Abstract
Fetal valproate syndrome (FVS) is caused by in utero exposure to the drug sodium valproate. Valproate is used worldwide for the treatment of epilepsy, as a mood stabiliser and for its pain-relieving properties. In addition to birth defects, FVS is associated with an increased risk of autism spectrum disorder (ASD), which is characterised by abnormal behaviours. Valproate perturbs multiple biochemical pathways and alters gene expression through its inhibition of histone deacetylases. Which, if any, of these mechanisms is relevant to the genesis of its behavioural side effects is unclear. Neuroanatomical changes associated with FVS have been reported and, among these, altered serotonergic neuronal differentiation is a consistent finding. Altered serotonin homeostasis is also associated with autism. Here we have used a chemical-genetics approach to investigate the underlying molecular defect in a zebrafish FVS model. Valproate causes the selective failure of zebrafish central serotonin expression. It does so by downregulating the proneural gene ascl1b, an ortholog of mammalian Ascl1, which is a known determinant of serotonergic identity in the mammalian brainstem. ascl1b is sufficient to rescue serotonin expression in valproate-treated embryos. Chemical and genetic blockade of the histone deacetylase Hdac1 downregulates ascl1b, consistent with the Hdac1-mediated silencing of ascl1b expression by valproate. Moreover, tonic Notch signalling is crucial for ascl1b repression by valproate. Concomitant blockade of Notch signalling restores ascl1b expression and serotonin expression in both valproate-exposed and hdac1 mutant embryos. Together, these data provide a molecular explanation for serotonergic defects in FVS and highlight an epigenetic mechanism for genome-environment interaction in disease.
KEY WORDS: Serotonin, Fetal valproate syndrome, Zebrafish, Notch, Proneural gene, Hdac1
INTRODUCTION
Valproate (VPA) is a fatty acid derivative widely prescribed for its anticonvulsant, mood-stabilising and pain-relieving properties, but it has teratogenic and neuropsychiatric side effects upon in utero exposure, collectively termed fetal valproate syndrome (FVS). The underlying molecular cause of FVS is unknown, but candidate mechanisms are the dysregulation of transcription factors important for brain development, disruption of signal transduction pathways, inositol depletion and direct inhibition of epigenetic regulators such as the histone deacetylases (HDACs) (Chen et al., 1997; Detich et al., 2003; Einat et al., 2003; Marchion et al., 2005; Milutinovic et al., 2007; Phiel et al., 2001; Williams et al., 2002).
Fetal VPA exposure is associated with a 3- to 46-fold increased risk of autism spectrum disorder (ASD) (Bromley et al., 2013; Christensen et al., 2013; Dufour-Rainfray et al., 2011; Rasalam et al., 2005). Animal models of FVS display autism-like behaviours (Dufour-Rainfray et al., 2010; Kim et al., 2011; Yochum et al., 2008) and neuroanatomical abnormalities that are also reported in ASD (Ingram et al., 2000; Rodier et al., 1996). In these models, activity of the neurotransmitter serotonin (5HT) is altered, which has been implicated in the regulation of numerous behaviours, including social interaction (Ansorge et al., 2004; Patterson, 2006). Altered hippocampal and blood 5HT levels have been reported in animal models of FVS, which correlate with impaired 5HT neuronal differentiation (Dufour-Rainfray et al., 2010; Kuwagata et al., 2009; Miyazaki et al., 2005; Narita et al., 2002; Oyabu et al., 2013) and autism-like behaviours (Lin et al., 2013; Tsujino et al., 2007; Wang et al., 2013). Interestingly, in one of these rat models, treatment with a 5HT1A receptor agonist improved the abnormal behaviours, implying a deficit of 5HT signalling (Wang et al., 2013). By contrast, in the other study, VPA increased brain 5HT levels (Tsujino et al., 2007).
Significantly, 5HT is also implicated in autism pathogenesis. In ASD, 30% of subjects have elevated 5HT blood levels (Mulder et al., 2004; Schain and Freedman, 1961), central 5HT homeostasis is altered (Chugani et al., 1999; Chugani et al., 1997) and an association with stereotyped behaviour has been reported (Kolevzon et al., 2010; Sacco et al., 2010). Selective serotonin reuptake inhibitors (SSRIs) improve some manifestations of autism, including stereotypical behaviours (Hollander et al., 2003; McDougle et al., 2000), whereas depletion of the 5HT precursor tryptophan exacerbates these symptoms (Bauman et al., 2006). Genetic or pharmacological perturbation of the 5HT system is associated with autism-like behaviours in humans and in rodents (Bauman et al., 2006; Cook et al., 1997; Kane et al., 2012; Klauck et al., 1997; Nabi et al., 2004; Nakatani et al., 2009; Sutcliffe et al., 2005; Veenstra-VanderWeele et al., 2012). In particular, an allelic polymorphism of the serotonin transporter gene (SERT), which is a determinant of 5HT activity, is associated with ASD (Devlin et al., 2005). Furthermore, a mouse model of one of the human gain-of-function SERT genetic variants displays ASD-like behaviours and hyperserotonaemia (Veenstra-VanderWeele et al., 2012). Therefore, increases and decreases in central 5HT activity seem to produce common behavioural phenotypes, which is consistent with the view that autism can result from positive and negative changes in neurotransmitter signalling (Zoghbi and Bear, 2012).
TRANSLATIONAL IMPACT.
Clinical issue
The drug valproate is used worldwide as an anticonvulsant agent, as a mood stabiliser and for its pain-relieving properties. Valproate is teratogenic (interferes with early development) and fetal exposure causes fetal valproate syndrome (FVS), which is characterised by a spectrum of morphological, cognitive and behavioural deficits. Recent population-based epidemiological studies have highlighted the significantly increased risk of autism spectrum disorders (ASDs) in children exposed to valproate in utero. The in vivo mechanism of valproate action that is pertinent to its neuropsychiatric side effects is not clear. Multiple in vitro mechanisms have been described, including inhibition of histone deacetylases. Studies on animal models of FVS have identified biochemical and cellular perturbations of the central serotonergic system. Altered serotonin homeostasis is also a feature of idiopathic autism; therefore, uncovering the pathogenesis of serotonin deficits in FVS could reveal the molecular underpinnings of core behavioural abnormalities in autism.
Results
Zebrafish are highly suited to pharmacological and genetics approaches that can be combined to provide novel insights into disease mechanisms. In this article, the authors describe a zebrafish model of FVS that displays a failure of serotonergic differentiation in the brainstem in response to valproate treatment. They show that a critical proneural gene, ascl1b, is silenced by valproate through a mechanism that depends on inhibition of the histone deacetylase Hdac1. Their experiments further show that valproate unmasks tonic repression of the ascl1b gene by the Notch pathway. If the Notch pathway is blocked, valproate is no longer able to silence ascl1b. Importantly, restoration of Ascl1b in the presence of valproate rescues the expression of serotonin in the zebrafish brainstem.
Implications and future directions
This study shows that valproate represses expression of ascl1b, leading to defects in the serotonergic system in zebrafish. The failure in serotonergic differentiation in this new model is reminiscent of defects reported previously for mice exposed to valproate, suggesting that the mechanism unveiled herein is likely to provide a common molecular explanation for serotonergic abnormalities in this disorder. Indeed, the conservation of the differentiation pathways of serotonergic neurons in zebrafish and in humans suggests that these findings will be relevant to understanding the complex pathophysiology of FVS. More broadly, the authors highlight an epigenetic mechanism at work in an iatrogenic form of ASD that could also be relevant to idiopathic, common forms of autism.
5HT neurons in the hindbrain are derived from progenitors exposed to the signalling molecule sonic hedgehog (Shh) (Jessell, 2000). Serotonergic progenitor identity is characterised by expression of the transcription factors Nkx2.2, Foxa2 and Ascl1, all of which are required for 5HT neuronal differentiation (Briscoe et al., 1999; Jacob et al., 2007; Pattyn et al., 2004). Newly born 5HT neurons express post-mitotic determinants, including the transcription factor Pet1 (Hendricks et al., 2003) and subsequently the 5HT biosynthetic enzyme Tph2 (Zhang et al., 2004).
We investigated the molecular pathophysiology underlying serotonergic deficits in a zebrafish FVS model because this could provide mechanistic insight into the genesis of core autism behaviours. Importantly, hindbrain development and neuronal subtype diversity and serotonergic differentiation show strong conservation (Lillesaar, 2011). Furthermore, zebrafish exposed to VPA display morphological defects similar to those described in FVS, suggesting the validity of our system for modelling features of human FVS (Gurvich et al., 2005; Herrmann, 1993). We show that VPA specifically blocks hindbrain 5HT expression in zebrafish. Acting via Hdac1, VPA silences the zebrafish ortholog of mammalian Ascl1, ascl1b, by unmasking tonic Notch repression. Moreover, Ascl1b is sufficient to rescue 5HT expression in VPA-treated embryos.
RESULTS
VPA impairs central 5HT neuronal differentiation
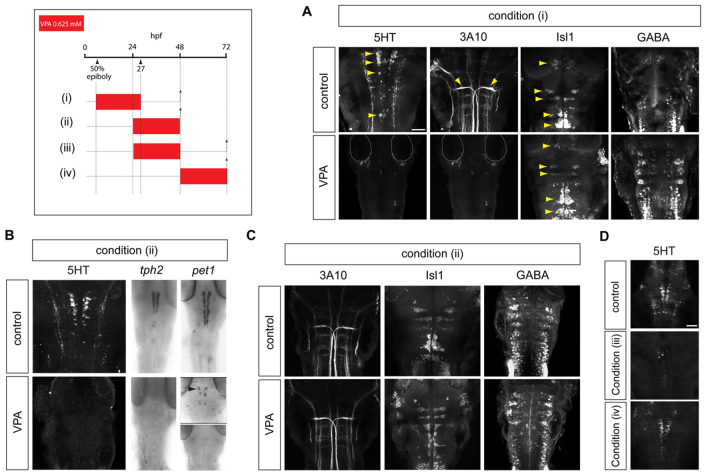
To assess the effect of VPA on brainstem development, we exposed zebrafish gastrulae at 50% epiboly to 0.625 mM VPA until 27 hours post-fertilisation (hpf), at which time the drug was removed and the embryos were allowed to develop until 48 hpf. Treatment from gastrulation was based on the heightened risk of teratogenicity in human infants exposed to VPA during the first trimester of pregnancy (Ornoy, 2009). Immunostaining for a range of hindbrain neuronal subtypes, specifically motor neurons, 5HT neurons, GABA-ergic neurons and Mauthner neurons, revealed a failure of 5HT neuronal differentiation marked by absence of 5HT expression (Fig. 1A). Additionally, Mauthner neurons, which express a neurofilament-associated antigen that is detected by the 3A10 monoclonal antibody, were also absent (Hatta, 1992; Schier et al., 1996) (Fig. 1A). Isl1-positive motor neurons and GABA-ergic neurons were present. The spatial distribution of motor neurons in VPA-treated embryos appeared subtly altered, suggesting migratory defects, but we did not pursue these changes further (Fig. 1A). Instead we focused on the striking serotonergic phenotype.
Fig. 1.
Effect of valproate exposure on the differentiation of serotonergic and other brainstem neuronal subtypes. The timing of drug treatment is indicated in this and all subsequent schematics by a coloured bar. Arrowheads mark the developmental stage of interest (hpf). Dashed lines with arrows indicate the developmental time point at which embryos were harvested. (A) Immunostaining for brainstem neuronal subtypes in 48 hpf zebrafish embryos exposed to VPA from 50% epiboly to 27 hpf [condition (i)] shows the failure of 5HT neuronal differentiation (n=22/22, P<0.0001), marked by 5HT (yellow arrowheads in control panel), and lack of Mauthner neurons (n=22/22), marked by the 3A10 anti-neurofilament monoclonal antibody (yellow arrowheads in control panel). Isl1+ motor neurons (yellow arrowheads in control and VPA panels) (n=10/10) and GABA-ergic neurons (n=10/10) persist. Scale bar: 50 μm. (B) Failure of serotonergic differentiation in embryos treated with VPA from 24-48 hpf [condition (ii)], as indicated by lack of 5HT (n=32/32, P<0.0001) and Tph2 expression. Pet1 (arrowhead) is severely reduced (n=3/15) or absent (n=12/15) in VPA-treated embryos. (C) Persistence of Mauthner, motor and GABA-ergic neurons in VPA treatment condition (ii). (D) At 72 hpf, embryos treated with VPA from 24-48 hpf [condition (iii)] show a limited recovery of 5HT expression (n=30/30). Treatment with VPA from 48-72 hpf [condition (iv)] does not affect serotonergic differentiation (n=30/30). hpf, hours post-fertilisation. Scale bar: 50 μm.
We speculated that exposure to VPA from gastrulation could affect common steps in the differentiation of multiple neuronal lineages. Therefore, we treated embryos with 0.625 mM VPA from 24-48 hpf and used appropriate markers to detect brainstem neuronal subtypes (Fig. 1B,C). Somatic motor, GABA-ergic and Mauthner neurons were still present (Fig. 1C), but there was a specific deficit of 5HT neuronal differentiation, marked by the absence of 5HT and tph2 expression (Fig. 1B). Moreover, there was an absence or severe reduction of pet1 expression, which indicates that VPA acts at a step proximal to or at the early stages of 5HT neuronal differentiation (Fig. 1B). Lower doses than this did not consistently lead to the loss of 5HT expression (see supplementary material Fig. S1).
Having established that brainstem 5HT expression was specifically lacking in embryos treated with VPA from 24 hpf, we addressed whether there was a delay in the differentiation of 5HT neurons. VPA was removed at 48 hours and embryos were analysed after a further 24 hours of incubation (Fig. 1D). There was limited recovery of 5HT expression at 72 hpf [mean number of 5HT neurons in controls=26.7±1.7 (s.d.), n=3; mean number of 5HT neurons in VPA-treated condition=1.8±1.3, n=4], which suggests that VPA does not merely retard the differentiation of 5HT neurons. We conclude that VPA specifically blocks the differentiation of 5HT neurons in embryos exposed to the drug between 24 and 48 hpf. This period coincides with the onset of differentiation of 5HT neurons between 25 and 30 hpf (Lillesaar et al., 2007). Because the post-mitotic differentiation of 5HT neurons is well underway from 48 hpf onwards (Lillesaar et al., 2007; McLean and Fetcho, 2004), we asked whether VPA could abolish 5HT expression after 48 hpf. VPA exposure between 48 hpf and 72 hpf had no effect on 5HT expression (Fig. 1D) (mean number of 5HT neurons=25.8±2.5, n=4). These data suggest that VPA acts on serotonergic progenitors, rather than on post-mitotic neurons.
To test whether specific neuronal subtypes are vulnerable to VPA application from 24 hpf because they only differentiate at or after 24 hpf, we extended the range of neuronal subtypes assayed. Cerebellar Purkinje neurons begin to differentiate from 3 dpf and are marked by expression of Parv7 (Bae et al., 2009). Wild-type embryos were incubated in VPA for a prolonged period, from 24 hpf to 4.5 dpf (see supplementary material Fig. S2). Immunostaining against Parv7 showed the persistence of this cell type in VPA-treated embryos (supplementary material Fig. S2). The differential sensitivity of 5HT and Purkinje neurons suggests that vulnerability to the effects of VPA is not linked directly to the timing of neuronal differentiation.
Inhibition of Hdac1 by VPA accounts for the failure of 5HT neuronal differentiation
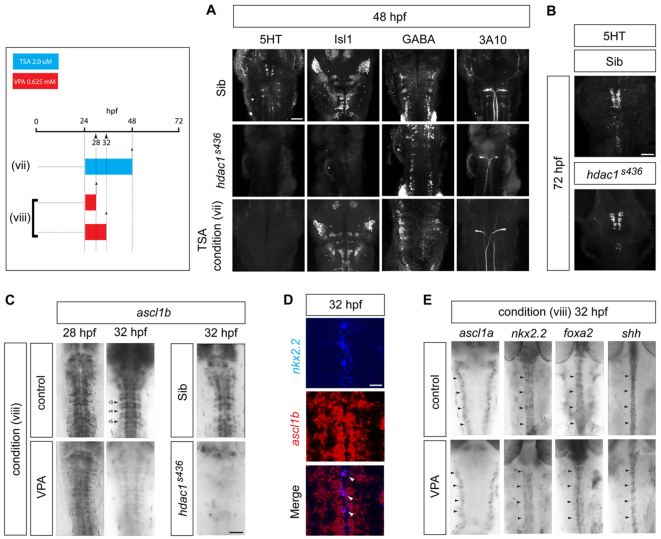
Next, we sought to identify the molecular pathway targeted by VPA in blocking 5HT neuronal differentiation. Previous studies have shown both in vivo and in vitro that VPA is an inhibitor of HDACs at therapeutic concentrations (Göttlicher et al., 2001; Gurvich et al., 2005; Kook et al., 2003; Phiel et al., 2001; Tremolizzo et al., 2002; Yildirim et al., 2003). To test the involvement of Hdac1, we used a zebrafish hdac1 mutant line, hdac1s436, that was previously isolated in a forward genetic screen (Noël et al., 2008). Immunostaining of hdac1s436 mutants revealed a lack of 5HT expression in the brainstem at 48 hpf (Fig. 2A). However, by 72 hpf there was a partial recovery of 5HT neuronal differentiation (Fig. 2B) that was more complete than in VPA-treated embryos at the same stage (Fig. 1D, middle panel) [mean number of 5HT neurons in control siblings (sibs)=31±2.5, n=4; mean number of 5HT neurons in mutants=25.3±5.4, n=4]. Apart from a severe reduction in the differentiation of somatic motor neurons, marked by Isl1 expression, which has been reported previously (Cunliffe, 2004), other neuronal subtypes, GABA-ergic and Mauthner neurons, appeared intact (Fig. 2A).
Fig. 2.
Failure of serotonergic differentiation in VPA-treated embryos is mimicked by blockade of Hdac1 activity and is associated with downregulation of ascl1b. (A) hdac1 mutation or pharmacological blockade mimics the effect of VPA on the differentiation of brainstem neuronal subtypes. At 48 hpf, hdac1s436 mutants lack 5HT neurons (n=15/15, P<0.0001) and show a severe depletion of Isl1+ motor neurons. Treatment with TSA from 24 to 48 hpf [condition (vii)] selectively affects 5HT expression (n=30/30, P<0.0001), but other neuronal subtypes are retained. Scale bar: 50 μm. (B) Partial recovery of 5HT neuronal differentiation in hdac1s436 mutants at 72 hpf (n=14/14). Scale bar: 50 μm. (C) Short duration (8 hour) exposure to VPA from 24 hpf [condition (vi)] leads to the downregulation of ascl1b expression (n=15/15), and similar loss of ascl1b expression is observed in hdac1s436 mutants (n=10/10). Rhombomeres (r) marked by arrowheads. Scale bar: 50 μm. (D) Double in situ hybridisation showing ascl1b expression in serotonergic progenitors, marked by expression of nkx2.2. Co-labelled cells are indicated by white arrowheads. Scale bar: 20 μm. (E) Short-duration VPA treatment [condition (viii)] does not affect the expression of the paralogous gene ascl1a, or other progenitor-expressed determinants of serotonergic fate, nkx2.2, foxa2 or shh (arrowheads).
To further test the involvement of HDACs in 5HT neuron production, wild-type embryos were treated with the HDAC inhibitor trichostatin A (TSA), a potent inhibitor of class I and class II HDACs (Yoshida et al., 1990). Pharmacological blockade of HDACs with TSA from 24-48 hpf recapitulated the effect of VPA treatment, and resulted in loss of 5HT expression at 48 hpf, with preservation of somatic motor, GABA-ergic and Mauthner cell differentiation (Fig. 2A). This suggests that the hdac1s436 neuronal phenotype differs from the effect of VPA most likely because of differences in the timing of Hdac1 inactivation. Moreover, the closely similar phenotypes that result from hdac1 mutation and VPA treatment are consistent with the idea that VPA blocks 5HT neuronal differentiation by inhibiting Hdac1.
VPA downregulates expression of the proneural gene ascl1b in serotonergic progenitors through a mechanism consistent with Hdac1 inhibition
Next we examined the expression of transcription factors that specify serotonergic progenitors. We reasoned that, because 5HT neurons are absent, any disruption of serotonergic fate determinants might occur before the first 5HT neurons are born. We therefore exposed 24 hpf embryos to VPA and analysed gene expression by in situ hybridisation 8 hours later. There was a striking reduction of ascl1b expression by 32 hpf that was not apparent at 28 hpf, just 4 hours earlier (Fig. 2C). Colocalisation of ascl1b and nkx2.2 in the hindbrain by fluorescent double in situ hybridisation confirmed the expression of ascl1b in serotonergic progenitors (Fig. 2D). Expression of the related gene ascl1a and other fate determinants, nkx2.2 and foxa2, and the signalling molecule shh were unchanged, which implies that the loss of ascl1b expression is not due to depletion of progenitors (Fig. 2E).
Further evidence implicating Hdac1 as the mediator of the effects of VPA on 5HT neurons is that, in hdac1s436 mutants, expression of ascl1b is also severely reduced, a finding that has been reported previously in a different hdac1-null mutant line (Fig. 2C) (Cunliffe, 2004).
In contrast to the downregulation of ascl1b, another bHLH proneural gene, ptf1a, which is required for the generation of cerebellar Purkinje neurons (Hori et al., 2008), was not decreased by VPA. Ptf1a expression in the cerebellar primordium is first detected at 48 hpf (Kani et al., 2010). We monitored ptf1a expression by in situ hybridisation for ptf1a transcripts in wild-type zebrafish embryos and by an eGFP reporter in a transgenic ptf1a-egfp line at 52 hpf in embryos continuously exposed to VPA from 24 hpf (see supplementary material Fig. S2) (Pisharath et al., 2007). The persistent ptf1a expression suggests that proneural genes are differentially sensitive to VPA and this is consistent with the continued differentiation of Purkinje neurons after VPA exposure (see supplementary material Fig. S2).
Notch signalling represses ascl1b in the presence of VPA
VPA has previously been reported to upregulate Notch signalling in a variety of systems (Greenblatt et al., 2007; Stockhausen et al., 2005). In zebrafish embryos this effect seems likely to be mediated by Hdac1 (Cunliffe, 2004). One possibility, therefore, was that Hdac1 was inhibiting the Notch pathway and thus decreasing the Notch-mediated inhibition of ascl1b via Her genes, which are the major effectors of Notch signalling in zebrafish (Cunliffe, 2004; Louvi and Artavanis-Tsakonas, 2006). A second possible mechanism is through direct upregulation of ascl1b by Hdac1 (Cunliffe, 2004; Harrison et al., 2011; Wang et al., 2009). Finally, Hdac1 repression of specific members of the Her family, in particular her6, which in turn represses ascl1b, is a third possibility (Cunliffe, 2004; Harrison et al., 2011).
her4 is a direct target of Notch signalling that is widely used as a readout of Notch activity (Takke and Campos-Ortega, 1999; Takke et al., 1999; Yeo et al., 2007). The downregulation of ascl1b in embryos following an 8-hour treatment with VPA from 24 hpf (Fig. 2C) was not accompanied by obvious elevation of her4 expression (Fig. 3A,B). Quantitative reverse-transcriptase PCR confirmed that there was a modest decrease in her4 expression relative to untreated control embryos (Fig. 3A). her6 is also a target of the Notch pathway and to date is the only Her gene reported to undergo negative regulation by Hdac1 (Cunliffe, 2004). In situ hybridisation for her6 and a panel of additional Her family members in wild-type embryos similarly treated with VPA also showed no difference in expression compared with controls (data not shown).
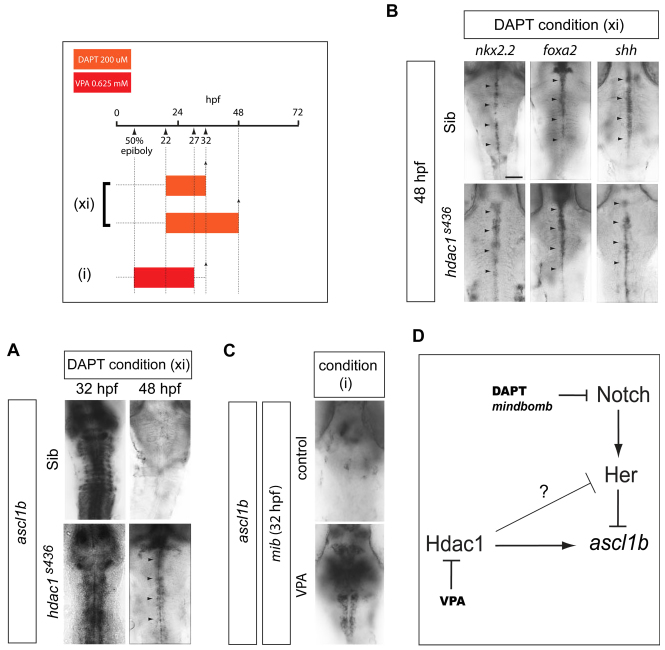
Fig. 3.
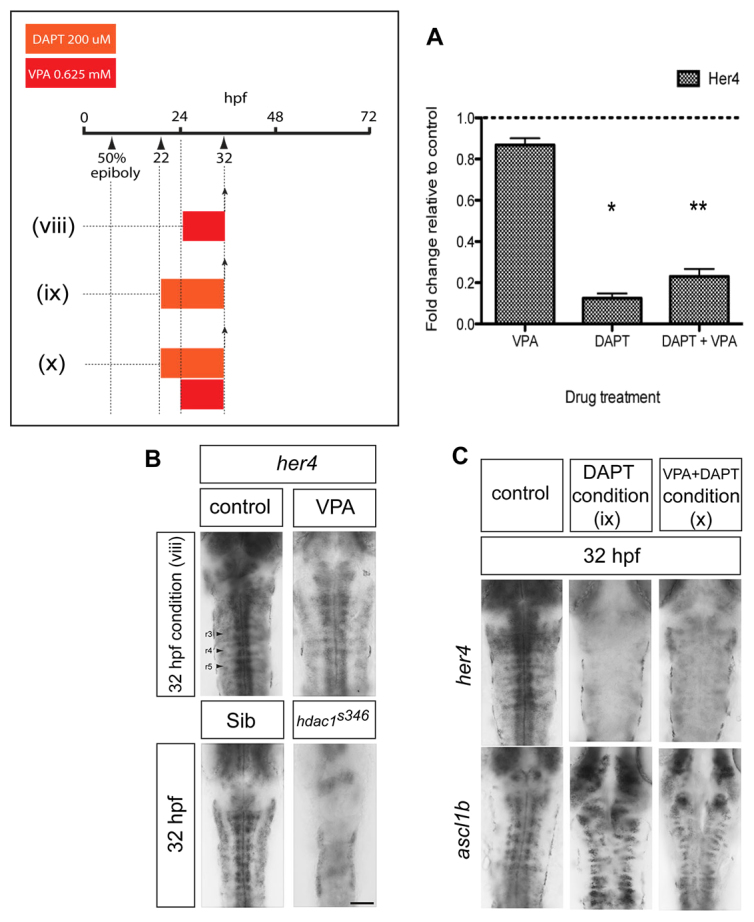
VPA treatment exposes cryptic transcriptional repression of ascl1b by basal levels of Notch signalling. (A) Quantitative reverse-transcriptase PCR showing that VPA mildly reduces her4 expression, whereas DAPT strongly reduces her4 expression to between 12% and 23% of control levels (*P=0.017, **P=0.001). (B) Short duration (8 hour) treatment with VPA [condition (viii)] does not upregulate her4 expression at 32 hpf (n=12/12). Downregulation of her4 expression in hdac1s436 mutant hindbrain at 32 hpf (n=5/5). Scale bar: 50 μm. (C) Concomitant blockade of Notch signalling using the γ-secretase inhibitor DAPT prevents the downregulation of ascl1b by VPA. Loss of her4 expression in DAPT-treated embryos [conditions (ix) and (x)] (upper panels) (n=35/35). Persistence of ascl1b expression in embryos treated with VPA and DAPT [condition (x)] (n=23/23).
To investigate the involvement of the Notch pathway in ascl1b repression by VPA, we inhibited Notch signalling over the same period and determined whether VPA could still repress ascl1b. Embryos at 22 hpf were treated with a small-molecule inhibitor of Notch signalling, the γ-secretase inhibitor DAPT, which is known to have potent activity in vivo in zebrafish (Geling et al., 2002; Imbimbo, 2008). DAPT treatment alone from 22-32 hpf effectively downregulated her4 expression, whereas ascl1b expression was maintained (Fig. 3A,C). Strikingly, following the addition of VPA at 24 hpf, her4 remained repressed and ascl1b expression persisted (Fig. 3A,C). Therefore, the repression of ascl1b by short-term exposure to VPA is prevented by concomitant blockade of Notch signalling, which implies that a basal level of Notch signalling represses ascl1b when Hdac1 activity is blocked.
To confirm that the repression of Ascl1b by VPA is not due to increased Notch signalling secondary to blockade of Hdac1 function, we analysed her4 expression in hdac1s436 mutants at 32 hpf (Fig. 3B). There was a marked reduction of her4 expression in mutant hindbrains, indicative of reduced Notch signalling (Fig. 3B). Therefore, VPA inhibition of Hdac1 does not repress ascl1b through the upregulation of Notch signalling, or via Hdac1-mediated repression of her4 or her6. Instead, Hdac1 acts independently of Notch activity to regulate ascl1b. Moreover, a parallel, tonic level of Notch signalling was sufficient to repress ascl1b transcription when Hdac1 was inhibited by VPA.
Hdac1 and Notch have opposing effects on ascl1b
To confirm the parallel roles for Hdac1 and Notch signalling, we tested whether DAPT could restore ascl1b expression in hdac1s436 mutants. hdac1s436 mutants and sibs were treated with DAPT from 22 hpf (Fig. 4A). At 32 hpf, sibs treated with DAPT showed strong expression of ascl1b, which was virtually absent by 48 hpf presumably because of depletion of the progenitor pool caused by the blockade of Notch signalling. Remarkably, DAPT restored ascl1b expression in hdac1s436 mutants, and expression was maintained at least up to 48 hpf (compare Fig. 2C and Fig. 4A). The other progenitor markers, nkx2.2, foxa2 and shh, were not altered by DAPT in either sibs or hdac1s436 embryos at 48 hpf (Fig. 4B). These data confirm that Hdac1 and Notch exert positive and negative regulatory effects, respectively, on ascl1b. Given the finding that DAPT could prevent the repression of ascl1b by VPA, the expression of ascl1b was then analysed in a Notch-signalling mutant, mindbomb (mib). These mutants lack an E3 ubiquitin ligase, which is crucial for Notch signalling (Itoh et al., 2003; Schier et al., 1996). Consistent with the data from chemical inhibition of Notch signalling, the expression of ascl1b was restored in 32 hpf mib mutants treated with VPA from 50% epiboly to 27 hpf (Fig. 4C).
Fig. 4.
Ascl1b transcriptional regulation by opposing Hdac1 and Notch activities. (A) Blockade of Notch signalling by DAPT in hdac1s436 mutants [condition (xi)] results in the recovery of ascl1b expression at 32 hpf and 48 hpf (arrowheads) (n=15/15 for both developmental stages combined). Arrowheads indicate the expression domain of ascl1b. (B) DAPT treatment of hdac1s436 mutants [condition (xi)] does not affect the expression of nkx2.2 (n=7/7), foxa2 (n=8/8) and shh (n=6/6) (arrowheads) at 48 hpf. Arrowheads indicate the expression domain of the respective genes in the ventral midline of the embryo. Scale bar: 50 μm. (C) VPA treatment of embryos on the Notch signalling mutant mindbomb (mib) background [condition (i)] is associated with recovery of ascl1b expression (n=11/11). (D) Model of transcriptional regulation of ascl1b by Hdac1 and the Notch pathway. Solid arrows indicate direct positive regulation. Lines with an orthogonal bar represent inhibition. The inhibitory arrow with a question mark above it, from Hdac1 to Her, takes account of the possibility that another Her gene (or another transcription factor of unknown identity) that represses ascl1b is in turn repressed by Hdac1.
Failure of 5HT neuronal differentiation in VPA-treated embryos is due to repression of ascl1b
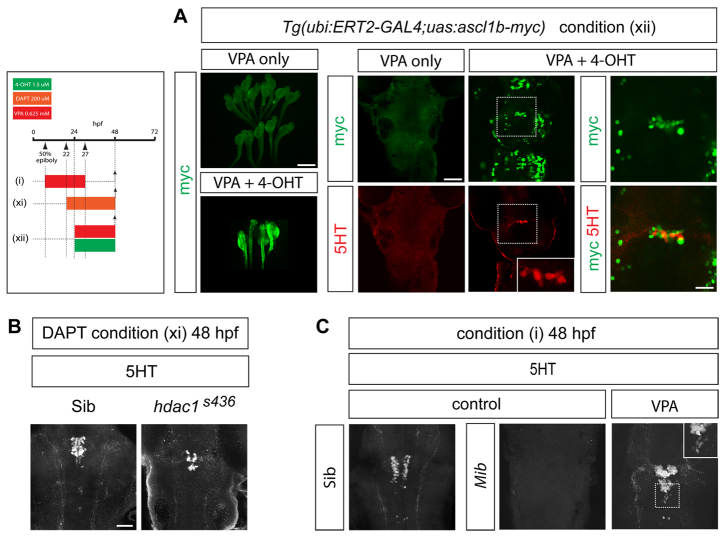
Finally, we asked whether replacement of ascl1b was sufficient to rescue 5HT neuronal differentiation in VPA-treated embryos. To test this, a plasmid encoding a Myc-tagged full-length Ascl1b (ascl1b-myc) under the control of UAS was injected into a stable transgenic zebrafish line, ubi:ERT2-GAL4 (Gerety et al., 2013), which allows the regulation of ascl1b-myc by 4-hydroxytamoxifen (4-OHT). In control embryos treated with VPA alone, Ascl1b-Myc could not be detected by immunostaining for Myc antigen (Fig. 5A) and 5HT-expressing neurons were absent. Addition of 4-OHT resulted in strong and widespread expression of Ascl1b-Myc and the rescue of 5HT neuronal differentiation in 16% of embryos (Fig. 5A, P=0.0058).
Fig. 5.
Ascl1b is sufficient to rescue serotonergic differentiation in VPA-treated embryos. (A) Mis-expression of Myc-tagged Ascl1b in VPA-treated transgenic embryos rescues 5HT neuronal differentiation. A stable transgenic line expressing ERT2-GAL4 under the control of the ubiquitin (ubi) promoter was injected at the one-cell stage with plasmid DNA encoding Ascl1b-Myc under the control of UAS. Embryos were treated with VPA with or without 4-hydroxytamoxifen (4-OHT) [condition (xii)]. Myc immunostaining in shown in green. The upper and lower left panels show low-power views (scale bar: 500 μm) of Myc-immunostained zebrafish embryos treated with VPA in the absence (upper panel) or presence (lower panel) of 4-OHT. Addition of 4-OHT leads to widespread expression of Ascl1b-Myc (bottom left panel). Panels to the right show high-power views (scale bar: 50 μm) of Myc (green) and 5HT (red) immunostaining in the hindbrain of transgenic embryos. Embryos treated with VPA alone fail to express 5HT (n=51/51). Addition of 4-OHT and induction of Ascl1b-Myc expression rescues 5HT expression in 8/51 VPA-treated embryos (P=0.0058). Upper right and bottom right panels are high-power (scale bar: 20 μm) views of the boxed areas in the panels immediately to the left. The bottom, middle panel inset shows a higher-power view of the 5HT-expressing cells in the boxed area. The bottom right panel shows merged channels representing Ascl1b-Myc (green) and 5HT (red) immunostaining. (B) In hdac1s436 mutants treated with DAPT [condition (xi)], there is a rescue of 5HT neuronal differentiation at 48 hpf (n=22/22, P<0.0001). Scale bar: 50 μm. (C) On the mib mutant background there is a recovery of 5HT neurons at 48 hpf in VPA-treated embryos [condition (i)] (n=12/12, P=0.0002). Inset shows a high-power image of the boxed region.
If repression of ascl1b is the reason for the failure of 5HT neuronal differentiation upon VPA exposure, then restoration of ascl1b expression in hdac1s436 and mib mutants should be sufficient to rescue 5HT expression. Indeed, immunostaining of DAPT-treated hdac1s436 embryos at 48 hpf revealed the rescue of 5HT expression in the raphe (Fig. 5B). We also exposed mib mutants to VPA from gastrulation (50% epiboly) to 27 hpf, and then incubated the embryos in normal medium until 48 hpf, at which time they were fixed and immunostained for 5HT. We had already established that an identical VPA regime abolishes 5HT expression on a wild-type background (Fig. 1A). Untreated mib mutants lacked 5HT neurons but, remarkably, 5HT neurons were generated in mib mutant embryos exposed to VPA (Fig. 5C). These findings confirmed that the loss of 5HT expression in VPA-treated embryos was not due to neuronal death, but instead was due to a block in their differentiation.
DISCUSSION
Using a zebrafish model of FVS, we have identified a molecular mechanism to explain the loss of 5HT neurons, which have been implicated in the neuropsychiatric manifestations of FVS. Two different VPA treatment regimes caused the failure of serotonergic differentiation, and the late-dosing regime (24-48 hpf) seemed to have greater selectivity for the serotonergic system than the early-dosing schedule (50% epiboly to 27 hpf). There is wide variation in the teratogenic dose of VPA in human and animal studies (Christensen et al., 2013; Ornoy, 2009), which makes direct comparisons of dosing regimes between different model organisms and with humans exposed to VPA in utero difficult to interpret. Nevertheless, the altered serotonergic differentiation in this zebrafish model is reminiscent of similar deficits that are found in rodent models, which correlate with autism-like behavioural abnormalities (Dufour-Rainfray et al., 2010; Dufour-Rainfray et al., 2011).
Recovery of ascl1b expression in VPA-treated hdac1s436 or mib mutant embryos is associated with rescue of 5HT neuronal differentiation. However, direct replacement of Ascl1b is sufficient to rescue 5HT expression in only 16% of embryos. Suboptimal timing or level of the ectopically expressed Ascl1b expression might explain the lower efficiency. Alternatively, non-physiological, persistent high-level expression of Ascl1b in post-mitotic neurons might account for the low rate of rescue (Cai et al., 2000). Nevertheless, taken together these findings indicate that the failure of 5HT neuronal differentiation in our model of FVS is most probably due to the loss of ascl1b expression. We cannot, however, exclude the possibility that VPA disrupts the expression of other, unknown, genes that are crucial for 5HT neuronal differentiation. We anticipate that the finding of downregulated expression of ascl1b in our zebrafish model will prompt closer analysis of rodent FVS models for changes in the expression of the mammalian ortholog Ascl1 in serotonergic progenitors. Ascl1 is known to be required for neuronal-subtype specification, which is separable from its better-known proneural function (Goridis and Brunet, 1999). In mammals, a requirement for Ascl1 in serotonergic differentiation has previously been demonstrated, including for the expression of 5HT itself, and this subtype-specification function cannot be substituted by other proneural genes (Jacob et al., 2009; Parras et al., 2002; Pattyn et al., 2004). Additionally, Ascl1 has been shown to be essential for the acquisition of noradrenergic traits in all mammalian central and peripheral neurons (Blaugrund et al., 1996; Guillemot et al., 1993; Hirsch et al., 1998). At least in part, Ascl1 regulates neurotransmitter phenotype in noradrenergic neurons in collaboration with Phox2 genes (Pattyn et al., 2000). However, Phox2 genes are not expressed in the mammalian serotonergic lineage. Instead, Ascl1 regulation of the zinc-finger transcription-factor-encoding gene Insm1 in post-mitotic serotonergic precursors is a crucial component of the mammalian serotonergic transcriptional programme (Jacob et al., 2009).
Using the HDAC inhibitor TSA and an hdac1 mutant line, we have provided evidence that the effect of VPA on serotonergic differentiation is mediated through Hdac1 inhibition. These findings are consistent with previous reports that VPA inhibits HDACs in vivo (Gurvich et al., 2005). Moreover, Hdac1 is one of the key molecular factors that regulate the transcription of ascl1b and these data are consistent with the previously identified positive regulatory role of Hdac1 (Cunliffe, 2004; Harrison et al., 2011). The regulation of ascl1b might be direct given that Hdac1 binds to the ascl1b promoter (Harrison et al., 2011). It has been proposed that Hdac1 promotes activation of ascl1b directly by participating in cycles of deacetylation and histone-acetyltransferase-dependent acetylation of transcription-unit-associated histones, and/or by maintaining the ascl1b promoter in a transcriptionally poised state (Wang et al., 2009). Nevertheless, we do not rule out the possibility that Hdac1 functions indirectly by inhibiting the expression of a transcriptional repressor of ascl1b (Fig. 4D).
A second transcriptional regulator of ascl1b that is unmasked by VPA is repressive regulation by the Notch pathway. It was shown previously that VPA upregulates Notch signalling and this is associated with increased expression of Notch effector genes (Greenblatt et al., 2007; Stockhausen et al., 2005). Therefore, we expected that repression of ascl1b by VPA might be mediated by enhanced Notch signalling. However, short-duration VPA treatment is sufficient to downregulate ascl1b without concomitantly enhancing Notch signalling. Consistent with this, the expression of a panel of Her genes, including her4, a target of Notch signalling, and her6, are not appreciably altered under these conditions. Nevertheless, our findings using chemical and genetic blockade of Notch function demonstrate that parallel Notch signalling participates in the VPA-mediated downregulation of ascl1b (Fig. 4D). Importantly, only a basal level of Notch signalling is required for VPA downregulation of ascl1b. Together, our findings lead to a revised view of ascl1b regulation based on a derepression model, because removal of the basal inhibitory Notch input when Hdac1 function is blocked by VPA is sufficient for ascl1b transcription.
The regulatory relationship between Hdac1 and the Notch pathway in the control of ascl1b expression reported in a previous study (Cunliffe, 2004) provided an opportunity to evaluate further whether VPA blockade of serotonergic differentiation occurs via Hdac1 inhibition. In hdac1 mutants, we found that her4 expression is reduced (Fig. 3B). Our result differs from the latter study that proposed increased Notch signalling in hdac1 mutants based on enhanced her6 expression (Cunliffe, 2004). A possible explanation for the difference is that her6 expression in hdac1 mutants is developmental-stage-dependent. The loss of ascl1b expression in hdac1s436 mutants and in VPA-treated embryos (Fig. 2C) occurs in the absence of enhanced Notch pathway activity during the period of treatment. This is consistent with Hdac1 inhibition as the salient in vivo mechanism of VPA. Moreover, these data are also consistent with direct regulation of ascl1b by Hdac1.
Because VPA has been reported to have a variety of modes of action, identifying which of these mechanisms is relevant in vivo for the behavioural manifestations of FVS is challenging. This is an important question, however, because identification of the molecular mechanism has potential therapeutic significance. The involvement of Hdac1 in the serotonergic deficit in FVS strongly suggests that an epigenetic mode of action could mediate at least some of the behavioural manifestations of FVS. This is not an isolated finding, because epigenetic contributions to other ASDs have been noted. Methyl-CpG-binding protein 2 (MECP2), which is implicated as the cause of the ASD-associated disease Rett syndrome contains a transcriptional repression domain, which physically interacts with the transcriptional co-repressor Sin3A (Amir et al., 1999). In turn, Sin3A recruits HDAC1 and HDAC2, which are believed to mediate transcriptional repression by MECP2 (Jones et al., 1998; Nan et al., 1998). More broadly, epigenetic modifications seem to regulate autism susceptibility; for example, autism is associated with duplications of 15q11-13, which is an imprinted region of the genome where DNA methylation status is associated with Prader-Willi syndrome and Angelman syndrome (Dykens et al., 2011). In conclusion, epigenetic regulation of gene expression seems to be an emerging point of convergence for environmental agents and hereditary factors in ASD pathogenesis.
MATERIALS AND METHODS
Zebrafish strains and husbandry
Zebrafish embryos were staged according to hpf and morphological criteria (Kimmel et al., 1995). mib mutant embryos were obtained by incrosses of heterozygote mib zebrafish. They were identified by the irregular appearance of the hindbrain, markedly reduced hindbrain ventricle and loss of early somite boundaries (Stickney et al., 2000; van Eeden et al., 1996). hdac1s436 mutants were generated by incrosses of heterozygote hdac1s436 zebrafish (Noël et al., 2008). Mutants were identified by their smaller CNS, narrow anterior rhombomeres, reduced midbrain ventricle, cleft eyes, retinal hypopigmentation, curled shape and, at 48 hpf, additionally by absent pectoral fins (Cunliffe, 2004). ubi:ERT2-GAL4 transgenic zebrafish were generated as described (Gerety et al., 2013).
Transgenic constructs and transient transgenic zebrafish
DNA encoding Ascl1b tagged with six copies of Myc at the C-terminus was made by gene synthesis (Genwiz). ascl1b-myc was subcloned into a 5× UAS plasmid containing a miniTOL2 backbone to facilitate genomic integration in zebrafish (Balciunas et al., 2006). Transient transgenic embryos were generated by co-injecting 10-20 pg of UAS:ascl1b-myc plasmid DNA with 25 pg of tol2 transposase mRNA into one-cell-stage embryos obtained by intercrossing UBI:ERT2-GAL4 transgenic zebrafish.
Reverse transcriptase PCR (RT-PCR) and primer sequences
Between 30 and 40 embryos at 32 hpf were used for each condition and experiments were performed in triplicate. At the end of the period of drug treatment embryos were placed in Trizol, homogenised, chloroform was added, the sample was centrifuged at 4°C, glycogen and isopropanol were added, the sample was then centrifuged again at 4°C, 75% ethanol was added, centrifugation at 4°C was repeated and the pellet was re-suspended in water. RNA purification was performed using the RNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions. cDNA synthesis was performed using the Superscript First-Strand Synthesis System for RT-PCR (Invitrogen), according to the manufacturer’s instructions. Quantitative RT-PCR was performed using the 7900HT Fast Real-Time PCR System (Applied Biosystems).
The following primers were used for RT-PCR: her4 5′-AGCAGCAGCCCGACTCCAGA-3′, 5′-GCTGACGGCCTCCTGCACAC-3′; beta-actin2 5′-CGAGCTGTCTTCCCATCCA-3′, 5′-TCACCAACGT -AGCTGTCTTTCTG-3′. Data was analysed using the Student’s t-test.
Immunohistochemistry and in situ hybridisation
For immunofluorescence and in situ hybridisation, embryos were fixed in 4% paraformaldehyde (PFA) either for 2-3 hours at room temperature or overnight at 4°C. For immunofluorescence, embryos were rinsed three times in 0.1% Tween in phosphate buffered saline (PBST), incubated for 20 minutes in 0.01 mg/ml proteinase K (Roche) in PBST/0.2% BSA/2% heat-inactivated goat serum solution (PBT), post-fixed in PFA, washed three times in PBT, blocked in PBT containing 1% DMSO, 0.5% Triton-X and 1% BSA (PBDT), and then incubated for 24 hours at 4°C in primary antibody in PBST. The following day, embryos were washed four times in PBT, blocked again in PBDT and then incubated in secondary antibody overnight in PBDT. Further washes in PBT were performed the next day, and embryos were then transferred to 70% glycerol and mounted under coverslips for viewing. Immunofluorescence staining was visualised by confocal microscopy (Leica TCS SP2). For single and double in situ hybridisation, embryos were processed as described (Lauter et al., 2011). Fluorescent signals were developed with Fast Blue and Fast Red. Differences between embryos were analysed using Fisher’s exact test.
The following antibodies were used: rabbit anti-5HT (1/500, Millipore), rabbit anti-GABA (1/500, Sigma), 3A10 mouse anti-neurofilament (1/25, DSHB), mouse anti-Parvalbumin7 (1/1000, gift from Dr Masahiko Hibi, Nagoya University, Japan), 4D5 mouse anti-Isl1 (1/45, DSHB), mouse anti-myc (1/500, Santa Cruz). The following riboprobes were used: foxa2 (Strähle et al., 1993), nkx2.2 (Barth and Wilson, 1995), shh (Krauss et al., 1993), her4 (Takke et al., 1999), her6, tph2 (also called tphR) (Teraoka et al., 2004), ascl1a, ascl1b (Allende and Weinberg, 1994), ptf1a (Zecchin et al., 2004). pet1 (IMAGE ID: 7000463) was subcloned from p-Express1 into pBluescript, then linearised with EcoRI (Roche) and transcribed using T7 Polymerase (Promega).
Drugs
Embryos were dechorionated manually prior to drug treatment. Sodium valproate (Sigma) was dissolved in water as a 2 M stock solution and was used at a final concentration of 0.625 mM. This dose is comparable to doses found to inhibit HDACs in vivo in other studies (Gurvich et al., 2005). Lower doses did not consistently abolish 5HT expression in the hindbrain (see supplementary material Fig. S1). DAPT (Calbiochem) was dissolved in DMSO as a 46 mM stock solution and used at 200 μM final concentration, which is similar to doses previously reported to block Notch signalling in zebrafish (Geling et al., 2002). A 5 mM TSA solution in DMSO (Sigma) was diluted to a final concentration of 2 μM in fish water prior to use, which is comparable to doses used in vivo to inhibit HDACs (Gurvich et al., 2005). 4-OHT (Sigma) was dissolved in ethanol at 12.5 mg/ml. Dilutions of 4-OHT were made to 1.5 μM in standard fish water just prior to use. In all drug conditions, controls were treated with an equivalent amount of vehicle diluted in fish water. Stock solutions of all these drugs were stored at −20°C.
Supplementary Material
Acknowledgments
We thank Johanna Fischer and Dr Elke Ober for providing reagents and hdac1s436 mutants, and Dr Alex Gould and Dr Vincent Cunliffe for advice. Mindbomb mutants were kindly provided by Dr Cristian Soza-Ried and Dr David Ish-Horowicz (Cancer Research UK). Anti-Parv7 antibody was a gift from Dr Masahiko Hibi. Zebrafish Her riboprobes were a gift from Dr Yi-Chuan Cheng. The zebrafish pet1 plasmid was a gift from Dr Laure Bally-Cuif. We thank Rawan Alsubaie and Milla Tomova for technical assistance.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
J.J., V.R. and J.B. conceived experiments and J.J., V.R. and S.M. performed experiments. J.J. and J.B. wrote the paper. S.C.C., N.S., S.S.G. and D.G.W. contributed expertise and/or reagents. D.J.M. and C.P.S. provided mib mutants.
Funding
This work was funded by an Autism Speaks Grant (#1299) to Alex Gould and J.B. and by the MRC (U117560541).
Supplementary material
Supplementary material available online at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.013219/-/DC1
References
- Allende M. L., Weinberg E. S. (1994). The expression pattern of two zebrafish achaete-scute homolog (ash) genes is altered in the embryonic brain of the cyclops mutant. Dev. Biol. 166, 509–530 [DOI] [PubMed] [Google Scholar]
- Amir R. E., Van den Veyver I. B., Wan M., Tran C. Q., Francke U., Zoghbi H. Y. (1999). Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188 [DOI] [PubMed] [Google Scholar]
- Ansorge M. S., Zhou M., Lira A., Hen R., Gingrich J. A. (2004). Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306, 879–881 [DOI] [PubMed] [Google Scholar]
- Bae Y. K., Kani S., Shimizu T., Tanabe K., Nojima H., Kimura Y., Higashijima S., Hibi M. (2009). Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Dev. Biol. 330, 406–426 [DOI] [PubMed] [Google Scholar]
- Balciunas D., Wangensteen K. J., Wilber A., Bell J., Geurts A., Sivasubbu S., Wang X., Hackett P. B., Largaespada D. A., McIvor R. S., et al. (2006). Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet. 2, e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth K. A., Wilson S. W. (1995). Expression of zebrafish nk2.2 is influenced by sonic hedgehog/vertebrate hedgehog-1 and demarcates a zone of neuronal differentiation in the embryonic forebrain. Development 121, 1755–1768 [DOI] [PubMed] [Google Scholar]
- Bauman M. L., Anderson G., Perry E., Ray M. (2006). Neuroanatomical and neurochemical studies of the autistic brain: current thought and future direction. In Understanding Autism (ed. Moldin S. O., Rubenstein J. L. R.), pp. 303–322 Boca Raton, FL: CRC Press; Taylor & Francis Group [Google Scholar]
- Blaugrund E., Pham T. D., Tennyson V. M., Lo L., Sommer L., Anderson D. J., Gershon M. D. (1996). Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers and Mash-1-dependence. Development 122, 309–320 [DOI] [PubMed] [Google Scholar]
- Briscoe J., Sussel L., Serup P., Hartigan-O’Connor D., Jessell T. M., Rubenstein J. L., Ericson J. (1999). Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature 398, 622–627 [DOI] [PubMed] [Google Scholar]
- Bromley R. L., Mawer G. E., Briggs M., Cheyne C., Clayton-Smith J., García-Fiñana M., Kneen R., Lucas S. B., Shallcross R., Baker G. A., Liverpool and Manchester Neurodevelopment Group (2013). The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J. Neurol. Neurosurg. Psychiatry 84, 637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Morrow E. M., Cepko C. L. (2000). Misexpression of basic helix-loop-helix genes in the murine cerebral cortex affects cell fate choices and neuronal survival. Development 127, 3021–3030 [DOI] [PubMed] [Google Scholar]
- Chen G., Yuan P., Hawver D. B., Potter W. Z., Manji H. K. (1997). Increase in AP-1 transcription factor DNA binding activity by valproic acid. Neuropsychopharmacology 16, 238–245 [DOI] [PubMed] [Google Scholar]
- Christensen J., Grønborg T. K., Sørensen M. J., Schendel D., Parner E. T., Pedersen L. H., Vestergaard M. (2013). Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309, 1696–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani D. C., Muzik O., Behen M., Rothermel R., Janisse J. J., Lee J., Chugani H. T. (1999). Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann. Neurol. 45, 287–295 [DOI] [PubMed] [Google Scholar]
- Chugani D. C., Muzik O., Rothermel R., Behen M., Chakraborty P., Mangner T., da Silva E. A., Chugani H. T. (1997). Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann. Neurol. 42, 666–669 [DOI] [PubMed] [Google Scholar]
- Cook E. H., Jr, Courchesne R., Lord C., Cox N. J., Yan S., Lincoln A., Haas R., Courchesne E., Leventhal B. L. (1997). Evidence of linkage between the serotonin transporter and autistic disorder. Mol. Psychiatry 2, 247–250 [DOI] [PubMed] [Google Scholar]
- Cunliffe V. T. (2004). Histone deacetylase 1 is required to repress Notch target gene expression during zebrafish neurogenesis and to maintain the production of motoneurones in response to hedgehog signalling. Development 131, 2983–2995 [DOI] [PubMed] [Google Scholar]
- Detich N., Bovenzi V., Szyf M. (2003). Valproate induces replication-independent active DNA demethylation. J. Biol. Chem. 278, 27586–27592 [DOI] [PubMed] [Google Scholar]
- Devlin B., Cook E. H., Jr, Coon H., Dawson G., Grigorenko E. L., McMahon W., Minshew N., Pauls D., Smith M., Spence M. A., et al. CPEA Genetics Network (2005). Autism and the serotonin transporter: the long and short of it. Mol. Psychiatry 10, 1110–1116 [DOI] [PubMed] [Google Scholar]
- Dufour-Rainfray D., Vourc’h P., Le Guisquet A. M., Garreau L., Ternant D., Bodard S., Jaumain E., Gulhan Z., Belzung C., Andres C. R., et al. (2010). Behavior and serotonergic disorders in rats exposed prenatally to valproate: a model for autism. Neurosci. Lett. 470, 55–59 [DOI] [PubMed] [Google Scholar]
- Dufour-Rainfray D., Vourc’h P., Tourlet S., Guilloteau D., Chalon S., Andres C. R. (2011). Fetal exposure to teratogens: evidence of genes involved in autism. Neurosci. Biobehav. Rev. 35, 1254–1265 [DOI] [PubMed] [Google Scholar]
- Dykens E. M., Lee E., Roof E. (2011). Prader-Willi syndrome and autism spectrum disorders: an evolving story. J. Neurodevelopmental Disorders 3, 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H., Yuan P., Gould T. D., Li J., Du J., Zhang L., Manji H. K., Chen G. (2003). The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J. Neurosci. 23, 7311–7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geling A., Steiner H., Willem M., Bally-Cuif L., Haass C. (2002). A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 3, 688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety S. S., Breau M. A., Sasai N., Xu Q., Briscoe J., Wilkinson D. G. (2013). An inducible transgene expression system for zebrafish and chick. Development 140, 2235–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goridis C., Brunet J. F. (1999). Transcriptional control of neurotransmitter phenotype. Curr. Opin. Neurobiol. 9, 47–53 [DOI] [PubMed] [Google Scholar]
- Göttlicher M., Minucci S., Zhu P., Krämer O. H., Schimpf A., Giavara S., Sleeman J. P., Lo Coco F., Nervi C., Pelicci P. G., et al. (2001). Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20, 6969–6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. Y., Vaccaro A. M., Jaskula-Sztul R., Ning L., Haymart M., Kunnimalaiyaan M., Chen H. (2007). Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist 12, 942–951 [DOI] [PubMed] [Google Scholar]
- Guillemot F., Lo L. C., Johnson J. E., Auerbach A., Anderson D. J., Joyner A. L. (1993). Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75, 463–476 [DOI] [PubMed] [Google Scholar]
- Gurvich N., Berman M. G., Wittner B. S., Gentleman R. C., Klein P. S., Green J. B. (2005). Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J. 19, 1166–1168 [DOI] [PubMed] [Google Scholar]
- Harrison M. R., Georgiou A. S., Spaink H. P., Cunliffe V. T. (2011). The epigenetic regulator Histone Deacetylase 1 promotes transcription of a core neurogenic programme in zebrafish embryos. BMC Genomics 12, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K. (1992). Role of the floor plate in axonal patterning in the zebrafish CNS. Neuron 9, 629–642 [DOI] [PubMed] [Google Scholar]
- Hendricks T. J., Fyodorov D. V., Wegman L. J., Lelutiu N. B., Pehek E. A., Yamamoto B., Silver J., Weeber E. J., Sweatt J. D., Deneris E. S. (2003). Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37, 233–247 [DOI] [PubMed] [Google Scholar]
- Herrmann K. (1993). Effects of the anticonvulsant drug valproic acid and related substances on the early development of the zebrafish (Brachydanio rerio). Toxicol. In Vitro 7, 41–54 [DOI] [PubMed] [Google Scholar]
- Hirsch M. R., Tiveron M. C., Guillemot F., Brunet J. F., Goridis C. (1998). Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development 125, 599–608 [DOI] [PubMed] [Google Scholar]
- Hollander E., Phillips A. T., Yeh C. C. (2003). Targeted treatments for symptom domains in child and adolescent autism. Lancet 362, 732–734 [DOI] [PubMed] [Google Scholar]
- Hori K., Cholewa-Waclaw J., Nakada Y., Glasgow S. M., Masui T., Henke R. M., Wildner H., Martarelli B., Beres T. M., Epstein J. A., et al. (2008). A nonclassical bHLH Rbpj transcription factor complex is required for specification of GABAergic neurons independent of Notch signaling. Genes Dev. 22, 166–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbimbo B. P. (2008). Therapeutic potential of gamma-secretase inhibitors and modulators. Curr. Top. Med. Chem. 8, 54–61 [DOI] [PubMed] [Google Scholar]
- Ingram J. L., Peckham S. M., Tisdale B., Rodier P. M. (2000). Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol. Teratol. 22, 319–324 [DOI] [PubMed] [Google Scholar]
- Itoh M., Kim C. H., Palardy G., Oda T., Jiang Y. J., Maust D., Yeo S. Y., Lorick K., Wright G. J., Ariza-McNaughton L., et al. (2003). Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 4, 67–82 [DOI] [PubMed] [Google Scholar]
- Jacob J., Ferri A. L., Milton C., Prin F., Pla P., Lin W., Gavalas A., Ang S. L., Briscoe J. (2007). Transcriptional repression coordinates the temporal switch from motor to serotonergic neurogenesis. Nat. Neurosci. 10, 1433–1439 [DOI] [PubMed] [Google Scholar]
- Jacob J., Storm R., Castro D. S., Milton C., Pla P., Guillemot F., Birchmeier C., Briscoe J. (2009). Insm1 (IA-1) is an essential component of the regulatory network that specifies monoaminergic neuronal phenotypes in the vertebrate hindbrain. Development 136, 2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M. (2000). Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 1, 20–29 [DOI] [PubMed] [Google Scholar]
- Jones P. L., Veenstra G. J., Wade P. A., Vermaak D., Kass S. U., Landsberger N., Strouboulis J., Wolffe A. P. (1998). Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19, 187–191 [DOI] [PubMed] [Google Scholar]
- Kane M. J., Angoa-Peréz M., Briggs D. I., Sykes C. E., Francescutti D. M., Rosenberg D. R., Kuhn D. M. (2012). Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS ONE 7, e48975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kani S., Bae Y. K., Shimizu T., Tanabe K., Satou C., Parsons M. J., Scott E., Higashijima S., Hibi M. (2010). Proneural gene-linked neurogenesis in zebrafish cerebellum. Dev. Biol. 343, 1–17 [DOI] [PubMed] [Google Scholar]
- Kim K. C., Kim P., Go H. S., Choi C. S., Yang S. I., Cheong J. H., Shin C. Y., Ko K. H. (2011). The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats. Toxicol. Lett. 201, 137–142 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- Klauck S. M., Poustka F., Benner A., Lesch K. P., Poustka A. (1997). Serotonin transporter (5-HTT) gene variants associated with autism? Hum. Mol. Genet. 6, 2233–2238 [DOI] [PubMed] [Google Scholar]
- Kolevzon A., Newcorn J. H., Kryzak L., Chaplin W., Watner D., Hollander E., Smith C. J., Cook E. H., Jr, Silverman J. M. (2010). Relationship between whole blood serotonin and repetitive behaviors in autism. Psychiatry Res. 175, 274–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook H., Lepore J. J., Gitler A. D., Lu M. M., Wing-Man Yung W., Mackay J., Zhou R., Ferrari V., Gruber P., Epstein J. A. (2003). Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J. Clin. Invest. 112, 863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S., Concordet J. P., Ingham P. W. (1993). A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75, 1431–1444 [DOI] [PubMed] [Google Scholar]
- Kuwagata M., Ogawa T., Shioda S., Nagata T. (2009). Observation of fetal brain in a rat valproate-induced autism model: a developmental neurotoxicity study. Dev. Neurosci. 27, 399–405 [DOI] [PubMed] [Google Scholar]
- Lauter G., Söll I., Hauptmann G. (2011). Two-color fluorescent in situ hybridization in the embryonic zebrafish brain using differential detection systems. BMC Dev. Biol. 11, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillesaar C. (2011). The serotonergic system in fish. J. Chem. Neuroanat. 41, 294–308 [DOI] [PubMed] [Google Scholar]
- Lillesaar C., Tannhauser B., Stigloher C., Kremmer E., Bally-Cuif L. (2007). The serotonergic phenotype is acquired by converging genetic mechanisms within the zebrafish central nervous system. Dev. Dyn. 236, 1072–1084 [DOI] [PubMed] [Google Scholar]
- Lin H. C., Gean P. W., Wang C. C., Chan Y. H., Chen P. S. (2013). The amygdala excitatory/inhibitory balance in a valproate-induced rat autism model. PLoS ONE 8, e55248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A., Artavanis-Tsakonas S. (2006). Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93–102 [DOI] [PubMed] [Google Scholar]
- Marchion D. C., Bicaku E., Daud A. I., Sullivan D. M., Munster P. N. (2005). Valproic acid alters chromatin structure by regulation of chromatin modulation proteins. Cancer Res. 65, 3815–3822 [DOI] [PubMed] [Google Scholar]
- McDougle C. J., Kresch L. E., Posey D. J. (2000). Repetitive thoughts and behavior in pervasive developmental disorders: treatment with serotonin reuptake inhibitors. J. Autism Dev. Disord. 30, 427–435 [DOI] [PubMed] [Google Scholar]
- McLean D. L., Fetcho J. R. (2004). Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J. Comp. Neurol. 480, 38–56 [DOI] [PubMed] [Google Scholar]
- Milutinovic S., D’Alessio A. C., Detich N., Szyf M. (2007). Valproate induces widespread epigenetic reprogramming which involves demethylation of specific genes. Carcinogenesis 28, 560–571 [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Narita N., Narita M. (2005). Maternal administration of thalidomide or valproic acid causes abnormal serotonergic neurons in the offspring: implication for pathogenesis of autism. Int. J. Dev. Neurosci. 23, 287–297 [DOI] [PubMed] [Google Scholar]
- Mulder E. J., Anderson G. M., Kema I. P., de Bildt A., van Lang N. D., den Boer J. A., Minderaa R. B. (2004). Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J. Am. Acad. Child Adolesc. Psychiatry 43, 491–499 [DOI] [PubMed] [Google Scholar]
- Nabi R., Serajee F. J., Chugani D. C., Zhong H., Huq A. H. (2004). Association of tryptophan 2,3 dioxygenase gene polymorphism with autism. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 125B, 63–68 [DOI] [PubMed] [Google Scholar]
- Nakatani J., Tamada K., Hatanaka F., Ise S., Ohta H., Inoue K., Tomonaga S., Watanabe Y., Chung Y. J., Banerjee R., et al. (2009). Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell 137, 1235–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X., Ng H. H., Johnson C. A., Laherty C. D., Turner B. M., Eisenman R. N., Bird A. (1998). Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389 [DOI] [PubMed] [Google Scholar]
- Narita N., Kato M., Tazoe M., Miyazaki K., Narita M., Okado N. (2002). Increased monoamine concentration in the brain and blood of fetal thalidomide- and valproic acid-exposed rat: putative animal models for autism. Pediatr. Res. 52, 576–579 [DOI] [PubMed] [Google Scholar]
- Noël E. S., Casal-Sueiro A., Busch-Nentwich E., Verkade H., Dong P. D., Stemple D. L., Ober E. A. (2008). Organ-specific requirements for Hdac1 in liver and pancreas formation. Dev. Biol. 322, 237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A. (2009). Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod. Toxicol. 28, 1–10 [DOI] [PubMed] [Google Scholar]
- Oyabu A., Narita M., Tashiro Y. (2013). The effects of prenatal exposure to valproic acid on the initial development of serotonergic neurons. Dev. Neurosci. 31, 202–208 [DOI] [PubMed] [Google Scholar]
- Parras C. M., Schuurmans C., Scardigli R., Kim J., Anderson D. J., Guillemot F. (2002). Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 16, 324–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H. (2006). Modeling features of autism in animals. In Understanding Autism (ed. Moldin S. O., Rubenstein J. L. R.), pp. 277–302 Boca Raton, FL: Taylor & Francis Group [Google Scholar]
- Pattyn A., Goridis C., Brunet J. F. (2000). Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol. Cell. Neurosci. 15, 235–243 [DOI] [PubMed] [Google Scholar]
- Pattyn A., Simplicio N., van Doorninck J. H., Goridis C., Guillemot F., Brunet J. F. (2004). Ascl1/Mash1 is required for the development of central serotonergic neurons. Nat. Neurosci. 7, 589–595 [DOI] [PubMed] [Google Scholar]
- Phiel C. J., Zhang F., Huang E. Y., Guenther M. G., Lazar M. A., Klein P. S. (2001). Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276, 36734–36741 [DOI] [PubMed] [Google Scholar]
- Pisharath H., Rhee J. M., Swanson M. A., Leach S. D., Parsons M. J. (2007). Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech. Dev. 124, 218–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasalam A. D., Hailey H., Williams J. H., Moore S. J., Turnpenny P. D., Lloyd D. J., Dean J. C. (2005). Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev. Med. Child Neurol. 47, 551–555 [DOI] [PubMed] [Google Scholar]
- Rodier P. M., Ingram J. L., Tisdale B., Nelson S., Romano J. (1996). Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J. Comp. Neurol. 370, 247–261 [DOI] [PubMed] [Google Scholar]
- Sacco R., Curatolo P., Manzi B., Militerni R., Bravaccio C., Frolli A., Lenti C., Saccani M., Elia M., Reichelt K. L., et al. (2010). Principal pathogenetic components and biological endophenotypes in autism spectrum disorders. Autism Res. 3, 237–252 [DOI] [PubMed] [Google Scholar]
- Schain R. J., Freedman D. X. (1961). Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J. Pediatr. 58, 315–320 [DOI] [PubMed] [Google Scholar]
- Schier A. F., Neuhauss S. C., Harvey M., Malicki J., Solnica-Krezel L., Stainier D. Y., Zwartkruis F., Abdelilah S., Stemple D. L., Rangini Z., et al. (1996). Mutations affecting the development of the embryonic zebrafish brain. Development 123, 165–178 [DOI] [PubMed] [Google Scholar]
- Stickney H. L., Barresi M. J., Devoto S. H. (2000). Somite development in zebrafish. Dev. Dyn. 219, 287–303 [DOI] [PubMed] [Google Scholar]
- Stockhausen M. T., Sjölund J., Manetopoulos C., Axelson H. (2005). Effects of the histone deacetylase inhibitor valproic acid on Notch signalling in human neuroblastoma cells. Br. J. Cancer 92, 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U., Blader P., Henrique D., Ingham P. W. (1993). Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 7, 1436–1446 [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. S., Delahanty R. J., Prasad H. C., McCauley J. L., Han Q., Jiang L., Li C., Folstein S. E., Blakely R. D. (2005). Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am. J. Hum. Genet. 77, 265–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takke C., Campos-Ortega J. A. (1999). her1, a zebrafish pair-rule like gene, acts downstream of notch signalling to control somite development. Development 126, 3005–3014 [DOI] [PubMed] [Google Scholar]
- Takke C., Dornseifer P., v Weizsäcker E., Campos-Ortega J. A. (1999). her4, a zebrafish homologue of the Drosophila neurogenic gene E(spl), is a target of NOTCH signalling. Development 126, 1811–1821 [DOI] [PubMed] [Google Scholar]
- Teraoka H., Russell C., Regan J., Chandrasekhar A., Concha M. L., Yokoyama R., Higashi K., Take-Uchi M., Dong W., Hiraga T., et al. (2004). Hedgehog and Fgf signaling pathways regulate the development of tphR-expressing serotonergic raphe neurons in zebrafish embryos. J. Neurobiol. 60, 275–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolizzo L., Carboni G., Ruzicka W. B., Mitchell C. P., Sugaya I., Tueting P., Sharma R., Grayson D. R., Costa E., Guidotti A. (2002). An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc. Natl. Acad. Sci. USA 99, 17095–17100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino N., Nakatani Y., Seki Y., Nakasato A., Nakamura M., Sugawara M., Arita H. (2007). Abnormality of circadian rhythm accompanied by an increase in frontal cortex serotonin in animal model of autism. Neurosci. Res. 57, 289–295 [DOI] [PubMed] [Google Scholar]
- van Eeden F. J., Granato M., Schach U., Brand M., Furutani-Seiki M., Haffter P., Hammerschmidt M., Heisenberg C. P., Jiang Y. J., Kane D. A., et al. (1996). Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development 123, 153–164 [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J., Muller C. L., Iwamoto H., Sauer J. E., Owens W. A., Shah C. R., Cohen J., Mannangatti P., Jessen T., Thompson B. J., et al. (2012). Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc. Natl. Acad. Sci. USA 109, 5469–5474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Lin H. C., Chan Y. H., Gean P. W., Yang Y. K., Chen P. S. (2013). 5-HT1A-receptor agonist modified amygdala activity and amygdala-associated social behavior in a valproate-induced rat autism model. Int. J. Neuropsychopharmacol. 16, 2027–2039 [DOI] [PubMed] [Google Scholar]
- Wang Z., Zang C., Cui K., Schones D. E., Barski A., Peng W., Zhao K. (2009). Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138, 1019–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. S., Cheng L., Mudge A. W., Harwood A. J. (2002). A common mechanism of action for three mood-stabilizing drugs. Nature 417, 292–295 [DOI] [PubMed] [Google Scholar]
- Yeo S. Y., Kim M., Kim H. S., Huh T. L., Chitnis A. B. (2007). Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev. Biol. 301, 555–567 [DOI] [PubMed] [Google Scholar]
- Yildirim E., Zhang Z., Uz T., Chen C. Q., Manev R., Manev H. (2003). Valproate administration to mice increases histone acetylation and 5-lipoxygenase content in the hippocampus. Neurosci. Lett. 345, 141–143 [DOI] [PubMed] [Google Scholar]
- Yochum C. L., Dowling P., Reuhl K. R., Wagner G. C., Ming X. (2008). VPA-induced apoptosis and behavioral deficits in neonatal mice. Brain Res. 1203, 126–132 [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kijima M., Akita M., Beppu T. (1990). Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 265, 17174–17179 [PubMed] [Google Scholar]
- Zecchin E., Mavropoulos A., Devos N., Filippi A., Tiso N., Meyer D., Peers B., Bortolussi M., Argenton F. (2004). Evolutionary conserved role of ptf1a in the specification of exocrine pancreatic fates. Dev. Biol. 268, 174–184 [DOI] [PubMed] [Google Scholar]
- Zhang X., Beaulieu J. M., Sotnikova T. D., Gainetdinov R. R., Caron M. G. (2004). Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 305, 217. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y., Bear M. F. (2012). Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 4, a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.