Abstract
We introduce the term ‘silent agonists’ to describe ligands that can place the α7 nicotinic acetylcholine receptor (nAChR) into a desensitized state with little or no apparent activation of the ion channel, forming a complex that can subsequently generate currents when treated with an allosteric modulator. KC-1 (5′-phenylanabaseine) was synthesized and identified as a new silent agonist for the α7 nAChR; it binds to the receptor but does not activate α7 nAChR channel opening when applied alone, and its agonism is revealed by co-application with the type II positive allosteric modulator PNU-120596 in the Xenopus oocyte system. The concise synthesis was accomplished in three steps with the C–C bonds formed via Pd-catalyzed mono-arylation and organolithium coupling with N-Boc piperidinone. Comparative structural analyses indicate that a positive charge, an H-bond acceptor, and an aryl ring in a proper arrangement are needed to constitute one class of silent agonist for the α7 nAChR. Because silent agonists may act on signaling pathways not involving ion channel opening, this class of α7 nAChR ligands may constitute a new alternative for the development of α7 nAChR therapeutics.
Keywords: Silent-agonist, Allosteric modulator, Ion-channel, Nicotinic receptor
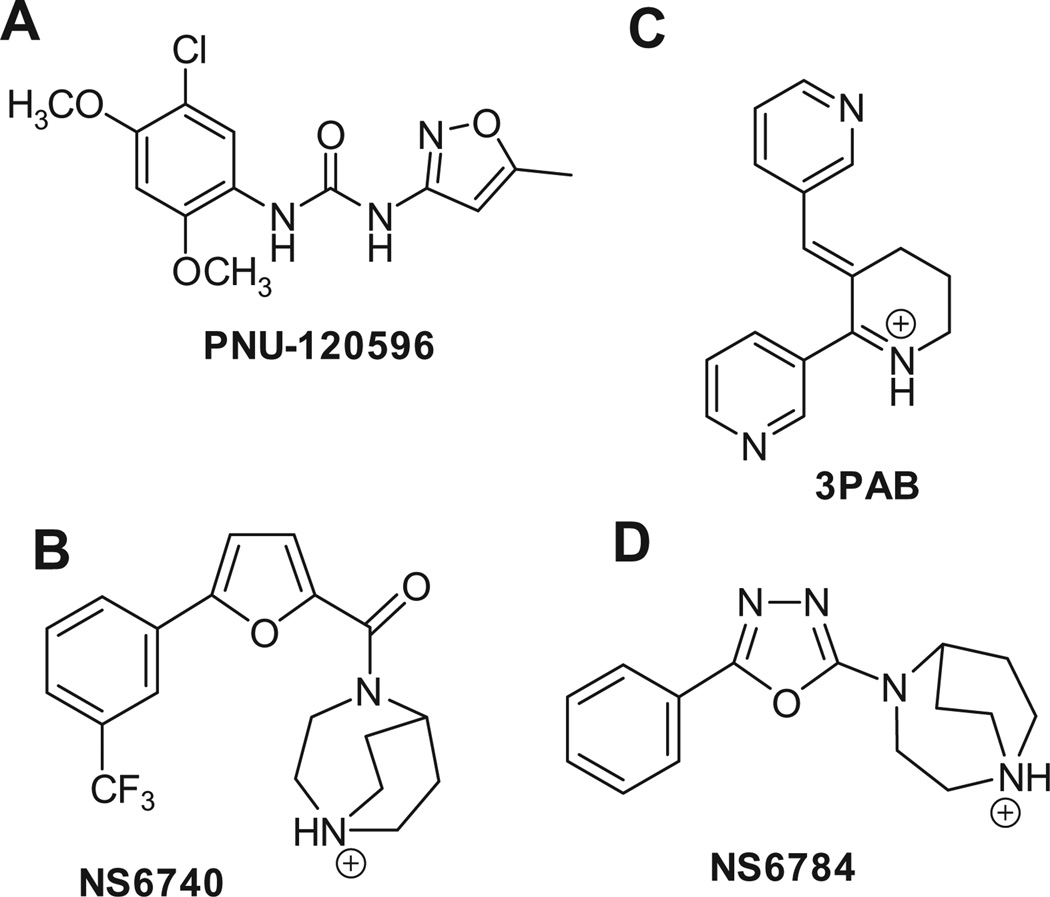
The α7 nicotinic acetylcholine receptor (α7 nAChR) is a homopentameric ligand gated ion channel that is distinguished from other nAChRs by its rapid desensitization and very low probability of channel opening.1 The α7 nAChR is currently a drug target for Alzheimer’s disease, schizophrenia and inflammatory disorders,2,3 and there is a great interest in development of α7-selective agonists4,5 and positive allosteric modulators (PAMs).6 α7 PAMs have been divided into type I and type II PAMs,7 and one well-characterized type II PAMs is PNU-1205967,8 (Fig. 1A). We have reported two forms of α7 desensitization. One is sensitive to type II PAMs such as PNU-120596, (termed Ds), and another is induced by strong episodes of activation and high occupancy and is insensitive to PNU-120596 (termed Di).9 The pharmacological relevance of desensitized states is likely to extend beyond their lack of ability to conduct an ion-current. There is growing evidence, especially in non-neuronal cells, that there is α7-mediated signal transduction under conditions when no ion channel activation can be detected.3,10–13 These findings make compounds that can put the nAChR into the Ds state, with little or no apparent agonism, of considerable potential interest from both mechanistic and therapeutic perspectives. We define a silent agonist14 as a molecule that does not activate or activates only very weakly α7 nAChR channel opening when used alone, inhibits the response of α7 nAChR to acetylcholine (ACh), and appears as an agonist when a type II PAM is used. NS-6740 is such an agent which, although inactive in an α7-sensitive model for cognitive improvement, has been shown to be effective at modulating the release of pro-inflammatory cytokines.13,15 Functionally, a silent agonist acts as a desensitizer when applied alone, inducing a significant amount of Ds and a channel activator in the presence of type II PAM. In this Letter we describe the design, synthesis and evaluation of a new compound that acts as a silent agonist.
Figure 1.
(A) Structure of type II PAM PNU-120596; (B and C) structures of α7 silent agonists NS-6740 and 3PAB; (D) structure of α7 agonist NS-6784.
Several observations led to a working model for the design of a silent agonist. Compound NS-6740 (Fig. 1B) has been characterized by Abbott and Neurosearch as a very weak agonist (<2% of the response of to ACh), whose agonist-like properties were revealed by adding the positive allosteric modulator PNU-120596.15 We found in our laboratories that 3PAB (Fig. 1C),16 also behaves as a silent agonist, leading to the idea that silent agonists may be groupable into structurally distinct families. To this end, we were intrigued with the divergent behavior of NS-6740 as a silent agonist and the structurally related NS-6784 acting as a typical α7 agonist. (Fig. 1D). Both compounds feature a cationic diaza [3.2.2] bicyclononane group, a central 2,5-disubstituted heterocyclic ring and a phenyl substituent at the 5-position of the central ring. Further, both compounds offer a hydrogen bond acceptor adjacent to the bicyclic ring system; an amide carbonyl in the case of NS-6740, or a nitrogen atom of the 1,2,4-oxadiazole central ring in NS-6784. We therefore initiated a synthesis of a molecule whose structural features and biological activity might shed further light on what constitutes a set of features that would confer silent agonism. We considered a minimal pharmacophore to feature a positively charged center, a central ring with hydrogen bonding capability, and a flanking aryl substituent with an angular relationship between these elements as embodied in the molecule KC-1 and the cartoon shown in Figure 2. One will note the core anabaseine portion of KC-1 is a non-selective nAChR agonist, and as will be presented we show phenyl substitution on the pyridine ring dramatically changes this profile.
Figure 2.
Putative pharmacophoric features for the KC-1/NS-6740 class of silent agonists.
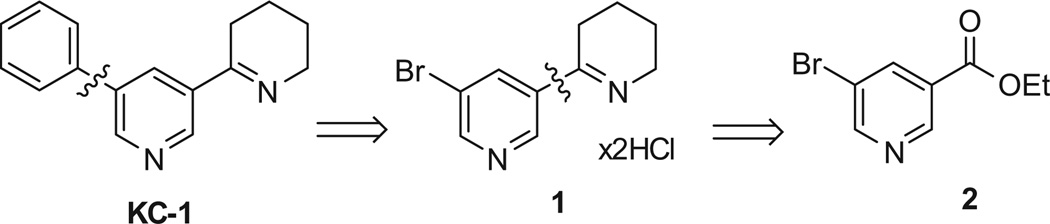
KC-1 (5′-phenylanabaseine) can be considered as an analog of anabaseine, and we decided to make KC-1 by adapting well-known anabaseine synthesis protocols. We first planned to prepare KC-1 from 5′-bromoanabaseine by organometallic coupling (Scheme 1). That approach would have allowed us flexibility in preparation of a series of KC-1 analogs. We envisaged preparing several grams of 5′-bromoanabaseine as its dihydrochloride salt (1) from bromonicotinic ethyl ester (2), by applying the Zoltewicz anabaseine synthetic protocol17 that we use in our lab. That approach is based on a mixed Claisen condensation of nicotinic ester and the amide enolate ion of N-aminomethyl protected 2-piperidone 3, followed by hydrolysis of the resulting sodium salt of the 3-nicotinoyl-2-piperidone intermediate in hot acid with concomitant decarboxylation and cyclic imine formation. Anabaseine is isolated by crystallization as its stable dihydrochloride salt.
Scheme 1.
Initial KC-1 retrosynthesis analysis.
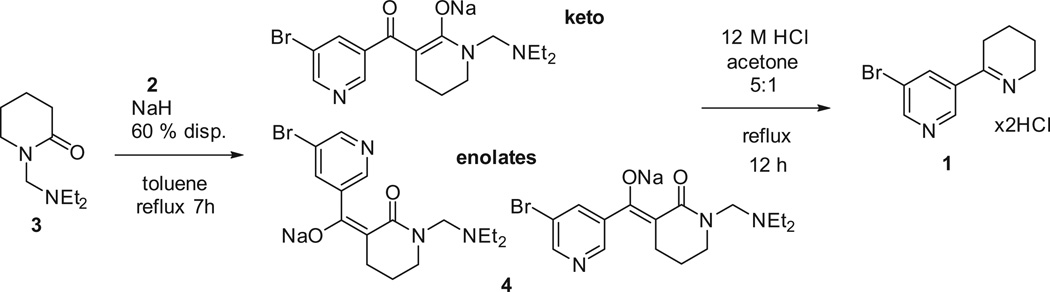
The reaction of 3-bromonicotinic ethyl ester (2) with N-protected 2-piperidone 316,17 and sodium hydride in toluene (Scheme 2) yielded the sodium salt 4 as a complex mixture of Z-and E-enolates and keto forms as supported by1H NMR, but it could not be isolated from the crude, so the crude product was subjected to the next step, hydrolysis with concentrated hydrochloric acid. The desired 5′-bromoanabaseine dihydrochloride (1) was formed in that reaction as shown by1H NMR, however extensive degradation was observed, many impurities were present, and the product couldn’t be obtained in a pure form. We thus tried to synthesize KC-1 from 5-phenylnicotinic ethyl ester (8) following the same protocol.
Scheme 2.
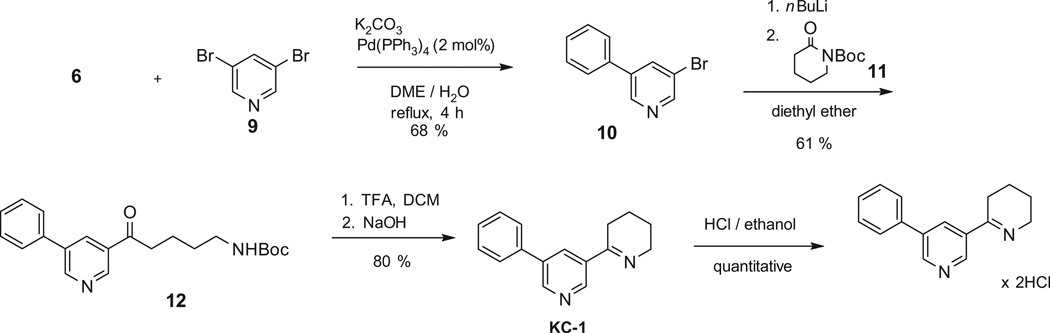
5-Phenylnicotic ethyl ester (8) is not commercially available and we prepared it from 3-bromonictotinic acid (5) by Suzuki coupling with phenylboronic acid (6), using palladium acetate as catalyst, and potassium phosphate as a base, in a 1:1 mixture of water and 2-propanol.18 The 5-phenylnicotinic acid was then reacted with thionyl chloride, followed by reaction with ethanol to give the product 8 in 63% yield from 5 (Scheme 3).
Scheme 3.
(a) Pd(OAC)2 4 mol %, K3PO4, 1:1 water/2-propanol, 80 °C, 7 h; (b) (1) SOCl2, reflux 4 h, (2) EtOH, reflux 3 h, 63% from 5.
The 5-phenylnicotinic ethyl ester (8) was then subjected to the mixed Claisen condensation with N-protected 2-piperidone 3, using the same methodology as presented in Scheme 2. Unfortunately, the intermediate sodium salt did not crystallize from the reaction mixture after removal of excess NaH by filtration, and again the crude was subjected to acidic hydrolysis. 5-Phenylcarboxylic acid 7, resulting from hydrolysis of unreacted ethyl ester 8 from Claisen condensation crystallized first, then the KC-1 salt, which was impure. The KC-1 salt was thus transformed with 1 M sodium hydroxide into its imine free-base form, purified by column chromatography, and then converted back into its dihydrochloride salt to yield the pure compound in 2% yield over two-steps from 5-phenylnicotine ethyl ester (8). The results of these two approaches suggest that the synthetic route to anabaseine is not highly tolerant of substitutions.
The third and most successful route to KC-1 employed addition of 5-phenylpyridynyl lithium generated with nBuLi from the bromide 10 to N-Boc protected 2-piperidone 11 in diethyl ether, followed by deprotection, ring closure, and dehydration (Scheme 4). Organometallic ring-opening reaction of N-alkoxycarbonyl lactams has been used to make cyclic imines by Giovanni et al.19 3-Bromo-5-phenylpyridine 10 was prepared from 3,5-dibromopyridine 9 by Suzuki coupling with phenylboronic acid 6, using 2 mol % (triphenylphosphine) palladium tetrakis and potassium carbonate in a mixture of dimethoxyethane and water in 68% yield following a patent procedure.20 The N-Boc 2-piperidone 11 was made from 2-piperidone following the reported procedure.19
Scheme 4.
Synthesis of KC-1 via an organolithium ring opening reaction.
The opening of the lactam ring in 11 by the anion of 10 formed by adding 10 into a solution of nBuLi in diethyl ether at −78 °C yielded the desired N-Boc-ω-aminoketone 12 in 61% yield. We also generated the anion of 10 using 2 equiv of t-BuLi in diethyl ether, and the desired product 12 was obtained in 31% yield. When n- BuLi was used in THF instead of diethyl ether, no product was formed. It has been reported that isopropyl magnesium chloride may be used for metal–halogen exchange instead of alkyl lithiums to avoid known side reactions of alkyl lithium with pyridines such as deprotonation, addition of nBuLi to pyridine ring, elimination of lithium bromide (to give pyridynes), reaction of 3-lithiopyridine with nBuBr, and even ring opening.21 However, in our case the use of isopropyl magnesium chloride resulted in a significantly lower yield (4%) of compound 12.
N-Boc-ω-aminoketone 12 was purified and treated with TFA followed by NaOH to give KC-1 in 80% yield after silica chromatography. The imine free base was then quantitatively transformed into its more stable dihydrochloride salt by treatment with hydrochloric acid in ethanol. Spectroscopic characterization of KC-1 (Supplementary data) was fully satisfactory.
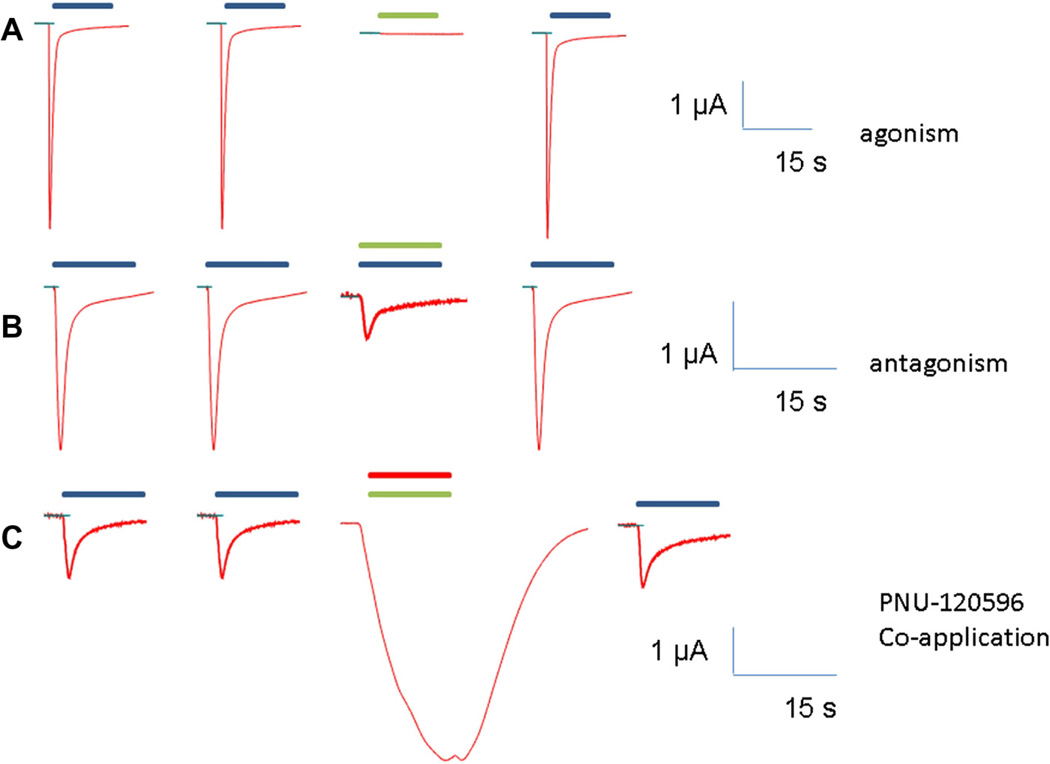
KC-1 was tested on human α7 nAChR receptors expressed in Xenopus laevis oocytes at room temperature using OpusXpress 6000 A (Molecular Devices, Sunnyvale, CA) as described previously.22 In brief, each oocyte received two initial controls of 60 µM acetylcholine (ACh), then experimental drug applications, followed by a 60 µM ACh post-control. Responses of α7 nAChR were calculated as peak current and net charge relative to preceding ACh controls to normalize the data (responses to ACh were normalized to 1), compensating for varying levels of receptors’ expression among oocytes. Data used were average from at least four cells given the same treatments. 100 µM application of KC-1 resulted in practically no response (Fig. 3A) (peak current: 0.004 ± 0.001, net charge 0.083 ± 0.009), showing that KC-1 does not act as a conventional α7 nAChR agonist. When 100 µM KC-1 was co-applied with 60 µM ACh, a diminished response to ACh was observed (Fig. 3B) (peak current: 0.17 ± 0.08, net charge 0.29 ± 0.06), indicating that KC-1 binds in the same binding site as ACh. Finally, when 100 µM KC-1 was co-applied with 10 µM PNU-120596 a very large response was observed (Fig. 3C) (peak current: 4.10 ± 0.68, net charge 17.8 ± 2.4), revealing that KC-1 favors the Ds state without ion channel opening, and thus acts as a silent agonist. Note that PNU-120596 has no detectable activity on its own with the α7 nAChR.8 The IC50 of KC-1 was estimated to be 41 ± 5 µM. In a control experiment we found that the α7-selective antagonist MLA (100 nM) was 100% effective at blocking responses to 100 µM KC-1 co-applied with 10 µM PNU-120596. Finally, controls showed equivalent responses to KC-1 with PNU-120596 or another type II PAM, TQS23 demonstrating that the activity of KC-1 is not PNU-120596 specific.
Figure 3.
Representative traces of the hα7 nAChR response to applications of KC-1. Blue bars represent duration of ACh applications, green bars the KC-1 application, and the red bar represents PNU-120596 application.
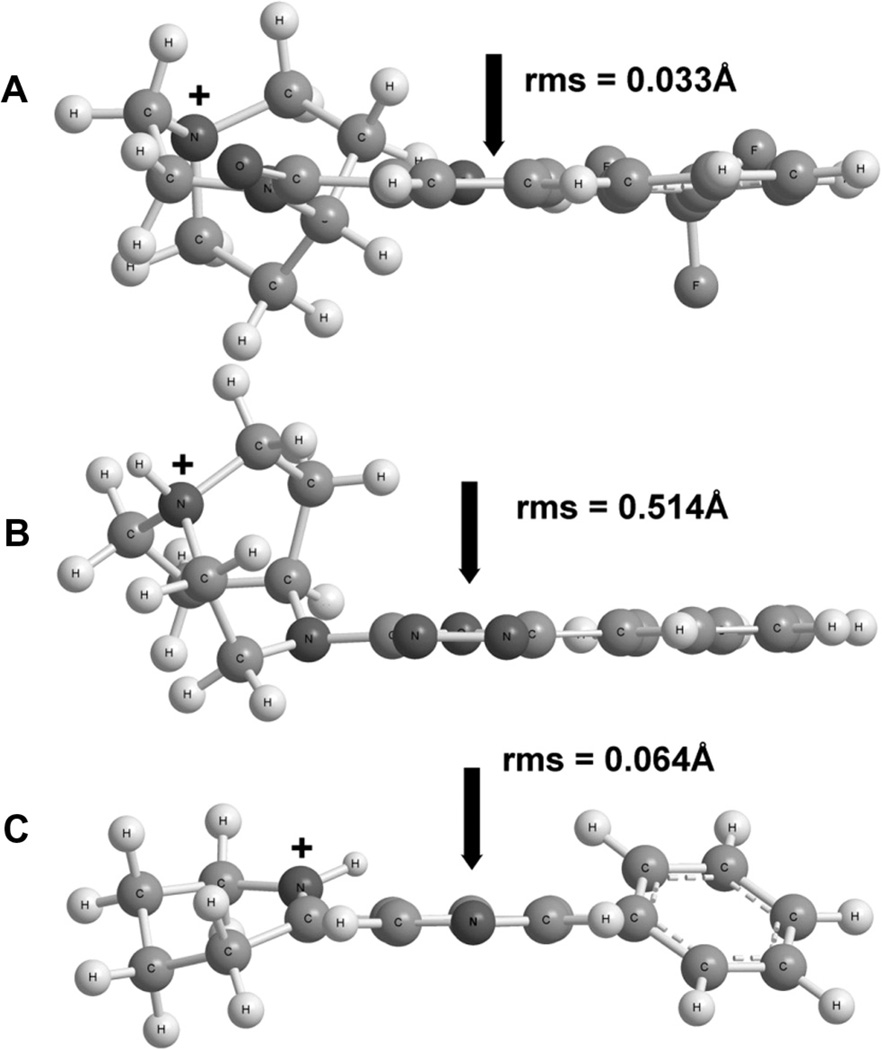
We confirmed that KC-1 was a silent agonist and sought to identify structural features which more closely resembled the silent agonist NS-6740 compared to agonist NS-6784. This was done with the recognition that with a small dataset we would not be able to develop a structure activity relationship in any quantitative sense; however the following observations were made and may be instructive. The structures of KC-1, NS-6740 and NS-6784 were first minimized with molecular mechanics, and local minima were identified by conformational searching over rotatable bonds. Identified minima were further optimized using DFT (B3LYP/6-31G*+ZPE analysis). We compared the lowest identified local minima for the compounds in terms of the distance between the charged nitrogen and putative hydrogen bond acceptor atoms and found no discernible differences. A noteworthy similarity between KC-1 and NS-6740, not shared with NS-6784 is placement of the charged group in a plane virtually coincident with the plane defining the central ring in the two molecules. This is shown in Figure 4, in which structures A (NS-6740) and C (KC-1) have respective deviations of the charged nitrogen from the plane of 0.033 and 0.064 Å, while structure B for NS-6784 has a 10-fold larger charge-to-plane deviation of 0.514 Å.
Figure 4.
Comparison of NS-6740 (A), NS-6784 (B) and KC-1 (C). The arrow indicates the position of the central aromatic ring, seen edge-on in these views. The ‘+’ symbol denotes the position of the charged nitrogen atom in each structure. The rms deviation refers to the deviation of the charged nitrogen from the central ring plane.
The difference in relationship between the position of the charged immonium/ammonium group and the aromatic rings may manifest in different binding modes or interactions for KC-1 and NS-6740 versus that of NS-6784, that lead to the observation of silent agonism or agonism respectively, for the two groups. These results provide the basis for further investigation and testing of KC-1 and analogs for the pharmacophore responsible silent agonists. For example, the conformational mobility between the pyridine and tetrahydropyridinyl rings leads to the possibility of syn-and anti-isomers with respect to the position of the pyridine and iminium nitrogens, each of which may have a different activity with the nAChR.
In summary, we synthesized KC-1 (5′-phenylanabaseine) as a designed silent agonist. This type II PAM-dependent activator allows selective control of the α7 nAChR in Xenopus oocytes converting a resting state into a Ds state. Silent agonists may find use as tools to study the function of α7 nAChR not involving ion conducting states. Ongoing studies are aimed at a robu st definition of the pharmacophore(s) that represent the requirements for silent agonists of the α7 nAChR.
Supplementary Material
Acknowledgments
Funding support from NIH Grant R01 GM057481. We thank Clare Stokes for much assistance in all of the technical aspects of the oocyte experiments. OpusXpress experiments were conducted by Shehd Abdullah Abbas Al Rubaiy, Matthew Kimbrell, and Lu Wenchi Corrie.
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2013.05.039.
References and notes
- 1.Williams DK, Peng C, Kimbrell MR, Papke RL. Mol. Pharmacol. 2012;82:746. doi: 10.1124/mol.112.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taly A, Corringer P, Guedin D, Lestage P, Changeux J. Nat. Rev. Drug Disc. 2009;8:733. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- 3.de Jonge WJ, Ulloa L., Br J. Pharmacol. 2007;151:915. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazurov A, Hauser T, Miller C. Curr. Med. Chem. 2006;13:1567. doi: 10.2174/092986706777442011. [DOI] [PubMed] [Google Scholar]
- 5.Horenstein NA, Leonik FM, Papke RL. Mol. Pharmacol. 2008;74:1496. doi: 10.1124/mol.108.048892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams DK, Wang J, Papke RL. Biochem. Pharmacol. 2011;82:915. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronlien JH, Hakerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. Mol. Pharmacol. 2007;72:715. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- 8.Hurst R, Hajos M, Raggenbass M, Wall T, Higdon N, Lawson J, Rutherford-Root K, Berkenpas M, Hoffmann W, Piotrowski D, Groppi V, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SJ. Neurosci. 2005;25:4396. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams DK, Wang J, Papke RL. Mol. Pharmacol. 2011;80:1013. doi: 10.1124/mol.111.074302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracey KJ. J. Clin. Invest. 2007;117:289. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arias HR, Richards VE, Nga D, Ghafoori ME, Le V, Mousa SA. Int. J. Biochem. Cell Biol. 2009;41:1441. doi: 10.1016/j.biocel.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Skok MV. J. Leukocyte Biol. 2009;86:1. doi: 10.1189/JLB.0209106. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen MS, Mikkelsen JD. J. Neuroimmunol. 2012;251:65. doi: 10.1016/j.jneuroim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 14.VanderGraaf P, VanSchaick E, Mathot R, Ijzerman A, Danhof M. J. Pharmacol. Exp. Ther. 1997;283:809. [PubMed] [Google Scholar]
- 15.Briggs CA, Gronlien JH, Curzon P, Timmermann DB, Ween H, Thorin-Hagene K, Kerr P, Anderson DJ, Malysz J, Dyhring T, Olsen GM, Peters D, Bunnelle WH, Gopalakrishnan M., Br J. Pharmacol. 2009;158:1486. doi: 10.1111/j.1476-5381.2009.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Papke RL, Horenstein NA. Bioorg. Med. Chem. Lett. 2009;19:474. doi: 10.1016/j.bmcl.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoltewicz J, Cruskie M. Org. Prep. Proced. Int. 1995;27:510. [Google Scholar]
- 18.Liu C, Yang W. Chem. Commun. 2009:6267. doi: 10.1039/b912364d. [DOI] [PubMed] [Google Scholar]
- 19.Giovannini A, Savoia D, Umanironchi A. J. Org. Chem. 1989;54:228. [Google Scholar]
- 20.Peters P, Olsen GN, Nielsen SF, Nielsen EO. 72,823. U.S. Patent. 2004
- 21.Trecourt F, Breton G, Bonnet V, Mongin F, Marsais F, Queguiner G. Tetrahedron. 2000;56:1349. [Google Scholar]
- 22.Papke RL, Stokes C. Methods. 2010;51:121. doi: 10.1016/j.ymeth.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. Mol. Pharmacol. 2007;72:715. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.