Abstract
Objectives
The current study examined five-year cognitive change in untrained African American and White participants from the ACTIVE study
Methods
Five year trajectories of memory, reasoning, visual processing speed/useful field of view, digit symbol substitution, and vocabulary were investigated. Education, health, gender, age and retest/practice effects were controlled for, and a missing data pattern mixture approach was used to adjust for dropout effects.
Results
After considering age, education health and gender, being African American uniquely explained 2% to 7% of the variance in cognitive performance. There were virtually no significant race differences in rates of change.
Discussion
Race-related results in the current study are consistent with previous research suggesting that social advantage factors like education have a stronger influence on level of performance than rate of change. The small remaining effects of being African American on performance levels likely reflect uncontrolled variation in factors like literacy and financial advantage.
Keywords: Aging, Cognition, Race/Ethnicity, Memory, Reasoning, UFOV, Vocabulary
It is well understood that race/ethnic disparities in health, broadly defined, exist throughout the human life span (e.g., Haas, Krueger & Rohlfsen, 2012). Cross-sectional studies suggest that there exist disadvantages in performance levels for older African Americans relative to Whites on a wide variety of cognitive screening and intelligence measures (e.g., Escobar, 1986; Fillenbaum, Heyman, Williams, Prosnitz, & Burchett, 1990; Heaton, Ryan, Grant, & Matthews, 1996; Kaufman, McLean, & Reynolds, 1988; Manly et al., 1998). More dynamically, however, there has been relatively little research that addresses similarity and differences in cognitive change rates for African American and White elders.
As older adults move into the later decades of life (e.g., 80s and 90s), growing evidence suggests that there is normative and accelerated cognitive decline (Ghisletta, Rabbitt, Lunn, & Lindenberger, 2012; Giambra, Arenberg, Zonderman, Kawas, & Costa, 1995; Lindenberger & Baltes, 1997; Singer Verhaeghen, Ghisletta, Lindenberger, & Baltes, 2003; Schaie, 1996), particularly in areas of cognition that are considered to be more fluid (Horn & Cattell, 1967) such as processing speed, working memory, attention, and even declarative memory and executive functioning (Baltes, 1993; Grady & Craik, 2000; Park et al., 2002). A question then arises whether this accelerated decline exists equally for African American and White individuals, or whether the life-long cognitive performance disadvantages reported for African American manifest themselves also in greater rates of decline.
It has been argued that late life cognitive differences between race groups reflect cumulative disadvantage and not differential rates of decline. For example, Byrd et al. (2006) found that early environmental factors (collected retrospectively) were significantly related to performance on neuropsychological tests. Less favorable early environments were correlated with poorer cognitive performance, even after adjusting for education. Similarly, in an AHEAD-based longitudinal study examining demographic and socioeconomic predictors of cognitive decline, non-Hispanic whites and non-Hispanic blacks evinced level differences (i.e., differences in average or initial level of performance) in cognition at a baseline assessment. Examining cognitive change, even after demographic and socioeconomic factors were considered, the rate of decline was less steep for non-Hispanic blacks than whites, resulting in diminished between-ethnicity differences to diminish with increasing age (Karlamangla et al., 2009).
Nonetheless, race differences in cognitive performance levels do manifest themselves in prevalence and incidence statistics for cognitive impairment. Diagnoses of Alzheimer’s disease, other types of dementia, and cognitive impairment are typically given when an individual’s cognitive performance is extremely low in comparison to a normative reference group.
Correspondingly, African Americans receive earlier and more frequent diagnoses of cognitive impairment or dementia (Inouye, Albert, Mohs, Sun, & Berkman, 1993; Manly et al., 1998; Whitfield, Weidner, Clark, & Anderson, 2002). In fact, overall, older African Americans have a not only been shown to have a greater prevalence of cognitive impairment and Alzheimer’s disease (Schwartz et al., 2004; Tang et al., 2001), but have also been shown to have greater physical disability (Bowen, 2009; Kelley-Moore & Ferraro, 2004; Mendes de Leon et al., 2005) and higher rates of mortality resulting from a variety of health conditions as well as shorter mean life expectancies (Hummer, 1996). What is not clear is whether these greater rates of impairment represent faster rates of decline, or simply that older adults enter late life at a lower level of functioning (i.e., closer to a threshold of impairment) due to cumulative life-long disadvantages in cognitive performance. This latter interpretation would be consistent with other studies that suggest that persons with lower education do not decline at a faster rate, but simply enter old age at lower cognitive levels (Zahodne et al., 2011).
Because of the late life disadvantages of older African Americans, relative to Whites, likely reflecting health and educational disparities, it has been proposed that level differences in cognition between African American and White elders should be attenuated after adjusting for lifetime disadvantage indicators such as socioeconomic status, health status, education, and gender (e.g., Aiken Morgan, Marsiske, & Whitfield, 2008; Jones, 2003; Manly et al., 1998; Manly, Jacobs, Touradji, Small, & Stern, 2002). Importantly, work done by Aiken Morgan et al. (2010) with the ACTIVE sample has shown that race-related test bias (i.e., differential test functioning for different race groups) was not a significant factor in the lower mean scores found in African Americans.
Extrapolating from physical/functional aging studies, one possible reason for accelerated cognitive decline for African Americans might in part be due to higher cardiovascular and comorbidity burden (Cooper et al., 2000). However, even when adjusting for socioeconomic status (SES), there is a lingering effect of race on some health outcomes (e.g., hypertension, diabetes, and arthritis; Williams, 1996; Whitfield et al., 2002). Zsembik & Peek (2001) hypothesized that since African Americans had greater prevalence of biological risk factors than Whites, race would operate on cognitive functioning indirectly through biological factors, particularly vascular diseases. Nonetheless, results indicated that race had a direct effect on cognition, after accounting for social and biological correlates. It is important to note that this direct race effect was smaller once the social and biological factors were added to the model, indicating that these factors account for some of the level differences (i.e., mean differences) seen on cognitive scores between African American and White older adults. It was concluded that the remaining race differences are most likely a result of further background variables such as quality of education and early life inequalities that were simply not accounted for in their study (Zsembik & Peek, 2001).
Education has been strongly implicated in the observed cognitive level differences observed in African American and White elders. For current cohorts of older adults, African Americans obtained fewer years of education (e.g., Snyder, 1993; Williams, 1999), and likely also experienced poorer quality (shorter years, impoverished study materials) of education in the early twentieth century (Bullock, 1967; Jones, 2003; Whitfield, 1996; Williams, 1999). There is somewhat conflicting literature about difference in health and physical functioning returns seen between races for increasing years of education. Farmer & Ferraro (2005) showed an interaction between race and education and race and employment status for self-rated health measures in older adults. This suggested that with increasing education levels, African Americans did show the same improvement on self-reported health measures as the White sample. However, in a recent study by Barnes et al. (2011), Blacks appeared to show greater returns than Whites in functional health for greater that 12 years of education, indicating that with each year of education beyond 12 years, there was significantly greater gain on the functional health outcomes for Blacks than for Whites.
The current study examined five-year cognitive change on a broad battery of psychometric measures in a subset of untrained African American and White participants from the ACTIVE study. The availability of five years of longitudinal data, on multiple cognitive measures, for both African American and White elders represents a major advantage of the ACTIVE data set over many previous studies. The ACTIVE (Advanced Cognitive Training in Independent and Vital Elderly) no-training control group (N=690) was employed, since intervention effects might alter naturally occurring rates of change. Further detail on the control group, in the context of the larger study, is provided in the Overview paper of this Special Issue of the Journal of Aging and Health. Only African American and White participants were included because representatives of other races were included in very low numbers (8 participants). ACTIVE provided a strong data set with which to study African American and White longitudinal trajectories, due to the study’s overall commitment to substantially represent African Americans, who comprised 27.5% of the sample. Because the larger ACTIVE clinical trial excluded persons of low cognitive status (described below), the educational distributions of the two white groups were more similar to one another than they would be in the American population as a whole. Consequently, ACTIVE permitted a comparison of differences in mean-level and rates of change in race groups that were selected to be relatively more educationally and cognitive similar than they might be in the population, as a function of the clinical trial inclusion/exclusion criteria. Nonetheless, factors like educational level and health differences were included in all analyses, as were gender and age. In ACTIVE, African American participants tended to be slightly younger at enrollment and slightly more female. Analyses also controlled for retest/practice effects and participant dropout effects (Lindenberger, Singer, & Baltes, 2002; Rabbitt, Diggle, Holland, & McInnes, 2004; Salthouse, 2010; Schaie, Labouvie & Barrett, 1973; Willis & Schaie, 1986)
Method
Participants
This paper utilized the African American and White no-training control group participants in ACTIVE. The control sample in ACTIVE (N = 690, M = 182; F = 508) has been extensively described elsewhere (e.g., Jobe et al., 2001; Ball et al., 2002). Table 1 shows the distribution of age, education, gender, and physical functioning by race/ethnicity categories. At time of enrollment in 1998–2000, participants were, on average, 74.05 years of age, and had an average of 13.37 years of education. With regard to the racial/ethnic distribution, 500 participants (72.46%) were white, 190 (27.54%) were African American. This study excluded the less than 1% of the total ACTIVE sample who reported themselves as members of other races or bi-/multi-racial As Table 1 also shows, there were significant racial/ethnic group differences in age, education, physical functioning, and proportion of sub-sample that was female, with African American participants tending to be younger (p < 0.05), less educated, have lower physical functioning, and contain a larger proportion of women (p < .001) than whites; effect sizes for the race differences tended to be small-to-medium (Cohen, 1998 ).
Table 1.
Characteristics of ACTIVE control participants at baseline.
| Total Sample (n = 690) | African Americans (n = 190) | Whites (n = 500) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Cohen’s d | t/χ2 | df | p | |
|
| ||||||||||
| Age | 74.05 | 6.06 | 73.26 | 5.75 | 74.34 | 6.15 | −0.18 | 2.10 | 688 | 0.036 |
| Years of Education | 13.37 | 2.70 | 12.74 | 2.44 | 13.61 | 2.76 | −0.33 | 4.05 | 383.841 | <.001 |
| Gender (% female) | 74.05 | - | 82.63 | - | 70.20 | - | - | 10.96 | 1 | <.001 |
| Physical Function | 69.00 | 24.64 | 63.53 | 23.84 | 71.08 | 24.64 | −0.31 | 3.61 | 680 | <.001 |
Note: T-tests show significance of the comparison between African American and White participants; for Gender, a corresponding chi-squared test was used. No covariate adjustment was employed.
Degrees of freedom and t-statistic were adjusted for non-homogeneity of variance.
The ACTIVE sample was drawn from older adults without dementia who were living independently in the community. Persons were excluded from participation if they had Mini Mental Status Examination (Folstein, Folstein & McHugh, 1975) scores less than 23, vision (Rubin & Salive, 1985) worse than 20/70 or health conditions such as history of stroke or low-survival cancers or had experienced substantial functional impairment with dressing, personal hygiene or bathing. Based on initial screening comparisons between those randomized and not-randomized in the total sample 379 of 2,454 Whites screened were excluded (15.4%) and 239 of 976 African Americans screened were excluded (24.5%). This represented a significantly higher exclusion rate for African American participants: χ2(df=1, N=3430) = 38.66, Cramer’s V = 0.11, p < .001. These comparisons could not be restricted to the current study sample only, since exclusion occurred prior to randomization.
Of the 690 elders included at baseline, there were 447 survivors and 246 dropouts at the fifth annual follow up. T-test comparisons of baseline characteristics of these two groups of participants revealed that, relative to dropouts, those assessed at Year 5 were younger (73.6 versus 74.9 years), reported higher levels of education (13.6 versus 13.0 years), had higher MMSE scores (27.5 versus 26.8), and included a higher percentage of females (68.3% versus 66.3%), and white participants (75.7% versus 63.0%; all significant at p < .05). Correspondingly, results that follow adjust for these covariates, and use a missing data pattern mixture approach (described below) to produce estimates combined across missing data mixture groups.
Procedure
Data at each occasion were collected over three sessions (as described in Jobe et al., 2001 and Ball et al., 2002). Two, two-hour sessions were conducted individually, and a third three-hour session was included in a group format. Assignment of measures to sessions was governed largely by prior practices and practical considerations. For example, some neuropsychological measures, like the Digit-Symbol Substitution task, that are more commonly given in one-on-one testing situations to maximize quality control were administered in single participant sessions. To reduce respondent burden, a goal was to distribute cognitive measures across several sessions, to minimize fatigue in any single session. Some sessions had to be individual in nature (e.g., those that included physical performance measures needed for other aspects of the ACTIVE study), and so cognitive tasks that were fit into those sessions also happened to be administered individually. Following baseline assessment, participants were re-evaluated, in a similar manner as during baseline, at 1-year follow up, 2-year follow up, 3-year follow up, and 5-year follow up. All available data from selected participants were used in the subsequent analyses.
Measures
This study reports performance in five cognitive domains used in ACTIVE. These domains employed traditional, well-studied laboratory tasks of cognitive and intellectual functioning. In the ACTIVE study, composites made up of multiple measures of memory, reasoning, and visual processing speed (Useful Field of View; UFOV; Ball, Owsley, Sloane, Roenker & Bruni, 1993) were considered proximal outcomes, because their chief function was to permit detection of memory, reasoning and speed training effects. In addition, measures of vocabulary (Ekstrom, French, Harman,& Derman, 1976) and digit-symbol substitution (Wechsler, 1981) were administered to permit the assessment of training effect breadth, and could be used to assess age-related change in widely studies cognitive measures thought to represent crystallized intelligence and perceptual/motor speed, respectively. Table 2 presents an overview of measures used. Further psychometric details about these instruments are given in other sources (e.g., Jobe et al., 2001 and Ball et al., 2002). For the composite of memory, reasoning, and speed, all constituent measures were Blom-normalized and standardized (Mean=0, standard deviation =1) across all occasions, and were then summed into unit weighted composites. As noted above, covariates of self-reported age, education, gender, and health (a general health rating scale from the SF-36 inventory; Ware & Sherbourne, 1992) were included in all conditional models.
Table 2.
Cognitive measures in ACTIVE
| Domain | Measure | Description | Source |
|---|---|---|---|
| Memory | Auditory Verbal Learning Test (AVLT) | Assessed older adults’ memory for lists of unrelated words. Participants were presented with recorded lists of 15 words presented at 2 second intervals, followed by a 2 minute recall period. Following five presentations of this list, participants were presented with a new list of 15 words and subsequently asked to recall words from the new list. Recall of the original list was then again assessed to measure learning. Finally recognition memory for words on the original list was evaluated. Score for word lists is the number of words correctly recalled. | Rey, 1941 |
| Hopkins Verbal Learning Test (HVLT) | A word list task, similar to AVLT. Lists were composed of 12 related words from familiar semantic domains (e.g., animals, vegetables). The original word list was presented as described for the AVLT, followed by a 2 minute recall period. Following three presentations of the list and an intervening unrelated activity, participants’ recognition memory for words on the original list was assessed. Score for word lists is the number of words correctly recalled. | Brandt, 1991 | |
| Rivermead Behavioral Memory Test: Prose Memory | Participants listened to tape recordings of 4–5 sentence paragraphs and then were given 3 minutes to write down as much of the story as they could recall. Score is the number of propositions correctly recalled, verbatim or in gist. | Wilson, Cockburn, & Baddeley, 1985 | |
| Reasoning | Word Series | The individual is shown a series of words that involve a pattern (e.g., months of year, days of week) and must identify the pattern and use the pattern to determine next item in the series. | Gonda & Schaie, 1985 |
| Letter Series | The pattern in a series of letters must be identified and used to determine the next item in the series | Thurstone & Thurstone, 1949 | |
| Letter Sets | Five sets of letters are shown with four sets following the same pattern. The set not following the pattern must be identified. | Ekstrom, French, Harman, & Derman, 1976 | |
| Visuospatial Speed | Useful Field of View | Speed of processing was assessed with the Useful Field of View (UFOV ®) test. This computer- administered measure provides an index of the stimulus duration needed to perform a variety of visual search tasks of increasing cognitive complexity at 75% correct. Each of four subtests (stimulus identification alone, divided attention, and selective attention) have a possible range from 16 to 500 msec, with the total score ranging from 48 to 1500 msec. The four subtests represent increments in difficulty, ranging from fairly simple (determining which of 2 objects—car or truck—appears in a fixation box in the center of a 17″ color computer touch screen) to fairly complex (judging which configuration of objects - 2 cars, 2 trucks, car and truck - appears in a fixation box, while simultaneously identifying the location of a peripheral target on the outside of a cluttered display). The score represents latency in msec. | Ball, Owsley, Sloane, Roenker & Bruni, 1993; Owsley, Ball, Sloane, Roenker & Brune, 1991 |
| Processing Speed | Digit Symbol | Participants see a template with nine symbols corresponding to nine digits. Participants are then given rows of digits with empty spaces below them; participants are asked to complete with as many matching symbols as they can in 90 seconds. The measure assesses attention and speed (motor, perceptual, visual scanning). | Wechsler, 1981 |
| Vocabulary | Crystallized Intelligences | Word recognition task in which participants must match a target word to one of four possible synonyms; | Ekstrom, French, Harman, & Derman, 1976 |
Analyses
Conditional growth models one each for reasoning, memory, UFOV, digit symbol, and vocabulary, were parameterized to control for the effects of background covariate variables (i.e., age, education, health, gender) and being African American on level and rate-of-change in cognitive functioning across the 5-year study period. This was done through implementation of a multi-level model (MLM) for change (Bryk & Raudenbush, 1992; Singer & Willett, 2003).
To examine any influence of missing data and participant attrition, a pattern-mixture model (PMM) approach (Hedeker & Gibbons, 1997) was also implemented. PMM allows for the examination of performance as a function of different patterns of missing data, although the current study presents final parameter estimates aggregated across missing and non-missing data patterns.
For each of the five cognitive outcomes (memory, reasoning, visual processing speed/useful field of view, digit-symbol substitution, vocabulary) a six-step hierarchical model building approach was adopted for each cognitive outcome variable. The steps involved estimating an unconditional null model (needed for later incremental fit and proportional variance explained calculations), followed by an unconditional growth model (estimating the linear and quadratic effects of time), followed by the addition of background covariates, a variable representing being African American, and finally Time by African American residual-centered product terms (Little, Bovaird & Widaman, 2006), to assess whether being African American moderated the slope of change, and are detailed in the appendix, which also shows the model fit results (including −2LL, AIC, within-person r2 and between-person r2) for each added predictor block and for each cognitive domain assessed. Following the penultimate product-term step, the model was re-estimated using missing data PMM. This step involved the introduction of a dummy coded predictor variable indicating whether or not an individual was present at the final measurement occasion. Product terms between this variable and the above described time functions and being African American were also estimated. Throughout the models, where product term interaction terms were included, they were residual centered (partialing out constituent main effects) to eliminate multicollinearity effects (Little, Bovaird & Widaman, 2006).
All models were estimated under the simplest assumptions about the repeated error structure over time (i.e., homoscedasticity and independence of errors) and diagonal random error structure (i.e., heteroscedasticity and independence of observations). The models were also estimated using the Maximum Likelihood (ML) method. The ability of a model to predict cognitive performance better than the baseline model (i.e., Deviance) was used as an index of Goodness of Fit. Improvements in predictability were determined by the amount of reduction of within-person residual variances and between-person intercept variances compared to the baseline model (Bryk & Raudenbush, 1992). Decreases in residual and intercept variances represent a proportional reduction of the prediction error, which is analogous to R2, and was used as an estimate of within-person and between-person effect sizes. The results summary that follows comes from the final model (Step 5) following implementation of pattern mixture model aggregation across missing and non-missing mixture groups.
Results
Investigation of proposed models
Prior to the proposed models, initial preliminary models (not detailed here) examined the unique effect of being African American, and whether being African American moderated the age effects, without inclusion of covariates. This step (included at the suggestion of an anonymous reviewer of the previous draft) provided an initial estimate of the raw bivariate association between being African American and the cognitive outcomes. The standardized beta for the effect of being African American was, for Digit Symbol: β = −0.30, for Memory β = −0.35, for Reasoning: β = −0.39, for Useful Field of View: β = −0.21, and for Vocabulary β = −0.50 (standard error = .05 for all outcomes; p < .001 for all). Being African American did not moderate either the linear or quadratic rates of change in any of these models. In these bivariate models, being African American uniquely explained between 4% (UFOV) and 25% (Vocabulary) of the variance. In subsequent proposed models, the effect of being African American was re-examined after controlling for covariates; the unique effect of being African American was much attenuated.
The final models, following implementation of pattern mixture model aggregation across missing and non-missing mixture groups, for each cognitive domain assessed are summarized in Table 3. In general, each model step yielded a significant improvement in model fit, and an increase in explained variance, with the exception of the final race-moderation step, which showed that being African American only significantly interacted with rate of change (quadratic) for the reasoning composite. In practical terms, being African American added relatively little to the models. Being African American uniquely accounted for between 2% and 7% of the between-person differences in cognition and, as noted, race groups did not evince significant differences in rates of linear or quadratic change. Despite the relatively unimportant role of race in these models, the models in general explained between 43% and 50% of the between person variability in cognition, and linear/quadratic change and retest effects explained between 8% and 21% of the within-person variability.
Table 3.
Multilevel Growth Models Examining Five-Year Cognitive Change
| Fixed Effects | Reasoning | Memory | UFOV | Digit Symbol | Vocabulary | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Predictor Variable | B | t | B | t | B | t | B | t | B | t |
| Within-person | ||||||||||
| Linear Time | 0.17 | 1.69 | −0.10 | −2.06* | −0.22 | −1.50 | −0.08 | −1.96* | 0.02 | 0.38 |
| Quadratic Time | 0.05 | 1.02 | −0.04 | −1.99* | −0.19 | −2.67** | −0.05 | −2.50** | 0.02 | 0.87 |
| Retest | 0.28 | 2.25* | −0.07 | −1.11 | 0.48 | 2.59** | 0.06 | 1.27 | 0.07 | 1.31 |
| Between-person | ||||||||||
| Age | −0.13 | −9.27** | −0.06 | −11.42** | −0.18 | −12.52** | −0.05 | −9.85** | 0.01 | 1.82 |
| Gender | 0.20 | 1.10 | 0.50 | 7.81** | −0.11 | −0.63 | 0.26 | 3.79** | 0.11 | 1.55 |
| Education | 0.29 | 9.13** | 0.09 | 8.53** | 0.13 | 4.24** | 0.07 | 6.18** | 0.14 | 11.30** |
| Health | 0.02 | 5.10** | 0.01 | 3.82** | 0.01 | 3.07** | 0.01 | 5.31** | 0.00 | 2.92** |
| African American (AA) | −1.49 | −6.12** | −0.62 | −6.88** | −1.15 | −4.45** | −0.59 | −6.26** | −0.75 | −7.89** |
| Interactions | ||||||||||
| Linear Time x AA | −0.11 | −1.47 | 0.03 | 0.89 | 0.12 | 1.09 | 0.02 | 0.82 | −0.01 | −0.28 |
| Quadratic Time x RaceAA | −0.07 | −2.13* | 0.03 | 1.74 | 0.08 | 1.79 | 0.02 | 1.49 | −0.01 | −0.52 |
|
| ||||||||||
| Random Effects | ||||||||||
|
| ||||||||||
| Predictor Variable | variance | Z | variance | Z | variance | Z | variance | Z | variance | Z |
|
| ||||||||||
| Linear Time | 0.03 | 4.70** | 0.00 | 3.85** | −0.03 | 3.51** | 0.00 | 5.14** | 0.00 | 0.97 |
| Quadratic Time | 0.00 | 0.31 | --a | 0.00 | --a | 0.00 | 0.00 | 0.51 | 0.00 | 2.53** |
Notes:
p < 0.05,
p < 0.01;
Variance too small to be estimated- The final Hessian matrix was not positive definite although all convergence criteria were satisfied. AA = African American (reference group is White); UFOV = Useful Field of View. In addition, all models included study site and an indicator of when in time data collection occurred (replicate). These covariates are not shown for presentation simplification. There were no hypotheses about site and replicate effects, but they were a substantial identifiable source of participant variation. These effects, along with degree of freedom and standard error information for each effect, are available upon request.
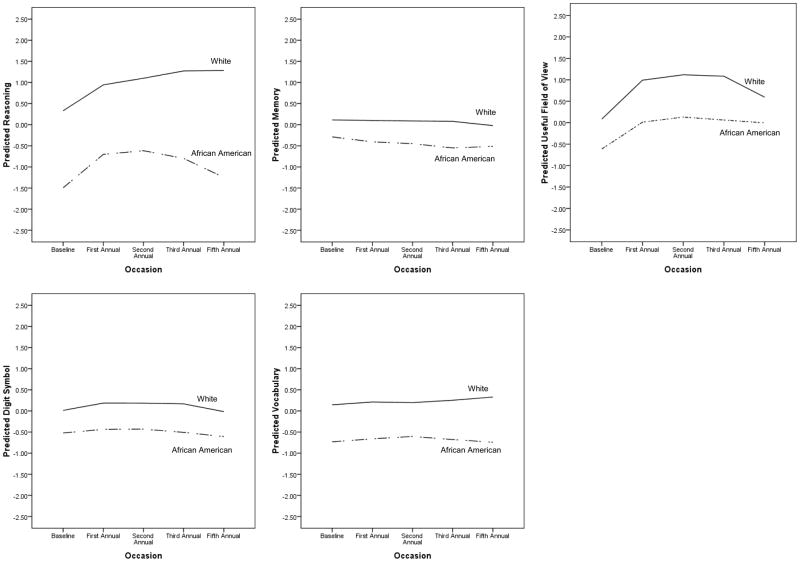
Looking across cognitive domains, in general there was relatively modest change over the five year period. Linear change was significant and negative only for the memory composite, although there was significant random variance (i.e., individual differences) in the linear slope for all abilities except for vocabulary. There was a negative quadratic time trend (accelerated decline in later years) for memory, visual processing speed/UFOV, and the digit symbol substitution test. There was also a significant positive retest effect for the reasoning and visual processing speed/UFOV measures. While the vocabulary measure had no fixed association with time, there was significant random variance in the quadratic trend, indicating that some individuals may have experienced relatively more decline than others. The model-implied linear and quadratic trajectories of the five cognitive domains, plotted separately for African American and White participants, are shown in Figure 1.
Figure 1.
Model estimated growth curves for 5-year cognitive change by racial group.
All covariates included to adjust for group differences in level showed some significant associations with the cognitive outcomes. Age was significantly and negatively associated with all cognitive measures except for vocabulary; women showed significantly better performance on the memory composite and the digit-symbol substitution measures. Education and Health were positively related to performance on all measures, while African American participants performed more poorly on all five cognitive outcomes.
As noted above, being African American did not moderate the linear or quadratic trends for any of the outcomes except for the reasoning composite, where African Americans evinced slightly more negative quadratic age change trajectories than Whites.
African American status as a moderator of rate of change
As a follow-up to the main analyses, several post-hoc multilevel growth models were parameterized and examined. These models examined the effects of age, race, and age by race interactions on five-year cognitive change, to answer the question of whether race might moderate rate of change differentially based on age (e.g., might race differences emerge in rate of change for the oldest participants?). Across all dependent variables only the Age x Linear Time interaction was significant: Digit Symbol, β = −0.003, t(505.27) = −3.62, p < .001; Memory: Age x Linear Time, β = −0.004, t(509.61) = −3.70, p < .001; Reasoning: Age x Linear Time, β = −0.008, t(492.21) = −3.69, p < .001; UFOV: Age x Linear Time, β = −0.012, t(542.22) = −3.73, p < .001; Vocabulary: Age x Linear Time, β = −0.002, t(480.69) = −2.771, p < .01. In all models, none of the previously reported results were substantially changed by the inclusion of these additional predictors (i.e., all fixed and random effects remained significant).
Discussion
In a sample of African American and White older adults selected for the ACTIVE clinical trial, race differences in level of performance on a variety of cognitive outcomes were relatively small in magnitude. With regard to the central question motivating the current paper, there was little evidence of race differences in rates of change over the five year period studied. Being African American accounted for only 2% to 7% of the individual differences in level of cognitive performance. The general trend of cognitive change over the five year period was negative quadratic change (initial increase followed by accelerated decline) for memory, visual processing speed/UFOV, and digit symbol substitution, but these trends were not moderated by being African American. In the sole exception, being African American appeared to slightly moderate individual differences in rates of change for reasoning, in the form of less accelerated quadratic decline for African American participants, even though the overall five year trajectory for reasoning was flat. Follow up analyses showed that higher age was associated with more negative linear change for all abilities studied, but this did not interact with being African American.
One criticism of the current findings likely relates to the positively selected nature of the sample. That is, to meet the inclusion criteria of the larger ACTIVE clinical trial, participants were required to perform at or above 23 on the MMSE. This had the effect of truncating the lower part of the cognitive status distribution, thereby serving to homogenize differences between race groups at enrollment. In addition, participants who reported extreme difficulty with two or more activities of daily living were also excluded. These selection factors clearly limit the generalizability of the obtained five-year changes to the larger population of older adults. An open question is whether these selection actors also account for the relatively modest five-year changes observed in this study. The inclusion/exclusion criteria for the ACTIVE study may not necessarily have eliminated all education and health effects, but they may have helped to minimize the confounding effects of education and health, since the groups were relatively equated at baseline (Aiken Morgan et al., 2010).
In the context of the selection filter imposed by the clinical trial inclusion factors, as well as the inclusion of a number of covariates in all models, especially education and health, it is surprising that significant race differences still persisted. The persistent race effect here, though small, likely reflects unmeasured cultural factors that influence cognitive performance (Zsembik & Peek, 2001; Leveille et al., 1998). It is also likely that the persistent race effect might reflect unspecified effects due to biological influences (e.g., genetics), racism, or unobserved heterogeneity (Clark, Anderson, Clark, & Williams, 1999; Jackson et al. 1996). The years of education variable included in the current study also likely fails to capture the true diversity of education for the cohorts included in this study, where “separate and unequal” was often true of public education for African American and Whites (see Manly et al., 2002). Because ACTIVE was a clinical trial, and did not have as its initial goal the discovery and explanation of race disparities in cognition, some variables that have been found important in explaining race differences in cognition were not available here. For example, in work by Mehta, Simonsick, Rooks, Newman, Pope, Rubin, and Yaffe (2004), literacy and financial adequacy, along with age, sex, and education, accounted for 86% of the black-white differences in mental status. This is consistent with the work of Manly et al (1998, 2002) and our own prior work with the ACTIVE pilot sample (Aiken Morgan, Marsiske & Whitfield, 2008), who also found that literacy accounted for much of the race differences cognition.
Another limitation of the study relates to the limited diversity of measures used to study cognitive change. Reasoning, memory and speed were heavily sampled measurement domains, since these were the targets of the ACTIVE cognitive interventions (Ball et al., 2002; Jobe et al., 2001). More crystallized (Horn & Cattell, 1967) or knowledge-based domains were not well represented in the study, with the exception of the vocabulary measure considered.
Nonetheless, the overarching finding with regard to race differences in longitudinal trends in the current study seems to be near-parallelism between the two racial groups. This is consistent with Allaire and Marsiske (1999), who reported that African Americans appeared to have parallel (linear) cross-sectional gradients to whites for most intellectual abilities; an exception was for measures of knowledge (e.g., verbal ability, everyday ‘facts’), where cross-sectional trends were more steeply negative for black participants. The current findings are also consistent with previous evidence that demographic variables that are associated with better performance in later life seem do not seem to confer advantages in terms of rate of decline. For example, Baltes (1997), and Lindenberger and Baltes (1997), reported that the negative age gradient found for a general measure of intellectual performance in the Berlin Aging Study applied equally well in two groups stratified by educational and social history; similar recent findings were reported by Zahodne et al. (2011) in the Victoria Longitudinal Study.
It is unclear how much some form of the cross-over effect (e.g., Elo & Preston, 1997), or the progressive narrowing of the mortality gap with age, might play a role in the small race effects observed in this study. Interpretively, this narrowing has been viewed as a reflection that, given ongoing health disparities and adverse living conditions for African Americans across the life span, only the fittest individuals are likely to reach old age (e.g., Jackson, 1980; Markides & Mindel, 1987; Manton, Stallard, & Wing, 1991) and age trends reflect this selective survivorship. Alternatively, it is also possible that, since recruiting and representing the oldest ages in a minority population is particularly difficult (Hogan and Robinson, 1993), the resulting sample becomes progressively more positively selected and biased as it relies on increasingly positively selected volunteers. These interpretations are only speculative in the current study (given study exclusion criteria, it was not possible to investigate the trajectories of the lowest functioning volunteers). It is clear, however, that ACTIVE enrolled a higher proportion of positively selected African Americans than Whites, given higher rates of exclusion for African Americans than for Whites (24.5% versus 15.4%).
One hypothesis for the cognitive level differences observed between African American and White elders is the cumulative disadvantage model (Byrd et al., 2006). The reduction of the unique effect of being African American (from 4–25% to 2–7%) after variables like education, age, health and gender were included in the model suggests that these variables might be possible mediators of apparent late-life race differences in cognition. The ability to examine cumulative disadvantage directly is weakened in ACTIVE, both due to the fact that one of the most salient variables, SES, was not collected in ACTIVE, and due to the fact that data from earlier points in the lifespan was not collected. A related study from ACTIVE, however, was able to collect current neighborhood socioeconomic position (SEP) data for most study participants from the 2000 Census (Sisco et al, 2012). That study found that current neighborhood SEP was uniquely associated with individual differences in cognitive level (especially verbal ability), even after controlling for education and verbal abilities as indicators of cumulative advantage; moreover, SEP was strongly and negatively related to being African American.
One typical problem in longitudinal investigators of cognition is the issue of selective attrition. Correspondingly, in this study, participants who survived until the five-year follow up tended to be younger (by 1.3 years, on average), more educated (by 0.6 years, on average), and perform slightly better on the MMSE (by 0.7 points). Returning participants were also more likely to be female and white. To reduce the influence of these biasing features, we included these covariates in all conditional models, and we also employed a missing data pattern mixture approach (Hedeker & Gibbons, 1997). This approach modeled the main effect of attrition status, as well as the interaction of attrition with time, being African American, and their interaction. These analyses were then used to produce the pooled parameter estimates shown in Table 3.
Taken together, the results of this study and previous investigations on sociocultural/ethnic group differences suggest that there may be a dissociation of race differences in level and slope of cognitive performance that closely parallels what has been reported with regard to education (e.g., Zahodne et al, 2011). With regard to level of performance, the lower performance of African American and lower educated elders in this study likely reflects the many potential sources of cumulative life diversity that tend to distinguish racial/ethnic minority members in American society (e.g., Whitfield et al., 2002). With regard to rate of longitudinal decline, at least in our sample of African American and White elders who met identical inclusion criteria for our clinical trial, the was no evidence that being African American conferred any particular disadvantage.
Acknowledgments
Mr. Dzierzewski and Ms. Thomas were supported by a grant from the National Institute of Aging (T32 AG020499), and Mr. Dzierzewski was also supported by a Ruth L. Kirchstein National Research Service Award (F31 AG032802). ACTIVE is supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew Senior Life (U01NR04507), Indiana University School of Medicine (U01NR04508), Johns Hopkins University (U01AG14260), New England Research Institutes (U01AG14282), Pennsylvania State University (U01AG14263), University of Alabama at Birmingham (U01AG14289), University of Florida (U01AG14276).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research, National Institute on Aging, or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Dr. Marsiske has received research support from Posit Science, Inc., in the form of site licenses for cognitive training programs for a different research project. Dr. Ball owns stock in the Visual Awareness Research Group and Posit Science, Inc., the companies that market the Useful Field of View Test and speed of processing training software now called Insight, and she serves as a member of the Posit Science Scientific Advisory Board. Dr. Rebok is an investigator with Compact Disc Incorporated for the development of an electronic version of the ACTIVE memory intervention.
Appendix
Modeling proceeded in six steps: (1) Null: Baseline model, estimated only a fixed and random intercept for cognitive functioning and serves as a comparison for later models; (2) Time Added: Linear and quadratic effects of time (coded as time since baseline), control variables (data collection site, replicate, and a dummy coded retest variable) were added. This model characterized the growth curves. The retest dummy code was set to 0 for first exposure (no previous experience) versus 1 for all subsequent exposures (following a procedure described in McArdle, Ferrer-Caja, Hamagami, & Woodcock, 2002), reflecting the consistent finding that retest/practice-related gains are usually largest between the first and second measurement; (3) Background Covariates Added: Background covariate variables were added (age, education, health, and gender); (4) African American Added: African American (AA; reference group = whites) was added. AA was entered last (in steps 4 and 5) to show the unique effect of AA (if any) after preceding covariates had been controlled, so as not to inflate the effect of AA status; (5) Time X African American interaction added: Time*AA product terms were added, to assess whether being African American moderated the slope of change.
Model fits at each hierarchical step are shown in the appendix table that follows:
Appendix Table.
Hierarchical fit information for growth models
| Fit Statistics | ||||
|---|---|---|---|---|
|
| ||||
| Model Steps | −2LL | AIC | r2w | r2b |
| Reasoning | ||||
| (1) Null | 9125.70 | 9131.70 | -- | -- |
| (2) Time addeda | 8818.28 | 8904.28 | 0.20 | 0.15 |
| (3) Background Covariates addedb | 8560.88 | 8654.88 | 0.20 | 0.42 |
| (4) AA added | 8507.15 | 8603.15 | 0.20 | 0.47 |
| (5) Time x AA addedc | 8502.96 | 8602.96 | 0.20 | 0.47 |
| (6) Pattern Mixture addedd | 8457.13 | 8569.13 | 0.20 | 0.50 |
| Memory | ||||
| (1) Null | 4845.59 | 4851.59 | -- | -- |
| (2) Time addeda | 4655.22 | 4741.22 | 0.14 | 0.10 |
| (3) Background Covariates addedb | 4354.82 | 4448.82 | 0.13 | 0.45 |
| (4) AA added | 4302.42 | 4398.42 | 0.13 | 0.49 |
| (5) Time x AA addedc | 4298.55 | 4398.55 | 0.14 | 0.49 |
| (6) Pattern Mixture addedd | 4250.66 | 4362.66 | 0.15 | 0.45 |
| Useful Field of View | ||||
| (1) Null | 10188.98 | 10194.98 | -- | -- |
| (2) Time addeda | 9925.58 | 10011.58 | 0.15 | 0.16 |
| (3) Background Covariates addedb | 9705.35 | 9799.35 | 0.14 | 0.42 |
| (4) AA added | 9683.81 | 9779.81 | 0.14 | 0.44 |
| (5) Time x AA addedc | 9679.93 | 9779.93 | 0.14 | 0.44 |
| (6) Pattern Mixture addedd | 9658.61 | 9770.61 | 0.15 | 0.45 |
| Digit Symbol | ||||
| (1) Null | 4314.82 | 4320.82 | -- | -- |
| (2) Time addeda | 4044.07 | 4130.07 | 0.22 | 0.12 |
| (3) Background Covariates addedb | 3823.42 | 3917.42 | 0.21 | 0.37 |
| (4) AA added | 3780.96 | 3876.96 | 0.21 | 0.41 |
| (5) Time x AA addedc | 3777.81 | 3877.81 | 0.21 | 0.41 |
| (6) Pattern Mixture addedd | 3750.40 | 3750.40 | 0.21 | 0.43 |
| Vocabulary | ||||
| (1) Null | 4035.39 | 4041.39 | -- | -- |
| (2) Time addeda | 3903.88 | 3989.88 | 0.08 | 0.16 |
| (3) Background Covariates addedb | 3726.55 | 3820.55 | 0.08 | 0.36 |
| (4) AA added | 3651.40 | 3747.40 | 0.08 | 0.43 |
| (5) Time x AA addedc | 3646.50 | 3746.50 | 0.08 | 0.43 |
| (6) Pattern Mixture addedd | 3631.68 | 3743.68 | 0.08 | 0.44 |
Notes: AA = African American (reference group is White); −2LL = −2 log likelihood; r2b = between-subjects pseudo R-squared, an estimate of the amount of between subjects variance (estimated from null model) explained by fixed and random predictors; r2w = within-subjects pseudo R-squared, an estimate of the amount of within subjects variance (estimated from null model) explained by fixed and random predictors;
Time includes linear and residual quadratic effects of time plus a dummy code indicating whether occasion is a retest;
Background covariates include: age, education, health, and gender.
Product terms for race by time (linear and quadratic) were included; these were residual-centered to eliminate collinearity with main effects
A dummy code representing attrition (1=present at fifth annual follow up; 0 = dropout) was included, as were its residual-centered interactions with race, time, and race-by-time interactions.
References
- Aiken Morgan AT, Marsiske M, Whitfield KE. Characterizing and explaining differences in cognitive test performance between African American and European American older adults. Experimental Aging Research. 2008;34(1):80–100. doi: 10.1080/03610730701776427. 10.1080/03610730701776427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken Morgan AT, Marsiske M, Dzierzewski J, Jones RN, Whitfield KE, Johnson KE, Cresci MK. Race-Related Cognitive Test Bias in the ACTIVE Study: A MIMIC Model Approach. Experimental Aging Research. 2010;36(4):426–452. doi: 10.1080/0361073X.2010.507427. 10.1080/0361073X.2010.507427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire JC, Marsiske M. Everyday cognition: Age and intellectual ability correlates. Psychology and Aging. 1999;14(4):627–644. doi: 10.1037//0882-7974.14.4.627. 10.1037/0882-7974.14.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske, Willis SL for ACTIVE Study Group. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA: The Journal of the American Medical Association. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Investigative Ophthalmology and Visual Science. 1993;34(3):110–23. Retrieved from http://www.iovs.org/content/34/11/3110.full.pdf+html. [PubMed] [Google Scholar]
- Baltes PB. The aging mind: Potential and limits. The Gerontologist. 1993;33(5):580–594. doi: 10.1093/geront/33.5.580. 10.1093/geront/33.5.580. [DOI] [PubMed] [Google Scholar]
- Baltes PB. On the incomplete architecture of human ontogeny: Selection, optimization, and compensation as foundation of developmental theory. American Psychologist. 1997;52:366–380. doi: 10.1037//0003-066x.52.4.366. 10.1037/0003-066X.52.4.366. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Hebert LE, Scherr PA, Evans DA, Mendes de Leon CF. Racial differences in the association of education with physical and cognitive function in older blacks and whites. Journal of Gerontology: Social Sciences. 2011;66B(3):354–363. doi: 10.1093/geronb/gbr016. 10.1093/geronb/gbr016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen ME. Childhood socioeconomic status and racial differences in disability: evidence from the Health and Retirement Study (1998–2006) Social science & medicine (1982) 2009;69(3):433. doi: 10.1016/j.socscimed.2009.06.006. 10.1016/j.socscimed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clinical Neuropsychology. 1991;5:125–142. 10.1080/13854049108403297. [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks: Sage; 1992. [Google Scholar]
- Bullock H. A history of Negro education in the south: from 1619 to the present. Cambridge: Harvard University Press; 1967. [Google Scholar]
- Byrd DA, Miller SW, Reilly J, Weber S, Wall TL, Heaton RK. Early Environmental Factors, Ethnicity, and Adult Cognitive Test Performance. The Clinical Neuropsychologist. 2006;20(2):243–260. doi: 10.1080/13854040590947489. 10.1080/13854040590947489. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist. 1999;54:805–819. doi: 10.1037//0003-066x.54.10.805. 10.1037/0003-066X.54.10.805. [DOI] [PubMed] [Google Scholar]
- Cohen JD. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- Cooper R, Cutler J, Desvigne-Nickens P, Fortmann SP, Friedman L, Havlik R, Thom T. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102(2):3137–3147. doi: 10.1161/01.cir.102.25.3137. 10.1161/01.CIR.102.25.3137. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman H, Derman D. Kit of Factor-Referenced Cognitive Tests. Princeton, NJ: Educational Testing Service; 1976. rev. ed. [Google Scholar]
- Elo IT, Preston SH. Racial and ethnic differences in american mortality at older ages. In: Martin L, Soldo B, editors. Racial and Ethnic Differences in the Health of Older Americans. Washington, DC: National Academy Press; 1997. pp. 10–43. [Google Scholar]
- Escobar JI. Use of the Mini-Mental State Examination (MMSE) in a community population of mixed ethnicity: Cultural and linguistic artifacts. Journal of Nervous and Mental Disease. 1986;174(10):607–614. doi: 10.1097/00005053-198610000-00005. 10.1097/00005053-198610000-00005. [DOI] [PubMed] [Google Scholar]
- Farmer MM, Ferraro KF. Are racial disparities in health conditional on socioeconomic status? Social Science and Medicine. 2005;60(1):191–204. doi: 10.1016/j.socscimed.2004.04.026. 10.1016/j.socscimed.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Fillenbaum GG, Heyman A, Williams K, Prosnitz B, Burchett B. Sensitivity and specificity of standardized screens of cognitive impairment and dementia among elderly Black and White community residents. Journal of Clinical Epidemiology. 1990;43(7):650–660. doi: 10.1016/0895-4356(90)90035-n. 10.1016/0895-4356(90)90035-N. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Rabbitt P, Lunn M, Lindenberger U. Two thirds of the age-based changes in fluid and crystallized intelligence, perceptual speed, and memory in adulthood are shared. Intelligence. 2012;40(3):260–268. 10.1016/j.intell.2012.02.008. [Google Scholar]
- Giambra LM, Arenberg D, Zonderman AB, Kawas C, Costa PT., Jr Adult life span changes in immediate visual memory and verbal intelligence. Psychology and Aging. 1995;10(1):123–139. doi: 10.1037//0882-7974.10.1.123. 10.1037/0882-7974.10.1.123. [DOI] [PubMed] [Google Scholar]
- Gonda J, Schaie KW. Schaie-Thurstone Mental Abilities Test: Word Series Test. Palo Alto, CA: Consulting Psychologists Press; 1985. [Google Scholar]
- Grady CL, Craik FIM. Changes in memory processing with age. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 224–231. [DOI] [PubMed] [Google Scholar]
- Haas SA, Krueger PM, Rohlfsen L. Race/ethnic and nativity disparities in later life physical performance: The role of health and socioeconomic status over the life course. Journals of Gerontology: B Psychological and Social Sciences. 2012;67B:238–248. doi: 10.1093/geronb/gbr155. 10.1093/geronb/gbr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Ryan L, Grant I, Matthews CG. Demographic influences on neuropsychological test performance. In: Grant I, Adams KM, editors. Neuropsychological assessment of neuropsychiatrie disorders. 2. New York: Oxford University Press; 1996. pp. 141–163. [Google Scholar]
- Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychological Methods. 1997;1:64–78. 10.1037/1082-989X.2.1.64. [Google Scholar]
- Hogan H, Robinson G. What the census bureau’s coverage evaluation programs tell us about differential undercount. Proceedings of the 1993 Research Conference on Undercounted Ethnic Populations; May 5–7, 1993.Washington, DC: US Census Bureau; 1993. http://www.census.gov/population/www/documentation/1993/conference.html. [Google Scholar]
- Horn JL, Cattell RB. Age differences in fluid and crystallized intelligence. Acta psychologica. 1967;26:107–129. doi: 10.1016/0001-6918(67)90011-x. [DOI] [PubMed] [Google Scholar]
- Hummer RA. Black–White differences in health and mortality: A review and conceptual model. The Sociological Quarterly. 1996;37(1):105–125. 10.1111/j.1533-8525.1996.tb02333.x. [Google Scholar]
- Inouye SK, Albert MS, Mohs R, Sun K, Berkman LF. Cognitive performance in high-functioning community-dwelling elderly population. Journal of Gerontology: Medical Sciences. 1993;48(4):M146–M151. doi: 10.1093/geronj/48.4.m146. 10.1093/geronj/48.4.M146. [DOI] [PubMed] [Google Scholar]
- Jackson JJ. Minorities and aging. Belmont, CA: Wadsworth; 1980. [Google Scholar]
- Jackson JS, Brown TN, Williams DR, Torres M, Sellers SL, Brown K. Racism and the physical and mental health status of African Americans: A thirteen year national panel study. Ethnicity and Disease. 1996;6:132–147. No doi. [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, Kleinman K. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. org/10.1016/S0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN. Racial bias in the assessment of cognitive functioning of older adults. Aging & Mental Health. 2003;7(2):83–102. doi: 10.1080/1360786031000045872. 10.1080/1360786031000045872. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of Cognitive Function in Late Life in the United States: Demographic and Socioeconomic Predictors. American Journal of Epidemiology. 2009;170(3):331–342. doi: 10.1093/aje/kwp154. 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, McLean JE, Reynolds CR. Sex, race, residence, region and educational differences on the 11 WAIS-R subtests. Journal of Clinical Psychology. 1988;44(2):231–248. doi: 10.1002/1097-4679(198803)44:2<231::aid-jclp2270440224>3.0.co;2-j. 10.1002/1097-4679(198803)44:2<231::AID-JCLP2270440224>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kelley-Moore JA, Ferraro KF. The black/white disability gap: persistent inequality in later life? Journal of Gerontology: Social Sciences. 2004;59(1):S34–S43. doi: 10.1093/geronb/59.1.s34. 10.1093/geronb/59.1.S34. [DOI] [PubMed] [Google Scholar]
- Leveille SG, Guralnik JM, Ferrucci L, Corti MC, Kasper J, Fried LP. Black/White differences in the relationship between MMSE scores and disability: The women’s Health and Aging Study. Journal of Gerontology: Psychological Sciences. 1998;53B:201–208. doi: 10.1093/geronb/53b.3.p201. 10.1093/geronb/53B.3.P201. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: Cross-sectional results from the Berlin Aging Study. Psychology and Aging. 1997;12(3):410–432. doi: 10.1037//0882-7974.12.3.410. 10.1037/0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Singer T, Baltes P. Longitudinal selectivity in aging populations: Separating mortality-associated versus experimental components in the Berlin Aging Study (BASE) Journals of Gerontology, Series B, Psychological Sciences and Social Sciences. 2002;57(6):474–482. doi: 10.1093/geronb/57.6.p474. 10.1093/geronb/57.6.P474. [DOI] [PubMed] [Google Scholar]
- Little TD, Bovaird JA, Widaman KF. On the Merits of Orthogonalizing Powered and Product Terms: Implications for Modeling Interactions Among Latent Variables. Structural Equation Modeling: A Multidisciplinary Journal. 2006;13:497–519. 10.1207/s15328007sem1304_1. [Google Scholar]
- Manly JJ, Jacobs DM, Sano M, Bell K, Merchant CA, Small SA, Stern Y. Cognitive test performance among nondemented elderly African Americans and whites. Neurology. 1998;50(5):1238–1245. doi: 10.1212/wnl.50.5.1238. Retrieved from http://www.neurology.org/content/50/5/1238.short. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and European American elders. Journal of the International Neuropsychological Society. 2002;8(3):341–348. doi: 10.1017/s1355617702813157. 10.1017.S135561770102015X. [DOI] [PubMed] [Google Scholar]
- Manton KG, Stallard E, Wing S. Analyses of black and white differentials in the age trajectory of mortality in two closed cohort studies. Statistics in Medicine. 1991;10:1043–1059. doi: 10.1002/sim.4780100705. 10.1002/sim.4780100705. [DOI] [PubMed] [Google Scholar]
- Markides KS, Mindel CI. Aging and ethnicity. Newbury Park, CA: Sage Publications; 1987. [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental psychology. 2002;38(1):115–142. 10.1037//0012-1649.38.1.115. [PubMed] [Google Scholar]
- Mendes de Leon CF, Barnes LL, Bienias JL, Skarupski KA, Evans DA. Racial disparities in disability: Recent evidence from self-reported and performance-based disability measures in a population-based study of older adults. Journal of Gerontology: Social Sciences. 2005;60(5):S263–S271. doi: 10.1093/geronb/60.5.s263. 10.1093/geronb/60.5.S263. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball K, Sloane ME, Roenker DL, Bruni JR. Visual/cognitive correlates of vehicle accidents in older drivers. Psychology and Aging. 1991;6:403–415. doi: 10.1037//0882-7974.6.3.403. 10.1037/0882-7974.6.3.403. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17(2):299–320. 10.1037/0882-7974.17.2.299. [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Holland F, McInnes L. Practice and Drop-Out Effects During a 17-Year Longitudinal Study of Cognitive Aging. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2004;59B(2):84–97. doi: 10.1093/geronb/59.2.p84. 10.1093/geronb/59.2.P84. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie tramatique. Archives de Psychologie. 1941;28:21. No doi. [Google Scholar]
- Rubin G, Salive M. Vision and Hearing. In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty EM, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. pp. 152–161. vol NIH Pub. No. 95-4009. [Google Scholar]
- Salthouse TA. Influence of Age on Practice Effects in Longitudinal Neurocognitive Change. Neuropsychology. 2010;24(5):563–572. doi: 10.1037/a0019026. 10.1037/a0019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW, Labouvie GV, Barrett TJ. Selective attrition effects in a fourteen-year study of adult intelligence. Journal of Gerontology. 1973;28(3):328–334. doi: 10.1093/geronj/28.3.328. 10.1093/geronj/28.3.328. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Intellectual development in adulthood: The Seattle longitudinal study. New York: Cambridge University Press; 1996. [Google Scholar]
- Schwartz BS, Glass TA, Bolla KI, Stewart WF, Glass G, Rasmussen M, Bandeen-Roche K. Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environmental Health Perspectives. 2004;112(3):314–320. doi: 10.1289/ehp.6727. 10.1289/chp.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford; New York: Oxford University Press; 2003. [Google Scholar]
- Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: Six-year longitudinal findings in the Berlin Aging Study (BASE) Psychology and Aging. 2003;18(2):318–331. doi: 10.1037/0882-7974.18.2.318. 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- Sisco SM, Marsiske M. Neighborhood Influences on Late Life Cognition in the ACTIVE Study. Journal of Aging Research. 2012 doi: 10.1155/2012/435826. 10.1155/2012/435826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder TD. Education characteristics of the population. In: Snyder TD, editor. 120 years of American education: a statistical portrait. Washington, DC: National Center for Education Statistics; 1993. pp. 5–24. [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. Retrieved from http://www.neurology.org/content/56/1/49.full.html. [DOI] [PubMed] [Google Scholar]
- Thurstone LL, Thurstone TG. Examiner manual for the SRA Primary Mental Abilities Test. Chicago: Science Research Associates; 1949. (Form 10–14) [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. Retrieved from http://www.jstor.org/stable/3765916. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Whitfield K. Studying cognition in older African-Americans: some conceptual considerations. Journal of Aging and Ethnicity. 1996;1(1):41–52. No doi. [Google Scholar]
- Whitfield KE, Weidner G, Clark R, Anderson NB. Sociodemographic diversity and behavioral medicine. Journal of Consulting and Clinical Psychology. 2002;70(3):463–481. doi: 10.1037//0022-006x.70.3.463. 10.1037/0022-006X.70.3.463. [DOI] [PubMed] [Google Scholar]
- Williams DR. Race/ethnicity and socioeconomic status: Measurement and methodological issues. International Journal of Health Services. 1996;26(3):483–505. doi: 10.2190/U9QT-7B7Y-HQ15-JT14. [DOI] [PubMed] [Google Scholar]
- Williams DR. Race, socioeconomic status, and health The added effects of racism and discrimination. Annals of New York Academy of Sciences. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- Willis SL, Schaie KW. Practical intelligence in later adulthood. In: Sternberg RJ, Wagner RK, editors. Practical intelligence: Nature and origins of competence in the everyday world. Cambridge, England: Cambridge University Press; 1986. pp. 236–268. [Google Scholar]
- Wilson BA, Cockburn J, Baddeley A. The Rivermead Behavioral Memory Test. Reading, England: Thames Valley Test Co; Gaylord, MI: National Rehabilitation Services; 1985. [Google Scholar]
- Zahodne LB, Glymour M, Sparks C, Bontempo D, Dixon RA, MacDonald SWS, Manly JJ. Education does not slow cognitive decline with aging:12-Year evidence from the Victoria Longitudinal Study. Journal of the International Neuropsychological Society. 2011;17:1039–1046. doi: 10.1017/S1355617711001044. 10.1017/S1355617711001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsembik BA, Peek MK. Race Differences in Cognitive Functioning Among Older Adults. Journal of Gerontology: Social Sciences. 2001;56B(5):S266–S274. doi: 10.1093/geronb/56.5.s266. 10.1093/geronb/56.5.S266. [DOI] [PubMed] [Google Scholar]