Abstract
Herpes simplex virus, varicella zoster virus, and pseudorabies virus are neurotropic pathogens of the Alphaherpesvirinae subfamily of the Herpesviridae. These viruses efficiently invade the peripheral nervous system and establish lifelong latency in neurons resident in peripheral ganglia. Primary and recurrent infections cycle virus particles between neurons and the peripheral tissues they innervate. This remarkable cycle of infection is the topic of this review. In addition, some of the distinguishing hallmarks of the infections caused by these viruses are evaluated in terms of their underlying similarities.
Keywords: neuroinvasion, neurotropism, neurovirulence, axon, neuron, sensory ganglion
INTRODUCTION
One of the defining characteristics of viruses belonging to the Herpesviridae family is the establishment of lifelong infections by means of a latency program. During latency the infection persists in a dormant state during which the viral genome is stably maintained but no viral particles are assembled. Neurons of the peripheral nervous system (PNS) host the latent infection for a subset of viruses belonging to the Alphaherpesvirinae, a subfamily of the Herpesviridae (Figure 1). The first demonstration of the neurotropic properties of these viruses came from a study of the veterinary pathogen, pseudorabies virus (PRV), published by Dr. Albert Sabin (145). Since that time, it is widely recognized that instillation of neurotropic herpesviruses such as herpes simplex virus (HSV-1, HSV-2) or PRV into rodents typically results in transmission of infection from the PNS to the central nervous system (CNS), producing a lethal encephalitis. Transmission from the PNS to the CNS occurs across synapse-linked neurons, and the resulting self-amplifying circuit-specific spread has been exploited to trace neural connections in the vertebrate nervous system (47, 158). How these neuroinvasive and neurovirulent properties relate to the more typically benign infections of natural hosts remains an open question. HSV-1 only rarely causes severe encephalitis in humans.
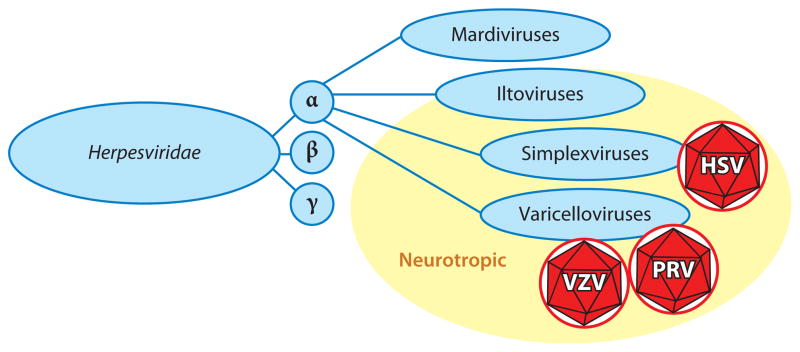
Figure 1.
The Alphaherpesvirinae subfamily consists of four genera. Two of the genera, the Simplexvirus and Varicellovirus, consist of human and veterinary pathogens that establish latency in peripheral neurons. Herpes simplex virus (HSV) is a simplexvirus, while varicella zoster virus (VZV) and pseudorabies virus (PRV) are varicelloviruses. At least one member of the Iltovirus genus is also proposed to establish latent infections in peripheral neurons (177), but the Mardivirus genus lacks neurotropism.
Productive infections consist of a cycle of transmission between latently infected neurons in ganglia of the PNS and the somatic cells to which they project. Virus particles never cross a synapse in recurrent infections of the natural host, and yet these viruses are exquisitely capable of doing just that. Why do these viruses maintain this devastating potential, and how is this property suppressed in the natural host, where disease is most often mild or nonexistent? This sets the neurotropic herpesviruses apart from other agents such as poliovirus, rabies virus, measles virus, and Japanese encephalitis virus, which are virulent upon entering the nervous system (175). Nevertheless, because of the high prevalence of these viruses in ourselves and our livestock, the low incidence of severe forms of disease takes a large toll. For example, HSV-1 pathogenesis is generally limited to cold sores but the virus is also the leading cause of infectious blindness in the United States (92). Reactivation of varicella zoster virus (VZV) produces shingles, which can transform into postherpetic neuralgia. And neonatal transmission of HSV-2 results in a high incidence of CNS and disseminated infections in newborns (72).
There are many outstanding questions regarding the neurotropic herpesvirus infectious cycle. “It must be explained, for example, why a virus causing no more than a cold sore in one person can produce fatal encephalitis in another.” This problem, which was articulated by Richard Johnson and Cedric Mims in 1968, remains relevant today (67). I review the current understanding of the neurotropic infectious cycle that has resulted from more than half a century of study.
TRANSMISSION FROM THE SITE OF EXPOSURE TO NERVE ENDINGS
Nerve endings are generally not exposed to the outside world. As such, the neurotropic herpesvirus infectious cycle exhibits dual tropism: replication in somatic cells such as epithelia, followed by transmission into neurons (Figure 2). How these viruses transmit between cell types is poorly understood. While Betaherpesvirinae and Gammaherpesvirinae can effect changes in cell tropism by modulating the composition of the infectious viral particle, there is currently no evidence that neuroinvasive herpesviruses employ a similar strategy (16, 149).
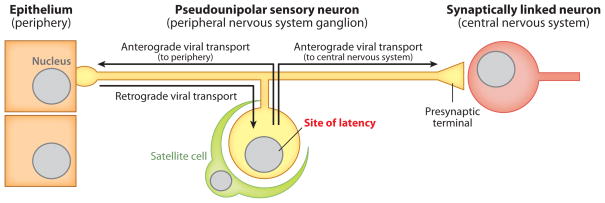
Figure 2.
Fundamentals of a neurotropic herpesvirus niche. Although the herpesviruses discussed in this article are referred to as neurotropic, these agents are more accurately described as dual tropic or multitropic. Primary infections begin in exposed tissues such as mucosal epithelia (left). Subsequent spread is normally restricted to innervating neurons resident in peripheral ganglia, where latent infections are maintained, following a single round of retrograde axon transport (middle). Reactivation results in the production of new viruses and the return to peripheral tissues (anterograde axon transport to epithelia). Severe disease associated with invasion of second-order neurons (anterograde axon transport to CNS) occurs only rarely in the natural host but may be frequent in secondary hosts (right). Anterograde and retrograde spread is dictated by the orientation of axonal microtubules and should not be confused with the direction of action potential propagation in the pseudounipolar neuron. Neurotropic herpesviruses can also infect cells of the circulatory system to varying degrees (not illustrated).
Despite the apparent lack of a neuron-specific tropism switch, viral effectors are required for neuroinvasion. The ICP34.5 protein of HSV-1 was described as a neurovirulence determinant due to its selective requirement for infections in animals (27). However, since its discovery, ICP34.5 has been recognized for its role in evading the host immune response (91, 101, 137). Although ICP34.5 is required for spread to neurons from the mouse cornea, for example, this is in large part due to loss of viral replication in the cornea itself (172). One factor that appears to specifically govern viral transmission into the nervous system without loss of viral replication at sites of peripheral inoculation is the PRV deubiquitinase activity housed within the amino terminus of the pUL36 (VP1/2) tegument protein (17, 89). All herpesviruses consist of a membrane envelope that contains an icosahedral capsid and a collection of additional proteins collectively referred to as the tegument, of which VP1/2 is one component. The capsid and tegument are deposited into cells upon fusion-mediated entry (Figure 3). How the deubiquitinase activity of VP1/2 contributes to the transmission of infection to the nervous system is currently unknown, but the phenotype of the mutant virus indicates that an innate barrier exists between epithelial tissues and innervating neurons that prevents viral transmission into the PNS. In this context, it is perhaps noteworthy that recent studies of poliovirus demonstrate that neuroinvasion is inefficient unless damage has been inflicted on the innervated tissue (86, 130). Whereas poliovirus is an enteric pathogen that infrequently invades the nervous system, the infectious cycles of HSV and PRV efficiently breach the epithelia-neuron barrier. Identifying the relevant substrates of the herpesvirus deubiquitinase will be required to better define the innate barrier function and how these viruses overcome it.
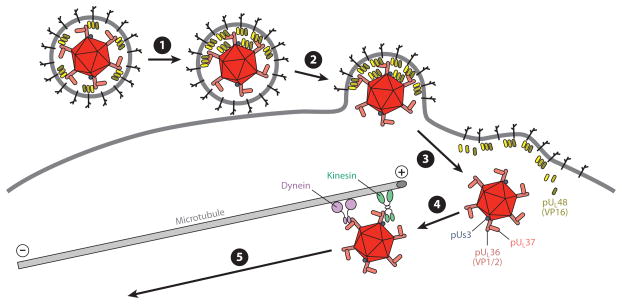
Figure 3.
Early events in herpesvirus infection. ❶ Virion contacts plasma membrane of a somatic cell or the axon membrane of a neuron. Tegument proteins redistribute and the virion orients with the bulk of its mass away from the cell. ❷ Fusion between the virion envelope and the cell membrane deposits the capsid and tegument proteins into the cytosol. ❸ The capsid releases from the majority of tegument and envelope proteins (including VP16), but a subset of inner tegument proteins (including pUs3, VP1/2, pUL37) remain capsid bound and together compose the retrograde transport complex. VP16, which enters the nucleus in somatic cells and promotes productive infection, may be lost upon entering a neuron owing to an inability to efficiently participate in retrograde axon transport. ❹ The retrograde transport complex traverses cortical actin (not illustrated) and associates with dynein and kinesin motors that in turn bind microtubules. Microtubules in axons are almost uniformly oriented, with plus-ends facing the axon terminals. ❺ Dynein dominates over kinesin activity, resulting in transport toward the minus-ends of microtubules and trafficking to the neural soma (retrograde transport).
DELIVERY OF VIRAL PARTICLES FROM AXON TERMINALS TO PERIPHERAL GANGLIA
Entering the Nerve Ending
Upon cell contact, three events are triggered in the HSV-1 virion: There is an internal restructuring of the tegument, the virion orients so that the dense pole of the particle faces away from the cell surface, and the fusion apparatus is triggered (54, 104, 109). Virion restructuring results in redistribution of tegument proteins in the virion that were initially symmetrically proportioned around the capsid to an asymmetric distribution, and its significance is unknown. Similar morphologic changes in HSV-1 virions can occur simply by allowing the particles to age (123). While the meaning of these findings can only be speculated on, they are suggestive of an internal virion trigger mechanism that is required at the moment of entry. Consistent with this, binding of HSV-1 virions to cells induces the disassociation of the pUL16 tegument protein from the capsid (109). This event is required for infection (108). Whether release of pUL16 from the capsid contributes to the larger morphological changes visualized in the tegument is currently unknown but seems likely.
How HSV-1 enters cells, including neurons, by membrane fusion is extensively reviewed elsewhere (31, 62, 70, 157). It should be noted here, however, that the principal HSV-1 envelope protein that triggers entry into cells is glycoprotein D (gD), which binds several cell membrane receptors including Nectin-1, HVEM (herpesvirus entry mediator), and 3-O-sulfated heparan sulfate (55, 114, 152). HSV-1 is dependent upon gD to enter cells, with Nectin-1 serving as the primary entry receptor on neurons and HVEM playing a supporting role (69, 77, 93, 164). However, the importance of gD in the neurotropic herpesviruses is not conserved. The prototypic varicellovirus, VZV, does not encode a gD homolog and instead may enter neurons through a different receptor interaction (160). In PRV, gD is dispensable for cell-cell spread and neuroinvasion, and gD mutant viruses spread independently of Nectin-1 and HVEM (25, 61, 134, 135, 140). Because PRV cannot produce extracellular plaque-forming units in the absence of gD, it can be inferred that PRV spread in the nervous system does not require the release of cell-free virions from infected cells.
Although all herpesviruses enter cells by membrane fusion, the fusion event can occur at either the plasma membrane or an endosomal membrane (111, 126). Several lines of evidence indicate that HSV-1 and PRV enter axon nerve endings by fusion at the plasma membrane: (a) entry is pH independent, (b) entry at the plasma membrane has been observed by transmission electron microscopy, and (c) capsids are not associated with fluid phase markers following entry (98, 125, 154).
Tegument Disassociation
Following fusion-mediated entry into cells, the herpesvirus envelope is lost and the tegument and capsid are deposited into the cytosol (Figure 3). A recent analysis of tegument protein associations with capsids based on resistance to detergent and salt extraction classified seven proteins as components of the inner tegument: pUL14, pUL16, pUL21, pUL36 (VP1/2), pUL37, pUs3, and ICP0 (139). The inner tegument consists of proteins in close juxtaposition to the capsid that likely are acquired prior to the final budding event that produces the enveloped virion. Although this study was not a comprehensive examination of all tegument proteins, it seems a reasonable first approximation that the identified proteins may remain bound to capsids upon entry as part of a retrograde transport complex. This is the case for at least three of these proteins. VP1/2 and pUL37 cotransport with capsids upon entry into sensory neurons according to fluorescence time-lapse imaging of HSV-1 and PRV infections (9, 96). The retention of VP1/2 and pUL37 on capsids postfusion is also evident by imaging fixed cell lines by electron microscopy and fluorescence methods (33, 58). The same approaches have confirmed that the pUs3 protein kinase is also cotransported with capsids following entry into neurons and nonneuronal cells infected with PRV (30, 58). Because tegument proteins that remain capsid bound may modulate retrograde transport, determining which proteins are retained on the capsid is of great interest. Consistent with this, a truncated version of VP1/2 that is poorly retained on HSV-1 capsids following entry fails to deliver genomes to nuclei (148). Of the seven recognized inner tegument proteins, four have been implicated in capsid delivery to the nucleus (Table 1).
Table 1.
Factors governing capsid delivery to the nucleus
| Step | Viral proteins | Cellular proteins | References |
|---|---|---|---|
| Passage through cortical actin? | gD | ROCK1 FAK PI3K Rho GTPases |
26, 28, 36, 52, 127, 129 |
| Retrograde microtubule transport | pUL35 (VP26)? | Dynein Dynactin |
7, 44–46 |
| Nuclear deliverya | pUL14 pUL36 (VP1/2) pUL37 ICP0 |
Proteasome | 33, 38, 39, 78, 143, 148, 181 |
| Nuclear docking and genome release | pUL6 (portal) pUL25 pUL36 (VP1/2) |
Importin-β Ran Nup214 Nup358hCG1 |
1, 2, 4, 14, 33, 68, 75, 122, 128, 131, 132, 138, 144 |
Factors listed are required for efficient delivery of capsids to the nucleus, and maybe involved in passage through cortical actin or microtubule transport.
HSV-1 and PRV tegument disassociation appears to be similar: both retain VP1/2 and pUL37 and remove pUL46 (VP11/12), pUL47 (VP13/14), pUL48 (VP16), and pUL49 (VP22) (9, 96). However, whereas removal of the last four tegument proteins appears to be efficient for PRV, HSV-1 retains small amounts of VP11/12 and VP16 on capsids postfusion (9). Although this seems to be a subtle difference between the two viruses, it may have relevance to the infectious cycle, as discussed below.
Cortical Actin
Binding of herpesvirus to cells triggers Rho GTPases that can in turn induce rearrangements in the actin cytoskeleton (28, 36, 129). There is increasing evidence that these signaling events may increase cell susceptibility to infection (reviewed in References 171 and 183). Whether these events pertain to infection at axon terminals has not been addressed. Applying cryo-electron tomography to image HSV-1 capsids deposited in the cytosol after fusion into synaptosomes, which are axon terminals severed from neurons, reveals capsids underneath the plasma membrane surrounded by dense meshworks of actin filaments (104). Although fusion-mediated entry occurred in less than one minute, cytosolic capsids remained near the plasma membrane for more than one hour. This suggests actin may be a barrier to initial infection.
Evidence that virus-induced signal cascades assist capsid translocation through cortical actin can be inferred from time-lapse imaging. Following contact with a terminal of an intact sensory axon, fluorescently tagged PRV capsids display motion that is biphasic (154). Initially, capsids move retrograde with slow kinetics as they enter the axon shaft. Fast retrograde transport consistent with dynein motion (see below) engages shortly thereafter. Capturing recordings of entry events remains challenging and has so far precluded an in-depth analysis of initial intracellular transport dynamics. Nevertheless, the available observations are consistent with navigation of capsids from the plasma membrane to microtubules via a directed process that may be promoted by alterations in axonal actin. In natural infections, nerve endings are generally not freely accessible to extracellular virions as they are in culture models of infection. Although highly speculative, the transmission of infection across epithelia-neuron contacts may promote virus-induced rearrangements of axon actin in a process analogous to the virological synapses reported for HIV and HSV (12, 115).
Retrograde Axon Transport
Time-lapse imaging of fluorescently tagged capsids of HSV-1 and PRV in cultured neurons has revealed that retrograde transport is a robust and sustained process that efficiently delivers capsids to the distant nucleus (9, 50, 94, 154). Transport occurs along microtubules at rates in excess of 1 μm s−1 (9, 80, 168). The fast retrograde axonal transport exhibited by HSV-1 and PRV capsids can be mediated only by active microtubule transport by the dynein motor complex (reviewed in References 71 and 170). Retrograde capsid motion is not continuous; brief reversals in transport direction occur in the axon (154). This transient anterograde motion implies the presence of a kinesin motor on the capsid in addition to the dynein motor complex. The presence of opposing microtubule motors is consistent with endogenous cellular cargoes as well as other viruses in axons (59, 147). Although not intuitive, the opposing pull of the kinesin motor may act to promote dynein retrograde transport of the capsid (6).
Purified extracellular virions stripped of their envelopes bind dynein and kinesin motors, and this binding is enhanced by extracting outer tegument proteins from the particles (139, 180). These findings implicate either the capsid or tegument proteins closely juxtaposed to the capsid surface as microtubule motor binding sites. Several genes encoding tegument proteins can be deleted without impeding retrograde axon transport, but the inner tegument proteins have critical roles in virion assembly, making the study of mutant viruses during the initial stages of neuron infection difficult (7). Among these, VP1/2 is considered a top candidate for recruiting microtubule motors to capsids. Antibodies directed against VP1/2 or truncation of the VP1/2 carboxyl-terminus interferes with capsid delivery to the nucleus and VP1/2 is required for microtubule transport during late infection in cell lines (33, 97, 148). In addition, VP1/2 carrying an amino acid change that confers temperature sensitivity dramatically accumulates in aggresomes at the nonpermissive temperature (1, 3). While this phenotype may simply result from an abundance of misfolded VP1/2 proteins inside cells, a recent model of virus retrograde transport posits that viruses may mimic protein aggregates to recruit dynein and effect their transport to the microtubule organizing center (MTOC) (176).
Viruses lacking the inner tegument proteins ICP0, pUL14, or pUL37 suffer from delayed delivery of capsids to the nucleus in cell lines (Table 1). Whether these proteins contribute to dynein recruitment and retrograde transport or another step in capsid delivery is also of great interest. The pUL37 tegument protein was examined in a nuclear delivery assay between nuclei in syncytia and in this context was dispensable (143). This finding indicates that pUL37 does not function at nuclear pore complexes (NPCs), but rather at an earlier step in infection: perhaps overcoming the cortical actin barrier or microtubule transport.
Dynein recruitment does not have to be mediated by a tegument protein. The pUL35 (VP26) capsid protein can bind dynein and was implicated in capsid retrograde transport (46). However, in the absence of VP26, PRV capsids transport at wild-type velocity, and transport of HSV-1 is unimpeded both in culture and in a mouse model of infection (7, 40, 44). Therefore, if VP26 binding to dynein is biologically relevant, it must be redundant with another dynein recruitment mechanism. Furthermore, de novo–assembled capsids isolated from the nucleus of infected cells do not bind to dynein in vitro, arguing that capsid proteins may not be involved in dynein recruitment; however, the possibility of posttranslational modifications to capsids in the cytosol cannot be ruled out (139, 180). While studies of HSV-1 and PRV have propelled our understanding of retrograde axon transport, a new neuron chamber infection model has the promise of extending these studies to VZV (103).
From Microtubule Organizing Center to Nuclear Pore Complexes
Minus-end-directed transport along microtubules is expected to end at the MTOC. However, HSV-1 capsids only transiently accumulate at the MTOC prior to moving to NPCs in the nuclear membrane (156). The MTOC is typically located adjacent to the nucleus. Whether the MTOC is close enough to allow subsequent passive diffusion of capsids to NPCs is debated. For adenovirus, capsid translocation from the MTOC to NPCs is dependent upon the nuclear export factor CRM1 (159). Whether CRM1 or a factor exported from the nucleus by CRM1 is necessary for proper adenovirus targeting is unknown, but the dependence on CRM1 argues for a facilitated process. Evidence of a plus-end kinesin motor activity associated with retrogradely trafficking HSV-1 and PRV capsids has led to the suggestion that movement from the MTOC to NPCs may be mediated by plus-end transport along perinuclear microtubules (45).
Nuclear Injection of the Viral Genome
By transmission electron microscopy, HSV-1 capsids are observed docked at NPCs following entry into cell lines (14, 156). The interactions between capsids and NPC proteins that mediate this coupling are not fully defined. Capsid docking is dependent on importin-β and Ran GTPase in an in vitro reconstituted model, and the Nup358 component of the NPC is required for capsid docking in intact cells (33, 131). Tegument components of the retrograde transport complex remain capsid bound once docked at nuclear pores, where the genome is released into the nucleus. Once docking occurs, the VP1/2 tegument protein is proteolytically processed, and this cleavage is required for subsequent release of the DNA genome from the capsid into the nuclear pore (68). A recent study determined that pUL37, and to a lesser extent VP1/2, is lost from docked capsids by 4 h postinfection (5). Whether this loss is of functional significance is not clear. Although the proteins may simply turn over after genome injection, their loss could be a functional consequence of VP1/2 proteolytic processing and genome release from the capsid. HSV-1 encoding a temperature-sensitive mutation in VP1/2 is not processed at the nonpermissive temperature and fails to release its genome upon NPC docking (14, 68, 75). VP1/2 is not expected to maintain the encapsidated genome, so its cleavage likely triggers a conformational change that allows escape of the DNA through the portal vertex of the capsid (122). VP1/2 is bound directly to the capsid surface through an interaction with the pUL25 capsid protein and additionally makes contact with the pUL17 and VP5 capsid proteins (23, 29, 132). Notably, pUL25 is required for stable genome encapsidation during assembly and packaging in the nucleus (107). Although deletion of UL25 prevents production of infectious virions, HSV-1-encoding UL25 mutations that are defective for genome injection after NPC docking have been isolated (138). In addition to binding VP1/2, pUL25 binds two NPC components: Nup214 and hCG1 (132). VP1/2 encodes a nuclear localization signal that is essential for productive infection (2). It will be of great interest to learn whether capsid-bound VP1/2 binds importin-β via the essential nuclear localization signal, and how this pertains to capsid docking and genome injection.
SELECTIVE LOSS OF VP16 DURING NEURONAL INFECTION
Once in the nucleus, a single viral genome can establish a replication compartment as an early step to viral amplification (76). In neurons of the natural host, this potential is suppressed and latency is instead established. The decision to replicate or enter latency is tied to the fate of the VP16 tegument protein. VP16 is a transactivator of immediate early gene expression that enters the nucleus of somatic cells in a complex with host cell factor C1 (HCF-C1) to promote productive infection (84, 85). Upon entering a cell, VP16 is removed from the capsid (Figure 3) (58). In the context of a neuron, this disassociation eliminates an obvious means to deliver VP16 to the neural soma upon entry at the distal axon (9, 96). Input VP16 may be otherwise unable to reach neuronal nuclei, as retrograde axon transport of cytosolic proteins targeted for nuclear import can be blocked in healthy neurons (60). These findings suggest that the neuron polar architecture is predisposed to favor latency establishment. However, VP16 expression in neural soma is not sufficient to trigger productive infection, indicating that there is a second barrier to VP16 nuclear delivery in neurons (150). This second block appears to be at the level of nuclear import. HCF-C1 is sequestered from the nucleus in neurons, which is expected to prevent VP16 nuclear import (81). These two properties functioning in tandem may serve as the primary determinant of latency establishment in neurons. In accordance with this model, VP16 is also a critical determinant for reactivation of HSV-1 from latency (165).
In the case of HSV-1, the presence of a small amount of capsid cotransported VP16 may help explain why, upon initial seeding of the nervous system, some neurons establish an acute productive infection that sends progeny virions back to peripheral innervated tissues, whereas other neurons become latently infected (15, 166). The difference in neuron fate could be attributed to small doses of VP16 delivered to the neural soma by variable numbers of retrogradely moving capsids. Consistent with such a model, VP16 was absent from capsids once docked at the nuclear membrane, indicating that this tegument protein is released from capsids following axon transport (9). Whether capsid-dependent trafficking of VP16 to nuclear pores requires HCF-C1 for subsequent nuclear import is unknown.
POSTREPLICATIVE SPREAD
Herpesvirus replication in the nucleus is followed by capsid assembly and genome encapsidation. There is a long-standing debate on how nucleocapsids egress to the cytosol, but current consensus supports a budding event through the inner nuclear membrane followed by a fusion event at the outer nuclear membrane (reviewed in References 66 and 110). Prior to envelopment at the trans-Golgi network (TGN), PRV capsids acquire inner tegument proteins, including VP1/2, pUL37, and pUs3 (57, 74). In fact, the composition of PRV capsids newly deposited into the cytosol, whether from extracellular virions or newly replicated from the nucleus, is remarkably similar. How capsids are differentially targeted to the nucleus early in infection and to the TGN postreplication is an open issue. Because the complement of tegument proteins that are capsid bound in the cytosol is not fully known during either stage of infection, differences in the composition of the capsid-tegument complexes may exist.
For HSV-1, this conundrum could be explained by observations that a subset of outer tegument proteins are acquired on capsids in the nucleus prior to reaching the cytosol; however, these observations are generally inconsistent. VP16 was detected by immunogold electron microscopy on capsids that had budded into the inner nuclear membrane (118). This reactivity was quite weak, however, and in several independent studies VP16 was not detected on purified nuclear capsids (139, 141, 180, 182). The pUL41 outer tegument protein (virion host shutoff, Vhs) also has mixed reports regarding whether it is present on nuclear capsids (139, 141, 180). Similar to reports on these outer tegument proteins, reports vary regarding the site of acquisition for HSV-1 inner tegument proteins, including VP1/2 and pUL37 (20, 124, 139, 169, 180).
In contrast to the varying reports for HSV-1, tegument proteins have generally not been detected on PRV nuclear capsids (57). However, the PRV VP1/2 tegument protein is expressed as multiple isoforms, one of which is a carboxyl-terminal fragment that associates with capsids in the nucleus and expedites nuclear egress (90). Although this new finding has not yet been examined in the context of HSV-1 infection, there is evidence of a carboxyl-terminal species of VP1/2 associated with nuclear HSV-1 capsids (139). Given the technical challenges inherent to these studies, a case can be argued for the need of side-by-side experiments comparing HSV-1 and PRV tegument acquisition to determine whether divergence in assembly pathways of these neurotropic herpesviruses truly exists. A report that HSV-1 uses gB and gH to egress from the nucleus, whereas a separate report found no role for these proteins in PRV nuclear egress, further emphasizes the need for direct comparative analysis to define the assembly and egress pathways used by these viruses (49, 73). This is especially important given that HSV-1 and PRV assembly and egress are essentially indistinguishable when viewed by transmission electron microscopy (56).
Transport to the Cytoplasmic Envelopment Site
While the carboxyl-terminal species of VP1/2 plays an accessory role in the egress of capsids from the nucleus, full-length VP1/2 is essential for cytoplasmic envelopment and egress (41, 53). In addition, time-lapse imaging has shown that UL36-null PRV capsids fail to transport along microtubules. UL37-null PRV envelopes in the cytoplasm poorly but retains vestigial intracellular transport (97). Because UL37-null PRV acquires capsid-bound VP1/2, the directed motion exhibited by this mutant supports a role for VP1/2 in microtubule transport that is enhanced by pUL37 (74). As discussed above, VP1/2 and pUL37 also contribute to capsid delivery to the nucleus, very likely by recruiting the dynein motor to capsids. Implicit in these observations is a puzzle. Capsid-VP1/2-pUL37-pUs3 complexes are present in the cytosol following both entry and postreplication (Figures 3 and 4), with both potentially recruiting the dynein motor, and yet the trafficking of these particles is quite different.
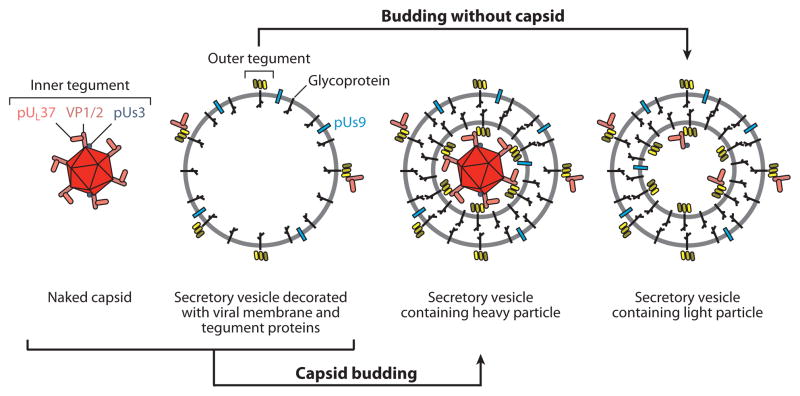
Figure 4.
Four types of cytoplasmic viral particles present during the egress stage of infection. Unenveloped (naked) capsids newly egressed from the nucleus acquire inner tegument proteins beginning with VP1/2, which binds capsids directly. The composition of these particles and the retrograde transport complex that is released into the cytosol following entry are similar, if not equivalent (see Figure 3). Outer tegument proteins associate primarily with viral membrane proteins resident in the biosynthetic pathway (secretory vesicle), which may also acquire some inner tegument proteins in the absence of capsids. Membrane proteins consist of glycoproteins and nonglycosylated membrane-associated proteins that include pUs9. Infectious virions (heavy particles) form when capsids bud into membranes decorated with viral membrane and outer tegument proteins and subsequently exocytose from the cell. Light particles are noninfectious secreted viral particles that consist of a viral envelope and tegument proteins but lack a capsid. The three membrane-associated intracellular particles expose membrane protein tails and tegument proteins to the cytosolic surface. Among the exposed proteins, pUs9 is enriched in the vesicle membrane surface and may direct trafficking of particles to the distal axon.
Two broad possibilities that could explain how capsids target to the nucleus during early infection and to the cytoplasmic envelopment site during egress require further examination. First, capsid-associated kinesin activities could be specifically enhanced during the egress stage of infection. Capsid-tegument complexes bind both dynein and kinesins (139). During retrograde axon transport, kinesin-based motion is weak and the predominating dynein-based motion results in capsid delivery to the neural soma (154). The presence of kinesin activity during retrograde axon transport is not intuitive but may be explained if kinesin and dynein recruitment also occurs during egress. In this scenario, capsid-associated kinesin activities could be enhanced to traffic the capsids to the site of envelopment. Second, capsids may recruit the dynein motor and move retrograde at both stages of infection. The presence of viral tegument and glycoproteins in the biosynthetic pathway during late infection could redirect capsid trafficking to the TGN for envelopment, as capsids move by dynein-based motion toward the MTOC. Similar to that observed in nonneuronal cell lines, capsids bud into the TGN in neurons and are released from the neural soma by exocytosis (113).
AXON TARGETING
In the absence of either gE, gI, or the pUs9 envelope proteins, PRV cannot transport through neural circuits in the anterograde direction (13, 18, 19, 21, 82, 83, 116, 117, 174). In cultured neurons the phenotype of the deletion mutants is not absolute, but PRV or HSV-1 lacking any one of these proteins is dramatically reduced for anterograde transmission in cultured neurons (24, 100, 155). When the genes for all three proteins are simultaneously deleted, anterograde transmission is eliminated (24). The gE, gI, and pUs9 viral envelope proteins coordinate the delivery of cytoplasmic viral particles from the neural soma into the axon. However, the requirement for these proteins in cultured axons is present only in neurons that have been cultured for prolonged periods: two weeks is typically used. In short-term cultures, anterograde axon transport of PRV is unimpeded in the absence of pUs9 (G. Smith & L.W. Enquist, unpublished data). Therefore, the barrier overcome by these proteins is slow to manifest following axon outgrowth and is consistent with the maturation of the axon initial segment (178).
The transmembrane domain and cytosolic tail of gE are dispensable for anterograde trafficking (167). This last point is remarkable for two reasons. First, gE is not structurally incorporated into virions in the absence of the transmembrane anchor and cytosolic tail. Second, the truncated protein is secreted. Taken together, these findings argue that gE is facilitating the delivery of cytoplasmic viral particles by interacting with extracellular proteins away from the viral particle.
Although the mechanisms by which these proteins function need to be further addressed, pUs9 appears to function in a way that is distinct from gE and gI. PRV lacking pUs9 is far more attenuated for anterograde transmission than are gE or gI mutants, yet the pUs9-null virus has no defect in plaque size, whereas gE-null and gI-null viruses do (24). The function of pUs9 is discussed further below.
Another puzzling facet to these proteins is that they are all selected against during serial passage in culture. PRV spontaneously loses gE expression during serial passage, and an extensive passage regime that was used to make an attenuated vaccine strain of PRV resulted in the deletion of all three genes. Similarly, isolates of HSV-1 with spontaneous mutations in gI or pUs9 have been reported (121, 161).
Anterograde Axon Transport
Trafficking of viral particles to the distal axon occurs by fast microtubule-based transport (153). In a remarkable case report of a nine-year-old boy that experienced herpetic pain from the sciatic nerve and zoster lesions six days later, a crude estimate of herpes anterograde spread in its natural setting was found to be consistent with the measured transport velocities of individual PRV capsids in culture neurons (162). This observation provides a unique validation of the use of neuron culture models in the study of herpesvirus infection. Unfortunately, studies of infection in neural culture models have resulted in a debate regarding the mechanics of anterograde axon transport. Two types of viral particle could potentially enter axons and engage in anterograde transport: cytosolic capsids that emerge from the nucleus (referred to as naked capsids owing to the absence of a membrane envelope) and enveloped virions in secretory vesicles resulting from the budding of naked capsids into the TGN. In both instances tegument proteins would be positioned to recruit motors and effect transport of the particle, whether it is a naked capsid or an enveloped virion (Figure 4). Prior to budding, naked capsids become decorated with inner tegument proteins, which together have the capacity to bind dynein and kinesin motors (139, 180). Upregulation of kinesin-bound motors would provide a hypothetical mechanism by which the distal axon could be targeted. On the other hand, enveloped virions are readily detected in neuronal cell bodies (22, 37, 113). Because enveloped virions reside in a membrane vesicle derived from the host biosynthetic pathway, these vesicles could intrinsically be targeted to the distal axon without the need for viral effectors. Alternatively, viral proteins, including tegument proteins and the cytosolic tails of envelope proteins, could serve as a platform to recruit microtubule motors to the cytosolic surface of the vesicle. Support for the latter comes from the observation that envelope and tegument proteins transport anterograde in axons in the absence of capsids (8, 37, 50, 112). In fact, tegument proteins transport to distal axons in advance of capsid-containing particles (96). Therefore, membrane-bound envelope-tegument complexes have the capacity to transport efficiently in axons. In further support of enveloped virion transport, TGN-derived vesicles containing viral particles isolated from HSV-1-infected cells exhibit kinesin-based motion in a reconstituted in vitro microtubule transport model (88).
Enveloped virions are observed by electron microscopy in axons in animal and culture models of infection, for both HSV-1 (32, 64, 79, 87, 99, 120) and PRV (24, 50, 51, 102), adding further support to the vesicle model of axon transport. However, this model was challenged by a study of HSV-1 in cultured human neurons that reported that all viral particles seen throughout the entire length of axons were naked capsids; enveloped virions were universally restricted to the neuronal cell body (136). This dramatic result had the explicit implication that capsids, or capsid-tegument complexes, directly recruited a kinesin motor independent of the neuronal secretory pathway. However, a subsequent reassessment of this finding concluded that the majority of capsids in axons were in fact enveloped but were restricted to axon terminals and varicosities along the axon (146). Therefore, the debate evolved into a discussion of why naked and enveloped viral particles were coresident in axons and which of these particles represented the species that transports anterograde in axons. Interested readers are directed to the many reports describing the debate that have presented differing viewpoints and have also provided descriptions of additional studies that contribute to aspects of the debate that go beyond the current overview (for example, see References 34 and 43).
Recently, work from several labs, including new electron microscopy studies and time-lapse imaging of viral particle composition during active anterograde transport (10, 65, 120, 179), has provided compelling data that enveloped capsids of HSV-1 are transported anterograde in axons. The only remaining question is whether naked capsids also transport anterograde in axons. Because the tegument and envelope of herpes virions are heterogeneous, with the copy number of protein species varying widely from one particle to the next, this question will be difficult to conclusively answer (37). Viral particles containing low amounts of a tagged envelope protein will often be scored as lacking an envelope, particularly with the added challenge of imaging the particles while they are moving at speeds in excess of 1 μm s−1. Although use of sensitive electron-multiplying charge-coupled device cameras coupled with bright light sources has allowed detection of dim emissions from rapidly moving viral particles in axons, detecting all viral particles by means of a heterogeneous antigen such as an envelope protein will likely require total internal reflection microscopy or a new technique of equivalent sensitivity to determine whether all capsids actively moving in axons are associated with envelope components (10). Several different investigations have provided additional insight into the nature of viral particle association with the transport vesicle. HSV-1-containing vesicles isolated from infected cell cytoplasm frequently do not contain a fully budded virion within the lumen of the vesicle, but instead have a capsid that is partially wrapped in the vesicle membrane (88). Similar structures are seen in axons of neurons infected with HSV-1 (146). Close inspection of transmission electron micrographs of PRV-containing vesicles in axons further hints at these structures (24). There is also functional evidence for axon transport of partially budded PRV (30). Although more studies are necessary to confirm the presence of stable budded intermediates serving as cargoes of kinesin-based anterograde transport, these preliminary observations may have implications for the spread of virions from axons to neighboring cells (see below). Moving forward, additional characterization of the transport vesicle hijacked by HSV-1 and PRV to reach the distal axon is needed. The cellular Vamp2 protein, which functions at presynaptic axon terminals, is often cotransported with HSV-1 and PRV transport vesicles and may indicate that virus-containing vesicles are predisposed to target the axon terminal (10). VP1/2 may be one protein that contributes to this targeting (151). In addition, the pUs9 protein is an intriguing candidate as a membrane-bound effector of anterograde axon transport (163).
A GFP-pUs9 fusion protein expressed by a recombinant of PRV cotransports with capsids to the distal axon, consistent with PRV anterograde transport occurring in a vesicle. Although this finding in itself is not unexpected, it is more notable that GFP-pUs9 was significantly diminished in extracellular viral particles. The reduction in GFP-pUs9 incorporation in extracellular particles relative to viral particles actively undergoing anterograde axon transport can easily be explained only if the GFP-pUs9 protein is enriched in the transport vesicle membrane that surrounds the viral particle (Figure 4). A mutation in GFP-pUs9 that prevents anterograde spread of PRV remained competent to move anterograde in axons, but capsids were no longer cotransported with the GFP signal. These findings provide a compelling case for pUs9 as an anterograde transport effector in neurons. Because pUs9 is dispensable for anterograde capsid transport in short-term neuron cultures, its contribution may be to overcome the barrier function of a mature axon initial segment. Alternatively, if pUs9 recruits a kinesin motor to effect anterograde transport, then redundant mechanisms of kinesin recruitment must be available in short-term neural cultures that become restricted as neurons mature.
Release from the Axon Terminal
Using chambered neuron culture models, investigators cannot detect release of free HSV-1 and PRV virions from axons of infected neurons (24, 106). This is due, not to a lack of anterograde transport in axons, but to virions remaining tethered to the axon surface after emerging from the axon (35). Infection by the surface-bound virions requires axon-cell contact and is dependent upon gB in the virions.
As PRV infection in cultured neurons progresses, the neurons display changes in electro-physiology (105). Action potentials fire at increased frequency and eventually neurons display synchronous firing. This electrical coupling of infected neurons is promoted by axon-axon fusion pores that form in part by the action of gB. Currently, no evidence indicates that the activity of gB that forms axon-axon fusion pores is the result of the population of gB present in virions at the axon surface. But if cell surface virions promote electrical coupling, it may hint at the presence of a pore between the axon and surface virion that would allow for subsequent connectivity upon fusion to a neighboring axon. In this context, the previously discussed observations that viral particles may not fully bud into transport vesicles could be relevant to electrical coupling if these structures are preserved after exocytosis. Changes in electrophysiology are predicted to cause the caustic itching (pruritis) exhibited during PRV infection, and a related phenomenon could underlie the severe pain (postherpetic neuralgia) often suffered following VZV reactivation.
Spread Between Neurons and to Distant Sites
As previously noted, herpes virions can egress from the cell bodies of neurons as well as from axons. The biological consequence of virion release from the neuron cell body in peripheral ganglia has broad implications in the pathogenesis of the neurotropic herpesviruses. In the simplest scenario, HSV or PRV would only transmit between neurons and the cells they innervate at the periphery by repeated cycles of anterograde and retrograde axonal transport (Figure 2). The tendency for HSV-1 lesions (herpes labialis, or cold sores) to creep from one reactivation episode to the next would be explained by lateral spread of infection in the mucosal epithelium, allowing for seeding of new neurons that innervate cells adjacent to the active lesion. This view is consistent with the presentation of cold sore lesions, which tend to be focal and exhibit limited dissemination in the innervated dermatome. This is in contrast to reactivated VZV infections, which are discussed below.
Spread of HSV-1 within sensory ganglia may be limited by satellite cells that surround each neuron. Although these support cells are susceptible to PRV and HSV-1, they generally do not appear to be productively infected (22, 32, 63, 87). Perhaps the best evidence for the absence of lateral spread within peripheral ganglia comes from an in vivo study of PRV infection (158). PRV inoculated into the anterior chamber of the rat eye transits by retrograde axon transport to neuron cell bodies in the superior cervical ganglion. Subsequent labeling of the subset of superior cervical ganglion neurons that project to the anterior chamber by retrograde labeling with wheat germ agglutinin conjugated to horseradish peroxidase demonstrated that PRV remained confined to the neurons projecting axons to the anterior chamber of the eye and did not spread to neighboring neurons projecting to other sites. These results provided compelling evidence that viral dissemination occurred only by circuit-specific transmission. However, the PRV strain used in this seminal study, PRV Bartha, is an attenuated vaccine strain. Whether these results hold true for wild-type isolates of PRV or HSV-1 has not, to the best of my knowledge, been examined. But available evidence argues that wild-type virus strains are not always subject to this restriction. HSV inoculated on the mouse flank results in a zosteriform spread throughout the innervated dermatome, which suggests HSV spreads between neurons in dorsal root sensory ganglia (DRG) before returning back to the skin (173). Applying PRV to the flank model does not produce zoster-like lesions in the skin, but nevertheless the virus transmits through the dermatome, indicating that PRV, like HSV, disseminates laterally within the peripheral ganglia (19). In contrast, the attenuated Bartha strain remains confined to the inoculation site in the skin and does not spread through the dermatome.
In contrast to HSV and PRV, VZV reactivation often produces infection throughout a dermatome, which is the prototypic shingles manifestation (herpes zoster). Observations of human DRG infected with VZV either from human patients or in a mouse xenograft model are consistent with productive infections of neurons and satellite cells (48, 142). Furthermore, satellite cell and neural syncytia in human DRG are also evident. Lateral spread in sensory ganglia would likely result in infection of sensory neurons that project to internal organs. These general visceral afferent neurons are similar to somatosensory pseudounipolar neurons and are coresident in sensory ganglia. Because visceral infections are not associated with cold sore eruptions, this would argue that HSV-1 does not randomly spread between neurons in sensory ganglia in adult humans. However, neonatal transmission of HSV-2 can produce life-threatening infections that involve disseminated infections in internal organs (72). VZV spread to internal organs can also occur, although this outcome is more common in immunocompromised individuals (11).
Disseminated infections can occur with HSV, PRV, and VZV by means of cell-associated viremia. While this is most prevalent in VZV infections, PRV transplacental spread occurs by monocytes that transmit the infection (119). HSV infections are generally not associated with cell-associated viremia, but in the absence of an interferon response, infection transmits efficiently to the liver and other organs (95, 133). Neonatal disseminated HSV-2 infections are also associated with cell-associated viremia (42).
CONCLUSIONS
Whereas many viruses transport retrogradely in axons of neurons, neuroinvasive herpesviruses are notable for their efficient entry into the nervous system and coordination of retrograde and anterograde trafficking at opposing stages of the infectious cycle. These properties make viruses such as HSV-1 candidates for the development of gene delivery vectors that target the nervous system, but also underlie the potential for these viruses to become highly virulent following dissemination into the central nervous system. Ongoing research will resolve how herpesvirus assembly and egress are coupled to neuroinvasion and pathogenesis, and may yield the tools to produce both novel gene vectors and antiviral treatments to combat severe forms of neuroinvasive disease.
SUMMARY POINTS.
Herpesviruses overcome multiple barriers to establish latent infections in the nervous system.
Tegument proteins retained on capsids likely recruit microtubule motors necessary for axon transport.
Several viral and cellular factors have been identified that contribute to capsid delivery to the nucleus and to our understanding of how and when these functions delineate steps in neuronal infection.
Removal of VP16 from capsids upon axon entry may promote establishment of latency in the infected neuron.
HSV and PRV effectively transmit between synapse-linked neurons, but productive recurrent infections in natural hosts do not involve synaptic transmission.
Neurologic responses such as pain and pruritis may indicate productive infection and spread within ganglia.
In addition to their characteristic neurotropism, HSV, PRV, and VZV share broad-tissue tropism, which accounts for replication in the mucosa, visceral organs, and cell-associated viremia.
Acknowledgments
Research in the author’s laboratory was funded by the National Institutes of Health. Many thanks go to Lynn W. Enquist and Gary Pickard for their helpful discussions. I apologize to those many investigators whose interesting work could not be cited because of page limits.
Glossary
- PNS
peripheral nervous system
- PRV
pseudorabies virus
- HSV
herpes simplex virus
- CNS
central nervous system
- VZV
varicella zoster virus
- MTOC
microtubule organizing center
- NPC
nuclear pore complex
- TGN
trans-Golgi network
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Abaitua F, Daikoku T, Crump CM, Bolstad M, O’Hare P. A single mutation responsible for temperature-sensitive entry and assembly defects in the VP1-2 protein of herpes simplex virus. J Virol. 2011;85:2024–36. doi: 10.1128/JVI.01895-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abaitua F, O’Hare P. Identification of a highly conserved, functional nuclear localization signal within the N-terminal region of herpes simplex virus type 1 VP1-2 tegument protein. J Virol. 2008;82:5234–44. doi: 10.1128/JVI.02497-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abaitua F, Souto RN, Browne H, Daikoku T, O’Hare P. Characterization of the herpes simplex virus (HSV)-1 tegument protein VP1-2 during infection with the HSV temperature-sensitive mutant tsB7. J Gen Virol. 2009;90:2353–63. doi: 10.1099/vir.0.012492-0. [DOI] [PubMed] [Google Scholar]
- 4.Addison C, Rixon FJ, Palfreyman JW, O’Hara M, Preston VG. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology. 1984;138:246–59. doi: 10.1016/0042-6822(84)90349-0. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal A, Miranda-Saksena M, Boadle RA, Kelly BJ, Diefenbach RJ, et al. Ultrastructural visualization of individual tegument protein dissociation during entry of herpes simplex virus 1 into human and rat dorsal root ganglion neurons. J Virol. 2012;86:6123–37. doi: 10.1128/JVI.07016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ally S, Larson AG, Barlan K, Rice SE, Gelfand VI. Opposite-polarity motors activate one another to trigger cargo transport in live cells. J Cell Biol. 2009;187:1071–82. doi: 10.1083/jcb.200908075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antinone SE, Shubeita GT, Coller KE, Lee JI, Haverlock-Moyns S, et al. The herpesvirus capsid surface protein, VP26, and the majority of the tegument proteins are dispensable for capsid transport toward the nucleus. J Virol. 2006;80:5494–98. doi: 10.1128/JVI.00026-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antinone SE, Smith GA. Two modes of herpesvirus trafficking in neurons: Membrane acquisition directs motion. J Virol. 2006;80:11235–40. doi: 10.1128/JVI.01441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antinone SE, Smith GA. Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis. J Virol. 2010;84:1504–12. doi: 10.1128/JVI.02029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antinone SE, Zaichick SV, Smith GA. Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. J Virol. 2010;84:13019–30. doi: 10.1128/JVI.01296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arvin AM. Varicellazoster virus. Clin Microbiol Rev. 1996;9:361–81. doi: 10.1128/cmr.9.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aubert M, Yoon M, Sloan DD, Spear PG, Jerome KR. The virological synapse facilitates herpes simplex virus entry into T cells. J Virol. 2009;83:6171–83. doi: 10.1128/JVI.02163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babic N, Klupp B, Brack A, Mettenleiter TC, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–84. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- 14.Batterson W, Furlong D, Roizman B. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J Virol. 1983;45:397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blyth WA, Harbour DA, Hill TJ. Pathogenesis of zosteriform spread of herpes simplex virus in the mouse. J Gen Virol. 1984;65(Pt. 9):1477–86. doi: 10.1099/0022-1317-65-9-1477. [DOI] [PubMed] [Google Scholar]
- 16.Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med. 2002;8:594–99. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- 17.Böttcher S, Maresch C, Granzow H, Klupp BG, Teifke JP, Mettenleiter TC. Mutagenesis of the active-site cysteine in the ubiquitin-specific protease contained in large tegument protein pUL36 of pseudorabies virus impairs viral replication in vitro and neuroinvasion in vivo. J Virol. 2008;82:6009–16. doi: 10.1128/JVI.00280-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brideau AD, Card JP, Enquist LW. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. J Virol. 2000;74:834–45. doi: 10.1128/jvi.74.2.834-845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brittle EE, Reynolds AE, Enquist LW. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J Virol. 2004;78:12951–63. doi: 10.1128/JVI.78.23.12951-12963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucks MA, O’Regan KJ, Murphy MA, Wills JW, Courtney RJ. Herpes simplex virus type 1 tegument proteins VP1/2 and UL37 are associated with intranuclear capsids. Virology. 2007;361:316–24. doi: 10.1016/j.virol.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Card JP, Levitt P, Enquist LW. Different patterns of neuronal injection after intracerebral infection of two strains of pseudorabies virus. J Virol. 1998;72:4434–41. doi: 10.1128/jvi.72.5.4434-4441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Card JP, Rinaman L, Lynn RB, Lee BH, Meade RP, et al. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13:2515–39. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardone G, Newcomb WW, Cheng N, Wingfield PT, Trus BL, et al. The UL36 tegument protein of herpes simplex virus 1 has a composite binding site at the capsid vertices. J Virol. 2012;86:4058–64. doi: 10.1128/JVI.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ch’ng TH, Enquist LW. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J Virol. 2005;79:10875–89. doi: 10.1128/JVI.79.17.10875-10889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ch’ng TH, Spear PG, Struyf F, Enquist LW. Glycoprotein D-independent spread of pseudorabies virus infection in cultured peripheral nervous system neurons in a compartmented system. J Virol. 2007;81:10742–57. doi: 10.1128/JVI.00981-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheshenko N, Liu W, Satlin LM, Herold BC. Focal adhesion kinase plays a pivotal role in herpes simplex virus entry. J Biol Chem. 2005;280:31116–25. doi: 10.1074/jbc.M503518200. [DOI] [PubMed] [Google Scholar]
- 27.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–66. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 28.Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BY, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174:1009–21. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coller KE, Lee JI, Ueda A, Smith GA. The capsid and tegument of the alphaherpesviruses are linked by an interaction between the UL25 and VP1/2 proteins. J Virol. 2007;81:11790–97. doi: 10.1128/JVI.01113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coller KE, Smith GA. Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus. Traffic. 2008;9:1458–70. doi: 10.1111/j.1600-0854.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat Rev Microbiol. 2011;9:369–81. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook ML, Stevens JG. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973;7:272–88. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copeland AM, Newcomb WW, Brown JC. Herpes simplex virus replication: roles of viral proteins and nucleoporins in capsid-nucleus attachment. J Virol. 2009;83:1660–68. doi: 10.1128/JVI.01139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curanovic D, Enquist L. Directional transneuronal spread of α-herpesvirus infection. Future Virol. 2009;4:591. doi: 10.2217/fvl.09.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curanovic D, Enquist LW. Virion-incorporated glycoprotein B mediates transneuronal spread of pseudorabies virus. J Virol. 2009;83:7796–804. doi: 10.1128/JVI.00745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Regge N, Nauwynck HJ, Geenen K, Krummenacher C, Cohen GH, et al. α-Herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J Cell Biol. 2006;174:267–75. doi: 10.1083/jcb.200510156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.del Rio T, Ch’ng TH, Flood EA, Gross SP, Enquist LW. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J Virol. 2005;79:3903–19. doi: 10.1128/JVI.79.7.3903-3919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delboy MG, Nicola AV. A pre-immediate-early role for tegument ICP0 in the proteasome-dependent entry of herpes simplex virus. J Virol. 2011;85:5910–18. doi: 10.1128/JVI.00267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delboy MG, Roller DG, Nicola AV. Cellular proteasome activity facilitates herpes simplex virus entry at a postpenetration step. J Virol. 2008;82:3381–90. doi: 10.1128/JVI.02296-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desai P, DeLuca NA, Person S. Herpes simplex virus type 1 VP26 is not essential for replication in cell culture but influences production of infectious virus in the nervous system of infected mice. Virology. 1998;247:115–24. doi: 10.1006/viro.1998.9230. [DOI] [PubMed] [Google Scholar]
- 41.Desai PJ. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J Virol. 2000;74:11608–18. doi: 10.1128/jvi.74.24.11608-11618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diamond C, Mohan K, Hobson A, Frenkel L, Corey L. Viremia in neonatal herpes simplex virus infections. Pediatr Infect Dis J. 1999;18:487–89. doi: 10.1097/00006454-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Rev Med Virol. 2008;18:35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- 44.Dohner K, Radtke K, Schmidt S, Sodeik B. Eclipse phase of herpes simplex virus type 1 infection: efficient dynein-mediated capsid transport without the small capsid protein VP26. J Virol. 2006;80:8211–24. doi: 10.1128/JVI.02528-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dohner K, Wolfstein A, Prank U, Echeverri C, Dujardin D, et al. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol Biol Cell. 2002;13:2795–809. doi: 10.1091/mbc.01-07-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Douglas MW, Diefenbach RJ, Homa FL, Miranda-Saksena M, Rixon FJ, et al. Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J Biol Chem. 2004;279:28522–30. doi: 10.1074/jbc.M311671200. [DOI] [PubMed] [Google Scholar]
- 47.Enquist LW, Husak PJ, Banfield BW, Smith GA. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 48.Esiri MM, Tomlinson AH. Herpes Zoster. Demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- 49.Farnsworth A, Wisner TW, Webb M, Roller R, Cohen G, et al. Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proc Natl Acad Sci USA. 2007;104:10187–92. doi: 10.1073/pnas.0703790104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feierbach B, Bisher M, Goodhouse J, Enquist LW. In vitro analysis of transneuronal spread of an alphaherpesvirus infection in peripheral nervous system neurons. J Virol. 2007;81:6846–57. doi: 10.1128/JVI.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Field HJ, Hill TJ. The pathogenesis of pseudorabies in mice following peripheral inoculation. J Gen Virol. 1974;23:145–57. doi: 10.1099/0022-1317-23-2-145. [DOI] [PubMed] [Google Scholar]
- 52.Frampton AR, Jr, Uchida H, von Einem J, Goins WF, Grandi P, et al. Equine herpesvirus type 1 (EHV-1) utilizes microtubules, dynein, and ROCK1 to productively infect cells. Vet Microbiol. 2010;141:12–21. doi: 10.1016/j.vetmic.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuchs W, Klupp BG, Granzow H, Mettenleiter TC. Essential function of the pseudorabies virus UL36 gene product is independent of its interaction with the UL37 protein. J Virol. 2004;78:11879–89. doi: 10.1128/JVI.78.21.11879-11889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuller AO, Santos RE, Spear PG. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989;63:3435–43. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–20. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 56.Granzow H, Klupp BG, Fuchs W, Veits J, Osterrieder N, Mettenleiter TC. Egress of alphaherpesviruses: comparative ultrastructural study. J Virol. 2001;75:3675–84. doi: 10.1128/JVI.75.8.3675-3684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granzow H, Klupp BG, Mettenleiter TC. The pseudorabies virus US3 protein is a component of primary and of mature virions. J Virol. 2004;78:1314–23. doi: 10.1128/JVI.78.3.1314-1323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Granzow H, Klupp BG, Mettenleiter TC. Entry of pseudorabies virus: an immunogold-labeling study. J Virol. 2005;79:3200–5. doi: 10.1128/JVI.79.5.3200-3205.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gross SP. Dynactin: coordinating motors with opposite inclinations. Curr Biol. 2003;13:R320–22. [PubMed] [Google Scholar]
- 60.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 61.Heffner S, Kovacs F, Klupp BG, Mettenleiter TC. Glycoprotein gp50-negative pseudorabies virus: a novel approach toward a nonspreading live herpesvirus vaccine. J Virol. 1993;67:1529–37. doi: 10.1128/jvi.67.3.1529-1537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heldwein EE, Krummenacher C. Entry of herpesviruses into mammalian cells. Cell Mol Life Sci. 2008;65:1653–68. doi: 10.1007/s00018-008-7570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill TJ, Field HJ. The interaction of herpes simplex virus with cultures of peripheral nervous tissue: an electron microscopic study. J Gen Virol. 1973;21:123–33. doi: 10.1099/0022-1317-21-1-123. [DOI] [PubMed] [Google Scholar]
- 64.Hill TJ, Field HJ, Roome AP. Intra-axonal location of herpes simplex virus particles. J Gen Virol. 1972;15:233–35. doi: 10.1099/0022-1317-15-3-253. [DOI] [PubMed] [Google Scholar]
- 65.Huang J, Lazear HM, Friedman HM. Completely assembled virus particles detected by transmission electron microscopy in proximal and mid-axons of neurons infected with herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology. 2011;409:12–16. doi: 10.1016/j.virol.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9:382–94. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- 67.Johnson RT, Mims CA. Pathogenesis of viral infections of the nervous system. N Engl J Med. 1968;278:23–30. doi: 10.1056/NEJM196801042780106. [DOI] [PubMed] [Google Scholar]
- 68.Jovasevic V, Liang L, Roizman B. Proteolytic cleavage of VP1-2 is required for release of herpes simplex virus 1 DNA into the nucleus. J Virol. 2008;82:3311–19. doi: 10.1128/JVI.01919-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karaba AH, Kopp SJ, Longnecker R. Herpesvirus entry mediator and nectin-1 mediate herpes simplex virus 1 infection of the murine cornea. J Virol. 2011;85:10041–47. doi: 10.1128/JVI.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karasneh GA, Shukla D. Herpes simplex virus infects most cell types in vitro: clues to its success. Virol J. 2011;8:481. doi: 10.1186/1743-422X-8-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10:854–65. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kimberlin DW, Whitley RJ. Neonatal herpes: What have we learned. Semin Pediatr Infect Dis. 2005;16:7–16. doi: 10.1053/j.spid.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 73.Klupp B, Altenschmidt J, Granzow H, Fuchs W, Mettenleiter TC. Glycoproteins required for entry are not necessary for egress of pseudorabies virus. J Virol. 2008;82:6299–309. doi: 10.1128/JVI.00386-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klupp BG, Fuchs W, Granzow H, Nixdorf R, Mettenleiter TC. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J Virol. 2002;76:3065–71. doi: 10.1128/JVI.76.6.3065-3071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knipe DM, Batterson W, Nosal C, Roizman B, Buchan A. Molecular genetics of herpes simplex virus. VI. Characterization of a temperature-sensitive mutant defective in the expression of all early viral gene products. J Virol. 1981;38:539–47. doi: 10.1128/jvi.38.2.539-547.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kobiler O, Brodersen P, Taylor MP, Ludmir EB, Enquist LW. Herpesvirus replication compartments originate with single incoming viral genomes. mBio. 2011;2(6):e00278–11. doi: 10.1128/mBio.00278-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kopp SJ, Banisadr G, Glajch K, Maurer UE, Grunewald K, et al. Infection of neurons and encephalitis after intracranial inoculation of herpes simplex virus requires the entry receptor nectin-1. Proc Natl Acad Sci USA. 2009;106:17916–20. doi: 10.1073/pnas.0908892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krautwald M, Fuchs W, Klupp BG, Mettenleiter TC. Translocation of incoming pseudorabies virus capsids to the cell nucleus is delayed in the absence of tegument protein pUL37. J Virol. 2009;83:3389–96. doi: 10.1128/JVI.02090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kristensson K, Ghetti B, Wisniewski HM. Study on the propagation of herpes simplex virus (type 2) into the brain after intraocular injection. Brain Res. 1974;69:189–201. doi: 10.1016/0006-8993(74)90001-8. [DOI] [PubMed] [Google Scholar]
- 80.Kristensson K, Lycke E, Röyttä M, Svennerholm B, Vahlne A. Neuritic transport of herpes simplex virus in rat sensory neurons in vitro. Effects of substances interacting with microtubular function and axonal flow [nocodazole, taxol and erythro-9-3-(2-hydroxynonyl)adenine] J Gen Virol. 1986;67:2023–28. doi: 10.1099/0022-1317-67-9-2023. [DOI] [PubMed] [Google Scholar]
- 81.Kristie TM, Vogel JL, Sears AE. Nuclear localization of the C1 factor (host cell factor) in sensory neurons correlates with reactivation of herpes simplex virus from latency. Proc Natl Acad Sci USA. 1999;96:1229–33. doi: 10.1073/pnas.96.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kritas SK, Nauwynck HJ, Pensaert MB. Dissemination of wild-type and gC-, gE- and gI-deleted mutants of Aujeszky’s disease virus in the maxillary nerve and trigeminal ganglion of pigs after intranasal inoculation. J Gen Virol. 1995;76:2063–66. doi: 10.1099/0022-1317-76-8-2063. [DOI] [PubMed] [Google Scholar]
- 83.Kritas SK, Pensaert MB, Mettenleiter TC. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky’s disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994;75:2319–27. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 84.La Boissière S, Hughes T, O’Hare P. HCF-dependent nuclear import of VP16. EMBO J. 1999;18:480–89. doi: 10.1093/emboj/18.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.La Boissière S, O’Hare P. Analysis of HCF, the cellular cofactor of VP16, in herpes simplex virus-infected cells. J Virol. 2000;74:99–109. doi: 10.1128/jvi.74.1.99-109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lancaster KZ, Pfeiffer JK. Limited trafficking of a neurotropic virus through inefficient retrograde axonal transport and the type I interferon response. PLoS Pathog. 2010;6:e1000791. doi: 10.1371/journal.ppat.1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LaVail JH, Topp KS, Giblin PA, Garner JA. Factors that contribute to the transneuronal spread of herpes simplex virus. J Neurosci Res. 1997;49:485–96. [PubMed] [Google Scholar]
- 88.Lee GE, Murray JW, Wolkoff AW, Wilson DW. Reconstitution of herpes simplex virus microtubule-dependent trafficking in vitro. J Virol. 2006;80:4264–75. doi: 10.1128/JVI.80.9.4264-4275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JI, Sollars PJ, Baver SB, Pickard GE, Leelawong M, Smith GA. A herpesvirus encoded deubiquitinase is a novel neuroinvasive determinant. PLoS Pathog. 2009;5:e1000387. doi: 10.1371/journal.ppat.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leelawong M, Lee JI, Smith GA. Nuclear egress of pseudorabies virus capsids is enhanced by a subspecies of the large tegument protein that is lost upon cytoplasmic maturation. J Virol. 2012;86:6303–14. doi: 10.1128/JVI.07051-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leib DA, Machalek MA, Williams BR, Silverman RH, Virgin HW. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc Natl Acad Sci USA. 2000;97:6097–101. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 93.Ligas MW, Johnson DC. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–94. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu WW, Goodhouse J, Jeon NL, Enquist LW. A microfluidic chamber for analysis of neuron-to-cell spread and axonal transport of an alpha-herpesvirus. PLoS ONE. 2008;3:e2382. doi: 10.1371/journal.pone.0002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luker GD, Prior JL, Song J, Pica CM, Leib DA. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J Virol. 2003;77:11082–93. doi: 10.1128/JVI.77.20.11082-11093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luxton GW, Haverlock S, Coller KE, Antinone SE, Pincetic A, Smith GA. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc Natl Acad Sci USA. 2005;102:5832–37. doi: 10.1073/pnas.0500803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luxton GW, Lee JI, Haverlock-Moyns S, Schober JM, Smith GA. The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport. J Virol. 2006;80:201–9. doi: 10.1128/JVI.80.1.201-209.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lycke E, Hamark B, Johansson M, Krotochwil A, Lycke J, Svennerholm B. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch Virol. 1988;101:87–104. doi: 10.1007/BF01314654. [DOI] [PubMed] [Google Scholar]
- 99.Lycke E, Kristensson K, Svennerholm B, Vahlne A, Ziegler R. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J Gen Virol. 1984;65:55–64. doi: 10.1099/0022-1317-65-1-55. [DOI] [PubMed] [Google Scholar]
- 100.Lyman MG, Feierbach B, Curanovic D, Bisher M, Enquist LW. PRV Us9 directs axonal sorting of viral capsids. J Virol. 2007;81:11363–71. doi: 10.1128/JVI.01281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma Y, Jin H, Valyi-Nagy T, Cao Y, Yan Z, He B. Inhibition of TANK binding kinase 1 by herpes simplex virus 1 facilitates productive infection. J Virol. 2011;86:2188–96. doi: 10.1128/JVI.05376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maresch C, Granzow H, Negatsch A, Klupp BG, Fuchs W, et al. Ultrastructural analysis of virion formation and anterograde intraaxonal transport of the alphaherpesvirus pseudorabies virus in primary neurons. J Virol. 2010;84:5528–39. doi: 10.1128/JVI.00067-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Markus A, Grigoryan S, Sloutskin A, Yee MB, Zhu H, et al. Varicellazoster virus (VZV) infection of neurons derived from human embryonic stem cells: direct demonstration of axonal infection, transport of VZV, and productive neuronal infection. J Virol. 2011;85:6220–33. doi: 10.1128/JVI.02396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maurer UE, Sodeik B, Grunewald K. Native 3D intermediates of membrane fusion in herpes simplex virus 1 entry. Proc Natl Acad Sci USA. 2008;105:10559–64. doi: 10.1073/pnas.0801674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McCarthy KM, Tank DW, Enquist LW. Pseudorabies virus infection alters neuronal activity and connectivity in vitro. PLoS Pathog. 2009;5:e1000640. doi: 10.1371/journal.ppat.1000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McGraw HM, Awasthi S, Wojcechowskyj JA, Friedman HM. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not Us9. J Virol. 2009;83:8315–26. doi: 10.1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McNab AR, Desai P, Person S, Roof LL, Thomsen DR, et al. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J Virol. 1998;72:1060–70. doi: 10.1128/jvi.72.2.1060-1070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meckes DG, Jr, Wills JW. Dynamic interactions of the UL16 tegument protein with the capsid of herpes simplex virus. J Virol. 2007;81:13028–36. doi: 10.1128/JVI.01306-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meckes DG, Jr, Wills JW. Structural rearrangement within an enveloped virus upon binding to the host cell. J Virol. 2008;82:10429–35. doi: 10.1128/JVI.01223-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mettenleiter TC, Klupp BG, Granzow H. Herpesvirus assembly: an update. Virus Res. 2009;143:222–34. doi: 10.1016/j.virusres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 111.Milne RS, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J Virol. 2005;79:6655–63. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miranda-Saksena M, Boadle RA, Aggarwal A, Tijono B, Rixon FJ, et al. Herpes simplex virus utilizes the large secretory vesicle pathway for anterograde transport of tegument and envelope proteins and for viral exocytosis from growth cones of human fetal axons. J Virol. 2009;83:3187–99. doi: 10.1128/JVI.01579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miranda-Saksena M, Boadle RA, Armati P, Cunningham AL. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J Virol. 2002;76:9934–51. doi: 10.1128/JVI.76.19.9934-9951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–36. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 115.Mothes W, Sherer NM, Jin J, Zhong P. Virus cell-to-cell transmission. J Virol. 2010;84:8360–68. doi: 10.1128/JVI.00443-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mulder W, Pol J, Kimman T, Kok G, Priem J, Peeters B. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE and gI. J Virol. 1996;70:2191–200. doi: 10.1128/jvi.70.4.2191-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mulder WAM, Jacobs L, Priem J, Kok GL, Wagenaar F, et al. Glycoprotein gE-negative pseudorabies virus has a reduced capability to infect second- and third-order neurons of the olfactory and trigeminal routes in the porcine central nervous system. J Gen Virol. 1994;75:3095–106. doi: 10.1099/0022-1317-75-11-3095. [DOI] [PubMed] [Google Scholar]
- 118.Naldinho-Souto R, Browne H, Minson T. Herpes simplex virus tegument protein VP16 is a component of primary enveloped virions. J Virol. 2006;80:2582–84. doi: 10.1128/JVI.80.5.2582-2584.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nauwynck HJ, Pensaert MB. Abortion induced by cell-associated pseudorabies virus in vaccinated sows. Am J Vet Res. 1992;53:489–93. [PubMed] [Google Scholar]
- 120.Negatsch A, Granzow H, Maresch C, Klupp BG, Fuchs W, et al. Ultrastructural analysis of virion formation and intraaxonal transport of herpes simplex virus type 1 in primary rat neurons. J Virol. 2010;84:13031–35. doi: 10.1128/JVI.01784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Negatsch A, Mettenleiter TC, Fuchs W. Herpes simplex virus type 1 strain KOS carries a defective US9 and a mutated US8A gene. J Gen Virol. 2011;92:167–72. doi: 10.1099/vir.0.026484-0. [DOI] [PubMed] [Google Scholar]
- 122.Newcomb WW, Booy FP, Brown JC. Uncoating the herpes simplex virus genome. J Mol Biol. 2007;370:633–42. doi: 10.1016/j.jmb.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Newcomb WW, Brown JC. Time-dependent transformation of the herpesvirus tegument. J Virol. 2009;83:8082–89. doi: 10.1128/JVI.00777-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Newcomb WW, Brown JC. Structure and capsid association of the herpesvirus large tegument protein UL36. J Virol. 2010;84:9408–14. doi: 10.1128/JVI.00361-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nicola AV, Hou J, Major EO, Straus SE. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol. 2005;79:7609–16. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77:5324–32. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78:7508–17. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O’Hara M, Rixon FJ, Stow ND, Murray J, Murphy M, Preston VG. Mutational analysis of the herpes simplex virus type 1 UL25 DNA packaging protein reveals regions that are important after the viral DNA has been packaged. J Virol. 2010;84:4252–63. doi: 10.1128/JVI.02442-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oh MJ, Akhtar J, Desai P, Shukla D. A role for heparan sulfate in viral surfing. Biochem Biophys Res Commun. 2010;391:176–81. doi: 10.1016/j.bbrc.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ohka S, Matsuda N, Tohyama K, Oda T, Morikawa M, et al. Receptor (CD155)-dependent endocytosis of poliovirus and retrograde axonal transport of the endosome. J Virol. 2004;78:7186–98. doi: 10.1128/JVI.78.13.7186-7198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ojala PM, Sodeik B, Ebersold MW, Kutay U, Helenius A. Herpes simplex virus type 1 entry into host cells: reconstitution of capsid binding and uncoating at the nuclear pore complex in vitro. Mol Cell Biol. 2000;20:4922–31. doi: 10.1128/mcb.20.13.4922-4931.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pasdeloup D, Blondel D, Isidro AL, Rixon FJ. Herpesvirus capsid association to the nuclear pore complex and viral DNA release involve the nucleoporin CAN/Nup214 and the capsid protein pUL25. J Virol. 2009;83:6610–23. doi: 10.1128/JVI.02655-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pasieka TJ, Collins L, O’Connor MA, Chen Y, Parker ZM, et al. Bioluminescent imaging reveals divergent viral pathogenesis in two strains of Stat1-deficient mice, and in αβγ interferon receptor-deficient mice. PLoS ONE. 2011;6:e24018. doi: 10.1371/journal.pone.0024018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peeters B, de Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peeters B, Pol J, Gielkens A, Moormann R. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J Virol. 1993;67:170–77. doi: 10.1128/jvi.67.1.170-177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Penfold MET, Armati P, Cunningham AL. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc Natl Acad Sci USA. 1994;91:6529–33. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Poon AP, Roizman B. Differentiation of the shutoff of protein synthesis by virion host shutoff and mutant γ134.5 genes of herpes simplex virus 1. Virology. 1997;229:98–105. doi: 10.1006/viro.1996.8425. [DOI] [PubMed] [Google Scholar]
- 138.Preston VG, Murray J, Preston CM, McDougall IM, Stow ND. The UL25 gene product of herpes simplex virus type 1 is involved in uncoating of the viral genome. J Virol. 2008;82:6654–66. doi: 10.1128/JVI.00257-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Radtke K, Kieneke D, Wolfstein A, Michael K, Steffen W, et al. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 2010;6:e1000991. doi: 10.1371/journal.ppat.1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rauh I, Mettenleiter TC. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–56. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Read GS, Patterson M. Packaging of the virion host shutoff (Vhs) protein of herpes simplex virus: two forms of the Vhs polypeptide are associated with intranuclear B and C capsids, but only one is associated with enveloped virions. J Virol. 2007;81:1148–61. doi: 10.1128/JVI.01812-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reichelt M, Zerboni L, Arvin AM. Mechanisms of varicellazoster virus neuropathogenesis in human dorsal root ganglia. J Virol. 2008;82:3971–83. doi: 10.1128/JVI.02592-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roberts AP, Abaitua F, O’Hare P, McNab D, Rixon FJ, Pasdeloup D. Differing roles of inner tegument proteins pUL36 and pUL37 during entry of herpes simplex virus type 1. J Virol. 2009;83:105–16. doi: 10.1128/JVI.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rode K, Dohner K, Binz A, Glass M, Strive T, et al. Uncoupling uncoating of herpes simplex virus genomes from their nuclear import and gene expression. J Virol. 2011;85:4271–83. doi: 10.1128/JVI.02067-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sabin AB. Progression of different nasally instilled viruses along different nervous pathways in the same host. Proc Soc Exp Biol. 1938;38:270–75. [Google Scholar]
- 146.Saksena MM, Wakisaka H, Tijono B, Boadle RA, Rixon F, et al. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J Virol. 2006;80:3592–606. doi: 10.1128/JVI.80.7.3592-3606.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salinas S, Bilsland LG, Henaff D, Weston AE, Keriel A, et al. CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog. 2009;5:e1000442. doi: 10.1371/journal.ppat.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schipke J, Pohlmann A, Diestel R, Binz A, Rudolph K, et al. The C-terminus of the large tegument protein pUL36 contains multiple capsid binding sites that function differently during assembly and cell entry of herpes simplex virus. J Virol. 2012;86:3682–700. doi: 10.1128/JVI.06432-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scrivano L, Sinzger C, Nitschko H, Koszinowski UH, Adler B. HCMV spread and cell tropism are determined by distinct virus populations. PLoS Pathog. 2011;7(1):e1001256. doi: 10.1371/journal.ppat.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sears AE, Hukkanen V, Labow MA, Levine AJ, Roizman B. Expression of the herpes simplex virus 1 alpha transinducing factor (VP16) does not induce reactivation of latent virus or prevent the establishment of latency in mice. J Virol. 1991;65:2929–35. doi: 10.1128/jvi.65.6.2929-2935.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shanda SK, Wilson DW. UL36p is required for efficient transport of membrane-associated herpes simplex virus type 1 along microtubules. J Virol. 2008;82:7388–94. doi: 10.1128/JVI.00225-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 153.Smith GA, Gross SP, Enquist LW. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci USA. 2001;98:3466–70. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith GA, Pomeranz L, Gross SP, Enquist LW. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proc Natl Acad Sci USA. 2004;101:16034–39. doi: 10.1073/pnas.0404686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Snyder A, Polcicova K, Johnson DC. Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons. J Virol. 2008;82:10613–24. doi: 10.1128/JVI.01241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sodeik B, Ebersold MW, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–21. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Spear PG, Manoj S, Yoon M, Jogger CR, Zago A, Myscofski D. Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry. Virology. 2006;344:17–24. doi: 10.1016/j.virol.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 158.Strack AM, Loewy AD. Pseudorabies virus: a highly specific transneuronal cell body marker in the sympathetic nervous system. J Neurosci. 1990;10:2139–47. doi: 10.1523/JNEUROSCI.10-07-02139.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Strunze S, Trotman LC, Boucke K, Greber UF. Nuclear targeting of adenovirus type 2 requires CRM1-mediated nuclear export. Mol Biol Cell. 2005;16:2999–3009. doi: 10.1091/mbc.E05-02-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci USA. 2010;107:866–71. doi: 10.1073/pnas.0913351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Szpara ML, Parsons L, Enquist LW. Sequence variability in clinical and laboratory isolates of herpes simplex virus 1 reveals new mutations. J Virol. 2010;84:5303–13. doi: 10.1128/JVI.00312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tannous R, Grose C. Calculation of the anterograde velocity of varicellazoster virions in a human sciatic nerve during shingles. J Infect Dis. 2011;203:324–26. doi: 10.1093/infdis/jiq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Taylor MP, Kramer T, Lyman MG, Kratchmarov R, Enquist LW. Visualization of an alphaherpesvirus membrane protein that is essential for anterograde axonal spread of infection in neurons. mBio. 2012;3(2):e00063–12. doi: 10.1128/mBio.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, et al. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thompson RL, Preston CM, Sawtell NM. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog. 2009;5:e1000352. doi: 10.1371/journal.ppat.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Thompson RL, Stevens JG. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology. 1983;131:171–79. doi: 10.1016/0042-6822(83)90543-3. [DOI] [PubMed] [Google Scholar]
- 167.Tirabassi RS, Townley RA, Eldridge MG, Enquist LW. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–64. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Topp KS, Meade LB, LaVail JH. Microtubule polarity in the peripheral processes of trigeminal ganglion cells: relevance for the retrograde transport of herpes simplex virus. J Neurosci. 1994;14:318–25. doi: 10.1523/JNEUROSCI.14-01-00318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Trus BL, Newcomb WW, Cheng N, Cardone G, Marekov L, et al. Allosteric signaling and a nuclear exit strategy: binding of UL25/UL17 heterodimers to DNA-filled HSV-1 capsids. Mol Cell. 2007;26:479–89. doi: 10.1016/j.molcel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: an ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 171.Van den Broeke C, Favoreel HW. Actin’ up: herpesvirus interactions with Rho GTPase signaling. Viruses. 2011;3:278–92. doi: 10.3390/v3040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Verpooten D, Feng Z, Valyi-Nagy T, Ma Y, Jin H, et al. Dephosphorylation of eIF2α mediated by the γ134.5 protein of herpes simplex virus 1 facilitates viral neuroinvasion. J Virol. 2009;83:12626–30. doi: 10.1128/JVI.01431-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Weeks BS, Ramchandran RS, Hopkins JJ, Friedman HM. Herpes simplex virus type-1 and -2 pathogenesis is restricted by the epidermal basement membrane. Arch Virol. 2000;145:385–96. doi: 10.1007/s007050050030. [DOI] [PubMed] [Google Scholar]
- 174.Whealy ME, Card JP, Robbins AK, Dubin JR, Rziha H-J, Enquist LW. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–97. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Whitley RJ, Gnann JW. Viral encephalitis: familiar infections and emerging pathogens. Lancet. 2002;359:507–13. doi: 10.1016/S0140-6736(02)07681-X. [DOI] [PubMed] [Google Scholar]
- 176.Wileman T. Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design? Annu Rev Microbiol. 2007;61:149–67. doi: 10.1146/annurev.micro.57.030502.090836. [DOI] [PubMed] [Google Scholar]
- 177.Williams RA, Bennett M, Bradbury JM, Gaskell RM, Jones RC, Jordan FT. Demonstration of sites of latency of infectious laryngotracheitis virus using the polymerase chain reaction. J Gen Virol. 1992;73:2415–20. doi: 10.1099/0022-1317-73-9-2415. [DOI] [PubMed] [Google Scholar]
- 178.Winckler B, Mellman I. Neuronal polarity: controlling the sorting and diffusion of membrane components. Neuron. 1999;23:637–40. doi: 10.1016/s0896-6273(01)80021-0. [DOI] [PubMed] [Google Scholar]
- 179.Wisner TW, Sugimoto K, Howard PW, Kawaguchi Y, Johnson DC. Anterograde transport of herpes simplex virus capsids in neurons by both separate and married mechanisms. J Virol. 2011;85:5919–28. doi: 10.1128/JVI.00116-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wolfstein A, Nagel CH, Radtke K, Dohner K, Allan VJ, Sodeik B. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic. 2006;7:227–37. doi: 10.1111/j.1600-0854.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 181.Yamauchi Y, Kiriyama K, Kubota N, Kimura H, Usukura J, Nishiyama Y. The UL14 tegument protein of herpes simplex virus type 1 is required for efficient nuclear transport of the alpha transinducing factor VP16 and viral capsids. J Virol. 2008;82:1094–106. doi: 10.1128/JVI.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yao F, Courtney RJ. Association of ICP0 but not ICP27 with purified virions of herpes simplex virus type 1. J Virol. 1992;66:2709–16. doi: 10.1128/jvi.66.5.2709-2716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zaichick SV, Bohannon KP, Smith GA. Alphaherpesviruses and the cytoskeleton in neuronal infections. Viruses. 2011;3:941–81. doi: 10.3390/v3070941. [DOI] [PMC free article] [PubMed] [Google Scholar]