Abstract
Kisspeptin (Kiss1) neurons in the rostral periventricular area of the third ventricle (RP3V) provide excitatory drive to gonadotropin-releasing hormone (GnRH) neurons to control fertility. Using whole cell patch clamp recording and single-cell (sc)RT-PCR techniques targeting Kiss1-CreGFP or tyrosine hydroxylase (TH)-EGFP neurons, we characterized the biophysical properties of these neurons and identified the critical intrinsic properties required for burst firing in 17β-estradiol (E2)-treated, ovariectomized female mice. One-fourth of the RP3V Kiss1 neurons exhibited spontaneous burst firing. RP3V Kiss1 neurons expressed a hyperpolarization-activated h-current (Ih) and a T-type calcium current (IT), which supported hyperpolarization-induced rebound burst firing. Under voltage clamp conditions, all Kiss1 neurons expressed a kinetically fast Ih that was augmented 3.4-fold by high (LH surge-producing)-E2 treatment. scPCR analysis of Kiss1 neurons revealed abundant expression of the HCN1 channel transcripts. Kiss1 neurons also expressed a Ni2+- and TTA-P2-sensitive IT that was augmented sixfold with high-E2 treatment. CaV3.1 mRNA was also highly expressed in these cells. Current clamp analysis revealed that rebound burst firing was induced in RP3V Kiss1 neurons in high-E2-treated animals, and the majority of Kiss1 neurons had a hyperpolarization threshold of −84.7 mV, which corresponded to the V½ for IT de-inactivation. Finally, Kiss1 neurons in the RP3V were hyperpolarized by μ- and κ-opioid and GABAB receptor agonists, suggesting that these pathways also contribute to rebound burst firing. Therefore, Kiss1 neurons in the RP3V express the critical channels and receptors that permit E2-dependent rebound burst firing and provide the biophysical substrate that drives the preovulatory surge of GnRH.
Keywords: RP3V, kisspeptin, burst firing, pacemaker current, T-type calcium channel
kisspeptin (kiss1) neurons in the the rostral periventricular area of the third ventricle (RP3V) project to GnRH neurons in the preoptic area (POA) (7) and have been identified as excitatory afferents to the gonadotropin-releasing hormone (GnRH) neurons (26). Kisspeptin is one of the most potent agonists and induces sustained firing in GnRH neurons (18, 39, 56). Although cell-attached recordings of firing rates of Kiss1 neurons in the RP3V have been reported (9, 11), there have been few studies characterizing the biophysical properties and molecular signature of these cells. A recent report documented the expression of an h-current (Ih) in RP3V neurons (40), but the underlying kinetic properties and channels intrinsic to these unique cells have not been characterized.
Single-action potential-generated calcium influx is sufficient to spark the release of classical neurotransmitters; however, burst firing or tetanic stimulation is required for the release of neuropeptides (1, 32, 46). Burst firing in many CNS neurons is generated primarily by the T-type calcium channel current (IT) (e.g., thalamic relay neurons), and the rhythmicity of the burst firing is dependent on the Ih (30, 53). Ih is mediated by the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel family, which includes channel subtypes 1–4. Ih depolarizes neurons from hyperpolarized states, raising the membrane potential into the range of IT activation (12, 13, 23, 30, 55). IT is mediated by the low-threshold voltage-gated calcium channels CaV3.1–3.3 (37). Upon activation, IT initiates a transient Ca2+-driven depolarization above the threshold of action potential initiation (i.e., a low threshold spike) (27, 49). This depolarization then drives the neuron to fire an ensemble (burst) of Na+-driven action potentials. Since burst firing facilitates neuropeptide release and a “preovulatory” release of kisspeptin would, in theory, depend on burst firing, we used in vitro recordings and single-cell (sc)RT-PCR of Kiss1 neurons in slice preparation to determine whether Kiss1 neurons in the RP3V exhibit burst firing and display the channels and currents necessary for this special property.
Critical for reaching a hyperpolarized membrane potential for de-inactivating CaV3 channels and activating HCN channels for initiating burst firing are a class of inwardly rectifying K+ channels that are activated by G protein-coupled receptors known as GIRK channels. Opioid receptors are coupled to GIRK channels, and activation of GIRK channels can facilitate rebound burst firing (4, 50). The vast majority of hypothalamic neurons express opioid receptors, and μ- and δ-opioid receptors are coupled to GIRK channels (21, 25, 28, 51, 57). Thus, we argue that rebound burst firing of Kiss1 neurons in the RP3V is facilitated by opioidergic afferents that operate through one or more of the classical opioid receptors expressed by these Kiss1 neurons. We know that the RP3V also expresses a sexually dimorphic, estrogen-sensitive population of endogenous opioid peptides, enkephalin and dynorphin, that activate primarily μ- and δ-opioid, and κ-opioid receptors, respectively (15, 47, 48). Therefore, we also tested the hypothesis that Kiss1 neurons in the RP3V are the direct targets for regulation by opioidergic afferents. We used whole cell voltage clamp recordings from Kiss1 neurons in slice preparation to assess the effects of opioid agonists on the excitability of Kiss1 neurons in the RP3V. In addition, we used scPCR to verify that the cognate opioid receptor transcripts are expressed in these Kiss1 neurons.
MATERIALS AND METHODS
Animals.
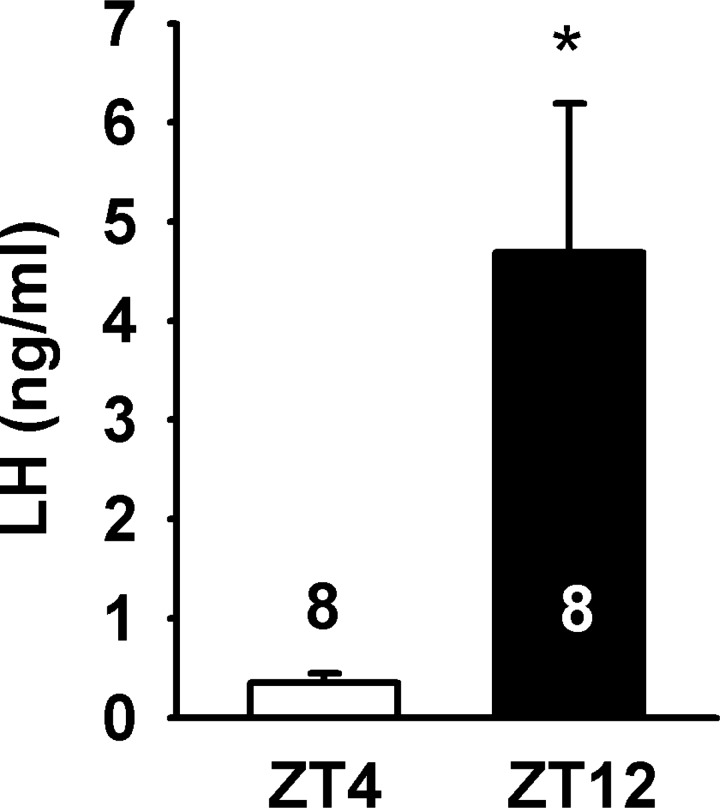
All procedures performed with animals were in accord with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by our local committee on animal care and use. Kiss1-CreGFP (C57BL6/J and S129 background) mice were produced by Dr. Robert Steiner and colleagues at the University of Washington (17), and tyrosine hydroxylase (TH)-EGFP mice (C57BL6/J) were produced by Dr. Kobayashi and colleagues at the Fukushima Medical University in Japan (33, 45). In each case, offspring that carried the transgene were identified by PCR on the genomic DNA extracted from tail biopsies. Transgenic animals were maintained as heterozygous by breeding with WT C57BL6/J mice. Animals were housed under constant temperature and light in a 12:12-h light-dark cycle with lights on at 0600 [zeitgeber time (ZT)0] and lights off at 1800 (ZT12). Food and water was provided ad libitum. Female animals between 6 and 30 wk of age were used for all experiments. Bilateral ovariectomies (OVX) were performed under inhalant isofluorane anesthesia. Carprofen (Rimadyl; Pfizer, NY) was given immediately after surgery at a dose of 4 mg/kg as an analgesic. Following OVX, animals were treated with 17β-estradiol benzoate (E2), demonstrated to induce an LH surge in CBB6 (EGFP-GnRH) mice (3). Five days (D5) after OVX, animals were treated with a subcutaneous injection of 0.25 μg E2 in sesame oil at ZT4 (lights on/off at ZT0/ZT12). On the following day (D6), animals were given a subcutaneous injection of 1.5 −2.0 μg of E2 in sesame oil. On the day of the induced surge (D7), E2-treated animals were euthanized at ZT4 (n = 8). E2-treated animals were also euthanized at ZT12 (n = 8). LH levels were 0.35 ± 0.10 ng/ml in the ZT4 E2-treated females (Fig. 1). In the ZT12 E2-treated animals, LH levels were 4.69 ± 1.5 ng/ml (Fig. 1). The low LH levels in the E2-treated animals at ZT4 vs. ZT12 are consistent with the presence of E2-mediated negative and positive feedback.
Fig. 1.
LH levels in two-dose 17β-estradiol (E2) treatment regimen of ovariectomized (OVX) Kiss1-CreGFP mice. LH (ng/ml) levels from serum of Kiss1-CreGFP mice at ZT4 (E2, n = 8) and ZT12 (n = 8). Lights went off at ZT12. LH levels of E2-treated animals at ZT12 (4.69 ± 1.5 ng/ml) were significantly higher than LH levels in E2-treated animals at ZT4 (0.35 ± 0.10 ng/ml); *P < 0.05 (Student's t-test).
Animals used for cell harvesting and for the majority of the electrophysiology experiments were treated with the surge-inducing dose of E2 as described above. The E2 injections (high E2) resulted in a uterine weight of 109.7 ± 4.1 mg; n = 28 (the mean uterine weight of proestrus animals in our colony is 135.4 ± 7.7 mg; n = 15). An additional group of animals used for electrophysiology was treated as above but received less E2 as a model for the diestrous stage of the estrus cycle. These animals received injections of 0.08 μg of E2 in sesame oil on D5 and D6 following OVX and were used for experiments on D7. The low-E2 injections (low-E2) resulted in a uterine weight of 56.1 ± 7.4 mg; n = 5 (the mean uterine weight of diestrus animals in our colony is 57.0 ± 2.9 mg; n = 24).
In the Kiss1-CreGFP mice, the fluorescent intensity of the POA neurons was dependent on the circulating E2 levels (as measured by the uterine weights). Therefore, low levels of E2 resulted in only faint Kiss1-GFP neurons. Since we were interested in comparing IT and Ih under low (diestrus type) and high (proestrus type) E2 states, and Kiss1 and Th mRNAs are coexpressed in RP3V neurons (8), these experiments were done with both Kiss1-CreGFP and TH-EGFP mice. The fluorescent intensity of TH-EGFP neurons is not dependent on circulating levels of E2.
For whole cell recording of IT and Ih in Kiss1 neurons during low (uterine weight <80 mg) and high (uterine weights >80 mg) E2 states, GFP-expressing cells in the RP3V area were patched, and the electrical activity was recorded for 30–40 min. After whole cell recording, the cell content was harvested for RT-PCR identification of Kiss1 and Th mRNA expressions (see below).
Radioimmunoassay for LH.
Radioimmunoassay (RIA) for mouse LH was performed by the Endocrine Technology and Support Lab at the Oregon National Primate Research Center (Oregon Health and Science University, Beaverton, OR) by using a traditional double-antibody RIA procedure described in Pau et al. (3) and Bosch et al. (36). The detection limit of the assay was 0.2 ng/ml. A mouse serum pool (ET mouse #4) was used in triplicate in each assay as a quality control. The interassay variation (CV) was 14.7% and the intra-assay CV was 3.8%.
Slice preparation.
On the day of experimentation, the animal was euthanized by decapitation. Trunk blood was collected, and the brain was removed from the skull. The brain stem was removed, and the resulting block was mounted on a cutting vibratome and submerged in ice-cold oxygenated (95% O2-5% CO2) high-sucrose artificial cerebral spinal fluid (aCSF) (in mM: 208 sucrose, 2 KCl, 1 MgCl, 1.25 NaH2PO4, 10 HEPES, 26 NaHCO3, 10 dextrose, 2 MgSO4, and 1 CaCl2). Two to three 220-μm slices were cut through the RP3V. Slices were transferred to an auxiliary chamber containing oxygenated aCSF at room temperature (in mM: 124 NaCl, 5 KCl, 1.44 NaH2PO4, 5 HEPES, 10 dextrose, 26 NaHCO3, 2 MgCl2, and 2 CaCl2) and allowed to recover for at least 1 h. The uteri were removed, trimmed of fat, blotted dry, and weighed. Weights were recorded as an indicator of E2 levels.
Cell harvesting and reverse transcription PCR.
Slices were individually visualized under a Leica inverted microscope to confirm GFP fluorescence. Two to three slices containing the RP3V were microdissected under a dissecting microscope. The tissue was digested with protease (from Streptomyces griseus, Sigma) at 37°C for 15 min and then washed three times with low-Ca2+ aCSF (1 mM Ca2+) and two times in aCSF. Using flame-polished glass Pasteur pipettes of decreasing sizes, slices were titurated and plated onto a 60-mm cell culture dish with a glass bottom. The cells were allowed to settle for 12 min until being moved to the harvesting microscope. Oxygenated aCSF at room temperature was constantly perfused into the dish at a rate of 2 ml/min. The cells were allowed to rest after perfusion began for at least 15 min. Fully intact, healthy cells that showed uniform fluorescence and were anchored to the glass plate were harvested. Harvesting was done by using a visualized patch-clamping with a standard glass pipette (1.5 mm OD/0.83 mm ID; World Precision Instruments, Sarasota, FL) that had been pulled to a 10-μm tip. Using the XenoWorks microinjector system (Sutter Instruments, Navato, CA), we applied gentle suction to pull the cell off of the plate. Collected cells were ejected from the pipette into a siliconized 0.5-ml tube containing a solution of 1× Invitrogen Superscript III Buffer, 15 U RNasin (Promega, Madison WI), 10 mM dithiothreitol (DTT), and diethylpyrocarbonate (DEPC)-treated water in a total of 5 μl (single cells) or 8 μl (pools of cells). Cells were harvested individually or as pools of 5 individual cells. Collection tubes were kept in a chilled metal block to avoid freeze/thaw cycling, but each completed pool of cells was frozen as soon as possible on dry ice and stored at −80°C until further processing. Cells were dissociated and harvested as we previously reported (3, 55).
Harvested cells were reverse transcribed according to the manufacturer's instructions (SuperScript III; Invitrogen, Carlsbad CA) and as described previously (3). The final products were stored at −20°C. Cells and controls underwent reverse transcription within 48 h of harvesting. The positive and negative controls included hypothalamic RNA samples that were subjected to reverse transcription with reverse transcriptase added (+RT) or without reverse transcriptase (−RT), respectively. After conversion to cDNA, individual cells and pools were confirmed to exhibit Kiss1 expression by amplifying Kiss1 mRNA using primers on a 1- to 2-μl template for 35 cycles (Table 1). PCR analysis of individual cells was used to determine the expression of Kiss1 mRNA and Th mRNA in GFP-Kiss1 neurons. PCR analysis of cell pools allowed determining a certain level of Kiss1 expression that was deemed consistent in all harvested pools used for further analysis. Pools that showed a faint or no Kiss1 expression were excluded from analysis. Control +RT and −RT tissue mRNAs were also run for Kiss1 expression to confirm there had been no contamination during the RT process. Each pool of cells was then evaluated using quantitative real-time PCR (qPCR).
Table 1.
Primer sequences for PCR
| Gene | Product Length, bp | Primer Sequence | BP No. | Accession No. | Slope | Efficiency, % | r2 |
|---|---|---|---|---|---|---|---|
| HCN1 | 136 | TTGCTGGCGTTATCACCAAG | 1527–1546 | NM_010408 | −3.25 | 100 | 0.96 |
| AGGGAGTAAAGACGACAGTAGG | 1662–1641 | ||||||
| HCN2 | 97 | ATGCTGCAAGACTTCCCCAGCG | 1423–1444 | NM_008226 | −3.27 | 100 | 0.97 |
| TGGCCTTGAAGAGCGCGAAC | 1591–1572 | ||||||
| HCN3 | 118 | TGCGGTGCTTGAGGAGTTCC | 1664–1682 | NM_008227 | −3.18 | 100 | 0.98 |
| ACTCGGCTCAGAGCGTTTC | 1781–1763 | ||||||
| HCN4 | 123 | CACTAAGGGCAACAAAGAGACC | 1929–1948 | NM_001081192 | −3.17 | 100 | 0.95 |
| AGTGAGTAGAGGCGGCAATAAG | 2051–2030 | ||||||
| CaV3.1 | 144 | TACTTCATCGCCCTCATGAC | 2935–2954 | NM_009783 | −3.37 | 98 | 0.95 |
| GTTGACAGGCAGCTGAATAC | 3059–3078 | ||||||
| CaV3.2 | 84 | GCAGCCATCCTCGTCAATAC | 2709–2728 | NM_021415 | −3.18 | 100 | 0.95 |
| GCTTATCTCCAGCGCGTTAG | 2773–2792 | ||||||
| CaV3.3 | 128 | TGGGCATTTTTGGCAAGAA | 965–973 | NM_001044308 | −3.42 | 96 | 0.99 |
| CAGTGCGGATGGCTGACA | 1093–111 | ||||||
| δ-OR | 123 | CCGGTACACCAAATTGAAGAC | 326–347 | NM_013622.3 | −3.22 | 100 | 0.98 |
| AACGGCCACGTTTCCATCAAG | 427–448 | ||||||
| κ-OR | 92 | GAGTGTGGACCGCTACATTGC | 82–101 | NM_011011.1 | −3.22 | 100 | 0.93 |
| TGGTGCCTCCAAGGACTATCG | 154–173 | ||||||
| μ-OR | 102 | CATGGCCCTCTATTCTATCG | 489–509 | NM_001039652.1 | −3.17 | 99 | 0.96 |
| ATGTTGGTGGCAGTCTTC | 572–590 | ||||||
| Kiss1 | 120 | TGCTGCTTCTCCTCTGT | 64–80 | NM_178260 | −3.41 | 97 | 0.99 |
| ACCGCGATTCCTTTTCC | 167–183 | ||||||
| TH | 131 | CAGCCCTACCAAGATCAAAC | 1266–1285 | NM_009377 | −3.10 | 100 | 0.99 |
| GTGTACGGGTCAAACTTCAC | 1377–1396 | ||||||
| β-Actin | 110 | AAGGCCAACCGTGAAAAGAT | 416–435 | NM_007393298 | −3.46 | 95 | 0.99 |
| GTGGTACGACCAGAGGCATA | 505–525 |
The sense (forward) primer is listed first, with the antisense (reverse) primer below. HCN, hyperpolarization-activated cyclic nucleotide-gated channel; CaV, voltage-gated calcium channel; OR, opioid recptor; Kiss1, kisspeptin; TH, tyrosine hydroxylase.
Primer design.
PCR primers were designed from the most recent National Center for Biotechnology Information on DNA and mRNA sequences using Clone Manager (Sci Ed Software, Cary, NC). Primers were designed to produce products between 75 and 200 base pairs (bp) and to be as efficient as possible. To distinguish cDNA from genomic DNA products, all primers crossed an intron/exon boundary. Only primers that demonstrated a single-peak melting curve and had efficiency between 95 and 100% were used (Table 1).
qPCR.
Quantitative analysis by PCR was performed using the Power Sybr Green Mastermix method as described previously (55) on an Applied Biosystems 7500 Fast real-time PCR system (Life Technologies, Carlsbad, CA). Primer efficiencies and data were analyzed as described previously (3, 55). All efficiencies are listed in Table 1. cDNA samples from Kiss1 neurons collected from the RP3V were run in duplicates, using 3.5–4 μl of cDNA per duplicate. Melting curves were analyzed for each reaction, and all genes were normalized to β-actin as a reference. One gene from each family (i.e., HCN1, CaV3.1, μ-opioid receptor) was then used as a “calibrator” to compare levels of mRNA among genes. Data were analyzed following the comparative ΔΔCT method described previously (38, 55) and are reported as relative amounts of mRNA expression to the calibrator. Data are expressed as means ± SE, and differences in gene expression among families of genes were analyzed by a one-way ANOVA (e.g., HCN, CaV3, opioid receptors).
Electrophysiological recording and cell harvesting.
Whole cell patch clamp recordings were conducted for GFP-tagged Kiss1 or TH neurons with the use of an Olympus BX51 (Olympus, Center Valley, PA) or a Zeiss Axioskop FS upright microscope (Carl Zeiss Microscopy, Thornwood, NY) equipped with fluorescence and infrared differential interference contrast (IR-DIC) imaging devices (17, 42, 54, 56). Patch pipettes (1.5-mm OD borosilicate glass; A-M Systems, Seattle, WA) were pulled on a Flaming/Brown puller (model P-97; Sutter Instrument, Novato, CA) and usually filled with the following internal solution (in mM): 128 potassium gluconate, 10 NaCl, 1 MgCl2, 11 EGTA, 10 HEPES, 4 ATP, and 0.25 GTP; adjusted to pH 7.3 with KOH; 290 mOsm. Pipettes filled with the above internal solution had a resistance of 2–3 MΩ. For loose-patch cell recordings, the patch pipettes were filled with aCSF, and a loose seal (20–60 MΩ seal resistance) was formed on the Kiss1 neurons to measure spontaneous action currents as previously described (17). For the whole cell configuration, access resistance was less than 25 MΩ and was 80% compensated when the recorded current was larger than 100 pA. Steady-state current/voltage (I/V) plots were constructed with step command potentials from −120 to −40 mV at an increment of 5 or 10 mV (with holding potential at −60 mV) and a duration of 0.5 or 1 s. Membrane input resistances of cells were calculated by measuring the slope of the I/V relationship curve between −70 and −50 mV. Postinhibitory rebound that caused low-threshold burst firing was examined at resting membrane potentials after a series of hyperpolarization of 1 s duration from −70 mV to −120 mV. The liquid junction potential of −10 mV was corrected for in all analyses. Consecutive current traces were filtered at 2–10 kHz and acquired at a sampling rate of 0.5–5k Hz. Standard whole cell patch clamp recording procedures and pharmacological testing were conducted as described previously (17, 42, 54, 56). Electrophysiological signals were amplified with an Axopatch 200B amplifier and digitized with a Digidata 1440A (Molecular Devices, Foster City, CA) or with an Axopatch 1D amplifier and digitized with a Digidata 1322 (Axon Instruments, Foster City, CA). Data were analyzed with Clampfit software (v. 9.0 or 10.0, Molecular Devices).
After recording, the cell was harvested by applying negative pressure until visualizing the cell content being gently aspirated into the tip of the recording pipette. The cell content was expelled into a 500-μl harvesting tube containing 5 μl of RT solution and stored at −80°C until further processing. All recorded cells that were harvested were reverse transcribed as described above. cDNA was analyzed for Kiss1 and Th mRNAs by using a 4-μl template in a 30-μl PCR reaction as described above. Cells that were negative to both Th and Kiss1 mRNA were further subjected to the detection of β-actin mRNA used as a positive control.
Drugs or chemicals.
TTA-P2 ({3,5-dichloro-N-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4-fluoropiperidin-4-ylmethyl]-benzamide}, a gift from Merck) was made up as a 10 mM stock solution in DMSO. ZD-7288 was purchased from Tocris Bioscience (Minneapolis, MN) and was made up as a stock of 100 mM in DMSO. TTX was purchased from Alomone Laboratories (Jerusalem, Israel) and was made up as 1 mM in stock in Milli-Q water.
Data analysis.
The firing pattern of individual neurons was determined with similar criteria as previously reported for RP3V Kiss neurons (11). Silent neurons had low spontaneous spiking activity (mean firing rate <0.5 Hz); tonic neurons fired regularly with a mean firing rate >4 Hz and a coefficient of variation (defined as SD/mean) of the interspike interval (ISI) <0.25. Burst firing was defined as a cluster of spikes occurring with an ISI of ≤250 ms and terminating with an ISI of ≥500 ms. Bursting neurons had more than 50% of their spikes occurring in bursts with intraburst frequency higher than 4 Hz. All other neurons were classified as irregular (CV greater than 0.25). Rebound burst firing was defined as the hyperpolarization-induced high-frequency (>10 Hz) firing with two or more spikes in a cluster. To be consistent, the maximal rebound burst firing frequency was determined from the first two spikes. Data were analyzed with Mini Analysis and Clampfit 9.2 software. Graphs were plotted using GraphPad Prism 4, Sigma Plot 8.0, and Macromedia Freehand 10 software. Comparisons between different treatments were performed using an unpaired Student's t-test or a one-way or two-way ANOVA with Bonferroni post hoc tests. Differences were considered significant if the probability of error was <5%. All data are presented as means ± SE.
RESULTS
Kiss1-GFP neurons in the RP3V area express Kiss1 mRNA.
Kiss1-CreGFP mice were used to target fluorescent Kiss1 neurons in the RP3V. Kiss1-GFP neurons in the RP3V area were dispersed, harvested individually, and subjected to RT-PCR to confirm Kiss1 mRNA expression. The analysis of 139 cells from five OVX E2-treated (surge model) females revealed that 94% expressed Kiss1 mRNA (Fig. 2A). Kiss1-positive cells were also tested for coexpression with Th mRNA, and 88% expressed Th mRNA in addition to Kiss1 mRNA. Adjacent nonfluorescent cells (n = 10) were negative for Kiss1 or Th mRNA. As reported previously (17), OVX oil-treated control animals had little or no GFP-labeled cells in the RP3V area. Therefore, only E2-treated (low and high dose) animals were used for cell harvesting and electrophysiological recordings.
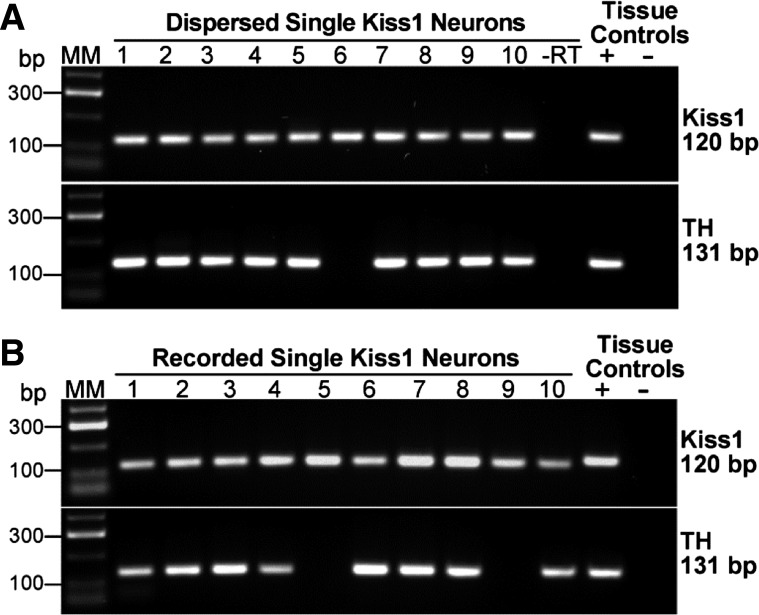
Fig. 2.
Single-cell RT-PCR identification of Kiss1 mRNA and Th mRNA. A: expression of kisspeptin (Kiss1) and tyrosine hydroxylase (Th) mRNA in Kiss1-CreGFP neurons in the periventricular POA. Representative gels illustrating coexpression of Kiss1 mRNA and Th mRNA in dispersed and harvested Kiss1-GFP neurons from high-E2 (surge-level)-treated animals. Expected sizes for the PCR products are 120 bp for Kiss1 and 131 bp for Th. As a negative control, cells reacted without reverse transcriptase (−RT) did not express any of the transcripts (a total of 6 cells were reacted −RT). POA tissue RNA was also included as a positive control (+RT) and negative control (−RT). MM, molecular markers. Kiss1 mRNA was expressed in 94% of Kiss1-GFP harvested neurons (n = 139); 88% of Kiss1-positive cells coexpressed Th mRNA. B: expression of Kiss1 and Th mRNA in Kiss1-CreGFP neurons harvested in the POA slice. Representative gels illustrating coexpression of Kiss1 and Th mRNA in Kiss1-GFP neurons harvested in the slice following whole cell recording for 30–40 min. Expected sizes for PCR products are 120 bp for Kiss1 and 131 bp for Th. POA tissue RNA was included as a positive control (+RT) and negative control (−RT). Kiss1 mRNA was expressed in 100% of Kiss1-GFP neurons (n = 27) that were located in the periventricular POA of high-E2-treated mice. Th mRNA was expressed in 75% of these neurons.
Identification of Kiss1 and Kiss1/TH dual phenotype in the slice.
To identify the Kiss1 and Th transcript from recorded Kiss1-GFP and TH-EGFP neurons, cells were harvested after recording for RT-PCR analysis. A total of 63 neurons from 10 Kiss1-GFP mice and 9 TH-EGFP mice were recorded and analyzed. In the high-E2 group (uterine weights >80 mg), we found that 100% (27/27) of fluorescently identified Kiss1-GFP neurons were positive for Kiss1 mRNA. 75% (20/27) of these Kiss1 mRNA-positive neurons (Kiss1-CreGFP mice) coexpressed Th mRNA (Fig. 2B). In addition, 60% (12/20) of the fluorescently identified TH-EGFP neurons were positive for Kiss1 mRNA. There was no difference among Kiss1 neurons that were recorded in the Kiss-GFP mice vs. the TH-EGFP mice in terms of resting membrane potential, cell membrane capacitance, input resistance, spike threshold, and IT and Ih amplitudes (Table 2). These parameters were also similar in Th-positive and Th-negative Kiss1 neurons. Therefore, the data from the Kiss-CreGFP and TH-EGFP mice were combined for further analysis.
Table 2.
Electrotonic properties of RP3V Kiss1+/TH+ neurons in high-E2-treated TH-EGFP and Kiss-Cre GFP mice
| Mice (cell no) | Cm (pF) | Rin (GΩ) | IT (pA) | Ih (pA) | AP Threshold (mV) | RMP (mV) |
|---|---|---|---|---|---|---|
| TH (n = 12) | 19.1 ± 2.6 | 2.7 ± 0.5 | 41.7 ± 10.1 | 46.0 ± 12.8 | −44.9 ± 0.7 | −54.6 ± 1.1 |
| Kiss (n = 14) | 18.5 ± 1.1 | 2.3 ± 0.6 | 44.0 ± 4.9 | 38.4 ± 4.9 | −45.1 ± 0.9 | −54.7 ± 0.6 |
Values are means ± SE. IT, T-type calcium channel current; Ih, hyperpolarization-activated h-current.
Spontaneous firing properties of RP3V Kiss1-CreGFP neurons.
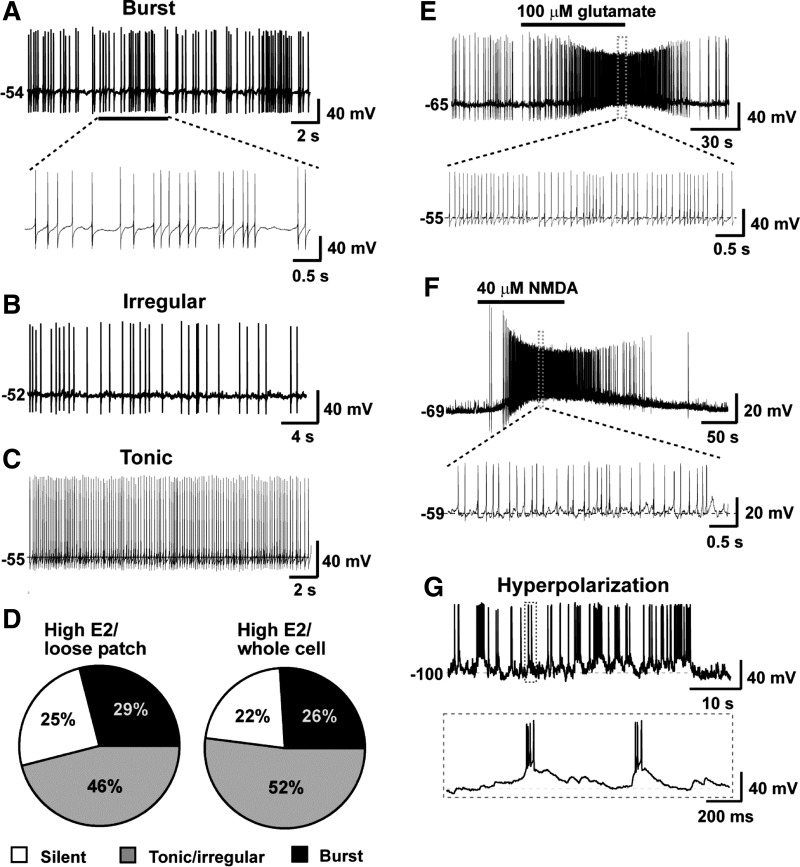
In the next series of experiments, we examined the spontaneous firing characteristics of identified RP3V Kiss1 Cre-GFP neurons from high-E2-treated animals in coronal slices by using whole cell electrophysiological recordings. We also did cell-attached recording for comparison (17). The expression of GFP was used to identify individual Kiss1 neurons. One to two cells were recorded in each coronal slice. Whole cell current clamp recordings revealed that the mean resting membrane potential of Kiss1 neurons in the RP3V was −54.7 ± 0.6 mV (n = 29) from high-E2 animals. The vast majority (78%) of Kiss1 neurons exhibited spontaneous firing at resting membrane potential that could be classified into three types of firing pattern: burst, tonic, and irregular (Fig. 3, A–C). Among the 77 neurons recorded (whole cell) from high-E2-treated animals, ∼52% displayed tonic/irregular firing; 26% exhibited burst firing; and 22% were silent during the recording (Fig. 3E). These firing patterns were consistent with those obtained from loose patch recordings, which showed 46% tonic/irregular firing, 29% burst firing, and 25% silent (n = 63; Fig. 3E). There was no correlation in firing pattern or frequency between cells recorded in the same slice. For example, we found tonic and irregular firing cells, silent and bursting cells, or tonic and bursting cells in the same slice. Moreover, bath-applied glutamate (100 μM) or NMDA (40 μM) depolarized (11.5 ± 4.3 mV in glutamate, 10.8 ± 3.2 mV in NMDA, n = 7) and increased the firing rate of all RP3V Kiss1 neurons examined (Fig. 3, E and F). Consistent with a previous report (9), the percentage of cells exhibiting spontaneous burst firing was low (26–29%), which indicates that synaptic input and activation of endogenous conductances for burst firing were missing. Indeed, when cells were hyperpolarized to −100 mV by constant current injection of −15 pA, rebound burst firing was induced (Fig. 3G). This implies that RP3V Kiss1 neurons may express the intrinsic conductances for postinhibitory rebound burst firing, and synaptic input may be critical for producing a hyperpolarizing stimulus.
Fig. 3.
Properties of spontaneous firing of Kiss1 neurons in the rostral periventricular area of the 3rd ventricle (RP3V) from E2-treated OVX mice. A–C: representative whole cell, current clamp recordings of spontaneous burst firing (A), irregular firing (B), and tonic firing (C) in RP3V Kiss1 neurons. D: summary of percentage of cells showing different firing pattern in whole cell and loose patch recordings. E and F: representative recordings showing glutamate- or NMDA-induced firing in Kiss1 neurons. G: hyperpolarization-induced spontaneous rebound burst firing recorded from a RP3V Kiss1 neuron. The cell was hyperpolarized by constant current injection of −15 pA.
Basic membrane properties and hyperpolarization recruited conductances in RP3V Kiss1 neurons.
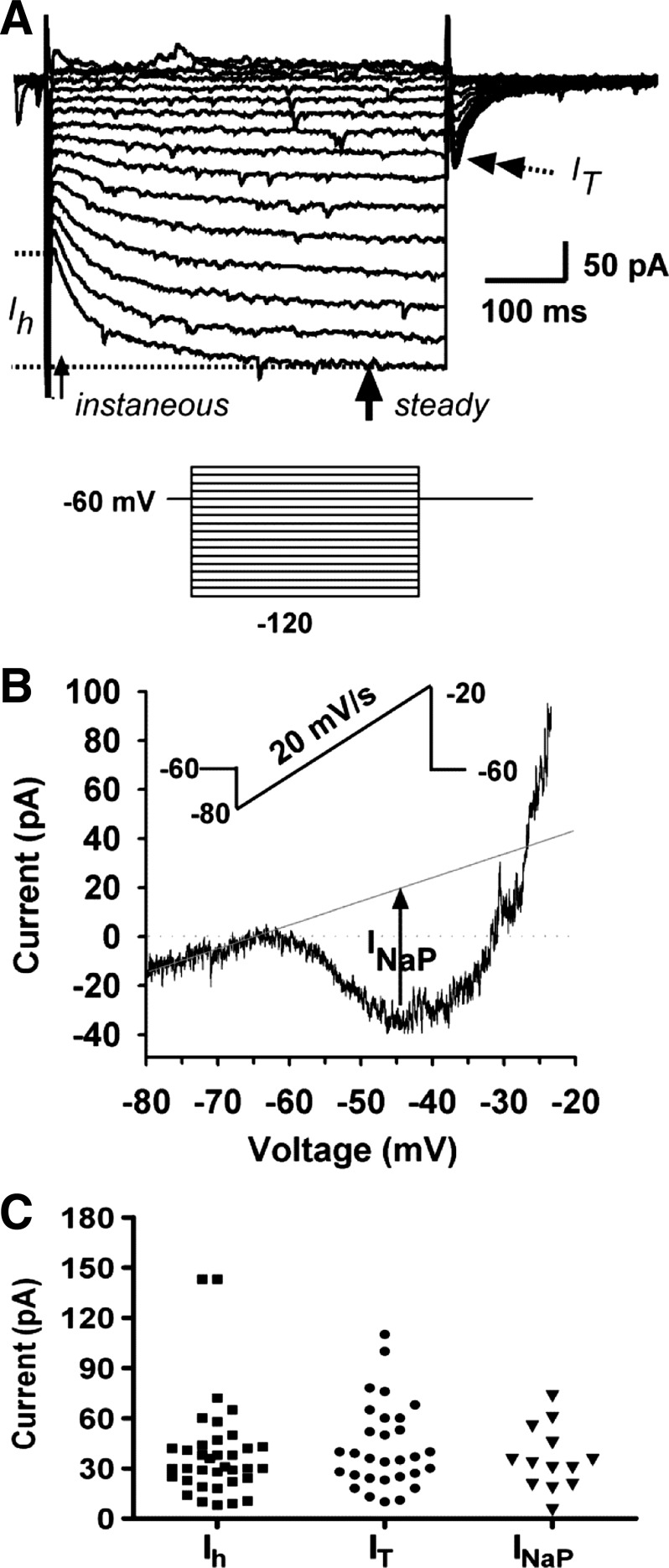
To examine whether RP3V neurons express intrinsic membrane conductances for burst firing, the basic membrane properties and the conductances recruited through hyperpolarization were examined in voltage clamp recordings from high-E2-treated animals. Since RP3V Kiss1 neurons express KATP channels that will affect the resting membrane potential as the cells are dialyzed with a low (2 mM) ATP internal solution (present findings) (14), the biophysical measurements of the Ih and IT were done under 4 mM ATP (internal) concentrations to minimize the effects of Kir6.2 (KATP) channel activity. In voltage clamp, a series of depolarization and hyperpolarization steps were delivered to examine the input resistance and the hyperpolarization-dependent Ih and IT that contribute to postinhibitory rebound burst in many neurons in the CNS (30, 53). Under voltage clamp conditions, both hyperpolarization-activated Ih and IT were evident in RP3V Kiss1 neurons (Fig. 4A). In the high-E2-treated animal, all fluorescently identified RP3V Kiss1 neurons displayed Ih greater than 5 pA at −120 mV (39.4 ± 4.9 pA, n = 36; Fig. 4C and Table 3). Eighty-three percent (30/36) of these neurons expressed IT with a mean amplitude of 42.94 ± 4.6 pA at −60 mV (Fig. 4C and Table 3); 11% (4/36) of them expressed A-type potassium currents (IA) (19.2 ± 4.8 pA, n = 4). Similarly for Kiss1 and TH dual-phenotype (Kiss+/TH+) neurons, all of them expressed an Ih, and 84% of them expressed IT (Table 3). Moreover, all of the Kiss1 neurons expressed a persistent sodium current (INaP) with a mean amplitude of 35.3 ± 5.2 pA (Fig. 4, B and C). The persistent INaP generated a significant inward current that contributed to the rebound burst firing (see discussion). The slope conductance between −70 and −50 mV was significantly increased from 0.74 ± 0.17 pS to 1.5 ± 0.2 pS by TTX (500 nM), indicative that TTX-sensitive, voltage-gated Na channels are responsible for this robust inward current (43).
Fig. 4.
Kiss1 neurons in the RP3V expressed intrinsic conductances for postinhibitory rebound burst firing. A: representative recording showing a series of hyperpolarizing/depolarizing pulses induced current (inset: voltage protocol: holding potential = −60 mV; steps from −45 to −120 mV, duration 500 ms). Single-head arrows indicate where instantaneous and steady-state hyperpolarization-activated h current (Ih) were measured. Steady-state current (Isteady state) was taken as an average from the arrow to the end of the pulse. Double-head arrow indicates peak T-type calcium current (IT), which was activated when the voltage was stepped back to −60 mV. B: representative recording showing persistent sodium current (INaP) in RP3V Kiss1 neurons. INaP was activated by a slow ramp of voltage (20 mV/s) from −80 to −20 mV (see inset protocol). Measurement of INaP was indicated as the difference between the inward peak at −50 mV and the extrapolated leak current from −80 mV (dashed line). C: scatter plot of amplitude distribution of the IT, Ih, and INaP that were larger than 5 pA in RP3V Kiss1 neurons. (IT: 42.9 ± 4.6 pA, n = 30; Ih: 39.4 ± 4.9 pA, n = 36; INaP: 35.3 ± 5.2 pA, n = 13, respectively). Ih and INaP were expressed in 100% of Kiss1 neurons; IT was expressed in 83% of cells.
Table 3.
Effects of high vs. low dose of E2 on expressions of IT and Ih in RP3V Kiss1 neurons
| Low E2 |
High E2 |
||||
|---|---|---|---|---|---|
| Cell type | Kiss1+/TH+ | Kiss1−/TH+ | Kiss1+/TH+ | Kiss1+/TH− | Kiss1−/TH+ |
| With IT | 3 (33%) | 1 (20%) | 26 (84%) | 4 (80%) | 2 (22%) |
| With Ih | 7 (78%) | 5 (100%) | 31 (100%) | 5 (100%) | 8 (89%) |
| Cell nos. | 10 | 5 | 31 | 5 | 9 |
E2, 17β-estradiol.
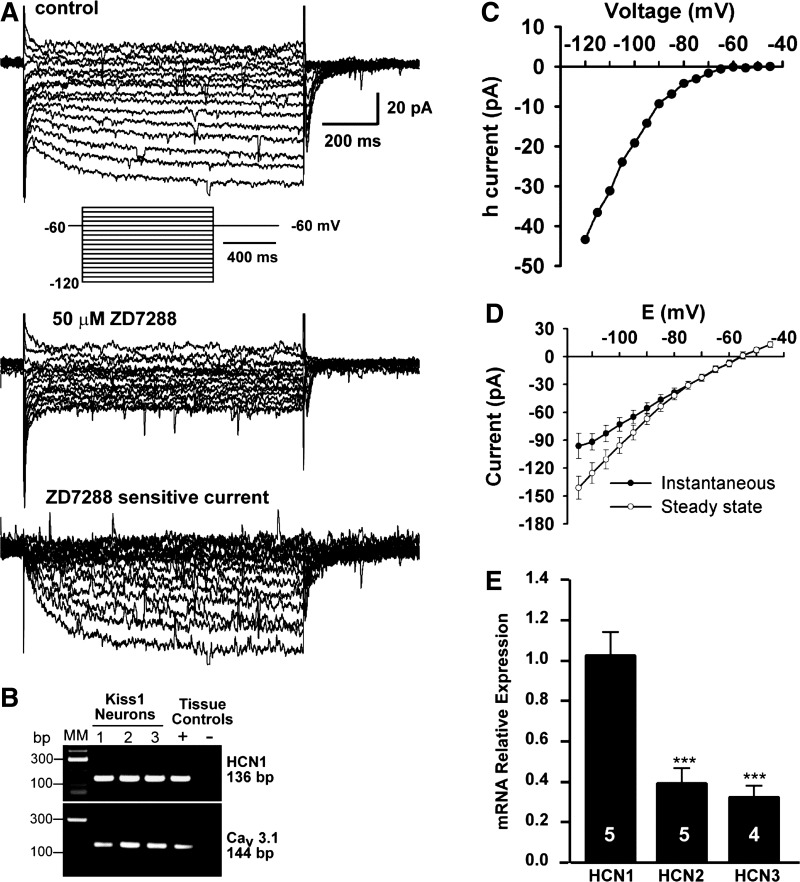
RP3V Kiss1/TH neurons express a rapid Ih that is primarily composed of HCN1.
Whole cell voltage clamp recordings revealed that all of the Kiss1 neurons in the RP3V from high-E2-treated animals expressed Ih (Fig. 5, A–D, and Table 3). Ih was recognized by its characteristic slowly developing inward current following hyperpolarizing voltage steps with the steady-state current reached early during the more hyperpolarized voltage step (Fig. 5A). Single-cell RT-PCR from recorded cells showed that the majority of these cells expressed HCN1 mRNA and CaV3.1 mRNA (Fig. 5B and see below). Ih was completely blocked in Kiss1 neurons by the selective blocker ZD-7288 (50 μM; Fig. 5A). Ih could be evoked in Kiss1 neurons at voltages up to −75 mV (Fig. 5, C and D). qPCR of Kiss1 neurons harvested from the RP3V demonstrated that these neurons express HCN1, -2, and -3 (Fig. 5E); HCN4 was undetectable (data not shown). The expression of HCN2 and HCN3 mRNAs was ∼30% of the HCN1 level. The Ih in Kiss1 neurons displayed fast activation kinetics with a τ = 84 ± 10 ms at −120 mV (Fig. 5A), consistent with the relatively high expression level of HCN1 mRNA (Fig. 5, B and E).
Fig. 5.
RP3V Kiss1 neurons express ZD-7288-sensitive Ih and mRNA for HCN channels. A: representative voltage-clamp recording of Ih with a hyperpolarizing voltage step protocol. Top: characteristic Ih are visible as slowly activated inward currents at hyperpolarized membrane potentials. Inset: voltage clamp protocol: Vhold = −60 mV; range from −40 to −120 mV, step size 5 mV, step duration 1 s. Middle: Ih is blocked by 50 μM ZD-7288, evidenced by lack of slowly developed inward current. Bottom: ZD-7288-sensitive current, which represents the isolated Ih. B: film image of representative Kiss1 neurons that expressed HCN1 and Cav3.1 transcripts following whole cell recording for 30–40 min. C: I/V relationship of Ih from A, showing voltage-dependent activation and inward rectification at increasingly hyperpolarized voltages. D: mean I/V relationship of 16 Kiss1 neurons showing instantaneous (filled circles) and steady-state (open circles) whole cell currents. Vhold = −60 mV. Protocol is indicated in Fig. 4A. The difference between instantaneous and steady-state I/V was due to activation of Ih. E: relative levels of mRNA for HCN1, -2, and -3 in pools of Kiss1 neurons within the RP3V (n = 4–5 animals, 3 pools/animal). Levels of HCN2 and HCN3 mRNA were significantly lower than HCN1 mRNA. Relative expression was calculated by ΔΔCT method and normalized to the mean ΔCT of HCN1. ***P < 0.001 for HCN2 vs. HCN1 and HCN3 vs. HCN1 (ANOVA).
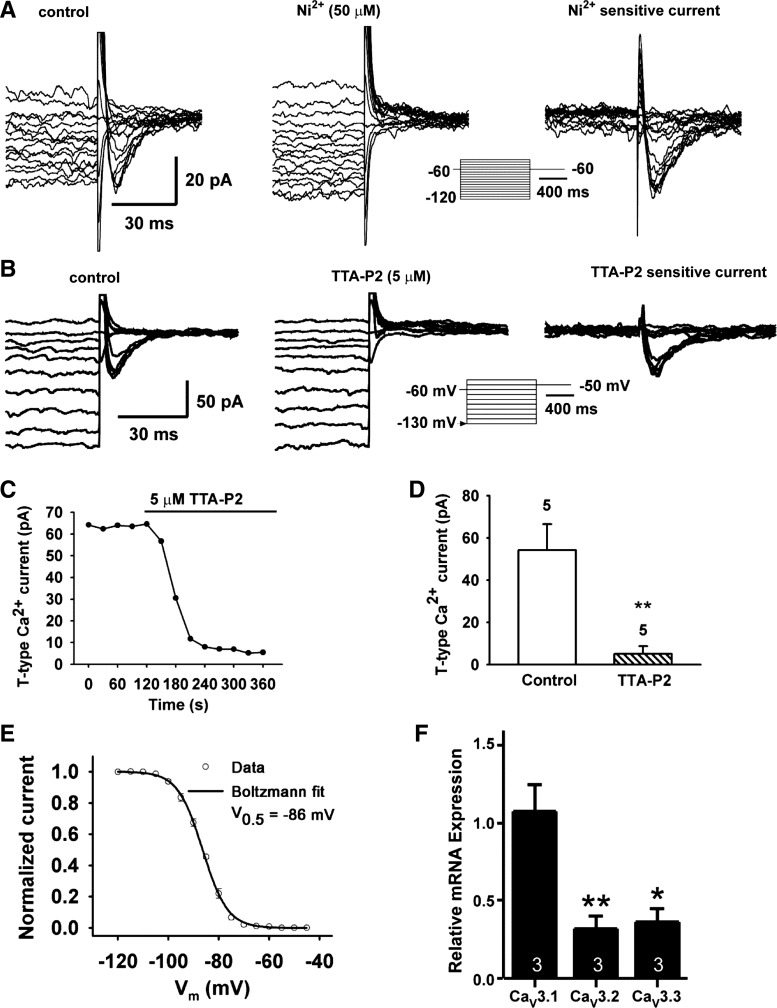
RP3V Kiss1/TH neurons express IT that is primarily composed of CaV3.1.
Whole cell voltage clamp recordings revealed that 80% of RP3V Kiss1 neurons from the high-E2-treated animals displayed an IT larger than 5 pA (fully recovered at −120 mV and activated at −60 mV; Figs. 4 and 6, A and B). All of these neurons also expressed Ih. The V1/2 for the recovery, as determined by fitting the data to a Boltzmann equation, was −86.4 ± 0.2 mV (Fig. 5E), which was similar to the V1/2 in GnRH neurons (55). Furthermore, the IT in these Kiss1 neurons was rapidly inactivated (at −60 mV, τ-fast = 19.4 ± 3.3 ms, τ-slow = 86.3 ± 29.5 ms, n = 8). IT in these Kiss1 neurons was blocked by 50 μM Ni2+ (Fig. 6A) and also by the selective IT blocker TTA-P2 (10). As shown in Fig. 6B, perfusing TTA-P2 (5 μM) blocked the IT within 3 min of bath application by 90.9 ± 5.3% (Fig. 6, C and D). Measurements by qPCR analysis of RP3V Kiss1 neuronal pools revealed that these neurons express primarily CaV3.1 mRNA and to a lesser extent CaV3.2 and CaV3.3 transcripts (Fig. 6F). CaV3.2 and CaV3.3 were significantly less abundant than CaV3.1 transcripts (P < 0.01 and P < 0.05, respectively). These data indicate that Kiss1 neurons in the RP3V preferentially express CaV3.1 channels, which is the T-type Ca2+ channel with the fastest kinetics. Despite lower expression, CaV3.2 and CaV3.3 were detectable in the pooled neurons, indicating that they may also contribute to IT in Kiss1 neurons in this region.
Fig. 6.
RP3V Kiss1 neurons express nickel- and TTA-P2-sensitive IT and mRNA for CaV3 channels. A: representative traces showing I-V relationship of IT before (left, control) and after exposure to 50 μM Ni2+ for 10 min (middle, Ni2+), and the subtracted Ni2+-sensitive IT (right) recorded from a RP3V Kiss1 neuron. Traces were truncated to highlight inward IT. The insert panel shows the voltage-clamp protocol used to measure IT. The pulse protocol consisted of 5-mV steps, from −40 to −120 mV, before returning to −60 mV. Pulses were of 1-s duration to ensure complete de-inactivation of the T-channel. B: representative traces showing I-V relationship of IT before (left, control) and after exposure to 5 μM TTA-P2 for 10 min (middle) and the subtracted, TTA-P2-sensitive IT (right) recorded from a RP3V Kiss1 neuron. Traces were truncated to highlight the inward IT following de-inactivation steps. Bottom: voltage-clamp protocol used to measure IT in Kiss1 neurons. C: analysis of time course of TTA-P2 blockade of IT in a Kiss1 neuron. D: summary of effects of TTA-P2 on maximum peak amplitude of IT at −50 mV. **P < 0.01 vs. control, with Student's unpaired t-test; n = 5 cells. Mean inhibition of IT by 5 μM TTA-P2 was 90.9 ± 5.3%. E: Boltzmann equation fit of the voltage-dependent de-inactivation of the T-channel. Half-maximum de-inactivation voltage is indicated: V1/2 = −86.4 ± 0.2 mV (n = 5, r2 = 0.999). F: levels of mRNA for Cav3.1, −3.2, and −3.3 derived from qPCR (n = 3 animals; 3 pools/animal). Relative expression was calculated by ΔΔCT method and normalized to mean ΔCT of CaV3.1. *P < 0.05 for CaV3.3 vs. CaV3.1; **P < 0.01, CaV3.2 vs. CaV3.1 (ANOVA).
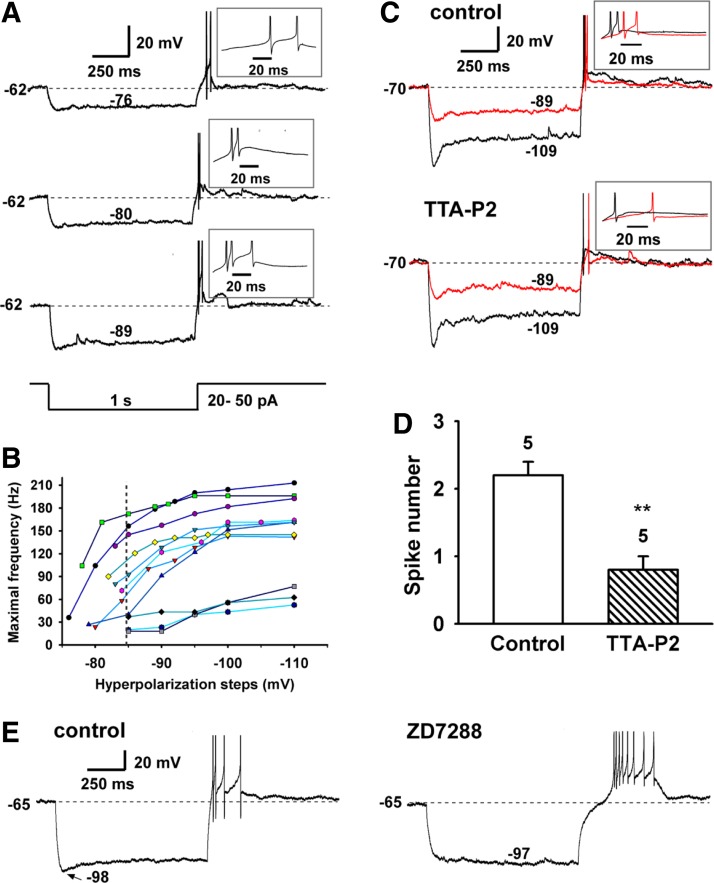
Hyperpolarization-induced rebound burst firing in RP3V Kiss1 neurons is dependent on expression of IT.
Since T-channels in RP3V Kiss1 neurons were recruited by hyperpolarization, we examined the ability of these neurons to generate rebound burst firing through current injection-induced hyperpolarization. As an example, the Kiss1 neuron in Fig. 7A had a resting membrane potential of −62 mV and fired irregularly at a mean frequency of 0.8 Hz. This cell had a T-current amplitude of 52 pA. The hyperpolarization threshold, defined as the minimum hyperpolarization (or current injection) required to induce rebound burst firing, was −76 mV (or −20 pA), and its corresponding burst firing rate determined by the first interspike interval was 36 Hz. Greater hyperpolarization to −80, −85, and −89 mV or current injection of −30, −40, and −50 pA induced higher firing frequencies of 104, 156, and 172 Hz, respectively (Fig. 7B). The burst firing frequency increased nonlinearly, with the largest changes near the hyperpolarization threshold (Fig. 7B). At resting membrane potential, we found that 82% of neurons had a hyperpolarization threshold of −84.7 ± 1.2 mV (n = 18), which, as predicted, is close to the V1/2 for de-inactivation of the T-channel. Next we examined the contribution of IT and Ih to the rebound burst firing. Rebound burst firing was clearly blocked by the selective Cav3 channel blocker TTA-P2 (Fig. 7, C and D), whereas rebound burst firing was not blocked by the HCN channel blocker ZD-7288 (Fig. 7E). However, blocking HCN channels did increase the delay for the initiation of first spike (Fig. 7E). Therefore the T-channel and h-channel play different roles in the generation of rebound burst firing.
Fig. 7.
Hyperpolarization-induced rebound burst firing in RP3V Kiss1 neurons. A: hyperpolarization-induced rebound burst firing from a Kiss1+/TH+ cell that expressed IT of 52 pA (activated at −60 mV) and had input resistances of 0.53 and 2.0 GΩ between −80 and −60 mV and between −70 and −50 mV, respectively. B: analysis of the effect of hyperpolarizing steps on maximal burst frequency in 11 cells. Dashed line, mean hyperpolarization threshold for burst firing of 82% of neurons. C: example recording showing that T-channel blocker TTA-P2 (5 μM) blocked the rebound burst firing. This cell had IT of 67 pA and Ih of 46 pA. Current injections of −30 and −90 pA yielded hyperpolarizations of −89 and −109 mV, respectively. D: summary of effects of TTA-P2 on spike number of rebound burst firing. **P < 0.01, paired Student's t-test. E: example recording showing that h-channel blocker ZD-7288 (50 μM) did not block the rebound burst firing but increased the delay to the first spike. This cell had IT of 30 pA and Ih of 38 pA. Current injection of −60 pA hyperpolarized the cell membrane to −98 mV.
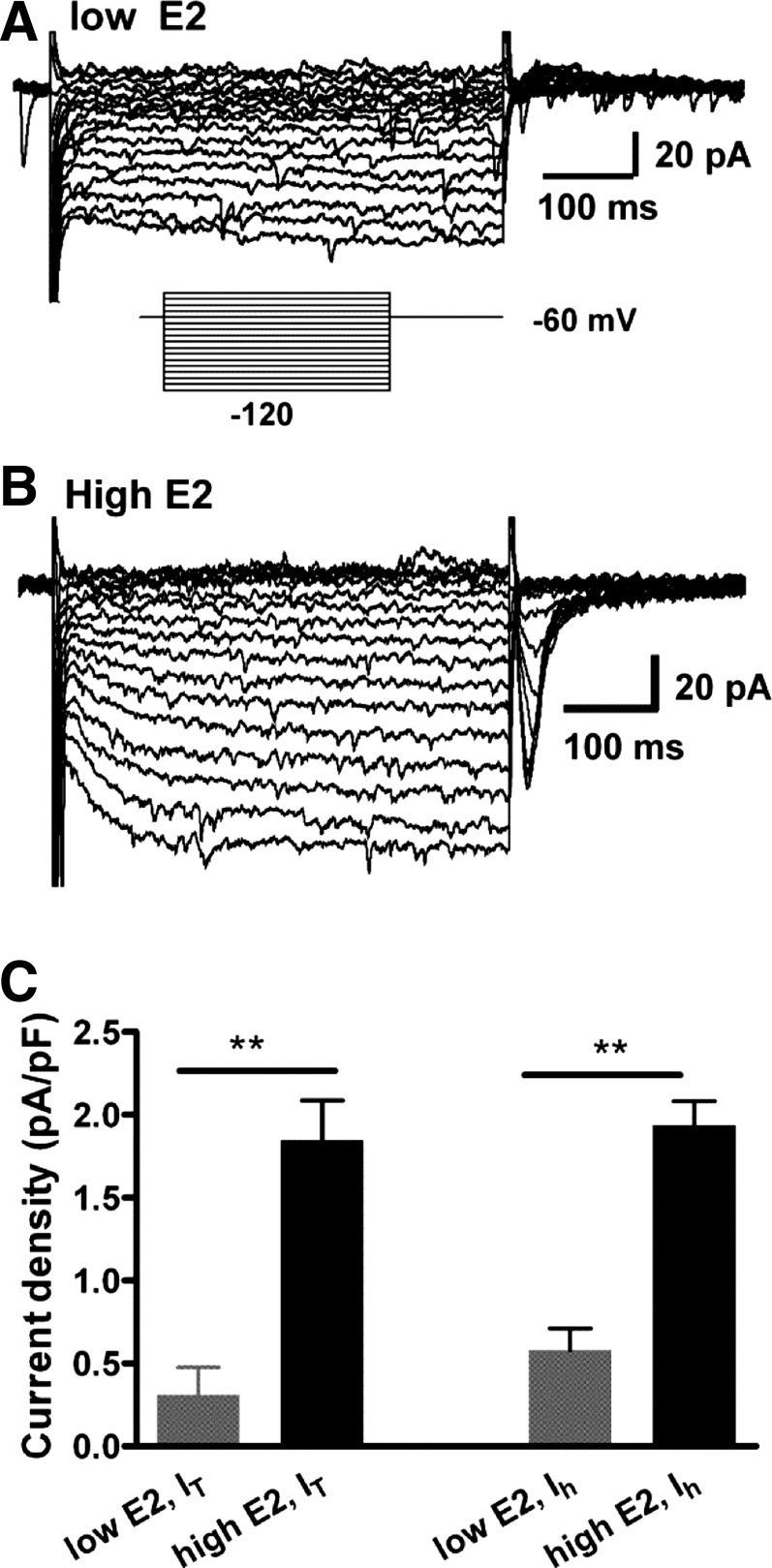
Expressions of IT and Ih are dependent on circulating levels of E2.
Our previous studies showed that the expression of T-type calcium channels and HCN channels in GnRH neurons are regulated by estrogens (2, 3, 55). Therefore, we examined the effects of low-E2 vs. high-E2 treatment on the expression of IT and Ih in RP3V Kiss1 neurons. To identify the Kiss1 neurons from low-E2-treated OVX females, both TH-EGFP and Kiss-GFP mice were used. For recordings from TH-EGFP mice, cells were targeted by EGFP fluorescence; for recordings from Kiss-GFP mice, cells were blindly targeted. All recorded neurons were harvested for RT-PCR identification of Kiss1 transcripts. A total of 18 neurons were recorded from the low-E2 group, and 10 of them were identified as Kiss1 mRNA positive. We found that, in the low-E2-group, few Kiss1+/TH+ neurons (3 of 10) expressed an IT (Table 3). The mean amplitude of the IT for all Kiss1-positive cells was 4.8 ± 2.4 pA (n = 10). The majority of Kiss1 neurons in the low-E2 group expressed an Ih (7 of 10) with a mean amplitude of 10.8 ± 2.7 pA (n = 10). In the high-E2 group, the vast majority of Kiss1-positive neurons (83%; total n = 36) expressed an IT (Table 3) with a mean amplitude of 34.8 ± 4.3 pA (n = 36) and an Ih of mean amplitude of 39.1 ± 4.4. pA (n = 36). We found that there was no difference between the low-E2 Kiss1 neurons and the high-E2 Kiss1 neurons in the resting membrane potential (−54.1.5 ± 1.5 mV, n = 9 in low-E2 vs. −54.7 ± 0.9 mV, n = 36 in high-E2) or the cell membrane capacitance (18.6 ± 1.5 pF, n = 10 in low-E2 vs. 18.4 ± 1.0 pF, n = 36 in high-E2). The IT density in the high-E2 group was sixfold higher than in the low-E2 group (1.85 pA ± 0.24 pA/pF vs. 0.31 ± 0.6 pA/pF, P < 0.01), and the Ih density in the high-E2 group was 3.4-fold higher than in the low-E2 group (1.94 pA ± 0.15 pA/pF vs. 0.58 ± 0.13 pA/pF, P < 0.01; Fig. 8). In contrast, only two of nine Kiss1−/TH+ neurons in the high-E2 group expressed an IT with an overall mean amplitude of 5.8 ± 5.8 pA (n = 9), whereas 89% (n = 8) of them expressed an Ih (Table 3) with mean amplitude of 20.8 ± 6.8 pA. Therefore, the IT is highly expressed in RP3V Kiss1 neurons and is exquisitely sensitive to E2, whereas the expression of the Ih is less sensitive to E2.
Fig. 8.
Expression of IT and Ih is estrogen state dependent in RP3V Kiss1 neurons. A and B: 2 representative recordings showing that IT and Ih greatly increased in high-E2-treated vs. low-E2-treated mice. C: summary of current density of IT and Ih in RP3V Kiss1 neurons from low-E2 and high-E2 animals. All cells were included for statistical analysis (n = 10 for low-E2 group, 5 animals; n = 36 for high-E2 group, 16 animals). **P < 0.01, unpaired Student's t-test.
Opioid peptides and GABAB agonist hyperpolarize RP3V Kiss1 neurons.
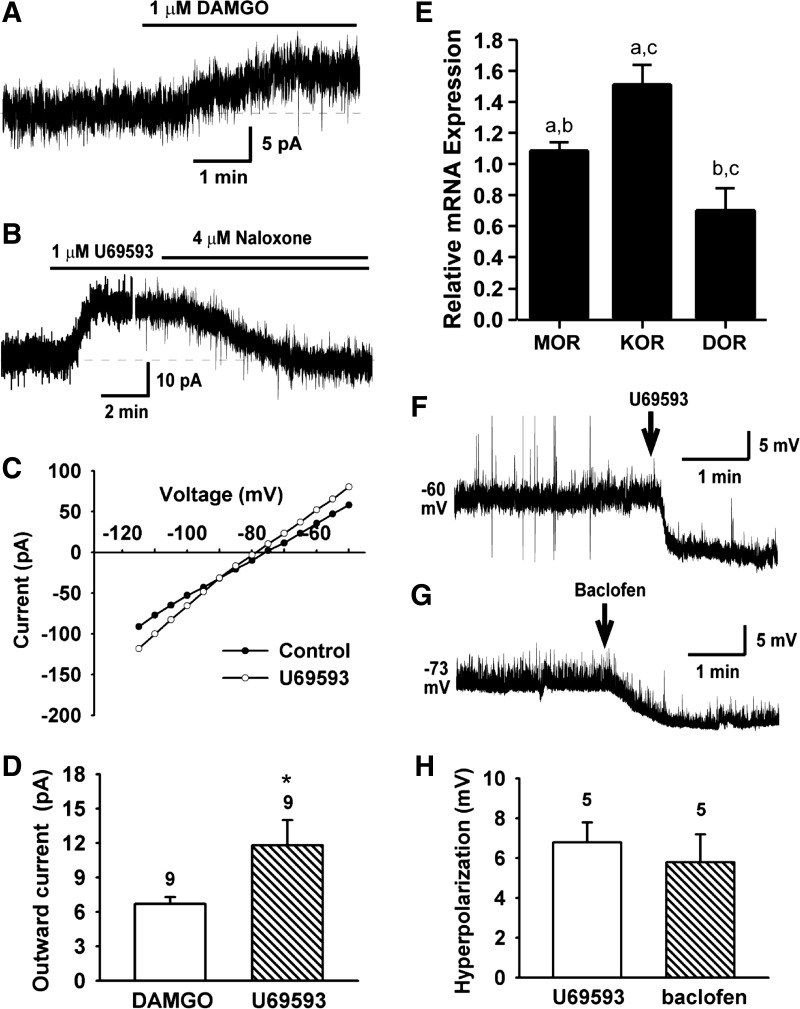
Clearly, the rebound burst firing is dependent on the hyperpolarization threshold. To search for possible metabotropic inhibitory inputs onto RP3V Kiss1 neurons, we examined responses of Kiss1 neurons to the μ- and κ-opioid receptor agonists by measuring the agonist-activated outward K+ currents at −60 mV using whole cell voltage clamp recordings (Fig. 9). In Kiss1 neurons in the RP3V (high-E2 group), the μ-opioid receptor agonist DAMGO (1 μM) induced a small outward current (Fig. 9A). The outward current reversed at EK+ and exhibited inward rectification (data not shown). Of the cells tested, 75% (9 of 12) responded to DAMGO with a mean evoked current of 6.7 ± 0.6 pA (Fig. 9D; n = 9). On the other hand, the κ-opioid receptor agonist U-69593 (1 μM) induced an outward current of 11.8 ± 2.2 pA (Fig. 9, B–D; n = 9) that exhibited inward rectification (Fig. 9C), indicative of the opening of GIRK channels. The effects were blocked by the nonselective opioid receptor antagonist naloxone (Fig. 9B). We also examined the κ-opioid receptor agonist U-69593 (1 μM) and GABAB receptor agonist baclofen (20 μM)-induced hyperpolarization. As shown in Fig. 9, F–H, U-69593 induced a hyperpolarization of 6.8 ± 1.0 mV (n = 5), and baclofen induced a similar hyperpolarization (5.8 ± 1.4 mV, n = 5). Therefore, RP3V Kiss1 neurons, like the vast majority of hypothalamic neurons (21, 23, 29), respond to opioid and GABAB agonists. Consistent with these electrophysiological observations, the qPCR analysis from pooled Kiss1 neurons confirmed that expression of the κ-opioid receptor mRNA was significantly greater than either the μ- or δ-receptor mRNA (Fig. 9E).
Fig. 9.
Effects of μ- and κ-opioid receptor and GABAB receptor agonists on Kiss1 neurons in the RP3V. A: in voltage-clamp and in the presence of TTX (0.5 μM), the selective μ-opioid receptor agonist DAMGO (1 μM) induced an outward current. Vhold = −60 mV. B and C: selective κ-opioid receptor agonist U-69593 (1 μM) induced an outward current (B), which reversed near −90 mV (C), indicating activation of a K+ current. Effects of U-69593 were antagonized by naloxone Vhold = −60mV. D: summary of outward K+ current amplitude induced by DAMGO and U-69593 at −60 mV (n = 9, *P < 0.05). E: relative expressions of μ-, κ-, and δ-opioid receptor mRNAs based on qPCR (n = 4–5 animals, 3 pools/animal). Relative expression was calculated by ΔΔCT method and normalized to mean ΔCT of μ-opioid receptors; a-a, b-b, P < 0.05; c-c, P < 0.001 (one-way ANOVA). F and G: selective κ-opioid receptor agonist U-69593 (1 μM)-induced and GABAB receptor agonist baclofen (20 μM)-induced hyperpolarization of RP3V Kiss1 neurons. H: summary results of U-69593- and baclofen-induced hyperpolarization.
DISCUSSION
Here, we profile the endogenous electrophysiological properties of Kiss1 neurons in the RP3V of the female mouse. We show that the T-type calcium current is crucial for rebound burst firing and that this phenomenon is estrogen dependent. A significant fraction of these neurons exhibit spontaneous burst firing, and the vast majority of these cells express the pacemaker currents Ih and IT. The Ih displayed rapid kinetics, consistent with the abundant expression of HCN1 channels, which was amplified by E2. The predominance of the T-type Ca2+ channel CaV3.1 corroborated the presence of a rapidly inactivating IT in these cells, which was increased manyfold by E2 treatment. The presence of a robust T-current is essential for the high-frequency rebound bursting that is manifested following a hyperpolarizing stimulus. Furthermore, both κ- and μ-opioid receptor agonists hyperpolarized Kiss1 neurons, which suggests that opioid synaptic input provides a portion of the stimulus required for reaching the hyperpolarization threshold necessary for recruitment of CaV3.1 channels. The Kiss1 neurons were also hyperpolarized by the GABAB agonist baclofen, which would further contribute to reaching the hyperpolarization threshold. Notably, the majority of the Kiss1 neurons in the RP3V expressed Th mRNA, which is consonant with the observation that these Kiss1 neurons in the RP3V exhibit rebound burst firing reminiscent of dopamine neurons in the arcuate nucleus (29).
Kiss1 neurons in the RP3V are similar to those in the arcuate nucleus (ARC) but differ in important ways. Kiss1 neurons in the ARC (so-called KNDy neurons) coexpress neurokinin B (NKB) and dynorphin (16, 34). In contrast, Kiss1 neurons in the RP3V express neither NKB nor dynorphin but express one or more classical transmitters including dopamine (8, 20, 34; present findings). We have previously shown that Kiss1 neurons in the ARC of mice and guinea pigs are either silent (∼50%) or show tonic/irregular firing (∼50%) (17, 42), and a report in a cross-bred kisspeptin-IRES-Cre × ROSA26-CAGS-τGFP mouse described similar findings (9). Although Kiss1 neurons in the ARC and RP3V exhibit ionotropic glutamate-dependent burst firing (17; present findings), one-quarter of the Kiss1 neurons in the RP3V exhibit spontaneous burst firing. This suggests that, although Kiss1 neurons in the ARC and RP3V express a common neuropeptide and share the capacity to exhibit burst firing in response to glutamate, Kiss1 neurons in these two regions exhibit different biophysical properties and/or synaptic inputs.
The “pacemaker” current (Ih) is required for rhythmic firing, and virtually all Kiss1 neurons in both the ARC and RP3V express this current (present findings; 17, 40, 42). Ih is a noninactivating, nonselective cation current. This current is activated at hyperpolarized membrane potentials, and it depolarizes the cell into the range required for activation of the low-threshold IT, which is responsible for generating a burst of action potentials. The activation kinetics of Ih are determined by the properties of the underlying channels, and the HCN1 channel exhibits the fastest kinetics (30, 44). The relative abundance of these subunits within a neuronal population can modulate the frequency and pattern of burst firing in these cells. The rapid depolarization mediated by HCN1 channels decreases interburst interval. Therefore, the kinetic properties of HCN1 endow RP3V Kiss1 neurons with the ability to generate rhythmic burst firing. Also contributing to the rhythmicity of burst firing in Kiss1 neurons is the persistent Na+ current, which is active in the range subthreshold to action potential generation (43). A similar persistent Na+ current is recognized to be important for generating a pacemaker potential in GnRH neurons in the telost (35). We found that Ih was regulated by E2 such that the whole cell current increased 3.4-fold with an E2 treatment that produced an LH surge in our mice, corroborating a recent report in cross-bred kisspeptin-IRES-Cre × ROSA26-CAGS-τGFP mice studied throughout the estrous cycle (40). Most likely, this is attributable to an E2-dependent increase in HCN channel expression, as is the case for E2 regulation of such channels in GnRH neurons (3).
Although Ih is responsible for the rhythmicity of burst firing, IT is the most critical for generating the depolarizing stimulus (low threshold spike) that supports an ensemble of action potentials (19, 12, 13). IT is a low-threshold activated Ca2+ current, whose primary physiological function is to generate burst firing (5, 37). Since the T-type Ca2+ (CaV3) channels are activated by low voltage, they open in a voltage range that induces a strong inward Ca2+ current. The primary difference between the various T-type Ca2+ channels is their rate of inactivation, with CaV3.1 exhibiting the fastest and CaV3.3 the slowest kinetics (24). In Kiss1 neurons in the RP3V, we found a rapidly inactivating, Ni2+- and TTA-P2-sensitive T-type current, consistent with the expression of CaV3.1 mRNA (10). The combination of the rapid kinetics of Ih and IT in Kiss1 neurons in the RP3V would support a higher frequency of burst firing. Previous studies have suggested that Kiss1 neurons in the RP3V must fire at a minimum of 5 Hz (spikes/s) to produce a sustained release of kisspeptin onto GnRH neurons (26), and our data indicate that Kiss1 neurons are capable of firing at much higher frequencies (Fig. 7B). Although a rapidly inactivating IT produced by CaV3.1 channels will produce a shorter duration of Ca2+ depolarization, CaV3.1 also de-inactivates more rapidly than other CaV3 channels, which would allow more CaV3.1 channels to be recruited during the hyperpolarization for the next burst. Remarkably, IT was increased sixfold with an E2 treatment that produced an LH surge in these mice. This would indicate that there is an increase in CaV3 channel density as we have previously demonstrated for the expression of these channels in GnRH neurons (22, 55). This means that, once the cells reach the hyperpolarization threshold for recruiting CaV3 channels, there is greater rebound burst of action potentials. Moreover, we deduce that high-frequency burst firing of Kiss1 neurons in the RP3V has the capacity to drive a sustained output from downstream synaptic targets and may explain the prolonged firing of GnRH neurons associated with the preovulatory surge (6).
Notably, we determined that Kiss1 neurons in the RP3V exhibit a hyperpolarization threshold that is close to the V1/2 for de-inactivation of the T-channel, which is the most critical for dictating the rebound burst firing frequency. GIRK channels are critical for driving the membrane potential into the hyperpolarized state necessary to de-inactivate the CaV3 channels underlying the T-current. The majority of hypothalamic neurons express GABAB and opioid receptors, which are coupled to GIRK channels, and Kiss1 neurons in the RP3V are no exception (present findings; 21, 25, 28, 51, 57). In fact, these cells express κ-, μ-, and δ-opioid receptor transcripts, and the vast majority of these cells respond to κ- and μ-agonists. The μ-opioid receptor agonist DAMGO induced an outward current in RP3V Kiss1 neurons, similar to other hypothalamic neurons (present findings; 21, 25, 28, 51). The κ-opioid receptor agonist U-69593 generated an even greater GIRK current, which correlated with the relative abundance of the κ- vs. μ-receptor transcripts in these neurons. GABAB and opioid receptors coupled to GIRK channels in these cells would help provide the hyperpolarization stimulus required to de-inactivate CaV3.1 channels. Also, opioidergic afferents may make direct contact with Kiss1 neurons in the RP3V. Met-enkephalin is coexpressed in 28–38% of Kiss1 neurons in the RP3V area of mice (41). Since met-enkephalin is a potent endogenous agonist for the μ-opioid receptor (52), and Kiss1 neurons respond to the enkephalin analog DAMGO, met-enkephalin may serve an autoregulatory role in hyperpolarizing Kiss1 neurons. Prodynorphin neurons are also abundant in the RP3V and much more prevalent in females than males (47). Moreover, Kiss1 neurons in the RP3V express the κ-opioid receptor (which mediates the effects of dynorphin), and these cells are robustly hyperpolarized by the κ-opioid receptor agonist U-69593. Thus, we infer that κ- and/or μ-receptor-dependent signaling combined with GABAB signaling to Kiss1 neurons in the RP3V provides some of the critical input required for de-inactivating CaV3 channels in Kiss1 neurons. Interestingly, A12 dopamine neurons in the ARC also express Ih and IT. They are hyperpolarized by μ-opioid and GABAB agonists and show similar rebound burst characteristics (29). Since the majority of Kiss1 neurons in the RP3V coexpress Th transcript, their electrophysiological and molecular signatures suggest that these neurons belong to an important class of hypothalamic parvocellular “pacemaker” neurons (29, 31).
In summary, we have characterized the endogenous conductances and channels critical for producing burst firing in the Kiss1 neurons in the RP3V. The majority of these neurons colocalized Th mRNA. Kiss1/TH neurons in the RP3V express a rapidly activating Ih, mediated primarily through HCN1 channels, and a T-type calcium current, mediated primarily through CaV3.1 channels. We have defined a hyperpolarization threshold that is critical for recruiting CaV3.1 channels for generating a high-frequency rebound burst firing. Importantly, the expression of both IT and Ih was augmented by an E2 treatment paradigm that generates an LH surge in the ovariectomized female. We have also discovered a robust hyperpolarizing response to GABAB as well as κ-, and to a lesser extent, μ-opioid receptor agonists, indicative of GABAergic synaptic input and input from opioidergic neurons, most plausibly dynorphin or met-enkephalin. Thus, the biophysical and molecular fingerprints of Kiss1 neurons in the RP3V are consistent with their putative role in generating the sustained, high-frequency burst firing required for driving the preovulatory GnRH surge.
GRANTS
Research reported in this publication was supported by National Institutes Health R01 Grants NS-38809 (M. J. Kelly), NS-43330 (O. K. Rønnekleiv), DK-68098 (M. J. Kelly and O. K. Rønnekleiv), and HD-049651 (R. A. Steiner). K. J. Tonsfeldt was supported by a NIH training grant (T32 NA AG-023477) fellowship and funds from the Department of Physiology and Pharmacology Steinberg Endowment. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.Z. and J.Q., electrophysiology experiments; K.J.T. and M.A.B., molecular biology and LH surge experiments. C.Z., K.J.T., M.A.B., J.Q., M.J.K. and O.K.R interpreted results and prepared figures; C.Z., K.J.T., M.J.K. and O.K.R. drafted manuscript; C.Z., K.J.T., M.A.B., J.Q., R.A.S., K.K., M.J.K., and O.K.R. edited and revised manuscript; C.Z., K.J.T., M.A.B., J.Q., R.A.S., K.K., M.J.K., and O.K.R. approved final version of the manuscript.
REFERENCES
- 1.Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. J exp Biol 139: 51–65, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Bosch MA, Hou J, Fang Y, Kelly MJ, Rønnekleiv OK. 17β-Estradiol regulation of the mRNA expression of T-type calcium channel subunits: role of estrogen receptor α and estrogen receptor β. J Comp Neurol 512: 347–358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch MA, Tonsfeldt KJ, Ronnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-estradiol. J Mol Cell Endocrinol 367: 85–97, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunton J, Charpak S. μ-Opioid peptides inhibit thalamic neurons. J Neurosci 5: 1671–1678, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationship of voltage-gated calcium channels. Pharmacol Rev 57: 411–425, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102: 15682–15687, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus: sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147: 5817–5825, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarkson J, Herbison AE. Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to GnRH hormones. J Neuroendocrinol 23: 293–301, 2011 [DOI] [PubMed] [Google Scholar]
- 9.de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology 153: 1–10, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC, Leresche N. Selective T-type calcium channel block in thalamic neurons reveals channelredundancy and physiological impact of ITwindow. J Neurosci 30: 99–109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ducret E, Gaidamaka G, Herbison AE. Electrical and morphological characteristics of anteroventral periventricular nucleus kisspeptin and other neurons in the female mouse. Endocrinolgy 151: 2223–2232, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Erickson KR, Rønnekleiv OK, Kelly MJ. Electrophysiology of guinea-pig supraoptic neurones: role of a hyperpolarization-activated cation current in phasic firing. J Physiol (Lond) 460: 407–425, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson KR, Rønnekleiv OK, Kelly MJ. Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendocrinology 57: 789–800, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Frazão R, Cravo RM, Donato J, Jr, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in kiss1 cell activity requires estrogen receptor α. J Neurosci 33: 2807–2820, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein A, Naidu A. Multiple opioid receptors: ligand selectivity profiles and binding site signatures. Mol Pharm 36: 265–272, 1989 [PubMed] [Google Scholar]
- 16.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148: 5752–5760, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Ronnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology 152: 4298–4309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25: 11349–11356, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol 68: 1373–1383, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of kiss1 gene expression in the brain of the rat. Endocrinology 148: 1774–1783, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kelly MJ, Loose MD, Rønnekleiv OK. Estrogen suppresses μ-opioid and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci 12: 2745–2750, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly MJ, Qiu J. Estrogen signaling in hypothalamic circuits controlling reproduction. Brain Res 1364: 44–52, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly MJ, Rønnekleiv OK. Electrophysiological analysis of neuroendocrine neuronal activity in hypothalamic slices. In: Methods in Neurosciences: Pulsatility in Neuroendocrine Systems, edited by Levine JE. San Diego: Academic, 1994, p. 47–67 [Google Scholar]
- 24.Klockner U, Lee JH, Cribbs LL, Daud A, Hescheler J, Pereverzev A, Perez-Reyes E, Schneider T. Comparison of the Ca2+ currents induced by expression of three cloned α1 subunits,α1G,α1H and α11, of low-voltage-activated T-type Ca2+ channels. Eur J Neurosci 11: 4171–4178, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology 136: 2341–2344, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Porteous R, d'Anglemont de Tassigny X, Colledge WH, Millar R, Petersen SL, Herbison AE. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci 31: 2421–2430, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242: 1654–1664, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Loose MD, Kelly MJ. Opioids act at μ-receptors to hyperpolarize arcuate neurons via an inwardly rectifying potassium conductance. Brain Res 513: 15–23, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Loose MD, Rønnekleiv OK, Kelly MJ. Membrane properties and response to opioids of identified dopamine neurons in the guinea pig hypothalamus. J Neurosci 10: 3627–3634, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lüthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron 21: 9–12, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Lyons DJ, Horjales-Araujo E, Broberger C. Synchronized network oscillations in rat tuberoinfundibular dopamine neurons: switch to tonic discharge by thyrotropin-releasing hormone. Neuron 65: 217–229, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Masterson SP, Li J, Bickford ME. Frequencey-dependent release of substance P mediates heterosynaptic potentiation of glutamatergic synaptic responses in the rat visual thalamus. J Neurophysiol 104: 1758–1767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushita N, Okada H, Yasoshima Y, Takahashi K, Kiuchi K, Kobayashi K. Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J Neurochem 82: 295–304, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29: 11859–11866, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oka Y. Tetrodotoxin-resistant persistent Na+ current underlying pacemaker potentials of fish gonadotrophin-releasing hormone neurones. J Physiol (Lond) 482: 1–6, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pau KYF, Orstead KM, Hess DL, Spies HG. Feedback effects of ovarian steroids on the hypothalamic-hypophyseal axis in the rabbit. Biol Repro 35: 1009–1023, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev 83: 117–161, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res 29: 2002–2007, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149: 1979–1986, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piet R, Boehm U, Herbison AE. Estrous cycle plasticity in the hyperpolarization-activated current Ih is mediated by circulating 17β-estradiol in preoptic area kisspeptin neurons. J Neurosci 33: 10828–10839, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porteous R, Petersen SL, Yeo SH, Bhattarai JP, Ciofi P, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Kisspeptin neurons co-express met-enkephalin and galanin in the rostral periventricular region of the female mouse hypothalamus. J Comp Neurol 519: 3456–69, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology 152: 1503–1514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Santoro B, Chen S, Lüthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci 20: 5264–5275, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawamoto K, Nakao N, Kobayashi K, Matsushita N, Takahashi H, Kakishita K, Yamamoto A, Yoshizaki T, Terashima T, Murakami F, Itakura T, Okano H. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci USA 98: 6423–6428, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shakiryanova D, Tully A, Hewes RS, Deitcher DL, Levitan ES. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat Neurosci 8: 173–178, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Simerly RB. Prodynorphin and proenkephalin gene expression in the anteroventral periventricular nucleus of the rat: sexual differentiation and hormonal regulation. Mol Cell Neurosci 2: 473–484, 1991 [DOI] [PubMed] [Google Scholar]
- 48.Simerly RB, McCall LD, Watson SJ. Distribution of opioid peptides in the preoptic region: immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. J Comp Neurol 276: 442–459, 1988 [DOI] [PubMed] [Google Scholar]
- 49.Tsien RW, Hess P, McCleskey EW, Rosenberg RL. Calcium channels: mechanisms of selectivity, permeation, and block. Annu Rev Biophys Biophys Chem 16: 265–290, 1987 [DOI] [PubMed] [Google Scholar]
- 50.Ulrich D, Huguenard JR. Gamma-aminobutyric acid type B receptor-dependent burst-firing in thalamic neurons: a dynamic clamp study. Proc Natl Acad Sci USA 93: 13245–13249, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner EJ, Rønnekleiv OK, Bosch MA, Kelly MJ. Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci 21: 2085–2093, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams JT, Egan TM, North RA. Enkephalin opens potassium channels on mammalian central neurones. Nature 299: 74–77, 1982 [DOI] [PubMed] [Google Scholar]
- 53.Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci 19: 235–263, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27: 10153–10164, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17β-Estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 29: 10552–10562, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28: 4423–4434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng SX, Bosch MA, Rønnekleiv OK. Mu-opioid receptor mRNA expression in identified hypothalamic neurons. J Comp Neurol 487: 332–344, 2005 [DOI] [PubMed] [Google Scholar]