Abstract
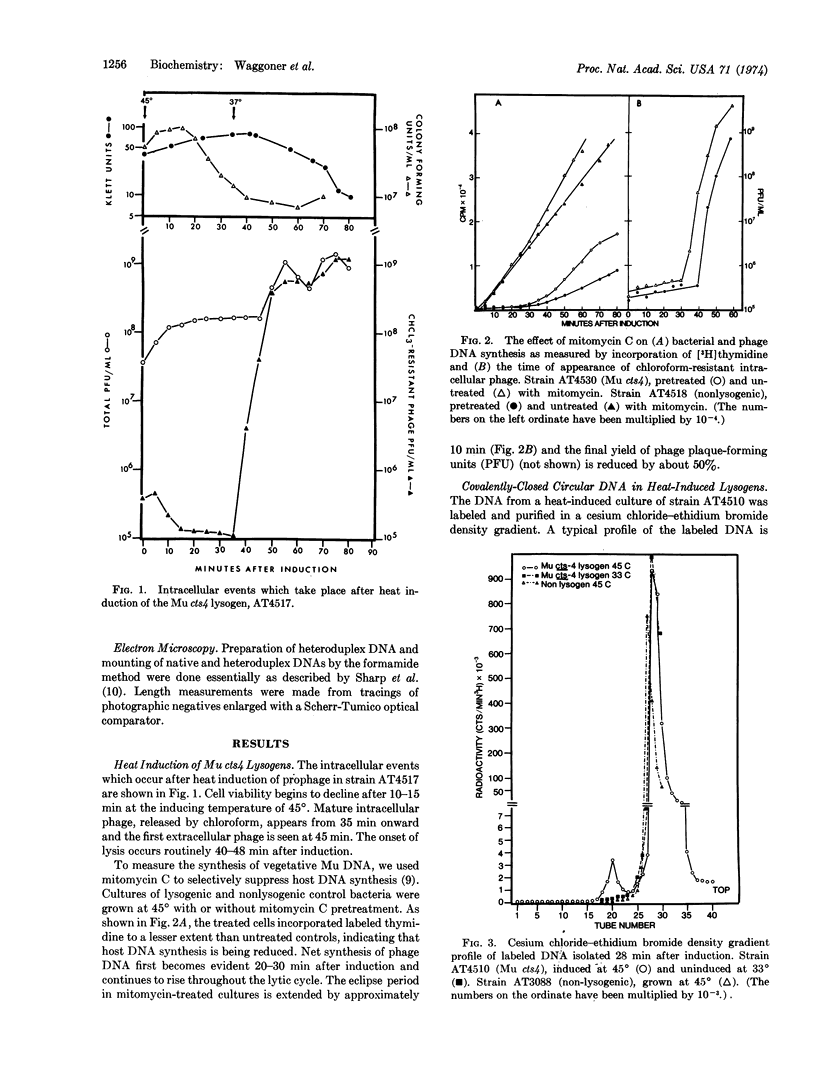
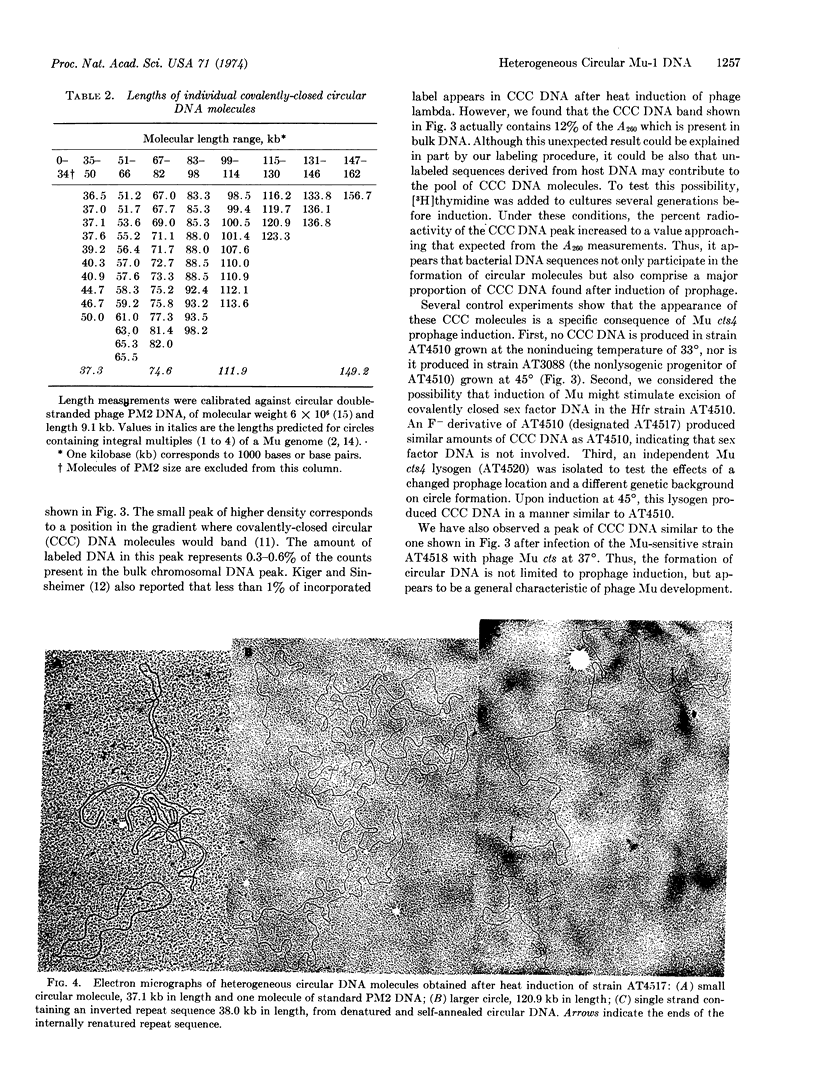
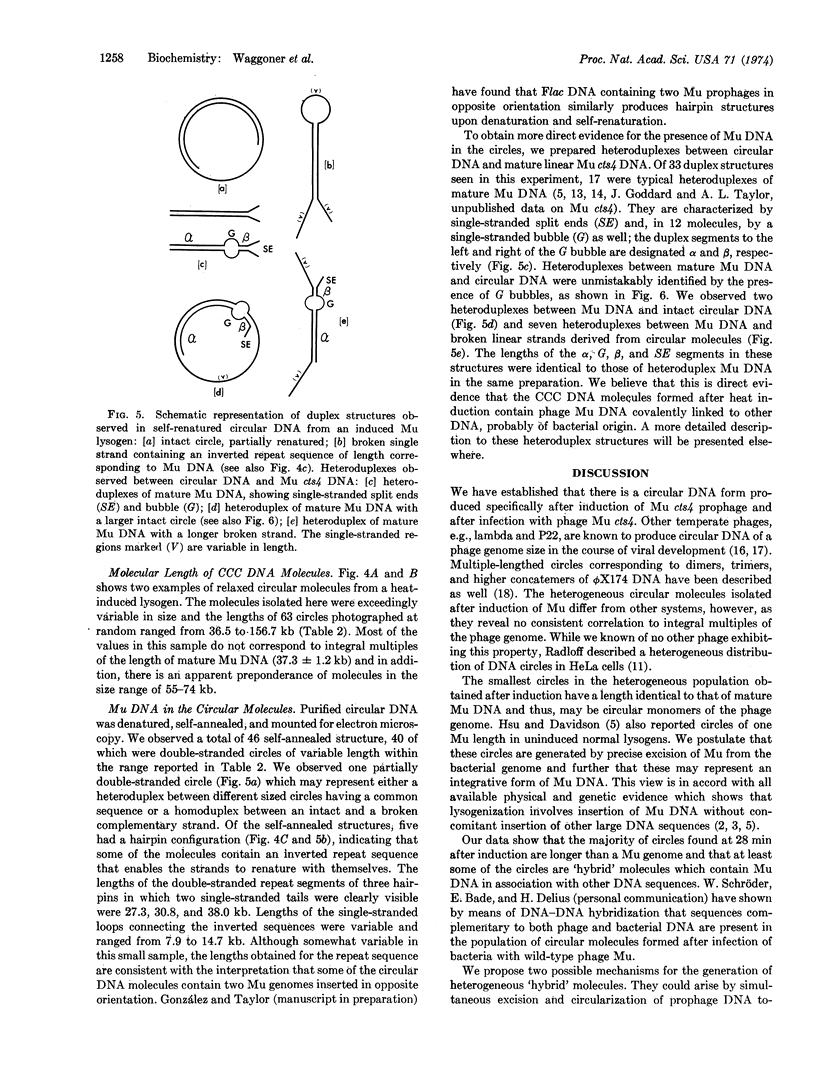
Covalently-closed circular DNA molecules are formed after induction of phage Mu cts4 and after infection with phage Mu cts4. The circular molecules obtained after induction have a molecular length range from 36.5 to 156.7 kilobases as measured by electron microscopic techniques. These heterogeneous molecules have no consistent correlation to exact multiples of a Mu genome equivalent (37.3 ± 1.2 kilobases). Direct evidence is given that these molecules contain phage Mu DNA that is covalently linked to other DNA sequences.
Keywords: cesium chloride-ethidium bromide gradients, electron microscopy, heat-inducible mutants
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J., Boram W., Bukhari A. I., Faelen M., Howe M., Metlay M., Taylor A. L., Toussaint A., Van de Putte P., Westmaas G. C. Summary of the genetic mapping of prophage Mu. Virology. 1973 Jul;54(1):90–92. doi: 10.1016/0042-6822(73)90117-7. [DOI] [PubMed] [Google Scholar]
- Bade E. G. Asymmetric transcription of bacteriophage Mu-1. J Virol. 1972 Dec;10(6):1205–1207. doi: 10.1128/jvi.10.6.1205-1207.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Daniell E., Abelson J., Kim J. S., Davidson N. Heteroduplex structures of bacteriophage Mu DNA. Virology. 1973 Jan;51(1):237–239. doi: 10.1016/0042-6822(73)90385-1. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. T., Davidson N. Structure of inserted bacteriophage Mu-1 DNA and physical mapping of bacterial genes by Mu-1 DNA insertion. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2823–2827. doi: 10.1073/pnas.69.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Sinsheimer R. L. Vegetative lambda DNA. IV. Fractionation of replicating lambda DNA on benzoylated-naphthoylated DEAE cellulose. J Mol Biol. 1969 Mar 28;40(3):467–490. doi: 10.1016/0022-2836(69)90166-1. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XV. Bacteriophage DNA synthesis in abortive infections with a set of conditional lethal mutants. J Mol Biol. 1967 Nov 28;30(1):69–80. doi: 10.1016/0022-2836(67)90244-6. [DOI] [PubMed] [Google Scholar]
- MACHATTIE L. A., THOMAS C. A., Jr DNA FROM BACTERIOPHAGE LAMBDA: MOLECULAR LENGTH AND CONFORMATION. Science. 1964 May 29;144(3622):1142–1144. doi: 10.1126/science.144.3622.1142. [DOI] [PubMed] [Google Scholar]
- Martuscelli J., Taylor A. L., Cummings D. J., Chapman V. A., DeLong S. S., Cañedo L. Electron microscopic evidence for linear insertion of bacteriophage MU-1 in lysogenic bacteria. J Virol. 1971 Oct;8(4):551–563. doi: 10.1128/jvi.8.4.551-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades M., Thomas C. A., Jr The P22 bacteriophage DNA molecule. II. Circular intracellular forms. J Mol Biol. 1968 Oct 14;37(1):41–61. doi: 10.1016/0022-2836(68)90072-7. [DOI] [PubMed] [Google Scholar]
- Rush M. G., Kleinschmidt A. K., Hellmann W., Warner R. C. Multiple-length rings in preparations of phi-X174 replicative form. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1676–1683. doi: 10.1073/pnas.58.4.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]




