Abstract
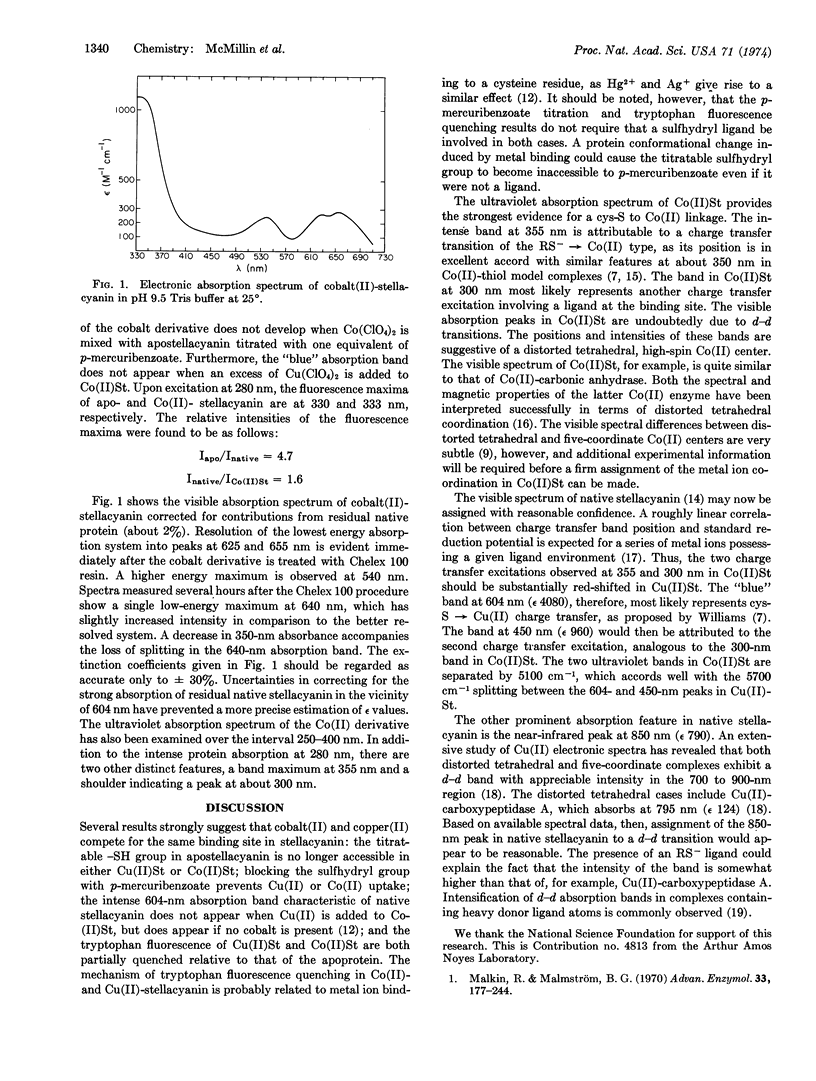
The cobalt(II) derivative of the “blue” copper protein stellacyanin has been prepared, and its visible-ultraviolet spectrum is reported. Tryptophan fluorescence quenching and p-mercuribenzoate titration results strongly suggest that Co(II) and Cu(II) compete for the same stellacyanin binding site and that a cysteine sulfur atom is coordinated in both cases. This interpretation is supported by the finding of an intense band at 355 nm in Co(II)-stellacyanin attributable to a charge transfer transition of the RS- → Co(II) type. The visible absorption spectrum of Co(II)-stellacyanin exhibits band maxima at 540, 625, and 655 nm. These bands are attributable to d-d transitions originating in a high-spin Co(II) center. It is suggested that a correspondence exists between charge transfer bands observed at 355 and 300 nm in the Co(II) derivative to those found at 604 and 450 nm in the native protein. It is concluded that the intense 604-nm peak in Cu(II)-stellacyanin is attributable to a cys-S → Cu(II) charge transfer transition.
Keywords: blue copper protein, metal replacement, electronic spectroscopy, cysteine ligand
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brill A. S., Bryce G. F. Cupric ion in blue proteins. J Chem Phys. 1968 May 15;48(10):4398–4404. doi: 10.1063/1.1668007. [DOI] [PubMed] [Google Scholar]
- Falk K. E., Reinhammar B. Visible and near-infrared circular dichroism of some blue copper proteins. Biochim Biophys Acta. 1972 Nov 28;285(1):84–90. doi: 10.1016/0005-2795(72)90182-1. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. The state of copper in stellacyanin and laccase from the lacquer tree Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):48–57. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- Morpurgo L., Finazzi-Agrò A., Rotilio G., Mondovì B. Studies of the metal sites of copper proteins. IV. Stellacyanin: preparation of apoprotein and involvement of sulfhydryl and tryptophan in the copper chromophore. Biochim Biophys Acta. 1972 Jul 21;271(2):292–299. doi: 10.1016/0005-2795(72)90203-6. [DOI] [PubMed] [Google Scholar]
- Ray W. J., Jr, Multani J. S. Characterization of the metal binding site of phosphoglucomutase by spectral studies of its cobalt(II) and nickel(II) complexes. Biochemistry. 1972 Jul 18;11(15):2805–2812. doi: 10.1021/bi00765a012. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. C., Root C. A., Wang R. H., Cerdonio M., Gray H. B. The nature of the ground states of cobalt(II) and nickel(II) carboxypeptidase A. Proc Natl Acad Sci U S A. 1973 Jan;70(1):161–163. doi: 10.1073/pnas.70.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]


