Abstract
The outcome of liver injury is determined by the success of repair. Liver repair involves replacement of damaged liver tissue with healthy liver epithelial cells (including both hepatocytes and cholangiocytes) and reconstruction of normal liver structure and function. Current dogma posits that replication of surviving mature hepatocytes and cholangiocytes drives the regeneration of liver epithelium after injury, whereas failure of liver repair commonly leads to fibrosis, a scarring condition in which hepatic stellate cells, the main liver-resident mesenchymal cells, play the major role. The present review discusses other mechanisms that might be responsible for the regeneration of new liver epithelial cells and outgrowth of matrix-producing mesenchymal cells during hepatic injury. This theory proposes that, during liver injury, some epithelial cells undergo epithelial-to-mesenchymal transition (EMT), acquire myofibroblastic phenotypes/features, and contribute to fibrogenesis, whereas certain mesenchymal cells (namely hepatic stellate cells and stellate cell-derived myofibroblasts) undergo mesenchymal-to-epithelial transition (MET), revert to epithelial cells, and ultimately differentiate into either hepatocytes or cholangiocytes. Although this theory is highly controversial, it suggests that the balance between EMT and MET modulates the outcome of liver injury. This review summarizes recent advances that support or refute the concept that certain types of liver cells are capable of phenotype transition (i.e., EMT and MET) during both culture conditions and chronic liver injury.
Keywords: hepatocytes, cholangiocytes, fibrosis, hepatic stellate cell, progenitors, regeneration
epithelial-mesenchymal transition (EMT) defines a biological process in which adherent epithelial cells acquire mesenchymal characteristics and become more migratory/invasive (29). The reverse process of EMT is mesenchymal-epithelial transition (MET), which involves a transformation of mesenchymal cells to acquire epithelial traits. Both EMT and MET involve complex molecular and cellular events that ultimately change the gene expression and phenotype of cells. Thus they demonstrate the inherent plasticity of cells that are capable of EMT/MET (11, 46, 67, 68).
Epithelial cells with apical-basolateral polarity usually bind to each other by tight intercellular junctions and form plates. During EMT, epithelial cells eventually lose these key features by changing the expression and distribution of proteins that mediate cell-cell contacts and reorganizing the cytoskeletal elements responsible for normal epithelial polarity. In addition, these epithelial cells start to gain mesenchymal characteristics including the capacity to migrate and invade the extracellular matrix; they also switch to rear-to-front polarity and begin to express distinct mesenchymal markers. This newly formed migratory/invasive phenotype is normally accompanied by induction of mesenchymal filaments, rearrangements of actin cytoskeletal proteins, increased production of enzymes that degrade extracellular matrix, and altered expression of specific microRNAs (15, 29, 39, 94). Such comprehensive changes in cell phenotype do not take place instantaneously. Instead, EMT/MET is composed of a carefully orchestrated series of events modulated at both transcriptional and posttranscriptional levels (77, 78). The term “partial EMT” is used to distinguish cells that have not yet completed EMT from those that have undergone a “full EMT.” Cells in partial EMT are in the intermediate stages of EMT, and as such, continue to express epithelial markers although mesenchymal markers have already been acquired (29). Partial EMT may occur in hepatocellular carcinomas (HCCs) and during liver fibrosis.
EMT has been categorized into three different subtypes based on the biological context in which it occurs: development and organogenesis (type 1); wound healing, tissue, regeneration and organ fibrosis (type 2); and carcinogenesis (type 3) (28, 29, 94). Detailed description of these three subtypes of EMT can be found in many reviews articles (1, 29, 94). In brief, type 1 EMT occurs during implantation, embryo formation, and organ development. It is responsible for the creation of mesoderm, endoderm, and neural crest cells. During embryonic development, the primitive epithelium gives rise to primary mesenchyme through EMT, which can be reinduced to form secondary epithelia by MET and may undergo further differentiation to generate different organs/tissues via subsequent cycles of EMT/MET (29). Type 1 EMT does not induce fibrosis. In contrast, type 2 EMT has been classically associated with fibrosis in adult organs such as kidney, liver, lung, heart, and intestine (29, 38). Type 2 EMT usually occurs during wound healing and organ regeneration. It is typically mediated by inflammatory signals and generally halts when repair is accomplished and inflammation diminishes. However, under persistent injury and inflammation, type 2 EMT can continue to generate myofibroblasts that accumulate and cause progressive fibrosis, which may lead to organ destruction. In recent years, the occurrence of type 2 EMT in vivo has been seriously challenged (33, 38, 60). Type 3 EMT occurs in cancer epithelial cells, which undergo genetic and epigenetic changes and thus are able to invade and metastasize via the circulation, thereby generating distant metastatic tumor. Type 3 EMT resembles type 1 EMT in that generation of epithelia, rather than fibrosis, is typically the ultimate outcome (15).
The focus of this review paper will be type 2 EMT in the context of liver disease, especially liver fibrosis. We will summarize major evidence for or against the concept that certain types of liver cells can undergo EMT/MET in vitro and in vivo and begin by discussing some of the signaling pathways that regulate EMT/MET in the liver.
Signaling Pathways Governing EMT
Many signaling pathways have been documented to induce EMT/MET in embryonic development, normal and transformed cell lines, organ regeneration/fibrosis, and/or cancer progression. These signaling pathways include transforming growth factor-β (TGF-β) superfamily, sonic hedgehog (Hh), Wnt, Notch, epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and many others (77, 89). A detailed discussion of these pathways is beyond the scope of this review. Rather we will focus on those signaling pathways that have been studied in liver-associated EMT.
TGF-β signaling is considered as the master regulator of EMT (89, 93) and has been shown to induce EMT in various types of cultured nonmalignant and malignant epithelial cells, including hepatocyte and cholangiocyte (5, 27, 39, 42, 87). Binding of TGF-β1 with type I and II TGF-β receptors induces phosphorylation of Smad2/3. Phosphorylated Smads recruit Smad4 and translocate to the nucleus where Smad complexes control transcription of several target genes, such as SNAI1 (snail), SNAI2 (slug), and twist, to further suppress epithelial genes and induce mesenchymal genes (27, 93). Besides Smad signaling, TGF-β has also been shown to promote EMT through activation of mitogen-activated protein (MAP) kinase, Rho GTPases, and phosphatidylinositol 3 (PI3)-kinase (86). Moreover, a member of the TGF-β superfamily that antagonizes TGF-β signaling, bone morphogenetic protein (BMP)-7, negatively regulates TGF-β-induced EMT in different types of organ injury, including liver fibrosis. Both pharmacological administration and genetic expression of BMP-7 have been shown to inhibit TGF-β-provoked EMT and thus attenuate liver fibrosis in animals with carbon tetrachloride (CCl4)-induced liver injury (21, 95).
Hh signaling is a key morphogenic pathway that controls fetal liver development and also plays an essential role in many types of adult liver injury (16, 54). Soluble Hh ligands (Sonic Hh, Indian Hh, and Desert Hh) secreted by Hh-producing cells interact with Patched (Ptc) receptor on Hh-responsive cells. This relieves the Ptc coreceptor, Smoothened (Smo), from Ptc-mediated repression. Activated Smo, in turn, transduces intracellular signals that culminate in the nuclear localization of Glioblastoma (Gli) family transcription factors (Gli1, Gli2, and Gli3). Hh signaling promotes EMT during fetal development and during metastasis of several adulthood cancers (40). There is growing evidence that the Hh pathway may also regulate EMT-like transitions in certain types of liver cells during adult liver injury. The most extensive data supporting this concept have been acquired by studies of hepatic stellate cells (HSC). Although HSCs are not generally considered to be epithelial cells, several recent publications demonstrate that at least a subpopulation of HSCs has both mesenchymal and epithelial features (which will be discussed in more detail below) (36, 49). Both pharmacological and genetic inhibition of Hh signaling have been shown to block an EMT-like transition that occurs as HSCs become myofibroblasts, reverting the cells to a more quiescent/epithelial phenotype (17, 49). Similar findings have been reported when Hh pathway activity is manipulated in cholangiocytes and malignant hepatocytes (12, 55). This collective evidence suggests that, like TGF-β, Hh signaling also modulates EMT/MET in cells that are involved in adult liver repair and regeneration.
Notch signaling is another highly conserved cell pathway that becomes activated during both development and adult tissue repair. It is a complex pathway, as evidenced by the fact that multiple Notch receptors (Notch 1–4) and ligands (Jagged and Delta-like) exist. Upon ligand binding, proteolytic cleavage of the Notch receptor occurs. This releases the Notch intracellular domain (NICD) from the cell membrane, and permits its translocation to the nucleus. In the nucleus, the NICD forms a transcription complex with DNA-binding protein RBP-J and induces the transcription of downstream target genes, including genes in the hairy and enhancer of split (Hes) family and hairy/enhancer-of-split related with YRPW (Hey) family (34). A role for Notch in EMT has been documented in embryogenesis, adult tissue repair, and carcinogenesis (7, 8, 30, 64, 79). More recently, evidence for Notch signaling in EMT has also been demonstrated in the liver. Notch inhibition by a γ-secretase inhibitor, DAPT, blocked an EMT-like transition in both primary mouse HSCs and a rat HSC cell line. The suppression of HSC activation ultimately attenuated CCl4-induced rat liver fibrosis (13, 85). Similarly, treating cholangiocytes with this γ-secretase inhibitor, or with Jagged1-neutralizing antibody, to block Notch signaling also diminished an EMT phenotype (43, 85). Conversely, overexpressing Notch1 induced EMT in a cholangiocarcinoma cell line (100). These recent findings suggest an important role of Notch signaling in EMT during liver injury.
Hypoxia and redox stress are common events during chronic liver disease progression. Indeed, hypoxia-related signaling was recently documented to promote type 3 EMT during the development of liver cancer. Hypoxia-inducible factor (HIF) is the major transcription factor responsible for hypoxic responses. Zhang et al. (97) reported that increased HIF-1α levels correlated with loss of the epithelial marker E-cadherin but increased expressions of the EMT-associated transcriptional factor SNAI1 and the mesenchymal markers N-cadherin and vimentin in human liver cancer samples. They extended these in vivo findings by studying two HCC cell lines and showed that hypoxia-stabilized HIF-1α promoted EMT by increasing SNAI1 transcription (97). Wnt/β-catenin signaling might further enhance hypoxia-induced EMT in HCC cell lines by cross-talking with HIF-1α (98). Hypoxia-induced EMT in HCC cells is also regulated by PI3-kinase/AKT pathway (23, 88). In addition, reactive oxygen species (ROS) produced during hypoxia may play a role in liver cancer-related EMT because attenuation of ROS by antioxidants suppressed TGF-β-induced EMT in HCC cells (31).
Besides the aforementioned pathways, EGF, PDGF, and other pathways have also been reported to regulate EMT in the context of liver disease. More importantly, these pathways cross talk with each other and thus form a complex network to control progression of EMT/MET and determine the cell fate. These signaling pathways usually induce and activate several EMT-related transcription factors such as SNAI1, SNAI2, Twist, zinc finger E-box binding homeobox 1 (ZEB1), Krüppel-Like Factor 8 (KLF8), Goosecoid, and fork-head box protein C2 (FOXC2) (29). Upregulation of these transcription factors can repress expression of E-cadherin and other junction proteins and thus promote EMT and biological functions mediated through EMT. For example, Rowe et al. (62) reported that specific deletion of hepatocyte derived SNAI1 reduced CCl4-induced liver fibrosis and inflammatory responses. Several groups demonstrated that Twist prompted HCC cell invasion, migration, and vasculogenic mimicry (71, 91).
Challenges in Identifying Cells Undergoing EMT
EMT is a transition process and cells that are in the intermediate stage of EMT are sometimes referred to as “transitioning cells” or cells that have undergone “partial EMT” (29, 94). Coexpression of epithelial and mesenchymal markers is often used as a criterion to identify epithelial cells that are becoming mesenchymal (i.e., undergoing EMT) at a time when they retain some of their epithelial-specific features. Typical epithelial markers usually include E-cadherin and cytokeratin, whereas common mesenchymal markers are N-cadherin, α-smooth muscle actin (α-SMA), collagen 1α1, vimentin, desmin, fibroblast-specific protein 1 (FSP1, also known as S100A4), SNAI1, SNAI2, and twist (94). However, it is important to keep in mind that an epithelial cell that is undergoing EMT may have not yet fully activated expression of mesenchymal genes. In addition, not every mesenchymal marker is expressed concomitantly (for example, quiescent HSCs express large amounts of desmin, but minimal levels of α-SMA and collagen); some mesenchymal markers, such as FSP1, may not be expressed by all types of mesenchymal cells (e.g., liver myofibroblasts) (56); and some mesenchymal markers lack specificity (e.g., vimentin) (24). Moreover, it is difficult to determine colocalization of two markers by immunohistochemistry. All these factors confound our efforts to identify a cell as being (or not being) in the midst of an EMT, especially in whole tissues. An additional challenge that we need to overcome is the fact that EMT and MET are reversible processes. Hence it is possible for a mesenchymal-type cell that was derived from an epithelial cell to regain its epithelial traits (1, 15).
To overcome the limitations of immunostaining, lineage tracing has been recently used to track cells undergoing EMT/MET. This strategy exploits cell-type-specific activation of gene-regulatory elements (usually based on the cre-lox system) to generate permanently expressed markers (e.g., LacZ or a fluorescent-labeled protein) that specifically label all the progeny of that cell type (37). Despite the enormous power of this technique, the utilization of this approach to study EMT/MET has generated conflicting evidence, as will be discussed in more detail below.
Evidence That Specific Liver Cell Types Can Undergo EMT/MET in Culture
Several types of liver cells (including hepatocytes, cholangiocytes, HSCs, and liver progenitor cells) have been shown to undergo EMT/MET or EMT/MET-like transition during culture. Multiple groups demonstrated that treating hepatocytes (either primary cells or cell lines) with TGF-β induced them to downregulate epithelial markers, such as albumin and E-cadherin, and upregulate mesenchymal markers, including SNAI1, α-SMA, collagen, and FSP1, and/or to gain migratory capacity (14, 48, 51, 95, 96, 102). This was further confirmed in primary cultures of human fetal hepatocytes by showing that TGF-β treatment induced SNAI1 and decreased E-cadherin expression, as well reorganized the actin cytoskeleton (9). Besides TGF-β, other proteins or chemicals, such as hepatitis C viral protein and organochlorine pesticides, have also been reported to induce EMT in hepatocytes (2, 103). Meyer et al. (47) recently also reported that hepatocytes can intrinsically undergo dedifferentiation in collagen monolayer culture, as evidenced by change in morphology and upregulation of mesenchymal markers (N-cadherin, vimentin, and collagen 1α1). However, they showed that this phenomenon does not reflect a classical TGF-β-mediated EMT because SNAI1 and E-cadherin were not involved. Hence, in hepatocytes, EMT mechanisms appear to depend on the specific trigger (cytokines, environment, etc.), and this will also determine the markers that can be used to characterize the particular EMT program.
Cholangiocytes (liver ductular cells) are another type of liver epithelial cell that has been shown to undergo EMT in the culture. Omenetti et al. (55) reported that cholangiocytes isolated from rats with biliary fibrosis induced by bile duct ligation (BDL) upregulated expression of FSP1 and downregulated expression of aquaporin-1, cytokeratin 7 (Krt7), and cytokeratin 19 (Krt19). Furthermore, they showed that an immature cholangiocyte line cocultured with myofibroblastic HSCs (MF-HSCs), or treated with conditioned medium from MF-HSCs, was induced to undergo complete EMT (i.e., repression of epithelial gene expression, induction of mesenchymal gene expression, and acquisition of a migratory phenotype). In addition, they demonstrated that blocking Hh signaling by use of a Hh ligand-neutralizing antibody blocked EMT in the cholangiocytes that had been treated with MF-HSC conditioned medium (53, 55). Several other groups also reported that cultured primary human/rodent cholangiocytes induced mesenchymal markers and/or became highly motile when treated with TGF-β (18, 26, 63, 65). Thus, as with hepatocytes, there is also strong evidence that ductular cells are capable of EMT in vitro.
HSCs are liver-resident pericytes that become myofibroblastic during many types of liver injury. As discussed below, published data indicate that HSCs express both epithelial and mesenchymal genes. Moreover, growing evidence supports the concept that HSCs are transitional cells with inherently high plasticity. These findings suggest that HSC might be capable of epithelial-mesenchymal/mesenchymal-epithelial-like transitions. This possibility is supported by the putative origin of HSC. At least three independent groups have reported that HSCs are derived from mesothelial/submesothelial cells during both liver development and adult liver injury (3, 4, 41, 44, 59). During development, mesothelial cells are derived from the epiblast (a tissue that gives rise to all three germ layers). Mesothelial cells express both mesenchymal and epithelial cell markers (50). Therefore, Choi et al. (15) suggested that quiescent HSCs might be transitional cells derived from epithelial cells that have undergone a partial EMT. Consistent with that concept, Kordes et al. (36) showed that a subpopulation of adult primary rat HSCs that expressed the progenitor marker, CD133, could be induced to become either myofibroblastic, or hepatocytic cell under different culture conditions. These observations suggest that some adult HSCs (which are generally considered to be mesenchymal-type cells) can undergo MET and regain epithelial features. During this MET-like process, HSCs-derived hepatocytic cells were demonstrated to express α-fetoprotein (AFP), a marker of immature hepatocytes, and albumin, a marker of both hepatoblasts and hepatocytes. These findings were subsequently confirmed in another laboratory in vivo by lineage tracing studies, which will be discussed subsequently (49, 90). Other published data also support the concept that liver HSCs can transition to become liver epithelial cells. Sicklick et al. (70), for example, reported coexpression of mesenchymal genes and epithelial markers (i.e., AFP and Krt19) in two clonally derived MF-HSCs cell lines. Later on, Choi et al. demonstrated that freshly isolated rat HSCs expressed some epithelial markers, including E-cadherin, Krt7, and Krt19, which were downregulated during HSC activation. Moreover, they reported that Hh inhibition restored the epithelial gene expression in cultured HSCs, thus suggesting a regulatory role for Hh in adult liver EMT (17). More recently, Michelotti et al. (49) used multiple approaches including flow cytometry, immunocytochemistry, in situ hybridization, and PCR analysis to demonstrate coexpression of epithelial markers (such as Krt19, albumin, AFP, Krt18) and mesenchymal markers (such as desmin, α-SMA), as well as some progenitor markers (such as Sox9, Nanog, Oct4), in quiescent and myofibroblastic primary mouse HSCs. They were also able to confirm those findings in two other clonal MF-HSCs cell lines, including LX-2, a widely used human MF-HSC cell line. However, because freshly isolated primary HSC already coexpress epithelial and mesenchymal markers, a complete EMT does not occur during their differentiation/activation to a more myofibroblastic phenotype in culture. Similarly, EMT inhibitors are not able to fully extinguish mesenchymal gene expression in cultured HSC, despite reverting the cells to a more epithelial phenotype. Thus we use the term “EMT/MET-like transition” when describing HSC plasticity to distinguish the process from traditional EMT/MET.
The concept that both cholangiocytes and HSCs are able to undergo an EMT/MET-like transition is intriguing because at least subpopulations of both cell types have been suggested and documented to contribute progenitor populations in the liver (10, 35, 66, 72, 76). Conigliaro et al. (19) recently reported that epithelial and mesenchymal liver cells (hepatocytes and HSCs) may arise from common progenitor isolated from embryonic livers. Their study showed that sca+ (a stem marker) murine progenitor cells coexpressed markers of epithelial and mesenchymal lineages and were able to trans-differentiate into both hepatocytes and HSCs under culture conditions after few passages. They further demonstrated that when these clonally derived progenitor cells were transplanted into normal liver they gave rise to both hepatocytes and HSCs in vivo, confirming that the in vitro findings were not mere artifacts of cell culture. This evidence that HSCs and liver epithelial cells derive from a common, multipotent progenitor is also supported by an earlier study that showed that hepatic progenitor cells (oval cells) coexpressed epithelial and mesenchymal markers and demonstrated that transplantation of these progenitor cells could repopulate injured livers (92).
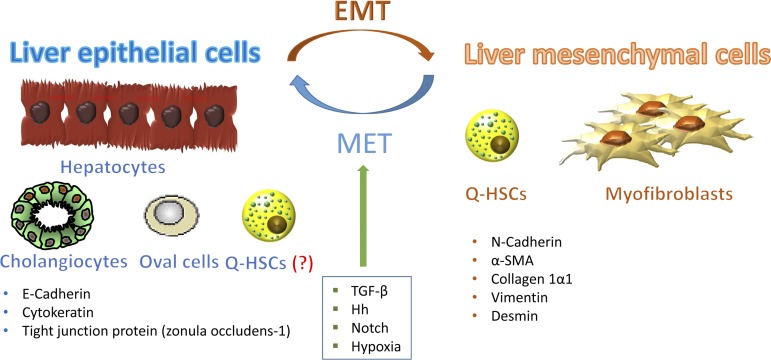
Thus there seems to be a fairly good amount of evidence showing that mature hepatocytes and cholangiocytes can transiently express markers of mesenchymal cells in vitro and that adult HSCs can also be induced to undergo EMT/MET-like transition under certain culture conditions. Moreover, some liver progenitor cells might be capable of EMT, both in vivo and in vitro, permitting them to repopulate the liver (Fig. 1). However, in vitro conditions do not necessarily always reflect situations that occur in vivo. Thus cell culture data cannot always be extrapolated to the in vivo conditions. Also, because cells that undergo EMT usually generate transitioning cell populations, it has been challenging to prove (or disprove) that any of these cell types actually undergoes EMT (or MET) during chronic liver injury. Consequently, it remains unclear/extremely controversial whether (and how) epithelial-mesenchymal transitions of resident liver cells might be involved in liver repair.
Fig. 1.
Resident liver cells are capable of undergoing epithelial-to-mesenchymal transition (EMT) and mesenchymal-to-epithelial transition (MET) under certain culture conditions. Hepatocytes, cholangiocytes, oval cells, and quiescent hepatic stellate cells (Q-HSCs) express epithelial markers such as E-cadherin, cytokeratin, and tight junction proteins. Some of these cells can undergo an EMT-like process and trans-differentiate into cells with a more mesenchymal phenotype [characterized by expression of N-cadherin, α-smooth muscle actin (α-SMA), collagen 1α1, vimentin, and/or desmin]. Some liver mesenchymal cells (e.g., quiescent and myofibroblastic hepatic stellate cells) can also differentiate into liver epithelial cells by MET. These processes are regulated by signaling pathways that become activated during liver injury, such as TGF-β, Hedgehog, Notch, and hypoxia-inducible factor.
Evidence For and Against the Occurrence of EMT in Adult Liver Injury
As mentioned earlier, it is inherently difficult to determine in situ whether a cell is undergoing EMT/MET. Early studies mostly used immunohistochemistry to assess the role of EMT in adult liver repair. There are several flaws/disadvantages of using this technique (15). First, it is technically challenging to demonstrate expression of several protein markers by any given cell at any point of time. Thus it is almost infeasible to acquire the knowledge of the comprehensive changes in the phenotype of single cells by this method. In addition, it is often difficult to determine by costaining whether one cell expresses both epithelial and mesenchymal markers. It is possible that the apparent coexpression of both markers simply reflects the fact that an adjacent cell expresses one of the markers. Last but not least, EMT/MET is a transitional process (rather than a static one), and even superb immunohistochemistry is incapable of capturing cell transition, which is generally considered as a critical criteria that a cell is undergoing EMT/MET.
Nevertheless, some useful information about EMT in liver injury has been obtained by studying human/animal samples with immunohistochemistry. For example, Rygiel et al. (63) reported that bile ductular cells coexpress ductular markers (Krt7 or Krt19) and FSP1, a mesenchymal marker, in human primary biliary cirrhosis, primary sclerosing cholangitis, and alcoholic liver disease. Similar findings were also reported by other groups who evaluated liver samples from patients with primary biliary cirrhosis and nonalcoholic fatty liver disease (55, 61, 73). Moreover, costaining for albumin (hepatocyte marker) and FSP1 was demonstrated in CCl4-induced murine liver fibrosis (95). Dooley et al. (21) also observed coexpression of transferrin (specific marker for hepatocyte) and collagen or SNAI1 in hepatitis B virus-infected patients liver samples.
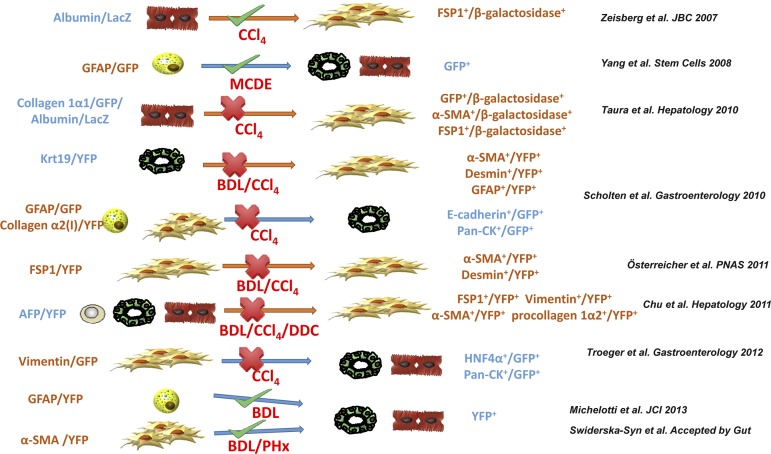
Lineage tracing is becoming a popular and powerful tool to overcome the limitations of immunostaining for identifying EMT. This technique genetically labels cells. Hence, the marker will be present in any progeny of the labeled cells. Zeisberg et al. (95) were the first to utilize this technique to suggest that hepatocyte-mediated EMT might provide an important source of fibrogenic myofibroblasts. These authors generated double transgenic (DTG) mice by crossing Albumin-Cre mice with Rosa26-floxstop-LacZ mice. In Albumin-Cre mice, expression of Cre-recombinase is under the control of albumin promoter. In Rosa36-floxstop-LacZ mice, the LacZ reporter gene is activated only after Cre-mediate excision of the floxed exon. Hence in the DTG mice only albumin(+) cells and their progeny were permanently marked with β-galactosidase. These mice were then examined to determine whether β-galactosidase ever colocalized with FSP1, a putative marker of fibroblasts. In healthy DTG mice, few FSP1-positive cells were detected. However, CCl4 treatment induced hepatic FSP1 expression and almost half of the FSP1 positive cells coexpressed β-galactosidase. The authors, therefore, concluded that hepatocyte-derived fibroblasts are an additional and significant lineage of mesenchymal cells that contribute to progression of liver fibrosis (95).
The second attempt to use lineage tracing to demonstrate EMT in adult liver injury bred glial fibrillary acidic protein (GFAP)-Cre mice with floxStopRepressorflox green fluorescent protein (GFP) transgenic mice (90). The resultant DTG mice expressed Cre-recombinase exclusively in cells that have activated transcription of GFAP. GFAP is a marker of HSCs and these DTG mice could be used to track the progeny of HSCs to determine whether HSCs undergo MET to generate mature liver epithelial cells. After methionine-choline-deficient, ethionine-supplemented diets, roughly one third of the mature-appearing albumin-positive hepatocytes and almost all of the liver ductular cells of these DTG mice expressed GFP. These data raised the intriguing possibility that hepatocytes, cholangiocytes, and HSCs are derived from common progenitors that are capable of EMT/MET during certain types of liver injury (15).
However, these two reports were seriously challenged by several lineage tracing studies that were published around 2010. Taura and coworkers (75) from Brenner's group bred collagen 1α1-GFP reporter mice with the Albumin-Cre-Rosa26-floxstop-LacZ-expressing reporter mice strain used by Zeisberg to simultaneously identify collagen-producing cells and cells derived from albumin(+) cells in the injured liver. However, unlike Zeisberg, they found no colocalization of FSP1 and β-galactosidase. Moreover, they were unable to detect any hepatocyte-derived collagen-expressing cells at different stages of CCl4-induced liver fibrosis.
Later, the same group generated multiple DTG mice to study EMT of cholangiocytes, and MET of HSCs, during liver injury (69). First Scholten et al. (69) crossed tamoxifen-inducible Krt19-CreERT mice with Rosa26f/f-YFP mice to trace the fate of cholangiocytes. In both BDL- and CCl4-induced liver fibrosis, the authors found no evidence of HSC/myofibroblast markers overlapping with the yellow fluorescent protein (YFP)-expressing Krt19+ progeny of cholangiocytes. They complemented these data by experiments with animals in which quiescent HSCs or myofibroblastic cells were labeled permanently by crossing mice that expressed Cre-recombinase under the control of GFAP or collagen α2(I) promoter with Rosa26f/f-mT/GFP or Rosa26f/f-YFP reporter mice, but they were unable to detect any colocalization of YFP+ cells with E-cadherin or pan-cytokeratin (a marker for cholangiocyte) either. Thus the authors concluded that EMT in cholangiocytes and MET in HSCs do not contribute to experimental hepatic fibrosis or liver regeneration.
A third study from the same group by Osterreicher et al. (56) demonstrated that FSP1 was not expressed by HSCs or type I collagen-producing fibroblasts. Rather, they found that FSP1-positive liver cells expressed macrophage markers, leading the authors to conclude that classical liver myofibroblasts do not express FSP1. Consistent with that concept, when FSP1-Cre mice were crossed with ROSA26-YFP mice, no colocalization of YFP with desmin or α-SMA was observed in either BDL- or CCl4-induced liver injury. This work challenged the reliability of FSP1 as a mesenchymal marker for studying EMT in the liver.
The fourth study that provided another piece of strong evidence against EMT during liver injury was performed by Chu et al. (18). These authors generated DTG mice by crossing AFP-Cre mice and Rosa26-YFP mice to tag all the epithelial cells in the liver (including hepatocytes, cholangiocytes, and oval cells) with YFP. Although they were able to detect EMT in cholangiocytes in vitro, they found no evidence that YFP colocalized with various putative mesenchymal markers, including FSP1, vimentin, α-SMA, or procollagen 1α2, in three different hepatic injury models. Hence the authors concluded that EMT by hepatocytes or cholangiocytes did not occur in vivo. Since this study was performed by a different group, Kisseleva and Brenner (33) concluded that EMT does not occur in vivo and has stated that “hepatic epithelial cells do not contribute to experimental liver fibrosis.” Later on, this claim was also supported by the work of Troeger et al. (80) reporting that vimentin-CreER marked myofibroblasts did not undergo MET.
Surprisingly, the battle over EMT has not ended. More recently, Michelotti et al. (49) reported that HSCs were capable of differentiating into hepatocytes and ductular cells by using the same type of lineage tracing techniques described above. In their recent article (49), α-SMA-Cre-ERT2 or GFAP-Cre-ERTM mice were crossed with Rosa-Stop-flox-YFP mice to trace the fate of HSCs after liver injury. After BDL, three types of YFP-positive cells were observed in fibrotic liver: stromal, hepatocytic, and ductular types. To confirm this finding, hepatocytes were isolated from these DTG mice after BDL and the YFP expression was evaluated directly by flow cytometry without using antibody. Interestingly, they found about 24–34% hepatocytes were YFP positive, consistent with a previous study that also showed a similar percentage of hepatocytes came from HSCs in injured livers (90). Moreover, they analyzed hepatocyte DNA by PCR to examine rearrangement of the Rosa26 locus and demonstrated that Cre-mediated recombination did occur in the tamoxifen-treated group. This piece of direct evidence for transgene rearrangement had not been provided by any of the previous studies that claimed to refute the existence of EMT during liver injury. Evidence for EMT/MET has also been observed recently by the same group using a partial hepatectomy (PHx) model (72a). This study found that, in tamoxifen-treated α-SMA-YFP mice, many progenitors, cholangiocytes, and up to 25% of hepatocytes were YFP+ by 48–72 h after PHx, indicating that liver epithelial cells were derived from α-SMA-YFP+ cells. What is particularly intriguing about these last two studies is that they identify HSCs (pericytes in the liver) as a resident population of inherently plastic cells which can be reprogramed by MET-EMT to replace adult liver epithelial cells, resembling Weinberg's “transitional breast cells” which coexpress epithelial and mesenchymal markers and are postulated to replenish breast cancer stem cell pools (25, 67).
It is difficult to reconcile all of the aforementioned contradictory results for and against the concept that liver cells undergo EMT in vivo (as summarized in Fig. 2). The fact that the conflicting data were generated by studying the same fibrosis models makes interpretation even harder. We will try to explore a few possibilities that may help to explain the discrepancies. First, like all other techniques, lineage tracing also has some pitfalls. The efficiency of Cre-mediated recombination is not perfect. For example, Scholten et al. (69) reported only 40% efficacy of Cre-recombination in their Krt19-Cre/YFP mice, so it is quite possible that EMT might have occurred in the nonlabeled Krt19+ cells. In fact, in an early study by Brenner and colleagues (45) that used DTG α-SMA/collagen 1α1 reporter mice to determine the relationship between α-SMA-expressing cells and collagen production following liver injury, around 50% of HSCs did not express either transgene whereas 7% only expressed α-SMA-red fluorescent protein, 14% only expressed collagen-enhanced green fluorescent protein, and 30% expressed both transgenes after 5 days in culture. Second, some of the putative “cell type-specific” markers used to create DTG mice are not really specific. For example, GFAP (a classical HSC marker) has also been reported to be expressed by ductular cells (90). Third, the liver injury models were examined at different, but limited numbers of, time points in different studies. Since EMT/MET are transitional processes, investigators who did not detect EMT/MET may have missed the window when cells were undergoing phenotypic transition, or the cells may have already finished transition and may even have transitioned back to their basal state. Fourth, lineage tracing still heavily relies on double immunohistochemistry to determine whether a cell coexpresses certain markers to decide whether it is undergoing EMT/MET. Consequently, the limitations of immunohistochemistry still apply to this advanced technique. In fact, employing only a few epithelial and mesenchymal markers might not be sufficient to truly determine the existence of EMT. It is also possible that some markers are too weak to be detected by current techniques or they might simply not be expressed in transitioning liver cells that express other kinds of markers. Finally, data in animal models may not exactly reflect the pathophysiological progression that occurs in human liver diseases. As mentioned earlier, immunohistochemistry has generated some compelling evidence supporting the concept that EMT/MET occur during various types of human liver injury. Given this, and all of the caveats about the limitations of currently available animal data, it is premature to conclude that EMT/MET do not occur in human liver injury. To better judge the occurrence of in vivo EMT/MET, we propose a few approaches that may need to be followed in the future lineage tracing studies. First, different types of injury models and different time points during/after injury should be examined in sufficient number of animals. Second, different detection approaches (such as direct immunofluorescence, antibody-mediated immunohistochemistry, isolation of different liver cell types, and examination of Cre recombination and YFP expression by PCR, flow cytometry, etc.) should be used in combination to compensate for each approach's shortcoming. Third, proper controls (both positive and negative) should always be included.
Fig. 2.
Summary of recently published lineage tracing studies that are for or against EMT. Different lineage tracing studies discussed in this review are summarized in chronological order of publication. Genetic labeling markers (promoters), injury models, detection methods, and conclusions for each study are shown. BDL, bile duct ligation; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; PHx, partial hepatectomy; HNF4α, hepatocyte nuclear factor 4α; MCDE, methionine-choline-deficient, ethionine-supplemented diet; Pan-CK, pan-cytokeratin.
EMT and Liver Carcinogenesis
Unlike type 2 EMT in fibrosis, the concept that EMT occurs in cancer is less controversial. There are fairly good amounts of evidence showing that EMT occurs in HCC and there are several review articles that discuss the role of EMT in liver cancers (52, 81). Here we will only briefly summarize some recent advances in this field. TGF-β was previously demonstrated to induce various types of HCC cell lines to acquire a mesenchymal phenotype with migratory capability (6, 22). Recently Gli1 (a Hh signaling transcription factor) and HAb18G/CD147 were found to mediate TGF-β driven EMT in HCC cells (83, 99), and HAb18G/CD147 expression in patient samples was positively correlated with mesenchymal markers and negatively related to epithelial markers (83). The positive role of Hh signaling in liver cancer cell EMT was also confirmed in a different study (12). Interestingly, the role of microRNAs (miRNAs) in HCC EMT has been rigorously studied as well. miRNA-216a/217 and miRNA-490-3p expression were reported to be upregulated in HCC tissues and also induced EMT in epithelial HCC cells (84, 101). On the other hand, several other miRNAs (such as miRNA-491, miRNA-612, and miRNA-200b) have been reported to inhibit or suppress EMT in HCC cells, and the expression of miRNA-491 and miRNA-612 was inversely associated with liver tumor differentiation and EMT (20, 74, 101). In fact, a tumor suppressor (p53) was demonstrated to suppress liver cancer EMT by upregulating miRNAs, including miR-200 and miR-192 family members (32). More information about the role of miRNAs in HCC can be found in a recent review (82). Despite all this progress that consistently demonstrates an important role for EMT in hepatocarcinogenesis, there are still many questions to be answered to definitely elucidate the role of EMT in the progression of premalignant human liver diseases, such as cirrhosis. Improved knowledge can lead to exploration of potential clinical targets for “EMT pathways” to improve the outcome of hepatocellular carcinoma.
Summary
EMT and MET were originally described as mechanisms for tissue construction during embryogenesis. Subsequently, they were suggested to play important roles during adult tissue remodeling responses, including fibrosis and cancer progression. Under certain cell culture conditions, there is no doubt that several types of resident adult liver cells and some liver progenitor cells are capable of undergoing EMT and/or MET (Fig. 1). These findings raise the possibility that EMT/MET might be involved in liver repair and regeneration. However, despite significant efforts made to determine whether EMT/MET occur during liver injury in vivo, this subject remains highly controversial and many of the data accumulated thus far are contradictory. A new concept called “escape program” or “escape reaction” was proposed to provide an alternative explanation for the complex phenotypical changes observed in EMT/MET (58, 60). This hypothesis was first described in malignant cells and suggested that such cells increase motility to escape dangers like oxidative stress, but do not fully differentiate into myofibroblasts (i.e., partial EMT might be a survival strategy) (57). Pinzani (60) introduced this concept to the liver field and he proposed that hepatocytes might also acquire a migratory phenotype during tissue injury to escape hostile microenvironment. The “escaping” hepatocytes either migrate to a less hostile microenvironment and reacquire their original epithelial phenotype, or undergo apoptosis if the hostile microenvironment persists (60). In any case, given the current level of confusion, further research is warranted to determine the significance of EMT/MET for liver repair. With the aid of more sophisticated techniques to map cell fate, as well advances in EMT research in other organs/tissues, the knowledge we gain might help us design novel diagnostic and therapeutic strategies to prevent and cure liver damage.
GRANTS
This work was supported by National Institutes of Health grant R01-DK077794 (A. M. Diehl).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.X. prepared figures; G.X. drafted manuscript; A.M.D. edited and revised manuscript; A.M.D. approved final version of manuscript.
REFERENCES
- 1.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119: 1438–1449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkari L, Gregoire D, Floc'h N, Moreau M, Hernandez C, Simonin Y, Rosenberg AR, Lassus P, Hibner U. Hepatitis C viral protein NS5A induces EMT and participates in oncogenic transformation of primary hepatocyte precursors. J Hepatol 57: 1021–1028, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Asahina K, Tsai SY, Li P, Ishii M, Maxson RE, Jr, Sucov HM, Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology 49: 998–1011, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 53: 983–995, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balli D, Ustiyan V, Zhang Y, Wang IC, Masino AJ, Ren X, Whitsett JA, Kalinichenko VV, Kalin TV. Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition. EMBO J 32: 231–244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertran E, Crosas-Molist E, Sancho P, Caja L, Lopez-Luque J, Navarro E, Egea G, Lastra R, Serrano T, Ramos E, Fabregat I. Overactivation of the TGF-beta pathway confers a mesenchymal-like phenotype and CXCR4-dependent migratory properties to liver tumor cells. Hepatology 2013. June 28. 10.1002/hep.26597 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 120: 4040–4054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caiado F, Carvalho T, Rosa I, Remedio L, Costa A, Matos J, Heissig B, Yagita H, Hattori K, da Silva JP, Fidalgo P, Pereira AD, Dias S. Bone marrow-derived CD11b+Jagged2+ cells promote epithelial-to-mesenchymal transition and metastasization in colorectal cancer. Cancer Res 73: 4233–4246, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Caja L, Bertran E, Campbell J, Fausto N, Fabregat I. The transforming growth factor-beta (TGF-beta) mediates acquisition of a mesenchymal stem cell-like phenotype in human liver cells. J Cell Physiol 226: 1214–1223, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Castilho-Fernandes A, de Almeida DC, Fontes AM, Melo FU, Picanco-Castro V, Freitas MC, Orellana MD, Palma PV, Hackett PB, Friedman SL, Covas DT. Human hepatic stellate cell line (LX-2) exhibits characteristics of bone marrow-derived mesenchymal stem cells. Exp Mol Pathol 91: 664–672, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, Arendt LM, Kuperwasser C, Bierie B, Weinberg RA. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA 108: 7950–7955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Lingala S, Khoobyari S, Nolta J, Zern MA, Wu J. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J Hepatol 55: 838–845, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Zheng S, Qi D, Zheng S, Guo J, Zhang S, Weng Z. Inhibition of Notch signaling by a gamma-secretase inhibitor attenuates hepatic fibrosis in rats. PLoS One 7: e46512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YL, Lv J, Ye XL, Sun MY, Xu Q, Liu CH, Min LH, Li HP, Liu P, Ding X. Sorafenib inhibits transforming growth factor beta1-mediated epithelial-mesenchymal transition and apoptosis in mouse hepatocytes. Hepatology 53: 1708–1718, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology 50: 2007–2013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SS, Omenetti A, Syn WK, Diehl AM. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol 43: 238–244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, Sudan DL, Sicklick JK, Michelotti GA, Rojkind M, Diehl AM. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol 297: G1093–G1106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 53: 1685–1695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conigliaro A, Amicone L, Costa V, De Santis Puzzonia M, Mancone C, Sacchetti B, Cicchini C, Garibaldi F, Brenner DA, Kisseleva T, Bianco P, Tripodi M. Evidence for a common progenitor of epithelial and mesenchymal components of the liver. Cell Death Differ 20: 1116–1123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding W, Dang H, You H, Steinway S, Takahashi Y, Wang HG, Liao J, Stiles B, Albert R, Rountree CB. miR-200b restoration and DNA methyltransferase inhibitor block lung metastasis of mesenchymal-phenotype hepatocellular carcinoma. Oncogenesis 1: e15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A, Breitkopf K, Weng H, Mertens PR. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology 135: 642–659, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Franco DL, Mainez J, Vega S, Sancho P, Murillo MM, de Frutos CA, Del Castillo G, Lopez-Blau C, Fabregat I, Nieto MA. Snail1 suppresses TGF-beta-induced apoptosis and is sufficient to trigger EMT in hepatocytes. J Cell Sci 123: 3467–3477, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Fu J, Chen Y, Cao J, Luo T, Qian YW, Yang W, Ren YB, Su B, Cao GW, Yang Y, Yan YQ, Shen F, Wu MC, Feng GS, Wang HY. p28GANK overexpression accelerates hepatocellular carcinoma invasiveness and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1alpha pathways. Hepatology 53: 181–192, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Galichon P, Hertig A. Epithelial to mesenchymal transition as a biomarker in renal fibrosis: are we ready for the bedside? Fibrogenesis Tissue Repair 4: 11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, Tam WL, Mani SA, van Oudenaarden A, Weinberg RA. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148: 1015–1028, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harada K, Sato Y, Ikeda H, Isse K, Ozaki S, Enomae M, Ohama K, Katayanagi K, Kurumaya H, Matsui A, Nakanuma Y. Epithelial-mesenchymal transition induced by biliary innate immunity contributes to the sclerosing cholangiopathy of biliary atresia. J Pathol 217: 654–664, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem 282: 22089–22101, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 119: 1417–1419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang J, Kim E, Kim W, Seong KM, Youn H, Kim JW, Kim J, Youn B. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines. J Biol Chem 288: 27343–27357, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HM, Haraguchi N, Ishii H, Ohkuma M, Okano M, Mimori K, Eguchi H, Yamamoto H, Nagano H, Sekimoto M, Doki Y, Mori M. Increased CD13 expression reduces reactive oxygen species, promoting survival of liver cancer stem cells via an epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol 19, Suppl 3: S539–S548, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, Alder H, Liu CG, Dejean A, Croce CM. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med 208: 875–883, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kisseleva T, Brenner DA. Is it the end of the line for the EMT? Hepatology 53: 1433–1435, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216–233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kordes C, Sawitza I, Gotze S, Haussinger D. Hepatic stellate cells support hematopoiesis and are liver-resident mesenchymal stem cells. Cell Physiol Biochem 31: 290–304, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Kordes C, Sawitza I, Muller-Marbach A, Ale-Agha N, Keitel V, Klonowski-Stumpe H, Haussinger D. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun 352: 410–417, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Kretzschmar K, Watt FM. Lineage tracing. Cell 148: 33–45, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest 121: 468–474, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamouille S, Subramanyam D, Blelloch R, Derynck R. Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell Biol 25: 200–207, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Maitah MY, Ahmad A, Kong D, Bao B, Sarkar FH. Targeting the Hedgehog signaling pathway for cancer therapy. Exp Opin Ther Targets 16: 49–66, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci USA 110: 2324–2329, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Eischeid AN, Chen XM. Col1A1 production and apoptotic resistance in TGF-beta1-induced epithelial-to-mesenchymal transition-like phenotype of 603B cells. PLoS One 7: e51371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Li J, Xiong J, Li M, Zhang Y, Zheng Q. Notch-dependent expression of epithelial-mesenchymal transition markers in cholangiocytes after liver transplantation. Hepatol Res 42: 1024–1038, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Loo CK, Wu XJ. Origin of stellate cells from submesothelial cells in a developing human liver. Liver Int 28: 1437–1445, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology 40: 1151–1159, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer C, Dzieran J, Liu Y, Schindler F, Munker S, Muller A, Coulouarn C, Dooley S. Distinct dedifferentiation processes affect caveolin-1 expression in hepatocytes. Cell Commun Signal 11: 6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer C, Liu Y, Kaul A, Peipe I, Dooley S. Caveolin-1 abrogates TGF-beta mediated hepatocyte apoptosis. Cell Death Dis 4: e466, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Kruger L, Premont R, Yang L, Syn WK, Metzger D, Diehl AM. Smoothened is a master regulator of adult liver repair. J Clin Invest 123: 2380–2394, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mutsaers SE. The mesothelial cell. Int J Biochem Cell Biol 36: 9–16, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Nitta T, Kim JS, Mohuczy D, Behrns KE. Murine cirrhosis induces hepatocyte epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology 48: 909–919, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogunwobi OO, Liu C. Therapeutic and prognostic importance of epithelial-mesenchymal transition in liver cancers: insights from experimental models. Crit Rev Oncol Hematol 83: 319–328, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Omenetti A, Bass LM, Anders RA, Clemente MG, Francis H, Guy CD, McCall S, Choi SS, Alpini G, Schwarz KB, Diehl AM, Whitington PF. Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology 53: 1246–1258, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol 54: 366–373, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest 118: 3331–3342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, Hardiman G, Karin M, Brenner DA. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci USA 108: 308–313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pani G, Galeotti T, Chiarugi P. Metastasis: cancer cell's escape from oxidative stress. Cancer Metastasis Rev 29: 351–378, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Pani G, Giannoni E, Galeotti T, Chiarugi P. Redox-based escape mechanism from death: the cancer lesson. Antiox Redox Signal 11: 2791–2806, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Macias D, Guadix JA, Munoz-Chapuli R. Contribution of mesothelium-derived cells to liver sinusoids in avian embryos. Dev Dyn 229: 465–474, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Pinzani M. Epithelial-mesenchymal transition in chronic liver disease: fibrogenesis or escape from death? J Hepatol 55: 459–465, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Robertson H, Kirby JA, Yip WW, Jones DE, Burt AD. Biliary epithelial-mesenchymal transition in posttransplantation recurrence of primary biliary cirrhosis. Hepatology 45: 977–981, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Rowe RG, Lin Y, Shimizu-Hirota R, Hanada S, Neilson EG, Greenson JK, Weiss SJ. Hepatocyte-derived Snail1 propagates liver fibrosis progression. Mol Cell Biol 31: 2392–2403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rygiel KA, Robertson H, Marshall HL, Pekalski M, Zhao L, Booth TA, Jones DE, Burt AD, Kirby JA. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab Invest 88: 112–123, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Saad S, Stanners SR, Yong R, Tang O, Pollock CA. Notch mediated epithelial to mesenchymal transformation is associated with increased expression of the Snail transcription factor. Int J Biochem Cell Biol 42: 1115–1122, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Sato Y, Harada K, Ozaki S, Furubo S, Kizawa K, Sanzen T, Yasoshima M, Ikeda H, Sasaki M, Nakanuma Y. Cholangiocytes with mesenchymal features contribute to progressive hepatic fibrosis of the polycystic kidney rat. Am J Pathol 171: 1859–1871, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sawitza I, Kordes C, Reister S, Haussinger D. The niche of stellate cells within rat liver. Hepatology 50: 1617–1624, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, Weinberg RA. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145: 926–940, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheel C, Weinberg RA. Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int J Cancer 129: 2310–2314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology 139: 987–998, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sicklick JK, Choi SS, Bustamante M, McCall SJ, Perez EH, Huang J, Li YX, Rojkind M, Diehl AM. Evidence for epithelial-mesenchymal transitions in adult liver cells. Am J Physiol Gastrointest Liver Physiol 291: G575–G583, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, Wang XH, Du J, Liu YX, Sun BC. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology 51: 545–556, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Suzuki A, Sekiya S, Onishi M, Oshima N, Kiyonari H, Nakauchi H, Taniguchi H. Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology 48: 1964–1978, 2008 [DOI] [PubMed] [Google Scholar]
- 72a.Swiderska-Syn M, Syn W, Xie G, Krüger L, Machado M, Karaca G, Michelotti GA, Choi SS, Premont RT, Diehl AM. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, Wang J, Witek RP, Fearing CM, Pereira TA, Teaberry V, Choi SS, Conde-Vancells J, Karaca GF, Diehl AM. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology 137: 1478–1488e8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tao ZH, Wan JL, Zeng LY, Xie L, Sun HC, Qin LX, Wang L, Zhou J, Ren ZG, Li YX, Fan J, Wu WZ. miR-612 suppresses the invasive-metastatic cascade in hepatocellular carcinoma. J Exp Med 210: 789–803, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, Brenner DA. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology 51: 1027–1036, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology 30: 1425–1433, 1999 [DOI] [PubMed] [Google Scholar]
- 77.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131–142, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 18: 99–115, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, Pradere JP, Friedman RA, Schwabe RF. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 143: 1073–1083e22, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Zijl F, Zulehner G, Petz M, Schneller D, Kornauth C, Hau M, Machat G, Grubinger M, Huber H, Mikulits W. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol 5: 1169–1179, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong CM, Kai AK, Tsang FH, Ng IO. Regulation of hepatocarcinogenesis by microRNAs. Front Biosci 5: 49–60, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z, Huang XF, Chen ZN, Bian H. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-beta signaling and is transcriptionally regulated by Slug. Oncogene 30: 4410–4427, 2011 [DOI] [PubMed] [Google Scholar]
- 84.Xia H, Ooi LL, Hui KM. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology 58: 629–641, 2013 [DOI] [PubMed] [Google Scholar]
- 85.Xie G, Karaca G, Swiderska-Syn M, Michelotti GA, Kruger L, Chen Y, Premont RT, Choi SS, Diehl AM. Cross-talk between notch and hedgehog regulates hepatic stellate cell fate. Hepatology 58: 1801–1813, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19: 156–172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu Q, Isaji T, Lu Y, Gu W, Kondo M, Fukuda T, Du Y, Gu J. Roles of N-acetylglucosaminyltransferase III in epithelial-to-mesenchymal transition induced by transforming growth factor beta1 (TGF-beta1) in epithelial cell lines. J Biol Chem 287: 16563–16574, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yan W, Fu Y, Tian D, Liao J, Liu M, Wang B, Xia L, Zhu Q, Luo M. PI3 kinase/Akt signaling mediates epithelial-mesenchymal transition in hypoxic hepatocellular carcinoma cells. Biochem Biophys Res Commun 382: 631–636, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14: 818–829, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Yang L, Jung Y, Omenetti A, Witek RP, Choi S, Vandongen HM, Huang J, Alpini GD, Diehl AM. Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers. Stem Cells 26: 2104–2113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, Peng WL, Wu JC. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology 50: 1464–1474, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology 47: 636–647, 2008 [DOI] [PubMed] [Google Scholar]
- 93.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24: 5764–5774, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119: 1429–1437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 282: 23337–23347, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Zhang J, Zhang H, Liu J, Tu X, Zang Y, Zhu J, Chen J, Dong L, Zhang J. miR-30 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochem Biophys Res Commun 417: 1100–1105, 2012 [DOI] [PubMed] [Google Scholar]
- 97.Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X, Dong J, Qian C. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor-1alpha in hepatocellular carcinoma. BMC Cancer 13: 108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C, Xie S, Chen C, Hu L, Xu S, Liang T. Wnt/beta-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1alpha signaling. Carcinogenesis 34: 962–973, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Zheng X, Vittar NB, Gai X, Fernandez-Barrena MG, Moser CD, Hu C, Almada LL, McCleary-Wheeler AL, Elsawa SF, Vrabel AM, Shire AM, Comba A, Thorgeirsson SS, Kim Y, Liu Q, Fernandez-Zapico ME, Roberts LR. The transcription factor GLI1 mediates TGFbeta1 driven EMT in hepatocellular carcinoma via a SNAI1-dependent mechanism. PLoS One 7: e49581, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou Q, Wang Y, Peng B, Liang L, Li J. The roles of Notch1 expression in the migration of intrahepatic cholangiocarcinoma. BMC Cancer 13: 244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Y, Li Y, Ye J, Jiang R, Yan H, Yang X, Liu Q, Zhang J. MicroRNA-491 is involved in metastasis of hepatocellular carcinoma by inhibitions of matrix metalloproteinase and epithelial to mesenchymal transition. Liver Int 33: 1271–1280, 2013 [DOI] [PubMed] [Google Scholar]
- 102.Zhu L, Wang L, Wang X, Luo X, Yang L, Zhang R, Yin H, Xie D, Pan Y, Chen Y. Hepatic deletion of Smad7 in mouse leads to spontaneous liver dysfunction and aggravates alcoholic liver injury. PLoS One 6: e17415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zucchini-Pascal N, Peyre L, de Sousa G, Rahmani R. Organochlorine pesticides induce epithelial to mesenchymal transition of human primary cultured hepatocytes. Food Chem Toxicol 50: 3963–3970, 2012 [DOI] [PubMed] [Google Scholar]