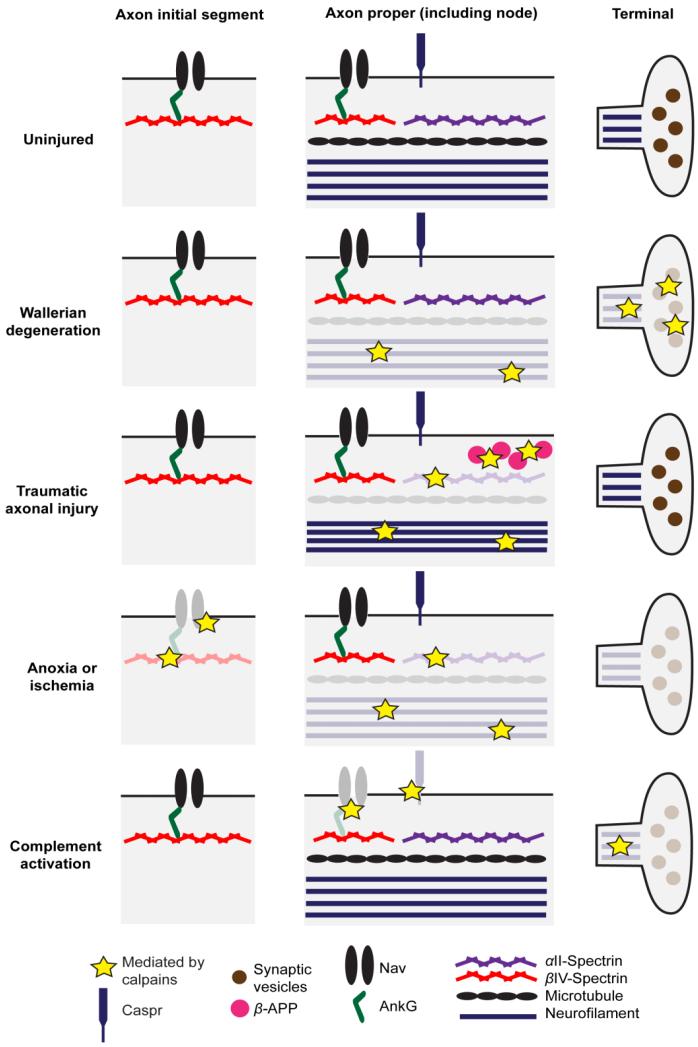
Fig. 2.
Axonal and synaptic pathology mediated by calpains in various injury models. In Wallerian degeneration, there is loss of microtubules and neurofilament in the axon proper, and loss of pre-terminal neurofilaments and synaptic vesicles. Prominent pathology after traumatic axonal injury includes αII-spectrin proteolysis, β-APP accumulation, microtubule loss, and neurofilament compaction. Based on these studies (Jafari et al., 1997, 1998; Maxwell and Graham, 1997), the number of neurofilaments early after axonal stretch injury appears to be extremely variable. An anoxic or ischemic insult is followed by AIS disassembly, αII-spectrin proteolysis, microtubule loss (Waxman et al., 1992), and neurofilament breakdown. There is also loss of preterminal neurofilaments and synaptic vesicles (Baxter et al., 2008; Tömböl et al., 2002). Finally, complement activation results in nodal and synaptic disruption. Calpains contribute to many, but not all, of these abnormalities. Color fading represents loss or proteolysis of the protein. Note that not all of the above proteins nor the mechanisms underlying the various abnormalities have been studied in each injury model. AnkG, ankyrinG; β-APP, β-amyloid precursor protein; Caspr, contactin-associated protein; Nav, voltage-gated Na+ channel
Care must be exercised when extrapolating these experimental findings to axons in vivo. First, cultures consist of developmentally young neurons in a “simplified” extracellular environment. Second, even though neurites are comprised of dendrites and axons (Touma et al., 2007), differentiating axons from dendrites was not considered when studying explant cultures. Third, the in vivo correlates of in vitro measures of neurite degeneration are not clear. Does loss of neurofilament immunolabeling in neurites represent GDC seen in vivo? Does fragmentation of tubulin immunostaining represent microtubule depolymerization? Finally, all the pharmacologic calpain inhibitors that were used in these experiments inhibit other proteases (Goll et al., 2003; Saatman et al., 1996).
Two early in vivo studies provide inconclusive evidence for a role of calpains in Wallerian degeneration. Couto et al. (2004) applied a calpain inhibitor (Mu-F-HF-FMK) directly to the optic nerve crush site in opossums. Four days post-crush, there is a slight, but statistically significant, increase in axons with preserved cytoskeleton (mean±SD: 76±3.7% versus 67±4.2% nontreated injured) and reduction in degenerating axons compared to injured animals without treatment. Experimental interpretation is limited by the modest effects and limited specificity of the inhibitor (Ma et al., 2013). Glass et al. (2002) studied sciatic nerve transection in mice. One h after transection, there is increased calpain activity, as measured by Ab38, in nerve homogenates; Ab38 specifically detects the calpain-cleaved αII-spectrin fragment (for more details, see section V.C1). The increase in Ab38 signal is not present when significant loss of NFL and β-tubulin occurs (between 24 and 48 h post-axotomy). The inability to detect an increase in Ab38 signal beyond 1 h post-transection is possibly due to the low baseline level of αII-spectrin in sciatic nerves or the instability of the fragment in the setting of robust and prolonged calpain activity (Ma et al., 2013). Pretreatment with intraperitoneally administered calpain inhibitor leupeptin, which is continued throughout the survival period, does not prevent NFL degradation 3 days post-sciatic nerve transection (Glass et al., 2002). As leupeptin has poor cellular permeability, it is possible that there was insufficient intra-axonal concentration (Ma et al., 2013).
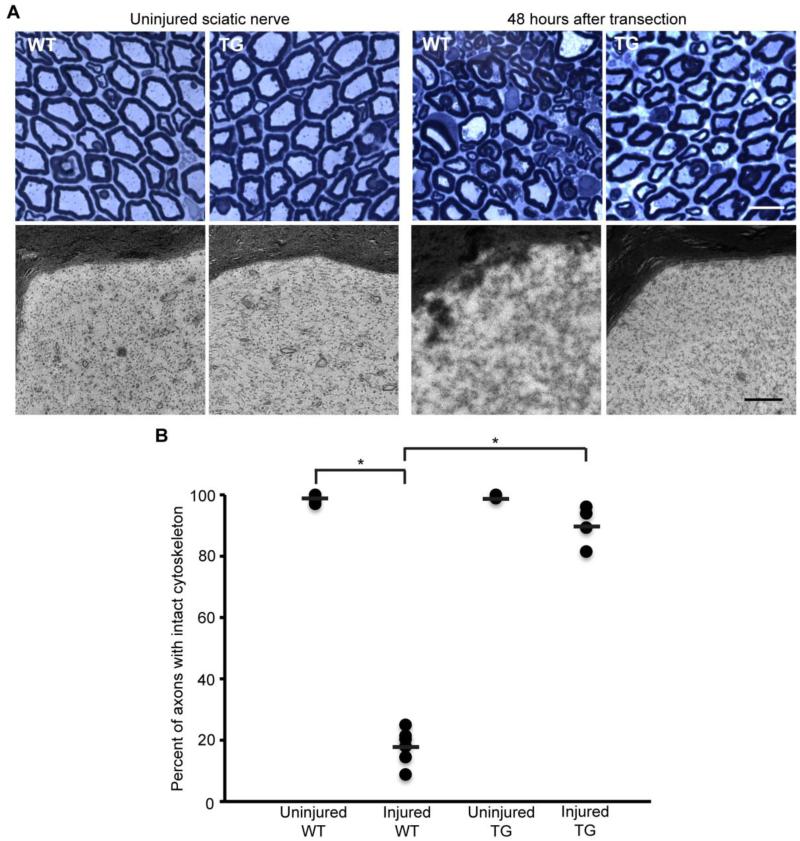
An in vivo study recently established that calpains are causally responsible for GDC (Ma et al., 2013). Based on the appearance of NFL fragments generated by calpain proteolysis, robust calpain activity first appears between 24 and 48 h after sciatic nerve transection and between 48 and 72 h after optic nerve transection. Calpastatin overexpression in transgenic mice reduces the proteolysis of NFL after sciatic and optic nerve transection, as well as the loss of NFH in sciatic nerves. It also provides robust ultrastructural protection of the axonal cytoskeleton, particularly neurofilaments, after sciatic nerve transection (Fig. 3). Ultrastructural protection of optic nerve axons is not observed however, likely due to the presence of “dark degeneration,” which obscures visualization of the cytoskeleton (Ma et al., 2013). “Dark degeneration” occurs when axons become filled with electron-dense material, and is commonly seen after transection of optic nerve axons (Bignami et al., 1981; Marques et al., 2003; Narciso et al., 2001). Neither the mechanism nor functional significance of “dark degeneration” is known. In this study, other cytoskeletal components were not examined. Nonetheless, this study confirms that GDC, in particular neurofilament proteolysis, is mediated by calpains.
Fig. 3.
Axonal cytoskeleton 48 h after sciatic nerve transection in wild-type and calpastatin-overexpressing transgenic mice. (A) Representative light and electron microscopic images of sciatic nerves from wild-type (WT) and transgenic (TG) mice. Scale bar=10 μm (light microscopy), 0.5 μm (electron microscopy). (B) Percentage of axons scored with intact cytoskeleton 48 h after sciatic nerve transection (n=7 WT and 4 TG). Each circle represents an individual nerve, while horizontal bars represent means. *p<0.0001. Protection of the axonal cytoskeleton in sciatic nerves of TG mice is nearly complete at 48 h post-transection. Reprinted from Ma et al. (2013), with permission from Elsevier.
In 2005, Kerschensteiner et al. (2005) coined the term “acute axonal degeneration” (AAD) to describe the rapid fragmentation of axons in regions immediately proximal and distal to the transection site. These investigators studied transgenic mice that express green fluorescent protein (GFP) in DRG axons traversing superficially through the spinal cord. After 10-20 min of quiescence, transection results in rapid (<5 min) fragmentation of 200-300 μm of axon segments at the cut ends, based on the fragmented appearance of intra-axonal GFP. Application of a calpain inhibitor cocktail to the transection site completely prevents this fragmentation (Kerschensteiner et al., 2005). Ultrastructural changes during AAD in optic nerves include condensation and misalignment of neurofilaments and fragmentation of microtubules (Knöferle et al., 2010), and ascribing some of these changes to calpains would be an intriguing next step. As AAD is temporally and spatially dissimilar to Wallerian degeneration, the importance of calpains in two different injury paradigms suggests that calpain-mediated degeneration may be a generalizable mechanism of axonal pathology.
In summary, calpains are important contributors to Wallerian degeneration, particularly neurofilament proteolysis. The causal mechanisms underlying axolemmal pathology, microtubule disruption, and “dark degeneration” still require further exploration. Axonal degeneration is likely multifactorial, and, in addition to pathologic calpain activity, may include Ca2+ dependent non-calpain mechanisms, the UPS, or autophagy (Beirowski et al., 2010; Knöferle et al., 2010; Yang et al., 2007; Zhai et al., 2003).
B. Axonal swelling, neurofilament compaction, and secondary axotomy after traumatic axonal injury
B.1. Pathophysiology of traumatic axonal injury
In the mammalian CNS, immediate severing or transection of axons at the moment of trauma, known as primary axotomy, is an irreversible process and is thought to only occur at the most severe mechanical strains (Maxwell et al., 1997; Povlishock and Katz, 2005). Instead, after blunt TBI, injured axons do not typically demonstrate primary axotomy, but evidence suggests that a biochemical cascade is triggered within axons, ultimately disrupting their continuity (secondary axotomy; Jafari et al., 1997; Maxwell and Graham, 1997; Povlishock et al., 1997; Povlishock and Katz, 2005; Saatman et al., 2003). In addition, blunt TBI disrupts axonal transport, leading to focal accumulation of vesicles and organelles, or axonal swellings (Büki and Povlishock, 2006). These swellings, also called spheroids or varicosities, are almost universally observed in CNS neurodegenerative diseases (Beirowski et al., 2010; Coleman, 2005). As secondary axotomy and axonal swellings are potentially preventable or reversible, they are a major focus of TBI researchers.
Support for secondary axotomy after mechanical strain comes from in vivo investigations. Within 1 h of fluid percussion injury in cats, intra-axonal accumulations of an anterograde tracer, horseradish peroxidase (HRP) conjugated to wheat germ agglutinin, occurs in labeled efferent fibers (Erb and Povlishock, 1988; Povlishock et al., 1983). Accumulations first show continuity with proximal and distal intra-axonal peroxidase profiles (axonal swellings). Over several hours, more of these accumulations appear to be disconnected from their distal profiles (axonal bulbs). A similar progression is observed after injury in rats (Kelley et al., 2006; Singleton et al., 2002). It is possible that continued anterograde transport of HRP may occur distal to a site of complete anterograde transport disruption, resulting in its disappearance from the distal segment and the illusion of axonal disconnection (Erb and Povlishock, 1988); however, secondary axotomy is also supported by ultrastructural analysis (Erb and Povlishock, 1988; Povlishock et al., 1983). Delayed axonal disconnection appears to be temporally and spatially linked to axonal swellings. It is important to note that it is the presence of axonal swellings that focuses the investigator on that segment of axon, and it is not known if secondary axotomy occurs at non-swollen sites.
An attractive hypothesis is that transport disruption is due to microtubule loss or depolymerization (Maxwell, 1996; McCracken et al., 1999; Saatman et al., 2003). Within 5 min after moderate to severe fluid percussion injury in cats, axons with altered axolemmal permeability in the ventral pontomedullary region display microtubule loss (Pettus and Povlishock, 1996). Fifteen min after optic nerve stretch in the guinea pig, there is a dramatic loss of microtubules (Maxwell and Graham, 1997). Importantly, internodal axonal swellings containing significantly reduced numbers of microtubules develops within 2 h of injury. In addition, mechanical strain has been proposed to physically rupture microtubules, leading to transport disruption (Tang-Schomer et al., 2012, 2010). Despite the strong circumstantial evidence, a direct link between microtubule loss and/or rupture to axonal swellings and transport disruption has not been established.
In addition to microtubule loss, neurofilament compaction has been suggested to impair axoplasmic transport (Smith and Meaney, 2000). When neurofilament subunits polymerize to form filaments, the central rod domains are normally masked by the phosphorylated sidearms. Binding of the antibody RMO-14, which is a commonly used histologic marker of TAI, to the rod domain of NFM suggests that proteolysis or dephosphorylation of the sidearm has occurred (Buki et al., 2003; Maxwell et al., 1997; Povlishock et al., 1997). Because the sidearms contribute to interneurofilament spacing, RMO-14 immunostaining likely indicates neurofilament compaction (Povlishock et al., 1997; Stone et al., 2001), and has been observed after human TBI (Stone et al., 2001) and in animal models of TAI (Buki et al., 2003; Stone et al., 2001). As RMO-14 immunoreactive axons in the pyramidal tract do not stain with β-APP nor have evidence of organelle pooling after impact acceleration head injury in rats (Stone et al., 2001), neurofilament compaction as a causal mechanism for transport disruption has been questioned (Ma et al., 2012b). In the setting of blunt TBI, the functional consequence of proteolysis or dephosphorylation of neurofilament sidearms remains unknown.
B.2. Role of calpains in traumatic axonal injury
Axonal calpain activity is elevated within minutes after experimental TAI (Büki et al., 1999; Saatman et al., 2003). Calpain proteolysis of αII-spectrin, measured by Ab38 immunolabeling, occurs in axons in the pyramidal tracts and medial lemnisci within 15 min of impact acceleration head injury in rats (Büki et al., 1999). After optic nerve stretch in the rodent, an in vivo model that isolates mechanical strain primarily to axons, Ab38 signal is detectable 20-30 min post-injury (Ma et al., 2012a; Saatman et al., 2003). As tubulin and MAPs are known calpain substrates (Billger et al., 1988; Fischer et al., 1991; Johnson et al., 1989), early calpain activity following injury makes calpains attractive candidates for the mechanisms underlying TAI pathology.
Calpains have been implicated in neurofilament compaction and impaired axonal transport after TAI. As early as 15 min after impact acceleration head injury, Ab38 immunoreactivity consistently colocalizes with RMO-14, suggesting that neurofilament compaction is temporally and spatially linked to pathologic calpain activity (Büki et al., 1999). Calpain inhibitor studies have provided further support for the causal role of calpains in TAI pathology (Table 2; Fig. 2). ALLN reduces neurite beading 60-90 min after fluid shear stress injury to dissociated forebrain neurons (Kilinc et al., 2009). Mechanical strain is not limited to axons, however, as there is increased calpain activity diffusely in the soma at the earliest time point (5 min post-injury). A single 30 mg/kg intravenous (IV) dose of MDL-28170 30 min prior to impact acceleration head injury in rats reduces β-APP and RMO-14 staining in brainstem fiber tracts 2 h post-injury (Buki et al., 2003). MDL-28170 also decreases β-APP labeling in the corpus callosum up to 7 d after fluid percussion injury (Ai et al., 2007). ALLM administered via continuous arterial infusion throughout the entire survival period reduces axonal swellings and/or bulbs in the corpus callosum and other white matter tracts 24 h after cortical impact injury (Posmantur et al., 1997), although quantification is not reported. Care must be taken when interpreting findings from TBI models in which the mechanical forces may not exclusively act upon axons (e.g., in vitro fluid shear stress, fluid percussion injury, impact acceleration head injury, and cortical impact). It is difficult to tease apart primary versus secondary injury to axons. Theoretically, a drug, when given systemically, may directly protect non-axonal structures, which in turn reduce the secondary injury seen within axons. In addition, neither of the inhibitors used in the in vivo studies (MDL-28170 and ALLM) is completely specific for calpains (Markgraf et al., 1998; Posmantur et al., 1997). Even though these studies suggest that MDL-28170 or ALLM may be beneficial after human TBI, their limitations make establishing a mechanistic link between intra-axonal calpain activity and TAI pathology tenuous.
Table 2. Effect of calpain inhibitors on axonal pathology in TBI and TAI models.
| Model | Inhibitor(s) | Effect of Calpain Inhibitor(s) | Reference |
|---|---|---|---|
| Fluid shear stress; dissociated forebrain neurons |
ALLN (3 μM) | Reduces neurite beading (phase contrast microscopy) 60-90 min post-injury. |
Kilinc et al., 2009 |
| Impact acceleration head injury |
MDL-28170 (30 mg/kg) IV 30 min before injury |
Reduces β-APP and RMO-14 immunostaining of axons in the corticospinal tract and medial longitudinal fasciculus at 2 h post-injury. |
Buki et al., 2003 |
| Fluid percussion injury |
MDL-28170 IV (various dosing strategies) |
Inhibitor effect varies depending on dosing strategy and post-injury survival duration. Inhibitor reduces β-APP immunostaining in the corpus callosum. Although its effect on CAP is less consistent, inhibitor partially normalizes CAP amplitude and latency of first and second major peaks (corresponding to myelinated and unmyelinated axons, respectively). Amplitude correlates with number of axons generating action potentials, while latency represents velocity of action potential propagation. |
Ai et al., 2007 |
| Controlled cortical impact |
ALLM IA bolus and infusion (total dose ~2.7 μmoles) |
Reduces axonal swellings, bulbs, and “breaks” in white matter tracts (NFL immunostaining) 24 h post-injury. |
Posmantur et al., 1997 |
| Optic nerve stretch in vivo |
MDL-28170 IV bolus and infusion (total dose 67.5 mg/kg) |
No improvement in retrograde transport of Fluoro-gold 4 days post-injury. |
Ma et al., 2012a |
| Optic nerve stretch in vivo |
Calpastatin overexpression |
Improves retrograde transport of Fluoro- gold 4 days post-injury. |
Ma et al., 2012b |
| Impact acceleration head injury |
MDL-28170 (30 mg/kg) IV 30 min before injury |
Reduces the length, but not the thickness, of HRP-labeling of axons in the corticospinal tract 2 h post-injury. No effect observed in the medial longitudinal fasciculus. |
Czeiter et al., 2009 |
β-APP, β-amyloid precursor protein; CAP, compound action potential; HRP, horseradish peroxidase; NFL, neurofilament light; IA, intra-arterial; IV, intravenous
To overcome these potential limitations, calpastatin overexpression was used in conjunction with the rat optic nerve stretch model (Ma et al., 2012b). Calpastatin overexpression within retinal ganglion cell axons partially preserves retrograde transport of Fluoro-gold™. In contrast, intensive therapy with MDL-28170 is ineffective in the same injury paradigm, possibly due to the short duration of treatment or inadequate intra-axonal concentration (Ma et al., 2012a).
The contribution of calpains specifically to microtubule loss or secondary axotomy has not been experimentally investigated. Nonetheless, the evidence in total suggests that calpains are responsible for key aspects of TAI, such as neurofilament compaction, and anterograde and retrograde transport disruption.
B.3. Commonalities and differences between Wallerian degeneration and TAI
In the previous sections, I limited my discussion of axonal degeneration to axonal transection models and emphasized transport disruption and neurofilament compaction in TAI models. This distinction is somewhat artificial but does reflect the different focus of nerve biologists and traumatologists. Evidence of transport disruption and degeneration is almost universally present after axonal injury irrespective of injury model. For example, in vivo rat optic nerve crush or transection results in the appearance of swellings close to the lesion site by 6-10 h post-injury, followed by their appearance ~5 and ~15 mm distal to the lesion site at 24 and 48 h post-injury, respectively (Beirowski et al., 2010). Even though swellings result from both axonal transection and TAI, there are key spatiotemporal differences. The centrifugal progression and delayed appearance of axonal swellings during Wallerian degeneration sharply contrast with experimental TAI models, where swellings first appear in presumably nonaxotomized axons within 30-60 min (Erb and Povlishock, 1988; Povlishock et al., 1983; Singleton et al., 2002). These spatiotemporal differences may be due to variations in the localization, degree, or duration of calpain activity. Concurrent or sequential non-calpain mechanisms may also contribute to these differences.
There are likely key mechanistic differences between Wallerian degeneration and TAI. Apoptotic effectors, in particular caspases, have been implicated in TAI (Büki and Povlishock, 2006; Büki et al., 2000; Chen et al., 2004; Stone et al., 2002) but they are unlikely to mediate degeneration of the transected axon (Finn et al., 2000; Schoenmann et al., 2010; Whitmore et al., 2003). Inhibition of the UPS protects transected neurites and axons in vitro and in vivo (Zhai et al., 2003) but accelerated progression to secondary axotomy after in vitro neurite stretch injury (Staal et al., 2009). The reasons behind these potentially important differences have been unexplored.
C. Disruption of the morphology and function of specialized axonal domains
C.1. Calpain substrates in the axolemma and subaxolemmal cytoskeleton
Several transmembrane proteins, specifically Nav1.2 and the Na+-Ca2+ exchanger-3 (NCX3; Fig. 1), are calpain substrates under various pathological conditions. Their proteolysis may further contribute to injury cascades (Iwata et al., 2004; Pannaccione et al., 2012). In addition to directly proteolyzing transmembrane proteins, calpains may cleave the underlying subaxolemmal cytoskeleton, thereby disrupting the localization and/or function of various transmembrane proteins.
Calpain proteolyzes the Nav1.2 α-subunit in calcium ionophore-treated cortical cultures, in an in vitro TBI model, and after controlled cortical impact in mice (Schoch et al., 2013; von Reyn et al., 2009). Nav1.2 α-subunit contains the tetrodotoxin (TTX) binding site, selectivity filter, channel pore, and structural elements responsible for activation and inactivation. Inactivation refers to the process by which an open voltage-gated Na+ channel closes even in the presence of maintained depolarization. Calpain proteolysis occurs at two predicted sites: within the intracellular I-II loop and II-III loop (von Reyn et al., 2009). There is possibly a third cleavage site but this is uncertain. As demonstrated in vitro, the channel fragments may remain in the plasma membrane but the functionality of the remaining cleaved channel is not known (von Reyn et al., 2009). However, it has been proposed that proteolysis of the α-subunit prevents Nav1.2 inactivation, resulting in Na+ influx and indirectly to persistent Ca2+ influx (Iwata et al., 2004). Particularly relevant to this review, stretching of isolated axons (MAP2-negative neurites) in cortical cultures results in proteolysis of the Nav1.2 α-subunit, which is blocked by generalized protease inhibition (Iwata et al., 2004). While recognizing the limitations of protein size estimation by western blot, the sizes of Nav1.2 α-subunit fragments generated by calpain activity (von Reyn et al., 2009) appear to differ from those resulting from isolated axonal stretch, suggesting that calpains may not solely be responsible for the proteolysis of the α-subunit in this model. Nonetheless, a proposed feed-forward pathway linking axonal stretch to sustained elevations in intra-axonal [Ca2+] via proteolysis of the Nav1.2 α-subunit (Iwata et al., 2004), is intriguing.
In normal physiology, plasmalemmal NCX, of which there are three known isoforms (NCX1, NCX2, and NCX3), couples Na+ influx to Ca2+ extrusion (forward mode). NCX1 immunoreactivity is detectable in myelinated axons in the sciatic nerve, optic nerve, and spinal cord, but not in the corpus callosum (Steffensen et al., 1997). All three isoforms are detectable in a small subset of axons and terminals in the rat hippocampus and cortex (Minelli et al., 2007). Of the three, NCX3 is a calpain substrate (Bano et al., 2005). Calpain proteolysis of NCX3 after focal brain ischemia and in glutamate-treated cerebellar cultures (Bano et al., 2005) or AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionate) receptor-stimulated hippocampal neuronal cultures (Araújo et al., 2007) results in 58-60 kDa fragments. After brain ischemia, NCX1 is proteolyzed by an unidentified protease, but NCX2 is not proteolyzed (Bano et al., 2005).
The function of the calpain-proteolyzed NCX3 is controversial. Early data suggests that the NCX3 is inactivated by calpains, and that its inactivation contributes to Ca2+ dysregulation and neuronal death (Bano et al., 2005). However, using patch-clamp technique in whole-cell configuration, Pannaccione et al. (2012) demonstrated a strong causal association between calpain proteolysis of NCX3 and increased NCX currents in the reverse mode of operation (couples Ca2+ influx to Na+ extrusion), suggesting that cleavage generates a hyperfunctional form of the antiporter. Axoplasmic [Na+] are acutely elevated after injury, driving the NCX to work in reverse mode (see section VI), so a hyperfunctional NCX3 may further exacerbate the axoplasmic [Ca2+] rise.
Of the spectrin subunits, αII, βII, and βIV-spectrin are calpain substrates (Fig. 1; Glantz et al., 2007; Harris and Morrow, 1990; Roberts-Lewis et al., 1994; Schafer et al., 2009). Commonly used as a sensitive and specific way to assay for calpain activity (Czogalla and Sikorski, 2005; Roberts-Lewis et al., 1994), αII-spectrin proteolysis is observed after nerve transection (Glass et al., 2002), TAI (Ma et al., 2012a; Saatman et al., 2003), and anoxic insult to nerves (Jiang and Stys, 2000). Two commonly used antibodies (i.e., Ab37 and Ab38) specifically recognize the calpain cleaved form of αII-spectrin by binding to the newly formed carboxyl-terminus of the ~150-kDa amino-terminal fragment (Roberts-Lewis et al., 1994). The preferred site of calpain proteolysis is between residues Tyr1176 and Gly1177, immediately adjacent to the calmodulin-binding domain of αII-spectrin (Glantz et al., 2007). Proteolyzed αII-spectrin significantly retains its actin binding and crosslinking activity in vitro, but these activities are disrupted in the presence of calmodulin (Harris and Morrow, 1990). Bovine βII-spectrin is proteolyzed by calpains into 165- and 125-kDa fragments in the presence of calmodulin. Concurrent proteolysis of both αII- and βII-spectrin causes fragmentation of the spectrin heterodimer and permanently impairs spectrin binding to actin regardless of calmodulin. Furthermore, calpain digest of αII/βII-spectrin inhibits its in vitro association with ankyrin-independent membrane binding sites (Hu and Bennett, 1991). Additional calpain cleavage sites in αII- and βII-spectrin have been identified (Glantz et al., 2007). For βIV-spectrin, calpain proteolysis yields a ~45 kDa fragment (Schafer et al., 2009). Together with cleavage of ankG (reportedly by calpains to generate ~72 and 95 kDa fragments), βIV-spectrin proteolysis likely disrupts AIS morphology. In addition, spectrin proteolysis may contribute to increased axolemmal permeability, thereby contributing to further Ca2+ influx in a feed-forward spiral (Czeiter et al., 2009).
Whether or not actin is a calpain substrate is controversial. Even though some investigators did not observe actin proteolysis by calpains (Goll et al., 1991; Siman and Noszek, 1988), there is evidence suggesting that actin may be a calpain substrate (Fig. 1). In vitro digest of purified actin isolated from bovine lens (43 kDA; isoform not specified) with m-calpain generates ~40 and 42 kDa fragments (Yoshida et al., 1984). Constitutive apoptosis in human neutrophils results in proteolysis of β-actin between residues Val43 and Met44, resulting in a ~38 kDa carboxyl-terminal fragment; proteolysis is inhibited by ALLN application (Brown et al., 1997). In cultured apoptotic neurons, generation of ~40 and 41 kDa actin fragments is reduced by ALLN or ALLM (Villa et al., 1998). Amino-terminal sequencing of the ~40 kDa carboxyl-terminal fragment determined that proteolysis occurs after the Arg residue corresponding to residue 37 in β-actin (Villa et al., 1998) and γ–actin (based on the sequence in Khaitlina (2001)). Directly microsequencing the ~41 kDa fragment from its amino-terminal end is not possible, but by using Lys-C digestion, high-performance liquid chromatography, and sequencing, the investigators confirmed that it is an actin fragment; the 2 peptides sequenced are common to α, β, and γ–actin. All 6 actin isoforms contain the two proteolytic sites (based on the sequences in Khaitlina (2001)). The last two studies suggest but do not definitively establish that calpains directly cleave actin. Apart from their broad specificity (Goll et al., 2003), ALLM and ALLN reduce apoptosis (Brown et al., 1997; Villa et al., 1998); multiple pathologic cascades may be active in a dying cell, one of which may be a yet unidentified protease1 that directly cleaves actin. The consequence of actin proteolysis is not known. The deoxyribonuclease I (DNase I) binding loop (residues 39-51) may be important for polymerization and stability of F-actin (Khaitlina and Strzelecka-Golaszewska, 2002). Although its relevance to the axon is unclear, disruption of this binding site has been proposed to interfere with actin binding to, and inhibition of, DNase 1, which degrades deoxyribonucleic acid (DNA) during apoptosis (Villa et al., 1998). It is not known whether actin acutely undergoes proteolysis in primarily axonal injury models, or whether actin proteolysis contributes to the disruption of the subaxolemmal cytoskeleton.
Of the calpain substrates in the axolemma (Nav1.2 and NCX) and subaxolemmal cytoskeleton (actin, spectrin, and ankyrin), there is reasonably strong evidence for calpain proteolysis only of αII-spectrin (Ma et al., 2012a; Saatman et al., 2003), βIV-spectrin (Schafer et al., 2009), and Nav1.2 (Schoch et al., 2013) in axons in mature animals. It is challenging to isolate the effects of intra-axonal calpains from calpain activity in the neuronal soma and dendrites. This task is made easier by the availability of antibodies for immunohistochemistry that selectively recognize the calpain-cleaved fragment (αII-spectrin; Roberts-Lewis et al., 1994), or the almost exclusive localization to axons of proteins (isoforms or splice variants) such as βIV-spectrin and Nav.1.2 (Berghs et al., 2000; Komada and Soriano, 2002; Westenbroek et al., 1989). An important next step is to identify the functional consequences of calpain proteolysis of these substrates within the axon.
C.2. Evidence of calpain involvement in the disruption of specialized domains
As calpain substrates include the subaxolemmal cytoskeleton and transmembrane proteins, pathologic calpain activity can have far-ranging effects. I will now discuss the evidence supporting intra-axonal calpains as key mediators of the morphological disruption of specialized domains—AIS, node, and terminal (Table 3; Fig. 2). In addition, their contribution to axonal and synaptic dysfunction will be examined.
Table 3. Effect of calpain inhibitors on axonal pathology from anoxia, ischemia, complement activation, and α-latrotoxin.
| Model | Inhibitor(s) | Effect of Calpain Inhibitor(s) | Reference |
|---|---|---|---|
| OGD; cortical neuronal culture |
|
Preserves βIV-spectrin labeled AIS 24 h post-OGD. IC50 for MDL-28170 = ~10 μM. |
Schafer et al., 2009 |
| MCAO (90 min) in vivo |
MDL-28170 IV bolus and infusion (total dose 40 mg/kg) |
Preserves βIV-spectrin and Nav labeled AIS in infarcted cortex 24 h post-MCAO. |
Schafer et al., 2009 |
| Anoxia± reoxygenation; optic nerves ex vivo |
|
Reduces αII-spectrin proteolysis (western blot), presumably in axons. No improvement in CAP (area under the CAP). |
Jiang and Stys, 2000 |
| Anoxia or OGD; optic nerves ex vivo |
|
Reduces NFM and NFH proteolysis, and NFH dephosphorylation (western blot). No improvement in CAP (area under the CAP). |
Stys and Jiang, 2002 |
| OGD; extensor digitorum longus preparation from mice that express YFP in motor neurons |
Calpain inhibitor VI (100 μM) |
Reduces NMJ denervation (loss of YFP fluorescence) 120 min post-OGD. |
Talbot et al., 2012 |
| Anti-GD1a antibody with complement; intramuscular axons in ex vivo triangularis sterni muscle |
AK295 (100 μM) | Preserves immunostaining for Nav1.6, ankG, and Caspr at nodes and paranodes, and immunostaining for NF at NMJs. No improvement in perineural currents at nodes or terminals. |
McGonigal et al., 2010 |
| Anti-GD1a antibody with complement; desheathed phrenic nerves ex vivo |
AK295 (100 μM) | Preserves immunostaining for Nav1.6 and Caspr, but not ankG, at nodes and paranodes. No improvement in CAP amplitude. |
McGonigal et al., 2010 |
|
α-latrotoxin; NMJs in hemidiaphragm preparations |
Calpeptin (50 μg/ml or 138 μM) |
Preserves NFH immunostaining and “cytoskeletal bundles” in NMJs. Synaptic vesicle depletion is less pronounced with calpain inhibitor. |
O’Hanlon et al., 2003 |
| Anti-GQ1b antibody with complement; NMJs in hemidiaphragm preparations |
|
Preserves NFH immunostaining and “cytoskeletal bundles” in NMJs. Does not reduce mitochondrial damage. No effect on synaptic vesicle depletion. |
O’Hanlon et al., 2003 |
AIS, axon initial segment; ankG, ankyrin G; CAP, compound action potential; Caspr, contactin-associated protein; MCAO, middle cerebral artery occlusion; Nav, voltage-gated Na+ channel; NF, neurofilament; NFH, neurofilament heavy; NFM, neurofilament medium; NMJ, neuromuscular junction; OGD, oxygen glucose deprivation
The AIS consists of a unique cytoskeleton comprised of βIV-spectrin and ankG (Schafer et al., 2009), which are required for Na+ channel clustering (Komada and Soriano, 2002; Zhou et al., 1998). Six h after middle cerebral artery occlusion (MCAO), the number of cortical neurons with intact immunostaining for Na+ channel, ankG, and βIV-spectrin at the AIS is significantly reduced (Schafer et al., 2009). Calpain proteolysis of βIV-spectrin and ankG occurs early (3 h post-occlusion), and this has been proposed to disrupt AIS morphology. Treatment with MDL-28170 preserves the βIV-spectrin staining at the AIS 24 h post-MCAO. Interestingly, for unclear reasons, MCAO does not disrupt the organization of nodes, which share a similar molecular composition to the AIS (Schafer et al., 2009). In primary cortical neurons exposed to oxygen glucose deprivation (OGD), MDL-28170 and calpastatin overexpression preserve βIV-spectrin immunostaining of the AIS (Schafer et al., 2009). Photolytic uncaging of Ca2+ at the AIS in live neurons was performed in vitro using a cell permeant acetoxymethyl ester derivative of the caged compound o-nitrophenyl EGTA. This results in a decrease in immunofluorescence intensity of the CAM neurofascin-186 at the AIS, implicating local calpain activity. The evidence in total suggests that calpains localized to the AIS are responsible for AIS disassembly.
Using a model of anti-GD1a antibody directed complement activation, the role of calpains in the disruption of nodal morphology and function was examined (McGonigal et al., 2010). The ganglioside GD1a is present in the node and motor nerve terminal, sites that correspond to those predicted to be affected in motor axonal forms of Gullain-Barré syndrome. In this model, GD1a is enriched in axons through using the GD3 synthase knockout mice; their axons are particularly vulnerable to anti-GD1a antibody mediated complement activation. A consequence of complement activation is the formation of bi-directional non-specific ion and water pores (membrane attack complex pores) on the plasma membrane. In intramuscular axons in ex vivo triangularis sterni muscle preparations treated with anti-GD1a antibody and complement, immunoreactivity for Nav1.6, ankG, and Caspr is decreased at nodes and paranodes, and this loss is blunted by AK295. However, AK295 does not ameliorate the loss of perineural currents at the node and motor nerve terminal, which indicates an inability to generate Na+ and K+ currents. The same laboratory also measured compound action potentials (CAPs) in experimentally desheathed phrenic nerve trunks exposed to anti-GD1a antibody and complement (McGonigal et al., 2010). Despite modest protection of nodal and paranodal morphology, injury-induced decrement of CAP amplitude is not reduced by AK295. CAP amplitude correlates with number of axons generating action potentials. The failure of the axolemma to maintain ionic homeostasis, when punctured by membrane attack complexes, was suggested to be the critical factor in mediating axonal conduction block (McGonigal et al., 2010).
Similarly, pharmacologic calpain inhibitors do not provide electrophysiological protection (CAP) to optic nerves exposed to ex vivo anoxia-reoxygenation or OGD (Jiang and Stys, 2000; Stys and Jiang, 2002). As removing extracellular Ca2+ is completely protective of CAP, other Ca2+–dependent pathways, such as phospholipases, phosphatases, or kinases, were suggested as potential factors in mediating electrophysiological deterioration. The disruption of action potential propagation may be due to multiple interdependent axonal factors, e.g., loss or mislocalization of nodal proteins, dysregulated activity of ion channels, and perturbation of ionic homeostasis, as well as extra-axonal factors. Inhibiting intra-axonal calpains may be inadequate for preserving such a complex process as action potential propagation but is likely a necessary component of any effective therapeutic strategy to maintain nodal function after injury.
In addition to contributing to nodal disassembly, calpains have been causally linked to the degeneration of the axon terminal in ex vivo and in vivo injury models. Extensor digitorum longus (EDL) muscle preparations from transgenic mice whose motor neurons express yellow fluorescent protein (YFP) were exposed to simulated ischemia/reperfusion (Talbot et al., 2012). Pre-treatment with calpain inhibitor VI [N-(4-fluorophenylsulphonyl)-L-valyl-L-leucinal] preserves neuromuscular junction (NMJ) innervation, assessed by YFP fluorescence. Morphological, including ultrastructural, protection of NMJs by calpain inhibitors was demonstrated in several additional injury models (O’Hanlon et al., 2003). NMJs in hemidiaphragm preparations were treated with anti-GQ1b ganglioside antibodies and complement, or the spider neurotoxin, α-latrotoxin—both form non-selective pores within the target membrane. Both calpeptin (carbobenzyloxy-Leu-normleucinal) and calpain inhibitor V (Mu-Val-HPh-CH2F) reduce the loss of NFH immunostaining and “cytoskeletal bundles” in the motor nerve terminal. The in vivo role of calpains in the NMJ was characterized using sciatic nerve transection in transgenic mice overexpressing calpastatin (Ma et al., 2013). Degeneration of the presynaptic terminal and synaptic dysfunction are observed early after nerve transection. Calpastatin overexpression preserves the morphology of preterminal axons and terminals (neurofilament and synaptic vesicle immunostaining) (Ma et al., 2013). However, there is no difference in compound muscle action potential (CMAP) amplitudes after nerve transection between wild-type and transgenic animals. As early impairment of CMAP likely represents synaptic dysfunction rather than failure of action potential conduction, CMAP amplitudes are expected to correlate with number of functioning synapses. In all of these injury models, calpains mediate key aspects of synaptic terminal degeneration; the cytoskeleton seems particularly vulnerable. Like nodes, the electrophysiologic function of synaptic terminals cannot be protected solely by calpain inhibition.
In summary, calpains are causally linked to the morphological degeneration of the AIS, nodes, and synaptic terminals. However, no study to date has demonstrated any improvement in electrophysiological outcome measures specifically of injured axons using calpain inhibition. In contrast, improvement of electrophysiological outcome measures (CAP amplitude and latency, tail sensory nerve action potential amplitude, long-term potentiation) with pharmacologic calpain inhibitors has been reported in animal models of TBI, taxol-induced sensory neuropathy, and Alzheimer’s disease (Ai et al., 2007; Trinchese et al., 2008; Wang et al., 2004). Calpain activity in the neuronal somata, dendrites, glial cells, or other structures may be one of many important pathologic mechanisms. Given the complexity of these injury models, the systemic administration of calpain inhibitors, and the lack of improvement in axonal-specific electrophysiological outcomes by calpains, it is unlikely that the electrophysiological protection seen in these animal models can be attributed solely to inhibition of intra-axonal calpains. Calpain inhibition is probably a component of any therapeutic strategy that fully protects axonal and synaptic function after injury.
VI. Mechanisms of Ca2+ entry into axoplasm
As calpains require elevated [Ca2+] for activity, the sources and mechanisms of Ca2+ entry into the axoplasm are particularly relevant to this review (Fig. 4). Most studies implicate the source of elevated [Ca2+] to be extracellular, while others point to intracellular Ca2+ stores. Because of the challenges of manipulating and measuring [Ca2+] in vivo, most of the studies have been performed in vitro or ex vivo, which limits generalizability to a living adult animal. In the following, I will discuss the routes of Ca2+ entry into the axoplasm in several injury models: neurite/axon transection, neurite/axon stretch, in vitro and in vivo TBI, and ischemia/anoxia. Studies that directly measure cation concentrations will be emphasized here.
Fig. 4.
Mechanism of Ca2+entry into the axoplasm after transection, mechanical strain, or anoxic/ischemic insult. Mechanisms by which axoplasmic [Ca2+] become elevated likely vary according to injury model (designated by arrow type). Note that several of the mechanisms have not been adequately studied in various injury models.
Based on experiments demonstrating protection of compound action potential with channel antagonists, N-type Ca2+ channels may contribute to Ca2+ influx during anoxic injury (Fern et al., 1995; Imaizumi et al., 1999)
AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionate; Cav, voltage-gated Ca2+ channel; Nav, voltage-gated Na+ channel; NCX, Na+-Ca2+ exchanger; TBI, traumatic brain injury.
As a whole, neurite/axon transection studies provide limited information about the sources of axoplasmic Ca2+. In one study, axoplasmic [Ca2+] was measured in pharmacologically treated-transected axons in the time frame coinciding with AAD, and not Wallerian degeneration (Knöferle et al., 2010). Topical application of a cocktail of amiloride (T-type Cav blocker), amlodipine (L-/N-type Cav blocker), and NBQX (1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium salt hydrate; AMPA receptor blocker) prevents in vivo AAD after axon transection in the optic nerve (Knöferle et al., 2010). Without these antagonists, axoplasmic [Ca2+] rapidly rises within 30 sec, although normal resting [Ca2+] is quickly restored over the next 60 sec. The authors suggested that the pharmacologic inhibitors also blunt the early rise in [Ca2+] (Knöferle et al., 2010) but results of any statistical analysis were not provided. Manipulating Ca2+ influx while directly measuring axoplasmic [Ca2+] during Wallerian degeneration has not been reported. George et al. (1995) assessed neurite degeneration, which is dependent on elevated intra-axonal [Ca2+]. Chelating extracellular Ca2+ or applying L-type Cav channel inhibitors reduces degeneration of transected DRG neurites, assessed 4 days post-injury. Pharmacological inhibition of N-type Ca2+ channels or TTX-sensitive Na+ channels is ineffective. Given the caveat that TTX-resistant Na+ channels are present on 10-15% of immature DRG cell bodies (Kostyuk et al., 1981), it is not clear if extracellular Na+ significantly contributes to axoplasmic [Ca2+] elevation. Extracellular Ca2+ influx, likely through L-type channels, appears to be a major contributor to the axoplasmic [Ca2+] elevation in Wallerian degeneration and possibly AAD.
Studies using severe axonal stretch models strongly implicate extracellular Ca2+ and Na+ in the elevation of axoplasmic [Ca2+]. In a model where neurites from N-Tera2cl/D2 neurons are stretched 75% beyond their initial lengths, an immediate rise in intra-axonal [Ca2+] is observed but can be reduced if extracellular Ca2+ or Na+ is removed or if neurons are pretreated with inhibitors to Nav channels (TTX), P/Q- and N-type Cav channels (ω–conotoxin MVIIC), or NCX (bepridil) (Wolf et al., 2001). In another study, stretching axons of rat primary cortical neurons to 70-75% beyond their initial lengths results in an immediate rise in axoplasmic [Ca2+], followed by a decrease toward resting [Ca2+] at 2 min post-stretch, and a subsequent secondary rise in [Ca2+] over the next 60 min (Iwata et al., 2004). Preinjury treatment with TTX completely abolishes the Ca2+ elevation, measured at 2, 20, and 60 min post-injury, whereas treatment starting at 5 min post-injury reduces the secondary [Ca2+] rise only at 60 min compared with injured nontreated cultures. Pre- or post-injury treatment with a protease inhibitor cocktail, which inhibits serine and cysteine proteases, metalloproteases, trypsin, and others, only reduces the 60 min [Ca2+] elevation. Based on these results, Iwata et al. (2004) proposed a model by which Na+ entry through mechanically sensitive Na+ channels triggers an increase in intra-axonal Ca2+ via opening of Cav channels and reversal of the NCX. Furthermore, the authors suggested that proteolysis of the Nav1.2 α-subunit results in loss of channel inactivation and additional Na+ influx, which further drives Ca2+ influx. These two studies together implicate axolemmal ion channels and exchangers, specifically TTX-sensitive Na+ channel, Cav channel, and NCX, as the predominant mechanisms underlying axoplasmic [Ca2+] elevation.
In contrast, the increase in axoplasmic [Ca2+] following mild neurite stretch injury originates principally from intracellular stores (Staal et al., 2010). The investigators deformed neurite bundles from rat cortical neurons using a fluid pulse, generating a transient 1-6% increase in original neurite length. Stretch injury induces a biphasic increase in intra-axonal [Ca2+], with the larger, initial peak occurring within 1-3 sec of injury. The immediate rise in intra-axonal [Ca2+] after stretch is not altered when Ca2+ is removed from the bath solution, suggesting that the early [Ca2+] rise is due to Ca2+ release from intracellular stores (Staal et al., 2010); however, the relevant intracellular compartments have not been identified. The smaller and slightly delayed peak is reduced in Ca2+-free media, suggesting that part of the [Ca2+] elevation in this injury model may be dependent on Ca2+ influx. Axoplasmic [Ca2+] elevation is primarily due to Ca2+ release from intracellular stores after mild axonal stretch injury, and influx of extracellular Ca2+ and Na+ after more severe axonal stretch.
In vitro and in vivo TBI models have been utilized to address the phenomenon of mechanoporation, or the mechanically induced creation of physical defects in the plasma membrane. Mechanoporation of the axolemma will be the focus here. For this section and Fig. 4, the in vitro TBI model, where the entire neuron is exposed to mechanical strain, is distinguished from in vitro models where strain is isolated to neurites. The [Ca2+] rise in neurites and influx of extracellularly applied membrane-impermeable 480 Da Lucifer yellow ammonium salt within minutes of applying fluid shear stress to chick forebrain neurons is reduced by post-injury application of poloxamer 188, whose mechanism of action is thought to be sealing damaged cell membranes (Kilinc et al., 2009, 2008). In this model, the entire neuron, as evidence by increased calpain activity in the soma and neurites (Kilinc et al., 2009), is injured so the source of axoplasmic Ca2+ and 480 Da tracer could partially be due to disruption of the plasmalemma surrounding the soma, as well as axolemmal mechanoporation. In two in vitro models, where mechanical strain is isolated to neurites, axolemmal permeability to a 570 Da fluorescent tracer (Alexa 488 hydrazide) is not observed early after injury (<1 h; Smith et al., 1999; Staal et al., 2007). The reason for this discrepancy is unclear.
Further support for the existence of axolemmal mechanoporation comes from in vivo TBI studies. First, ultrastructural evidence of mechanoporation, using electron microscopy, is observed after lateral acceleration of the head in non-human primates (Maxwell et al., 1993). Second, axolemmal permeability to the ~44 kDa HRP after TBI has been reported (Pettus et al., 1994). After infusing HRP in the intrathecal space, moderate fluid percussion injury (2.0-2.8 atm) results in axolemmal permeability to HRP as early as 5 min after injury. It is intriguing that treatment with MDL-28170 prior to impact acceleration head injury results in decreased permeability to HRP 2 h post-injury (Table 2; Czeiter et al., 2009). Post-injury calpain activity, possibly via spectrin proteolysis, may further worsen axolemmal permeability in a feed forward fashion (Czeiter et al., 2009).
Mechanisms of Ca2+ and Na+ entry into the axoplasm have been more thoroughly studied in anoxic and ischemic axons2 than in other injury models. The interested reader is referred to more comprehensive reviews (Stys, 2005, 2004). During ischemia, axoplasmic Ca2+ rises from a presumed baseline of ~100 nM to the tens of micromolar (Stys, 2005). In ex vivo nerves exposed to simulated ischemia, elevation of axoplasmic [Ca2+] predominantly results from influx of extracellular Ca2+ with a smaller contribution from internal stores (Nikolaeva et al., 2005). Recently, glutamate receptors have been implicated (Ouardouz et al., 2009a, 2009b).
Ca2+ influx from the extracellular environment predominantly occurs via reversal of the NCX, driven by axonal Na+ accumulation through TTX-sensitive Na+ channels, in anoxic optic nerves (Stys and Lopachin, 1998) and chemically ischemic dorsal roots (Petrescu et al., 2007). Depolarization also stimulates the NCX to operate in reverse mode (Stys, 2004). These two studies show no or inconsistent effects on axoplasmic [Ca2+] or [Ca]3 with L-type Cav channel inhibitors (Petrescu et al., 2007; Stys and Lopachin, 1998). Another study provides some support for a role for L-type Cav channels in Ca2+ influx in anoxic optic nerves by demonstrating that the inhibitor diltiazem ameliorates the anoxia-induced decrease in extracellular [Ca2+] (Brown et al., 2001). Axoplasmic Ca2+ was not directly measured, however. Besides extracellular Ca2+ influx, Ca2+ release from internal stores is also important. When optic nerves are bathed in Ca2+-free artificial cerebrospinal fluid, the Ca2+ contribution from internal stores during simulated ischemia originates from the endoplasmic reticulum and mitochondria, and its release is dependent on influx of extracellular Na+ (Nikolaeva et al., 2005). Even in the absence of axonal depolarization, Na+ accumulation may drive the mitochondrial NCX to export Ca2+ into the axoplasm, positively modulate ryanodine receptors, and promote IP3 generation by phospholipase C. The studies together support that in anoxic and ischemic injury, axoplasmic [Ca2+] elevation is predominantly due to TTX-sensitive Na+ channels and plasmalemmal NCX (operating in reverse mode), with a smaller contribution from internal Ca2+ stores.
The importance of excitotoxicity during anoxic and ischemic injury has recently gained more momentum. Glutamate, the main excitatory neurotransmitter in the mammalian CNS (Stys, 2005), may directly contribute to the rise of axoplasmic [Ca2+]. During anoxic injury to dorsal column slices, glutamate is released from the axoplasm and oligodendrocytes likely via reversal of Na+-dependent glutamate transport (Li et al., 1999). Glutamate is also released from optic nerves undergoing OGD (Tekkök et al., 2007). Overactivation of kainite and AMPA glutamate receptors, which are present on glia, is believed to exacerbate white matter injury by directly disrupting glia (Ouardouz et al., 2006; Stys, 2005). However, these receptors have recently been detected on myelinated axons in dorsal columns (Ouardouz et al., 2009a, 2009b). Even though the specific mechanisms of Ca2+ entry into the axoplasm vary depending on subunit composition of the glutamate receptors, they may include Ca2+ influx through the glutamate receptor, downstream activation of L-type Ca2+ channels (via influx of Na+ and localized depolarization), and release from IP3-and ryanodine-dependent intracellular stores. The specific contribution of glutamate receptors localized on axons to axoplasmic [Ca+] elevation and axonal pathology from anoxia and ischemia still remains to be characterized.
Neuromuscular junctions have also been studied during simulated ischemia. Motor nerve terminals are more sensitive to ischemia than axons and muscle fibers (Mäkitie and Teräväinen, 1977; Tömböl et al., 2002). EDL preparations were harvested from transgenic mice expressing YFP in motor neurons and their axons (Talbot et al., 2012). Chelating bath Ca2+ or adding pharmacologic inhibitors for plasma membrane NCX, P/Q-type Cav channels, L-type Cav channels, or Na+,K+,2Cl− co-transporter reduces the degeneration of EDL terminals, as assessed by YFP fluorescence, 120 min post-OGD. Even though intra-terminal [Ca2+] was not measured in the EDL preparation, elevated intracellular [Ca2+] within levator auris longus NMJs during OGD was observed. Given that NMJ degeneration is likely associated with increased intracellular [Ca2+], this study suggests that many of the mechanisms of Ca2+ entry into the axon terminal are shared with the axon fiber.
Taken together, these studies highlight the heterogeneity of mechanisms for [Ca2+] elevation. The sources and mechanisms of Ca2+ entry into the axoplasm and terminal depend on the injury and model system. Directly preventing Ca2+ elevation in human diseases may require different and/or multiple therapeutic interventions. As Ca2+-dependent non-calpain pathways may be important in Wallerian degeneration (Zhai et al., 2003), TAI (Staal et al., 2010), and ischemia (Stys, 2005), blunting the axoplasmic Ca2+ rise may confer additional benefits to calpain inhibition alone. Nonetheless, increased activity of axoplasmic calpains, which is downstream of elevated [Ca2+], is deleterious across many injury paradigms and remains a therapeutic target for many human diseases.
VII. Concluding Remarks
There has been growing awareness of the contribution of axonal pathology to the morbidity and mortality of many human diseases, including CNS and PNS trauma, cerebral ischemia, neurodegenerative disorders, and peripheral neuropathies. Axonal dysfunction and degeneration in these seemingly disparate disorders likely share common molecular injury mechanisms, one of which is dysregulated intra-axonal calpain activity. This review has attempted to bring together research findings from various disciplines to facilitate a more unified understanding of the pathologic roles played by calpains in axons and to highlight gaps in our current knowledge. Significant progress has been made over the past decade, yet much work still remains. The identification of the intra-axonal calpain contribution to morphological, electrophysiological, and functional abnormalities, particularly in vivo, is lacking in many injury models. In particular, our understanding of the downstream consequences of calpain proteolysis of specific substrates in axons remains vague. As we move forward, elucidating these effects in axons may be critically important as the outcome of substrate proteolysis may depend on their subcellular localization. Despite these shortcomings, it is clear that inhibition of intra-axonal calpains is likely a necessary component of any effective therapeutic strategy for a wide spectrum of human diseases.
HIGHLIGHTS.
Axonal injury and degeneration significantly contribute to outcome in many diseases.
Transection, stretch, ischemia, and complement activate intra-axonal calpains.
Calpain activity disrupts axonal transport, cytoskeleton, and specialized domains.
Calpain substrates include the Na+ channel, Na+-Ca2+ exchanger, and neurofilament.
Inhibition of intra-axonal calpains is likely beneficial for many human diseases.
ACKNOWLEDGEMENTS
I wish to acknowledge Catherine M. Kopil (University of Pennsylvania) for her invaluable help with technical editing, and to thank Steven S. Scherer (University of Pennsylvania), Toby A. Ferguson (Temple University), Robert W. Neumar (University of Michigan), Amy L. Boore, and Robert J. Lee (University of Pennsylvania) for critical reading of the manuscript. This work was supported by National Institutes of Health grant K08NS055880 (MM).
The funding source had no involvement in the conduct of the research or the preparation of the manuscript.
ABBREVIATIONS
- AAD
acute axonal degeneration
- AIS
axon initial segment
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionate
- ankG
ankyrinG
- β-APP
β-amyloid precursor protein
- CAM
cell adhesion molecule
- CAP
compound action potential
- Caspr
contactin-associated protein
- Cav
voltage-gated Ca2+ channel
- CMAP
compound muscle action potential
- CNS
central nervous system
- CSS1
common small subunit 1
- CSS2
common small subunit 2
- DIC
differential interference contrast
- DNA
deoxyribonucleic acid
- DNase I
deoxyribonuclease I
- DRG
dorsal root ganglia
- dSarm
sterile α/Armadillo/Toll-Interleukin receptor homology domain protein
- EDL
extensor digitorum longus
- EGTA
Ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid
- GDC
granular disintegration of the axonal cytoskeleton
- GFP
green fluorescent protein
- HRP
horseradish peroxidase
- IA
intra-arterial
- IP
intraperitoneal
- IV
intravenous
- Kv
voltage-gated K+ channel
- MAP
microtubule-associated protein
- MCAO
middle cerebral artery occlusion
- Nav
voltage-gated Na+ channel
- NCX
Na+-Ca2+ exchanger
- NFH
neurofilament heavy
- NFL
neurofilament light
- NFM
neurofilament medium
- Nmat-1
nicotinamide mononucleotide adenyl transferase 1
- NMJ
neuromuscular junction
- NrCAM
neuronal cell adhesion molecule
- OGD
oxygen glucose deprivation
- PNS
peripheral nervous system
- SCG
sympathetic superior ganglia
- SCI
spinal cord injury
- TAI
traumatic axonal injury
- TBI
traumatic brain injury
- TTX
tetrodotoxin
- Ube4b
ubiquitination factor E4B
- UPS
ubiquitin-proteasome system
- YFP
yellow fluorescent protein
- ZNRF1
zinc and ring finger 1
Footnotes
The caspase inhibitor Z-VAD-FMK (carbobenzoxy-Val-Ala-Asp-fluoromethyl ketone; 100 μm) inhibits nuclear condensation, but not actin proteolysis, in human neutrophil cultures undergoing constitutive apoptosis (Brown et al., 1997).
These ex vivo studies often use white matter tissue taken acutely from the adult animal. In contrast, for example, axonal stretch studies typically use neuronal cultures. When comparing these investigations, it is important to recognize that the presence and density of channels and receptors may be developmentally regulated (Brown et al., 2001), or differ between white matter regions in the same animal (Tekkök et al., 2007).
Electron probe X-ray microanalysis measures total (free plus bound) concentrations of elements (Stys and Lopachin, 1998).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ai J, Liu E, Wang J, Chen Y, Yu J, Baker AJ. Calpain inhibitor MDL-28170 reduces the functional and structural deterioration of corpus callosum following fluid percussion injury. J Neurotrauma. 2007;24:960–78. doi: 10.1089/neu.2006.0224. [DOI] [PubMed] [Google Scholar]
- Amos LA, Schlieper D. Microtubules and MAPs. Adv Protein Chem. 2005;71:257–98. doi: 10.1016/S0065-3233(04)71007-4. [DOI] [PubMed] [Google Scholar]
- Araújo IM, Carreira BP, Pereira T, Santos PF, Soulet D, Inácio  , et al. Changes in calcium dynamics following the reversal of the sodium-calcium exchanger have a key role in AMPA receptor-mediated neurodegeneration via calpain activation in hippocampal neurons. Cell Death Differ. 2007;14:1635–46. doi: 10.1038/sj.cdd.4402171. [DOI] [PubMed] [Google Scholar]
- Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol. 2006;291:C1159–71. doi: 10.1152/ajpcell.00207.2006. [DOI] [PubMed] [Google Scholar]
- Arthur JSC, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–81. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam M, Andrabi SS, Sahr KE, Kamath L, Kuliopulos A, Chishti AH. Disruption of the mouse μ-calpain gene reveals an essential role in platelet function. Mol Cell Biol. 2001;21:2213–20. doi: 10.1128/MCB.21.6.2213-2220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–85. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Baxter B, Gillingwater TH, Parson SH. Rapid loss of motor nerve terminals following hypoxia-reperfusion injury occurs via mechanisms distinct from classic Wallerian degeneration. J Anat. 2008;212:827–35. doi: 10.1111/j.1469-7580.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Nógrádi A, Babetto E, Garcia-Alias G, Coleman MP. Mechanisms of axonal spheroid formation in central nervous system Wallerian degeneration. J Neuropathol Exp Neurol. 2010;69:455–72. doi: 10.1097/NEN.0b013e3181da84db. [DOI] [PubMed] [Google Scholar]
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–92. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- Berdowski J, Berg RA, Tijssen JGP, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–87. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel J-M, et al. βIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1001. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertipaglia I, Bourg N, Richard I, Pahlman A-K, Andersson L, James P, Carafoli E. A proteomic study of calpain-3 and its involvement in limb girdle muscular dystrophy type 2a. Cell Calcium. 2009;46:356–63. doi: 10.1016/j.ceca.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Bevers MB, Neumar RW. Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab. 2008;28:655–73. doi: 10.1038/sj.jcbfm.9600595. [DOI] [PubMed] [Google Scholar]
- Bevers MB, Lawrence E, Maronski M, Starr N, Amesquita M, Neumar RW. Knockdown of m-calpain increases survival of primary hippocampal neurons following NMDA excitotoxicity. J Neurochem. 2009;108:1237–50. doi: 10.1111/j.1471-4159.2008.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevers MB, Ingleton LP, Che D, Cole JT, Li L, Da T, et al. RNAi targeting μ-calpain increases neuron survival and preserves hippocampal function after global brain ischemia. Exp Neurol. 2010;224:170–7. doi: 10.1016/j.expneurol.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A, Dahl D, Nguyen BT, Crosby CJ. The fate of axonal debris in Wallerian degeneration of rat optic and sciatic nerves. Electron microscopy and immunofluorescence studies with neurofilament antisera. J Neuropathol Exp Neurol. 1981;40:537–50. doi: 10.1097/00005072-198109000-00005. [DOI] [PubMed] [Google Scholar]
- Billger M, Wallin M, Karlsson J-O. Proteolysis of tubulin and microtubule-associated proteins 1 and 2 by calpain I and II. Difference in sensitivity of assembled and disassembled microtubules. Cell Calcium. 1988;9:33–44. doi: 10.1016/0143-4160(88)90036-x. [DOI] [PubMed] [Google Scholar]
- Bloom GS, Schoenfeld TA, Vallee RB. Widespread distribution of the major polypeptide component of MAP 1 (Microtubule-associated protein 1) in the nervous system. J Cell Biol. 1984;98:320–30. doi: 10.1083/jcb.98.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–50. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- Brown AM, Westenbroek RE, Catterall WA, Ransom BR. Axonal L-type Ca2+ channels and anoxic injury in rat CNS white matter. J Neurophysiol. 2001;85:900–11. doi: 10.1152/jn.2001.85.2.900. [DOI] [PubMed] [Google Scholar]
- Brown SB, Bailey K, Savill J. Actin is cleaved during constitutive apoptosis. Biochem J. 1997;323:233–7. doi: 10.1042/bj3230233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büki A, Povlishock JT. All roads lead to disconnection? – Traumatic axonal injury revisited. Acta Neurochir (Wien) 2006;148:181–94. doi: 10.1007/s00701-005-0674-4. [DOI] [PubMed] [Google Scholar]
- Büki A, Siman R, Trojanowski JQ, Povlishock JT. The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J Neuropathol Exp Neurol. 1999;58:365–75. doi: 10.1097/00005072-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Büki A, Okonkwo DO, Wang KKW, Povlishock JT. Cytochrome c release and caspase activation in traumatic axonal injury. J Neurosci. 2000;20:2825–34. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buki A, Farkas O, Doczi T, Povlishock JT. Preinjury administration of the calpain inhibitor MDL-28170 attenuates traumatically induced axonal injury. J Neurotrauma. 2003;20:261–8. doi: 10.1089/089771503321532842. [DOI] [PubMed] [Google Scholar]
- Cheever TR, Ervasti JM. Actin isoforms in neuronal development and function. Int Rev Cell Mol Biol. 2013;301:157–213. doi: 10.1016/B978-0-12-407704-1.00004-X. [DOI] [PubMed] [Google Scholar]
- Chen X-H, Siman R, Iwata A, Meaney DF, Trojanowski JQ, Smith DH. Long-term accumulation of amyloid-β, β-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol. 2004;165:357–71. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–98. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR. Wallerian degeneration, Wlds, and Nmnat. Annu Rev Neurosci. 2010;33:245–67. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–7. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- Couto LA, Narciso MS, Hokoç JN, Martinez AMB. Calpain inhibitor 2 prevents axonal degeneration of opossum optic nerve fibers. J Neurosci Res. 2004;77:410–9. doi: 10.1002/jnr.20170. [DOI] [PubMed] [Google Scholar]
- Czeiter E, Büki A, Bukovics P, Farkas O, Pál J, Kövesdi E, et al. Calpain inhibition reduces axolemmal leakage in traumatic axonal injury. Molecules. 2009;14:5115–23. doi: 10.3390/molecules14125115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czogalla A, Sikorski AF. Spectrin and calpain: a ‘target’ and a ‘sniper’ in the pathology of neuronal cells. Cell Mol Life Sci. 2005;62:1913–24. doi: 10.1007/s00018-005-5097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Guyton MK, Butler JT, Ray SK, Banik NL. Activation of calpain and caspase pathways in demyelination and neurodegeneration in animal model of multiple sclerosis. CNS Neurol Disord Drug Targets. 2008;7:313–20. doi: 10.2174/187152708784936699. [DOI] [PubMed] [Google Scholar]
- Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. Axon physiology. Physiol Rev. 2011;91:555–602. doi: 10.1152/physrev.00048.2009. [DOI] [PubMed] [Google Scholar]
- Donat JR, Wiśniewski HM. The spatio-temporal pattern of Wallerian degeneration in mammalian peripheral nerves. Brain Res. 1973;53:41–53. doi: 10.1016/0006-8993(73)90765-8. [DOI] [PubMed] [Google Scholar]
- Duflocq A, Le Bras B, Bullier E, Couraud F, Davenne M. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol Cell Neurosci. 2008;39:180–92. doi: 10.1016/j.mcn.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Dutt P, Croall DE, Arthur JSC, De Veyra T, Williams K, Elce JS, Greer PA. m-Calpain is required for preimplantation embryonic development in mice. BMC Dev Biol. 2006;6:3. doi: 10.1186/1471-213X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb DE, Povlishock JT. Axonal damage in severe traumatic brain injury: an experimental study in cat. Acta Neuropathol. 1988;76:347–58. doi: 10.1007/BF00686971. [DOI] [PubMed] [Google Scholar]
- Fern R, Ransom BR, Waxman SG. Voltage-gated calcium channels in CNS white matter: role in anoxic injury. J Neurophysiol. 1995;74:369–77. doi: 10.1152/jn.1995.74.1.369. [DOI] [PubMed] [Google Scholar]
- Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci. 2000;20:1333–41. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer I, Romano-Clarke G, Grynspan F. Calpain-mediated proteolysis of microtubule associated proteins MAP1B and MAP2 in developing brain. Neurochem Res. 1991;16:891–8. doi: 10.1007/BF00965538. [DOI] [PubMed] [Google Scholar]
- Friedrich P, Papp H, Halasy K, Farkas A, Farkas B, Tompa P, Kása P. Differential distribution of calpain small subunit 1 and 2 in rat brain. Eur J Neurosci. 2004;19:1819–25. doi: 10.1111/j.1460-9568.2004.03313.x. [DOI] [PubMed] [Google Scholar]
- Gaskin F, Cantor CR, Shelanski ML. Biochemical studies on the in vitro assembly and disassembly of microtubules. Ann N Y Acad Sci. 1975;253:133–46. doi: 10.1111/j.1749-6632.1975.tb19197.x. [DOI] [PubMed] [Google Scholar]
- Geddes JW, Saatman KE. Targeting individual calpain isoforms for neuroprotection. Exp Neurol. 2010;226:6–7. doi: 10.1016/j.expneurol.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564–74. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- George EB, Glass JD, Griffin JW. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci. 1995;15:6445–52. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz SB, Cianci CD, Iyer R, Pradhan D, Wang KKW, Morrow JS. Sequential degradation of αII and βII spectrin by calpain in glutamate or maitotoxin-stimulated cells. Biochemistry. 2007;46:502–13. doi: 10.1021/bi061504y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD. Wallerian degeneration as a window to peripheral neuropathy. J Neurol Sci. 2004;220:123–4. doi: 10.1016/j.jns.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Glass JD, Culver DG, Levey AI, Nash NR. Very early activation of m-calpain in peripheral nerve during Wallerian degeneration. J Neurol Sci. 2002;196:9–20. doi: 10.1016/s0022-510x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Ransom BR. New light on white matter. Stroke. 2003;34:330–2. doi: 10.1161/01.str.0000054048.22626.b9. [DOI] [PubMed] [Google Scholar]
- Goll DE, Dayton WR, Singh I, Robson RM. Studies of the α-actinin/actin interaction in the Z-disk by using calpain. J Biol Chem. 1991;266:8501–10. [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Goodman SR. Discovery of nonerythroid spectrin to the demonstration of its key role in synaptic transmission. Brain Res Bull. 1999;50:345–6. doi: 10.1016/s0361-9230(99)00098-2. [DOI] [PubMed] [Google Scholar]
- Guroff G. A neutral, calcium-activated proteinase from the soluble fraction of rat brain. J Biol Chem. 1964;239:149–55. [PubMed] [Google Scholar]
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–79. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Hamakubo T, Kannagi R, Murachi T, Matus A. Distribution of calpains I and II in rat brain. J Neurosci. 1986;6:3103–11. doi: 10.1523/JNEUROSCI.06-11-03103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AS, Morrow JS. Calmodulin and calcium-dependent protease I coordinately regulate the interaction of fodrin with actin. Proc Natl Acad Sci U S A. 1990;87:3009–13. doi: 10.1073/pnas.87.8.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S, Doi N, Kitamura F, Sorimachi H. Stomach-specific calpain, nCL-2/calpain 8, is active without calpain regulatory subunit and oligomerizes through C2-like domains. J Biol Chem. 2007;282:27847–56. doi: 10.1074/jbc.M703168200. [DOI] [PubMed] [Google Scholar]
- Hu R-J, Bennett V. In vitro proteolysis of brain spectrin by calpain I inhibits association of spectrin with ankyrin-independent membrane binding site(s) J Biol Chem. 1991;266:18200–5. [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22:341–53. [PubMed] [Google Scholar]
- Imaizumi T, Kocsis JD, Waxman SG. The role of voltage-gated Ca2+ channels in anoxic injury of spinal cord white matter. Brain Res. 1999;817:84–92. doi: 10.1016/s0006-8993(98)01214-1. [DOI] [PubMed] [Google Scholar]
- Iwata A, Stys PK, Wolf JA, Chen X-H, Taylor AG, Meaney DF, Smith DH. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci. 2004;24:4605–13. doi: 10.1523/JNEUROSCI.0515-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari SS, Maxwell WL, Neilson M, Graham DI. Axonal cytoskeletal changes after non-disruptive axonal injury. J Neurocytol. 1997;26:207–21. doi: 10.1023/a:1018588114648. [DOI] [PubMed] [Google Scholar]
- Jafari SS, Nielson M, Graham DI, Maxwell WL. Axonal cytoskeletal changes after nondisruptive axonal injury. II. Intermediate sized axons. J Neurotrauma. 1998;15:955–66. doi: 10.1089/neu.1998.15.955. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Stys PK. Calpain inhibitors confer biochemical, but not electrophysiological, protection against anoxia in rat optic nerves. J Neurochem. 2000;74:2101–7. doi: 10.1046/j.1471-4159.2000.0742101.x. [DOI] [PubMed] [Google Scholar]
- Job D, Fischer EH, Margolis RL. Rapid disassembly of cold-stable microtubules by calmodulin. Proc Natl Acad Sci U S A. 1981;78:4679–82. doi: 10.1073/pnas.78.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GVW, Jope RS, Binder LI. Proteolysis of tau by calpain. Biochem Biophys Res Commun. 1989;163:1505–11. doi: 10.1016/0006-291x(89)91150-9. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. The applied neuropathology of human spinal cord injury. Spinal Cord. 1999;37:79–88. doi: 10.1038/sj.sc.3100807. [DOI] [PubMed] [Google Scholar]
- Kamakura K, Ishiura S, Sugita H, Toyokura Y. Identification of Ca2+-activated neutral protease in the peripheral nerve and its effects on neurofilament degeneration. J Neurochem. 1983;40:908–13. doi: 10.1111/j.1471-4159.1983.tb08072.x. [DOI] [PubMed] [Google Scholar]
- Kamakura K, Ishiura S, Suzuki K, Sugita H, Toyokura Y. Calcium-activated neutral protease in the peripheral nerve, which requires μM order Ca2+, and its effect on the neurofilament triplet. J Neurosci Res. 1985;13:391–403. doi: 10.1002/jnr.490130306. [DOI] [PubMed] [Google Scholar]
- Kar P, Samanta K, Shaikh S, Chowdhury A, Chakraborti T, Chakraborti S. Mitochondrial calpain system: an overview. Arch Biochem Biophys. 2010;495:1–7. doi: 10.1016/j.abb.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Keith C, DiPaola M, Maxfield FR, Shelanski ML. Microinjection of Ca++-calmodulin causes a localized depolymerization of microtubules. J Cell Biol. 1983;97:1918–24. doi: 10.1083/jcb.97.6.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BJ, Farkas O, Lifshitz J, Povlishock JT. Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and Wallerian degeneration. Exp Neurol. 2006;198:350–60. doi: 10.1016/j.expneurol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–7. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Khaitlina SY. Functional specificity of actin isoforms. Int Rev Cytol. 2001;202:35–98. doi: 10.1016/s0074-7696(01)02003-4. [DOI] [PubMed] [Google Scholar]
- Khaitlina SY, Strzelecka-Golaszewska H. Role of the DNase-I-binding loop in dynamic properties of actin filament. Biophys J. 2002;82:321–34. doi: 10.1016/S0006-3495(02)75397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilinc D, Gallo G, Barbee KA. Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp Neurol. 2008;212:422–30. doi: 10.1016/j.expneurol.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Kilinc D, Gallo G, Barbee KA. Mechanical membrane injury induces axonal beading through localized activation of calpain. Exp Neurol. 2009;219:553–61. doi: 10.1016/j.expneurol.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilinc D, Peyrin J-M, Soubeyre V, Magnifico S, Saias L, Viovy J-L, Brugg B. Wallerian-like degeneration of central neurons after synchronized and geometrically registered mass axotomy in a three-compartmental microfluidic chip. Neurotox Res. 2011;19:149–61. doi: 10.1007/s12640-010-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011;124:314–23. doi: 10.1161/CIRCULATIONAHA.111.018820. [DOI] [PubMed] [Google Scholar]
- Kinbara K, Ishiura S, Tomioka S, Sorimachi H, Jeong S-Y, Amano S, et al. Purification of native p94, a muscle-specific calpain, and characterization of its autolysis. Biochem J. 1998;335:589–96. doi: 10.1042/bj3350589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A. 2010;107:6064–9. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, Soriano P. βIV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–48. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König N, Raynaud F, Feane H, Durand M, Mestre-Francès N, Rossel M, et al. Calpain 3 is expressed in astrocytes of rat and Microcebus brain. J Chem Neuroanat. 2003;25:129–36. doi: 10.1016/s0891-0618(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. Ankyrin G. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270:2352–9. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Veselovsky NS, Tsyndrenko AY. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons—I. Sodium currents. Neuroscience. 1981;6:2423–30. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Letourneau PC. Actin in axons: stable scaffolds and dynamic filaments. Results Probl Cell Differ. 2009;48:65–90. doi: 10.1007/400_2009_15. [DOI] [PubMed] [Google Scholar]
- Li S, Mealing GAR, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci. 1999;19:RC16, 1–9. doi: 10.1523/JNEUROSCI.19-14-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfvenberg L, Backman L. Calpain-induced proteolysis of β-spectrins. FEBS Lett. 1999;443:89–92. doi: 10.1016/s0014-5793(98)01697-4. [DOI] [PubMed] [Google Scholar]
- Ma H, Fukiage C, Kim YH, Duncan MK, Reed NA, Shih M, et al. Characterization and expression of calpain 10. A novel ubiquitous calpain with nuclear localization. J Biol Chem. 2001;276:28525–31. doi: 10.1074/jbc.M100603200. [DOI] [PubMed] [Google Scholar]
- Ma M, Li L, Wang X, Bull DL, Shofer FS, Meaney DF, Neumar RW. Short-duration treatment with the calpain inhibitor MDL-28170 does not protect axonal transport in an in vivo model of traumatic axonal injury. J Neurotrauma. 2012a;29:445–51. doi: 10.1089/neu.2011.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Shofer FS, Neumar RW. Calpastatin overexpression protects axonal transport in an in vivo model of traumatic axonal injury. J Neurotrauma. 2012b;29:2555–63. doi: 10.1089/neu.2012.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Ferguson TA, Schoch KM, Li J, Qian Y, Shofer FS, et al. Calpains mediate axonal cytoskeleton disintegration during Wallerian degeneration. Neurobiol Dis. 2013;56:34–46. doi: 10.1016/j.nbd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni RB, Cambiazo V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol Rev. 1995;75:835–64. doi: 10.1152/physrev.1995.75.4.835. [DOI] [PubMed] [Google Scholar]
- Mahajan VB, Skeie JM, Bassuk AG, Fingert JH, Braun TA, Daggett HT, et al. Calpain-5 mutations cause autoimmune uveitis, retinal neovascularization, and photoreceptor degeneration. PLoS Genet. 2012;8:e1003001. doi: 10.1371/journal.pgen.1003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkitie J, Teräväinen H. Ultrastructure of striated muscle of the rat after temporary ischemia. Acta Neuropathol. 1977;37:237–45. doi: 10.1007/BF00686885. [DOI] [PubMed] [Google Scholar]
- Malik MN, Fenko MD, Iqbal K, Wisniewski HM. Purification and characterization of two forms of Ca2+-activated neutral protease from calf brain. J Biol Chem. 1983;258:8955–62. [PubMed] [Google Scholar]
- Malik MN, Ramaswamy S, Tuzio H, Shiekh AM, Fenko MD, Wisniewski HM, Howard RG. Micromolar Ca2+ requiring protease from human platelets: purification, partial characterization and effect on the cytoskeletal proteins. Life Sci. 1987;40:593–604. doi: 10.1016/0024-3205(87)90374-2. [DOI] [PubMed] [Google Scholar]
- Marcilhac A, Raynaud F, Clerc I, Benyamin Y. Detection and localization of calpain 3-like protease in a neuronal cell line: possible regulation of apoptotic cell death through degradation of nuclear IκBα. Int J Biochem Cell Biol. 2006;38:2128–40. doi: 10.1016/j.biocel.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Markgraf CG, Velayo NL, Johnson MP, McCarty DR, Medhi S, Koehl JR, et al. Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke. 1998;29:152–8. doi: 10.1161/01.str.29.1.152. [DOI] [PubMed] [Google Scholar]
- Marques SA, Taffarel M, Martinez AMB. Participation of neurofilament proteins in axonal dark degeneration of rat’s optic nerves. Brain Res. 2003;969:1–13. doi: 10.1016/s0006-8993(02)03834-9. [DOI] [PubMed] [Google Scholar]
- Mata M, Kupina N, Fink DJ. Calpain II in rat peripheral nerve. Brain Res. 1991;564:328–31. doi: 10.1016/0006-8993(91)91471-c. [DOI] [PubMed] [Google Scholar]
- Maxwell WL. Histopathological changes at central nodes of Ranvier after stretch-injury. Microsc Res Tech. 1996;34:522–35. doi: 10.1002/(SICI)1097-0029(19960815)34:6<522::AID-JEMT4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Graham DI. Loss of axonal microtubules and neurofilaments after stretch-injury to guinea pig optic nerve fibers. J Neurotrauma. 1997;14:603–14. doi: 10.1089/neu.1997.14.603. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Watt C, Graham DI, Gennarelli TA. Ultrastructural evidence of axonal shearing as a result of lateral acceleration of the head in non-human primates. Acta Neuropathol. 1993;86:136–44. doi: 10.1007/BF00334880. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–40. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- McCracken E, Hunter AJ, Patel S, Graham DI, Dewar D. Calpain activation and cytoskeletal protein breakdown in the corpus callosum of head-injured patients. J Neurotrauma. 1999;16:749–61. doi: 10.1089/neu.1999.16.749. [DOI] [PubMed] [Google Scholar]
- McGonigal R, Rowan EG, Greenshields KN, Halstead SK, Humphreys PD, Rother RP, et al. Anti-GD1a antibodies activate complement and calpain to injure distal motor nodes of Ranvier in mice. Brain. 2010;133:1944–60. doi: 10.1093/brain/awq119. [DOI] [PubMed] [Google Scholar]
- Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–30. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- Minelli A, Castaldo P, Gobbi P, Salucci S, Magi S, Amoroso S. Cellular and subcellular localization of Na+-Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium. 2007;41:221–34. doi: 10.1016/j.ceca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Narciso MS, Hokoç JN, Martinez AMB. Watery and dark axons in Wallerian degeneration of the opossum’s optic nerve: different patterns of cytoskeletal breakdown? An Acad Bras Cienc. 2001;73:231–43. doi: 10.1590/s0001-37652001000200008. [DOI] [PubMed] [Google Scholar]
- Nikolaeva MA, Mukherjee B, Stys PK. Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J Neurosci. 2005;25:9960–7. doi: 10.1523/JNEUROSCI.2003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Lewis SA, Cowan NJ. The microtubule binding domain of microtubule-associated protein MAP1B contains a repeated sequence motif unrelated to that of MAP2 and tau. J Cell Biol. 1989;109:3367–76. doi: 10.1083/jcb.109.6.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien ET, Salmon ED, Erickson HP. How calcium causes microtubule depolymerization. Cell Motil Cytoskeleton. 1997;36:125–35. doi: 10.1002/(SICI)1097-0169(1997)36:2<125::AID-CM3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, et al. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci. 2006;26:5230–9. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hanlon GM, Humphreys PD, Goldman RS, Halstead SK, Bullens RWM, Plomp JJ, et al. Calpain inhibitors protect against axonal degeneration in a model of anti-ganglioside antibody-mediated motor nerve terminal injury. Brain. 2003;126:2497–509. doi: 10.1093/brain/awg254. [DOI] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–4. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Malek S, Coderre E, Stys PK. Complex interplay between glutamate receptors and intracellular Ca2+ stores during ischaemia in rat spinal cord white matter. J Physiol. 2006;577:191–204. doi: 10.1113/jphysiol.2006.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Coderre E, Basak A, Chen A, Zamponi GW, Hameed S, et al. Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors. Ann Neurol. 2009a;65:151–9. doi: 10.1002/ana.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Coderre E, Zamponi GW, Hameed S, Yin X, Trapp BD, Stys PK. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann Neurol. 2009b;65:160–6. doi: 10.1002/ana.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannaccione A, Secondo A, Molinaro P, D’Avanzo C, Cantile M, Esposito A, et al. A new concept: Aβ1-42 generates a hyperfunctional proteolytic NCX3 fragment that delays caspase-12 activation and neuronal death. J Neurosci. 2012;32:10609–17. doi: 10.1523/JNEUROSCI.6429-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–7. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- Peles E, Salzer JL. Molecular domains of myelinated axons. Curr Opin Neurobiol. 2000;10:558–65. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Perlmutter LS, Siman R, Gall C, Seubert P, Baudry M, Lynch G. The ultrastructural localization of calcium-activated protease “calpain” in rat brain. Synapse. 1988;2:79–88. doi: 10.1002/syn.890020111. [DOI] [PubMed] [Google Scholar]
- Perlmutter LS, Gall C, Baudry M, Lynch G. Distribution of calcium-activated protease calpain in the rat brain. J Comp Neurol. 1990;296:269–76. doi: 10.1002/cne.902960207. [DOI] [PubMed] [Google Scholar]
- Perrot R, Berges R, Bocquet A, Eyer J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol. 2008;38:27–65. doi: 10.1007/s12035-008-8033-0. [DOI] [PubMed] [Google Scholar]
- Petrescu N, Micu I, Malek S, Ouardouz M, Stys PK. Sources of axonal calcium loading during in vitro ischemia of rat dorsal roots. Muscle Nerve. 2007;35:451–7. doi: 10.1002/mus.20731. [DOI] [PubMed] [Google Scholar]
- Pettus EH, Povlishock JT. Characterization of a distinct set of intra-axonal ultrastructural changes associated with traumatically induced alteration in axolemmal permeability. Brain Res. 1996;722:1–11. doi: 10.1016/0006-8993(96)00113-8. [DOI] [PubMed] [Google Scholar]
- Pettus EH, Christman CW, Giebel ML, Povlishock JT. Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J Neurotrauma. 1994;11:507–22. doi: 10.1089/neu.1994.11.507. [DOI] [PubMed] [Google Scholar]
- Pietsch M, Chua KCH, Abell AD. Calpains: attractive targets for the development of synthetic inhibitors. Curr Top Med Chem. 2010;10:270–93. doi: 10.2174/156802610790725489. [DOI] [PubMed] [Google Scholar]
- Posmantur R, Kampfl A, Siman R, Liu SJ, Zhao X, Clifton GL, Hayes RL. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience. 1997;77:875–88. doi: 10.1016/s0306-4522(96)00483-6. [DOI] [PubMed] [Google Scholar]
- Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Becker DP, Cheng CLY, Vaughan GW. Axonal change in minor head injury. J Neuropathol Exp Neurol. 1983;42:225–42. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Marmarou A, McIntosh T, Trojanowski JQ, Moroi J. Impact acceleration injury in the rat: evidence for focal axolemmal change and related neurofilament sidearm alteration. J Neuropathol Exp Neurol. 1997;56:347–59. [PubMed] [Google Scholar]
- Rasband MN. Composition, assembly, and maintenance of excitable membrane domains in myelinated axons. Semin Cell Dev Biol. 2011;22:178–84. doi: 10.1016/j.semcdb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK. Currently evaluated calpain and caspase inhibitors for neuroprotection in experimental brain ischemia. Curr Med Chem. 2006;13:3425–40. doi: 10.2174/092986706779010342. [DOI] [PubMed] [Google Scholar]
- Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:173–89. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- Ray SK, Samantaray S, Smith JA, Matzelle DD, Das A, Banik NL. Inhibition of cysteine proteases in acute and chronic spinal cord injury. Neurotherapeutics. 2011;8:180–6. doi: 10.1007/s13311-011-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Lewis JM, Savage MJ, Marcy VR, Pinsker LR, Siman R. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci. 1994;14:3934–44. doi: 10.1523/JNEUROSCI.14-06-03934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23:863–73. doi: 10.1002/(sici)1097-4598(200006)23:6<863::aid-mus4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Murai H, Bartus RT, Smith DH, Hayward NJ, Perri BR, McIntosh TK. Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc Natl Acad Sci U S A. 1996;93:3428–33. doi: 10.1073/pnas.93.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, Abai B, Grosvenor A, Vorwerk CK, Smith DH, Meaney DF. Traumatic axonal injury results in biphasic calpain activation and retrograde transport impairment in mice. J Cereb Blood Flow Metab. 2003;23:34–42. doi: 10.1097/01.WCB.0000035040.10031.B0. [DOI] [PubMed] [Google Scholar]
- Sandoval IV, Weber K. Calcium-induced inactivation of microtubule formation in brain extracts. Presence of a calcium-dependent protease acting on polymerization-stimulating microtubule-associated proteins. Eur J Biochem. 1978;92:463–70. doi: 10.1111/j.1432-1033.1978.tb12768.x. [DOI] [PubMed] [Google Scholar]
- Sato C, Nishizawa K, Nakayama T, Nakamura H, Yoshimura N, Takano E, Murachi T. Rapid proteolysis of brain MAP-1 related cytoskeleton-associated 350kd protein by purified calpain. Cell Struct Funct. 1986;11:253–7. doi: 10.1247/csf.11.253. [DOI] [PubMed] [Google Scholar]
- Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83:174–91. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Schád É , Farkas A, Jékely G, Tompa P, Friedrich P. A novel human small subunit of calpains. Biochem J. 2002;362:383–8. doi: 10.1042/0264-6021:3620383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Jha S, Liu F, Akella T, McCullough LD, Rasband MN. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J Neurosci. 2009;29:13242–54. doi: 10.1523/JNEUROSCI.3376-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer WW. Calcium-induced degeneration of axoplasm in isolated segments of rat peripheral nerve. Brain Res. 1974;69:203–15. doi: 10.1016/0006-8993(74)90002-x. [DOI] [PubMed] [Google Scholar]
- Schlaepfer WW, Lee C, Trojanowski JQ, Lee VM-Y. Persistence of immunoreactive neurofilament protein breakdown products in transected rat sciatic nerve. J Neurochem. 1984;43:857–64. doi: 10.1111/j.1471-4159.1984.tb12809.x. [DOI] [PubMed] [Google Scholar]
- Schlaepfer WW, Lee C, Lee VM-Y, Zimmerman U-JP. An immunoblot study of neurofilament degradation in situ and during calcium-activated proteolysis. J Neurochem. 1985;44:502–9. doi: 10.1111/j.1471-4159.1985.tb05442.x. [DOI] [PubMed] [Google Scholar]
- Schoch KM, von Reyn CR, Bian J, Telling GC, Meaney DF, Saatman KE. Brain injury-induced proteolysis is reduced in a novel calpastatin-overexpressing transgenic mouse. J Neurochem. 2013;125:909–20. doi: 10.1111/jnc.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, Arama E, Yaron A. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J Neurosci. 2010;30:6375–86. doi: 10.1523/JNEUROSCI.0922-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Yang C, Zhang L, Cook P, Pike B, Hill WD. Characterization of the bovine neurofilament NF-M protein and cDNA sequence, and identification of in vitro and in vivo calpain cleavage sites. Biochem Biophys Res Commun. 2004;325:619–25. doi: 10.1016/j.bbrc.2004.09.223. [DOI] [PubMed] [Google Scholar]
- Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, et al. Neurofascins are required to establish axonal domains for salutatory conduction. Neuron. 2005;48:737–42. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Shy ME, Garbern JY, Kamholz J. Hereditary motor and sensory neuropathies: a biological perspective. Lancet Neurol. 2002;1:110–8. doi: 10.1016/s1474-4422(02)00042-x. [DOI] [PubMed] [Google Scholar]
- Siman R, Noszek JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–87. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Siman R, Gall C, Perlmutter LS, Christian C, Baudry M, Lynch G. Distribution of calpain I, an enzyme associated with degenerative activity, in rat brain. Brain Res. 1985;347:399–403. doi: 10.1016/0006-8993(85)90208-2. [DOI] [PubMed] [Google Scholar]
- Singleton RH, Zhu J, Stone JR, Povlishock JT. Traumatically induced axotomy adjacent to the soma does not result in acute neuronal death. J Neurosci. 2002;22:791–802. doi: 10.1523/JNEUROSCI.22-03-00791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Meaney DF. Axonal damage in traumatic brain injury. Neuroscientist. 2000;6:483–95. [Google Scholar]
- Smith DH, Wolf JA, Lusardi TA, Lee VM-Y, Meaney DF. High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. J Neurosci. 1999;19:4263–9. doi: 10.1523/JNEUROSCI.19-11-04263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi H, Kinbara K, Kimura S, Takahashi M, Ishiura S, Sasagawa N, et al. Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence. J Biol Chem. 1995;270:31158–62. doi: 10.1074/jbc.270.52.31158. [DOI] [PubMed] [Google Scholar]
- Sorimachi H, Hata S, Ono Y. Expanding members and roles of the calpain superfamily and their genetically modified animals. Exp Anim. 2010;59:549–66. doi: 10.1538/expanim.59.549. [DOI] [PubMed] [Google Scholar]
- Staal JA, Dickson TC, Chung RS, Vickers JC. Cyclosporin-A treatment attenuates delayed cytoskeletal alterations and secondary axotomy following mild axonal stretch injury. Dev Neurobiol. 2007;67:1831–42. doi: 10.1002/dneu.20552. [DOI] [PubMed] [Google Scholar]
- Staal JA, Dickson TC, Chung RS, Vickers JC. Disruption of the ubiquitin proteasome system following axonal stretch injury accelerates progression to secondary axotomy. J Neurotrauma. 2009;26:781–8. doi: 10.1089/neu.2008.0669. [DOI] [PubMed] [Google Scholar]
- Staal JA, Dickson TC, Gasperini R, Liu Y, Foa L, Vickers JC. Initial calcium release from intracellular stores followed by calcium dysregulation is linked to secondary axotomy following transient axonal stretch injury. J Neurochem. 2010;112:1147–55. doi: 10.1111/j.1471-4159.2009.06531.x. [DOI] [PubMed] [Google Scholar]
- Stanton RA, Gernert KM, Nettles JH, Aneja R. Drugs that target dynamic microtubules: a new molecular perspective. Med Res Rev. 2011;31:443–81. doi: 10.1002/med.20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen I, Waxman SG, Mills L, Stys PK. Immunolocalization of the Na+-Ca2+ exchanger in mammalian myelinated axons. Brain Res. 1997;776:1–9. doi: 10.1016/s0006-8993(97)00868-8. [DOI] [PubMed] [Google Scholar]
- Stone JR, Singleton RH, Povlishock JT. Intra-axonal neurofilament compaction does not evoke local axonal swelling in all traumatically injured axons. Exp Neurol. 2001;172:320–31. doi: 10.1006/exnr.2001.7818. [DOI] [PubMed] [Google Scholar]
- Stone JR, Okonkwo DO, Singleton RH, Mutlu LK, Helm GA, Povlishock JT. Caspase-3-mediated cleavage of amyloid precursor protein and formation of amyloid β peptide in traumatic axonal injury. J Neurotrauma. 2002;19:601–14. doi: 10.1089/089771502753754073. [DOI] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–30. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Stys PK. General mechanisms of axonal damage and its prevention. J Neurol Sci. 2005;233:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Stys PK, Jiang Q. Calpain-dependent neurofilament breakdown in anoxic and ischemic rat central axons. Neurosci Lett. 2002;328:150–4. doi: 10.1016/s0304-3940(02)00469-x. [DOI] [PubMed] [Google Scholar]
- Stys PK, Lopachin RM. Mechanisms of calcium and sodium fluxes in anoxic myelinated central nervous system axons. Neuroscience. 1998;82:21–32. doi: 10.1016/s0306-4522(97)00230-3. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53(Suppl 1):S12–8. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- Talbot JD, David G, Barrett EF, Barrett JN. Calcium dependence of damage to mouse motor nerve terminals following oxygen/glucose deprivation. Exp Neurol. 2012;234:95–104. doi: 10.1016/j.expneurol.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Schomer MD, Patel AR, Baas PW, Smith DH. Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration. FASEB J. 2010;24:1401–10. doi: 10.1096/fj.09-142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Schomer MD, Johnson VE, Baas PW, Stewart W, Smith DH. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol. 2012;233:364–72. doi: 10.1016/j.expneurol.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkök SB, Ye Z, Ransom BR. Excitotoxic mechanisms of ischemic injury in myelinated white matter. J Cereb Blood Flow Metab. 2007;27:1540–52. doi: 10.1038/sj.jcbfm.9600455. [DOI] [PubMed] [Google Scholar]
- Tömböl T, Pataki G, Németh A, Hamar J. Ultrastructural changes of the neuromuscular junction in reperfusion injury. Cells Tissues Organs. 2002;170:139–50. doi: 10.1159/000046187. [DOI] [PubMed] [Google Scholar]
- Tompa P, Buzder-Lantos P, Tantos A, Farkas A, Szilágyi A, Bánóczi Z, et al. On the sequential determinants of calpain cleavage. J Biol Chem. 2004;279:20775–85. doi: 10.1074/jbc.M313873200. [DOI] [PubMed] [Google Scholar]
- Touma E, Kato S, Fukui K, Koike T. Calpain-mediated cleavage of collapsin response mediator protein(CRMP)-2 during neurite degeneration in mice. Eur J Neurosci. 2007;26:3368–81. doi: 10.1111/j.1460-9568.2007.05943.x. [DOI] [PubMed] [Google Scholar]
- Trinchese F, Fa’ M, Liu S, Zhang H, Hidalgo A, Schmidt SD, et al. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest. 2008;118:2796–807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill SM, Goldberg MP. Hypoxic injury of isolated axons is independent of ionotropic glutamate receptors. Neurobiol Dis. 2007;25:284–90. doi: 10.1016/j.nbd.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–79. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Villa PG, Henzel WJ, Sensenbrenner M, Henderson CE, Pettmann B. Calpain inhibitors, but not caspase inhibitors, prevent actin proteolysis and DNA fragmentation during apoptosis. J Cell Sci. 1998;111:713–22. doi: 10.1242/jcs.111.6.713. [DOI] [PubMed] [Google Scholar]
- von Reyn CR, Spaethling JM, Mesfin MN, Ma M, Neumar RW, Smith DH, et al. Calpain mediates proteolysis of the voltage-gated sodium channel α-subunit. J Neurosci. 2009;29:10350–6. doi: 10.1523/JNEUROSCI.2339-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghray A, Wang D-s, McKinsey D, Hayes RL, Wang KKW. Molecular cloning and characterization of rat and human calpain-5. Biochem Biophys Res Commun. 2004;324:46–51. doi: 10.1016/j.bbrc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Wakatsuki S, Saitoh F, Araki T. ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation. Nat Cell Biol. 2011;13:1415–23. doi: 10.1038/ncb2373. [DOI] [PubMed] [Google Scholar]
- Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos Trans R Soc London. 1850;140:423–9. [Google Scholar]
- Wang JT, Barres BA. Axon degeneration: where the Wlds things are. Curr Biol. 2012;22:R221–3. doi: 10.1016/j.cub.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M-S, Wu Y, Culver DG, Glass JD. Pathogenesis of axonal degeneration: parallels between Wallerian degeneration and vincristine neuropathy. J Neuropathol Exp Neurol. 2000;59:599–606. doi: 10.1093/jnen/59.7.599. [DOI] [PubMed] [Google Scholar]
- Wang MS, Davis AA, Culver DG, Wang Q, Powers JC, Glass JD. Calpain inhibition protects against Taxol-induced sensory neuropathy. Brain. 2004;127:671–9. doi: 10.1093/brain/awh078. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Black JA, Stys PK, Ransom BR. Ultrastructural concomitants of anoxic injury and early post-anoxic recovery in rat optic nerve. Brain Res. 1992;574:105–19. doi: 10.1016/0006-8993(92)90806-k. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Lindsten T, Raff MC, Thompson CB. The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ. 2003;10:260–1. doi: 10.1038/sj.cdd.4401147. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci. 2001;21:1923–30. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Zhong G, Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 2013;339:452–6. doi: 10.1126/science.1232251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L-S, Ksiezak-Reding H. Calpain-induced proteolysis of normal human tau and tau associated with paired helical filaments. Eur J Biochem. 1995;233:9–17. doi: 10.1111/j.1432-1033.1995.009_1.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN. βIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J Neurosci. 2004;24:7230–40. doi: 10.1523/JNEUROSCI.2125-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fukui K, Koike T, Zheng X. Induction of autophagy in neurite degeneration of mouse superior cervical ganglion neurons. Eur J Neurosci. 2007;26:2979–88. doi: 10.1111/j.1460-9568.2007.05914.x. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Murachi T, Tsukahara I. Degradation of actin and vimentin by calpain II, a Ca2+-dependent cysteine proteinase, in bovine lens. FEBS Lett. 1984;170:259–62. doi: 10.1016/0014-5793(84)81324-1. [DOI] [PubMed] [Google Scholar]
- Yu CG, Li Y, Raza K, Yu XX, Ghoshal S, Geddes JW. Calpain 1 knockdown improves tissue sparing and functional outcomes following spinal cord injury in rats. J Neurotrauma. 2013;30:427–33. doi: 10.1089/neu.2012.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M, Starling A. Calpains and disease. N Engl J Med. 2005;352:2413–23. doi: 10.1056/NEJMra043361. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, et al. Involvement of the ubiquitin-proteasome system in the early stages of Wallerian degeneration. Neuron. 2003;39:217–25. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]