Abstract
Dendritic spines are the primary postsynaptic sites of excitatory neurotransmission in the brain. They exhibit a remarkable morphological variety, ranging from thin protrusions, to stubby shapes, to bulbous mushroom shapes. The remodeling of spines is thought to regulate the strength of the synaptic connection, which depends vitally on the number and the spatial distribution of AMPA-type glutamate receptors (AMPARs). We present numerical and analytical analyses demonstrating that this shape strongly affects AMPAR diffusion. We report a pronounced suppression of the receptor exit rate out of spines with decreasing neck radius. Thus, mushroomlike spines become highly effective at retaining receptors in the spine head. Moreover, we show that the postsynaptic density further enhances receptor trapping, particularly in mushroomlike spines local exocytosis in the spine head, in contrast to release at the base, provides rapid and specific regulatory control of AMPAR concentration at synapses.
Introduction
The AMPA-type glutamate receptors (AMPARs) are responsible for fast responses at excitatory synapses, and various signaling pathways and numerous regulatory proteins modulate the properties of synaptic AMPARs (1,2). The number and density of AMPARs at postsynaptic sites is widely regarded to be a key determinant of synaptic strength and is affected during various neurodegenerative diseases (3). The amount of AMPARs at these synaptic sites is not fixed and dynamic changes in AMPARs underlie long-lasting changes in synaptic transmission such as long-term potentiation and long-term depression (3,4). It is therefore of fundamental importance to control the local abundance of AMPARs at synapses. At least three cellular processes govern the density and distribution of synaptic AMPA receptors: local endocytosis and exocytosis from intracellular compartments (5), lateral diffusion from the surface (6), and immobilization at synapses by anchoring at the postsynaptic density (PSD) (7). In most models, these different trafficking steps are all required to eventually reach the synaptic membrane, although the relevance of exocytosis on the spine membrane has remained controversial (8,9).
AMPAR-dependent excitatory sites are typically located on dendritic spines, small membranous protrusions located along the dendrite’s length. Dendritic spines contain a PSD, which contains scaffold proteins that regulate the clustering of surface AMPARs, and dedicated zones for endocytosis and exocytosis positioned just lateral to the PSD (5,7). Electron-microscopy studies have revealed a rich diversity of shapes that can roughly be divided into three shape categories: thin, filopodialike protrusions (thin spines); short spines without a well-defined spine neck (stubby spines); and spines with a large bulbous head (mushroom spines) (10). Previous work has reported on various correlations between changes in the spine morphology and functional parameters of synapses (11–15). For example, the spine head volume is correlated with the area of the PSD and the number of postsynaptic receptors (12,16,17). In addition, the spine neck has been related to the time constant of calcium compartmentalization and filtering of electrical potentials (18–20). Theoretical models confirmed that the spine neck can regulate diffusional coupling between spines and dendrites (21,22). Given that spine geometry influences intracellular dynamics, it is also possible that spine morphology directly affects the dynamics of AMPAR lateral diffusion to and from the synapse.
Previous theoretical work on AMPAR trafficking has mainly examined flat, two-dimensional geometries when surface diffusion, internal recycling, and the role of the PSD in AMPAR trapping (21,23–25) were studied. For example, it has recently been demonstrated that controlling the AMPAR/scaffold binding and unbinding rates in the PSD allows enrichment of AMPARs at postsynaptic sites (26). However, a quantitative model integrating the three-dimensional morphological variability of dendritic spines into mechanisms that control AMPAR trafficking at synapses is lacking. In this study, we build a quantitative model integrating the morphological variability of realistic spine geometries into mechanisms that control AMPAR trafficking at postsynaptic sites. Our stimulations allow, the direct observation of how two trapping mechanisms—the PSD and spine shape—work together to control the number and spatial distribution of AMPARs for the regulation of synaptic strength.
Methods
Modeling the surface diffusion of AMPA receptors
Electron microscopy of dendrites reports spines with a wide variety of shapes, ranging from thin spines with lengths of 2–4 μm and a radius of 0.1–0.5 μm, to mushroomlike spines that have a relatively thin neck with a radius of 0.05–0.2 μm and a large head with a radius of 0.5–1 μm (12,27). Other observed shapes such as the very thin filopodium to branched spines are rarer, and will not be considered explicitly although the same principles as those we present here apply to these shapes. The functional domain of the spines, associated with the electron-dense region in the PSD, is generally situated at the top of the spine. In this region of the spine, AMPA receptors can be immobilized via interactions with scaffold proteins. The parameterization of the shape that we will be using in this article effectively covers a large fraction of different spine shapes observed in experiments (27). The axisymmetric body that we use is shown in Fig. 1, and is parameterized as
| (1) |
where R is the maximal radial distance of the surface representing the radius of the head of the spine and B is a measure of the height of the spine. Varying A, B, and R allows one a transition from stubby, via thin, to mushroomlike morphologies. The bottom of the spine is connected to the dendritic membrane. Detailed information about this shape—including its metric tensor and Christoffel symbols—may be found in the Supporting Material.
Figure 1.
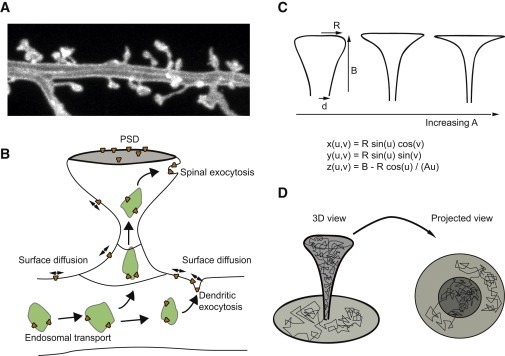
Modeling the surface diffusion of AMPA receptors in the dendritic spine. (A) Image of dendritic spines from cultured rat hippocampal neurons. (B) Illustration depicting the main processes involved in targeting receptors to the PSD. (C) Parameterization of spine morphology that we use in Figs. 2–4. The shape can be varied from stubby to elongated and mushroomlike. (D) Simulated trajectory of a membrane-bound receptor on the curved dendritic membrane. Whereas the local receptor density on the membrane is constant, a local enrichment in spines is misleadingly suggested in the projected view. This is analogous to what happens on cylindrical substrates (39). To see this figure in color, go online.
Random walk simulation on a curved surface
We simulate random walks as trajectories consisting of fixed-length steps in completely random directions. On a curved surface, both the selection of a random direction as well as traveling a fixed distance in this direction requires some careful thought. Naively picking a direction and step size on the surface will result in top-seeking motion as a result of a singularity in the parameterization of the surface. A truly random directional vector of unit length must be chosen subject to the constraint
(for rotationally symmetric systems, gij = 0 if i ≠ j). The length of this random vector in local coordinates u,v is thus
and
where ρ is picked randomly between 0 and 2π.
Next, we construct the geodesic curve parameterized by the arc length s (28), and apply a second-order approximation of the tangential plane,
| (2) |
where the first derivative is the unit tangent vector and the second-order correction is obtained by solving the local geodesic equation for the surface
| (3) |
with Γikl for the Christoffel symbols of the surface, and xi is the i-component of (see the Supporting Material for an explicit demonstration of this). Rewriting this to our parameterization, a step of size λ, i.e.,
in the local coordinates (u,v) is achieved by a shift in coordinates
where the index q labels the discrete iteration step number, and
| (4) |
The resultant diffusive motion is, by construction, locally Brownian (〈x2〉 = 4Dt) to second-order in the curvature (see the Supporting Material). In Fig. 1 D, we graph a sample trajectory of a single receptor on the spinal and dendritic membrane. In our simulations we measure the average number of steps N of length λ it takes to arrive at the absorbing boundary. These quantities can be related to an effective mean escape time through
| (5) |
where D is the diffusion constant.
Mean first-passage time calculations
To analyze the effects of geometry and curvature on the confinement of receptors theoretically, we calculate the mean first-passage time (MFPT). This MFPT may be considered a characteristic timescale for the diffusion in a system with absorbing boundaries. The literature abounds with treatises on how to compute it analytically (see, for instance, Berg and Purcell (29) and Linderman and Lauffenburger (30) and, more recently, Holcman and Triller (21) and Holcman and Schuss (22,31)) for geometries such as spheres and surfaces of revolution.
The MFPT W is obtained by solving the differential equation
| (6) |
where D is the two-dimensional constant and ∇2 is the Laplace-Beltrami operator. In general, the difficulty of solving Eq. 6 lies in the nonlinearity of the Laplace-Beltrami operator. This Laplace-Beltrami operator for the parameterization we have chosen can be derived analytically, as we discuss in the Supporting Material. For a flat Euclidian space, this operator reduces to the Laplace operator because there are no nonzero Christoffel symbols for flat geometries. In the trivial case of a particle starting at the center of a circle of radius R, the MFPT for being absorbed by the boundary of the circle is equal to R2/(4D). The Laplace-Beltrami operator for nontrivially curved surfaces such as those given by Eq. 1 may also be calculated analytically (30). We will solve the equation for the resultant MFPT (Eq. 6) numerically. To calculate the MFPT as was later shown in Fig. 3, we should solve Eq. 6 subject to the boundary conditions
| (7) |
where Ωref is the reflecting boundary and Ωabs is the absorbing boundary (see the Supporting Material).
Figure 3.
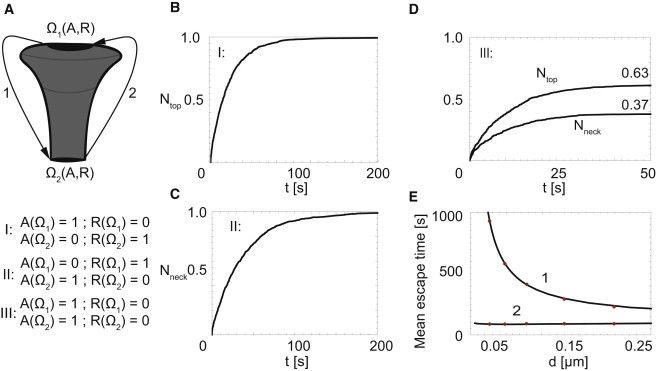
Shape-dependent asymmetry between capture and leakage dynamics. (A) A spinal surface, consisting of two equally sized absorbing regions, one at the base of the neck and one at the top of the spine (R = 1 μm, B = 4 μm, d = 0.12 μm, D = 0.1 μm2/s; see Fig. 1C). (B–D) At t = 0, the local receptor concentration is homogeneously distributed over the surface of the spine. (B) Time-evolution of capture at Ω1 upon rendering this region absorbing. (C) Time-evolution of capture at Ω2 upon rendering this region absorbing. (D) Time-evolution of capture at Ω1 and Ω2 upon rendering both regions absorbing. (E) Neck-size dependence of the mean escape time of receptors released at the reflecting boundary Ω1 (1) or Ω2 (2) and captured by the absorbing boundary Ω2 (1) or Ω1 (2), respectively. (Dots) Simulation results; (continuous line) analytical results based on MFPT. To see this figure in color, go online.
Results
Modeling diffusion on curved membranes
We model the lateral diffusion of surface AMPA receptors by simulating fixed step-length random walks of noninteracting hard-core particles on the curved surface of the dendritic membrane. On such curved surfaces, proper implementation of the random walks requires careful thought because both the selection of a random angle as well as implementing the fixed step length depends on the ambient geometry. We expand the surface around a point of departure up to second-order in the local curvature, a procedure that was proven to yield correct diffusive behavior provided that the step size is small compared to the smallest length-scale in curvature (see Methods and Christensen (28)). In rare cases, one may forego simulations and obtain analytical results for the MFPT (21,22,31). We present several cases where this could be obtained for our system, allowing validation of our simulation protocol (see Methods).
To properly capture the effects of the specific shape of a dendritic spine, while retaining a surface that is computationally manageable (i.e., that may be straightforwardly parameterized), we distill from the considerable variety of shapes (see Fig. 1 A) the most notable classes of spine morphologies, including thin, stubby, and mushroomlike (see Fig. 1 B (12,32,33)). We next generated trajectories of receptors diffusing across the surface of these various spine shapes and found that the local, time-averaged density of receptors is constant in equilibrium, as expected for diffusion. Nevertheless, an apparent local enrichment was observed in an orthogonal projection, resulting from an increased projected surface area perpendicular to the direction of observation.
Dendritic spine morphology controls AMPAR exit dynamics
We next tested whether the shape of dendritic spines can directly affect lateral diffusion of surface AMPA receptors. We therefore simulated the temporal distribution of the receptor density in various domains for one particular type of spine and its surrounding dendritic membrane as depicted in Fig. 2 A (R = 1 μm, B = 4 μm, d = 0.12 μm, D = 0.1 μm2/s; see Fig. 1 C). Fig. 2 B shows what happens following the release of 1000 receptors at the top of the spine at t = 0. For a mushroomlike spine, and realistic parameter values, it takes ∼500 s before all locally measured concentrations of receptors in the spine are equilibrated with the surrounding dendritic membrane (assuming a reflecting boundary positioned at a distance of 1 μm from the center of the base of the spine to prevent all receptors from leaving the simulation domain). This simulation shows that the shape in itself cannot retain gradients in receptor density indefinitely—at some point, it must come to a uniform equilibrium.
Figure 2.
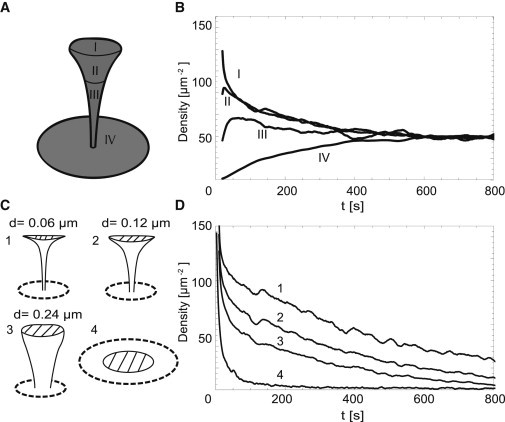
Spine shape influences exit dynamics of receptors. (A) Domains used to analyze density evolution in panel B (R = 1 μm, B = 4 μm, d = 0.12 μm, D = 0.1 μm2/s; see Fig. 1C). (B) Time evolution of receptor density in domains I–IV after the release of 1000 receptors at the top of the spine. Reflecting boundary was positioned at a distance of 1 μm from the center of the base of the spine. (C) Four different spine geometries used in panel D. (1–3: R = 1 μm, B = 4 μm, D = 0.1 μm2/s; 4: RPSD = 0.71 μm, R1 = 2.35 μm, D = 0.1 μm2/s; see Fig. 1C.) (D) Time evolution of receptor density (dashed area). One-thousand receptors were released in the center of this region at t = 0.
To examine how the time until equilibrium depends on the morphology of the spine, we measured the progression of receptor movements following the release of 1000 receptors in the region at the top of the spine (dashed region in Fig. 2 C) for three distinct spine shapes. The spine morphologies each have the same height and same total surface area in the head of the spine to ensure that any effect we measure reflects how the surface is arranged in space. The surface area of the neck is slightly larger for the wider necks. Keeping the combined surface area of neck and head constant and renormalizing the height of the spine yields qualitatively similar results. Increasing the size of the head, as it occurs in spine maturation, is not explicitly covered here—it would add a trivial increase in the residence time due to the increased surface area in the head. Fig. 2 D shows that spines containing a thin neck (mushroomlike spines) are able to retain elevated concentrations of receptors after release approximately five times longer than stubby shapes, and between 10 and 20 times longer than planar structures of the same total area. These results demonstrate that the overall shape of a spine, rather than just the area and neck length, controls the equilibration time of receptors and that mushroomlike spines have strongly reduced receptor exit rates.
Dendritic spine shape controls asymmetry between PSD capture and escape
To mediate synaptic transmission and intracellular signaling, AMPARs are positioned at the postsynapse through interactions with the postsynaptic density (PSD) (7,34–36). We therefore next investigated how the efficiency of AMPAR capturing at the PSD depends on the geometry of dendritic spines. We prepared a spinelike structure with an absorbing PSD region, as illustrated in Fig. 3 A, and simulate diffusive equilibration using three distinct settings:
-
1.
With an absorbing zone at the top of the spine and a reflecting structure at the base of the neck;
-
2.
With an absorbing zone at the bottom and a reflecting structure at the top of the spine; and
-
3.
With absorbing zones at both top and bottom.
At t = 0, 1000 receptors are equally distributed on the surface of the spine (R = 1 μm, B = 4 μm, d = 0.12 μm, D = 0.1 μm2/s; see Fig. 1 C), after which we record the temporal capture at the top of the spine (Fig. 3 B) and escape through the neck of the spine (Fig. 3 C), respectively. For an unbiased comparison, the absorbing PSD zone at the top and the one at the bottom are of equal perimeter size. The reflective boundary settings simply serve to focus our attention on either PSD capture or base escape, preventing exit elsewhere.
We observed a striking difference in timescales: receptor capture at the top occurs roughly twice as fast as leak-out at the bottom demonstrating the asymmetry induced by the specific morphology of the spine affects AMPA receptor distributions. Similarly, when—after equilibration—both boundaries are rendered absorbing (representing the realistic condition that receptors either escape or end up in the PSD, see Fig. 3 C), the flux into the PSD domain at the top is larger than that out of the spine at the base.
To further quantify these effects, we calculate the mean time it takes 1000 receptors starting at the top of the spine to escape through the base of the neck (red dots in Fig. 3 E (1)) or the time it takes for the reverse process to complete: we show receptors released at the reflecting base of the neck that end up captured in the PSD (red dots in Fig. 3 E (2)). We measure a strong dependence of the outflow of receptors on the neck radius: MFPT ∼ (neck radius)−0.7. Mushroomlike spines—those with the smallest neck diameters—are thus significantly more effective at retaining receptors. For this particular setting, we also computed the MFPT directly (solid black line in Fig. 3 E) to validate our numerical method. We find that when receptors are released homogeneously throughout the surface of the spine, the PSD together with the shape of a spine allows efficient capturing of AMPA receptors in the spine head.
Exocytosis in spines is more efficient than exocytosis on the dendritic shaft
Besides AMPAR surface diffusion and PSD trapping, intracellular trafficking and recycling of AMPARs is also important for synaptic strength (3,34). Here, AMPARs containing vesicles are transported toward the plasma membrane and released by exocytosis at specific sites, either at the dendrite shaft or in the dendritic spine (5,37). Given these two different locations of AMPAR exocytosis, we determine which mechanism is most efficient in concentrating AMPA receptors at the postsynaptic membrane. To quantify the process, we record the temporal evolution of the absorption of receptors in the PSD after the localized release of 1000 receptors, for different locations around the PSD (see Fig. 4 A, I–III). We employ similar simulation settings as before, allowing receptors to get absorbed at the PSD and diffuse freely elsewhere. To isolate the effect of morphology, we repeat this exercise for a planar geometry and for spines with different neck radii (d = 0.24 μm and d = 0.06 μm; see Fig. 1 C).
Figure 4.
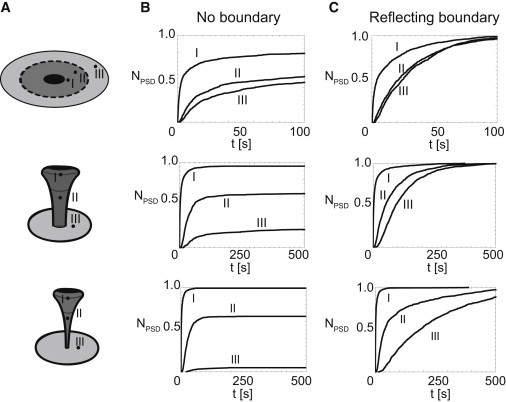
Position of exocytosis and shape of the spine strongly alter the dynamics and efficiency of receptor capturing by the PSD. (A) Three different geometries used to analyze effect of the exocytotic position (R = 1 μm, B = 4 μm, D = 0.1 μm2/s; see Fig. 1C). (B and C) Fraction of absorbed receptors over time for three different shapes after exocytosis at positions I, II, or III, as depicted in panel A. Reflecting boundaries were positioned at infinity (B) or at a distance of 1 μm from the center of the base of the spine (C). One-thousand receptors are released on various positions (close to the head of the spine (I), in the neck of the spine (II), and on a 0.5-μm distance from the centrum of the base of the spine on the dendritic membrane (III)). We consider three different shapes, these being a spine with a neck radius of 0.06 μm, 0.24 μm, and a planar shape. In the flat geometry the total surface area in the PSD/Spine/Dendrite is the same as in the spine, with a neck radius of 0.24 μm (release I at 1.2 μm, II at 2.2 μm, and III at 2.4 μm from the center). These figures show that, especially for mushroomlike spines, exocytosis close to the top of the spine results in a much faster and more efficient capturing of receptors. To see this figure in color, go online.
Fig. 4 B shows that release close to the PSD—especially for mushroomlike spines with a thin neck—results in nearly all receptors captured in the PSD, while for exocytosis in the neck of the spine substantially fewer receptors end up in the PSD. If the receptors are exocytosed 0.5 μm away from the base of the spine, almost none of them end up at the PSD. In short, release of AMPARs close to the PSD is markedly more efficient than release at the dendritic membrane. Thus, AMPAR exocytosis in the spine heads provides rapid and specific control of AMPA receptors concentration at the PSD. Importantly, this effect is not simply a result of the proximity; although the effect is still observed in a planar setting (Fig. 4 B), the outcome is much weaker. Because all surface areas are kept constant, the advantage of exocytosis within the spine head is a direct consequence of the specific spine geometry.
The location of exocytosis and the shape of the spine do not only influence the efficiency of AMPAR trafficking, they also influence the timescale for PSD capture. To quantify this effect we now employ a reflecting boundary at a distance 1 μm from the base of the spine and measure the time it takes to absorb the receptors in the PSD, again as a function of the location of the exocytosis. Fig. 4 C shows that in the presence of the reflecting boundary, all receptors are ultimately absorbed at the PSD. However, there is a remarkable difference in the timescale for this capture process. For a typical mushroomlike spine, >90% of the receptors are captured within seconds. For release in the neck, this takes 10 times as long, and for release at the base it may take hundreds or even thousands of seconds. Again, the effect strongly depends on the morphology of the spine. Thus, our modeling demonstrates that exocytosis on the spine membrane is a very effective way to deliver AMPA receptors to postsynaptic membranes.
Discussion
In this study we have developed a quantitative model integrating the morphological variability of realistic spine geometries into mechanisms that control AMPAR trafficking at postsynaptic sites. Our simulations reveal how two trapping mechanisms—anchoring at the PSD and diffusion on specific curved geometries—work together to control the number and spatial distribution of AMPARs for regulation of synaptic strength. The model demonstrates that AMPAR trafficking is a coordinated action among surface diffusion, local exocytosis, and trapping at the PSD within the geometric restrictions of individual dendritic spines.
We have examined the influence of spine morphology on three processes related to AMPAR trafficking:
-
1.
We determined the exit dynamics of surface receptors through the neck of the spine and found a steep power-law suppression of the rate of outflow of receptors as a function of neck size. Therefore, mushroomlike spines are more effective at retaining gradients in receptor concentration after local release within the spine head when compared to stubby or elongated spines. Recently, experiments on narrow membrane tubes have shown that the curvature of the membrane affects the lateral diffusivity (38) due to hydrodynamic interactions in thin cylindrical geometries. These effects are not included in our model. We may, however, speculate on their effect on the processes considered here: because small necks reduce the diffusivity, the effects will further increase the escape time, rendering the spine shape even more effective at retaining AMPAR gradients. In future work, we will include this effect by introducing a curvature-dependent diffusion coefficient.
-
2.
By considering the PSD in our measurement, we demonstrate that the timescale for an AMPAR entering the spine, and ending up at the PSD, may be up to an order-of-magnitude faster than the time of the reverse process, when a receptor is leaving the PSD and exiting the spine through the neck. Thus, the specific shape of the spine directly induces this asymmetric trafficking.
-
3.
Our simulations reveal that local exocytosis results in effective and rapid capture of AMPARs, particularly at curved geometries such as in mushroomlike spines. The model reveals that increasing the number of AMPAR at synapses is most effectively achieved through active transport of AMPAR-containing vesicles and subsequent release at local exocytotic sites in the spine head. The strong impact of morphology on receptor diffusion implies that in the interpretation of single-particle tracking and fluorescence-recovery-after-photobleaching experiments, the shape of the spine must be explicitly included.
Our model indicates that mushroomlike spines with local exocytotic sites adjacent to the PSD are privileged over others, because they can rapidly and efficiently regulate their synaptic AMPAR levels. The specific and high-affinity AMPAR/scaffold interactions within the PSD, in combination with the geometric shape of the mushroomlike spines and local exocytosis within the spine head, allow direct and rapid control over the amount of AMPARs at synaptic sites. In this way, each individual spine presents an isolated and asymmetric, but adaptive structure, which provides dendrites with a complex palette of regulatory options to efficiently control synaptic AMPAR level. Future work will be required to explicitly examine the effect of interactions between particles—which may lead to interesting crowding phenomena—and the dynamics within the PSD itself.
Acknowledgments
We thank Stefan Paquay, Bart van Lith, Eugene Katrukha, Max Adrian, and Paul van der Schoot for valuable discussions and Philipp Schätzle for sharing images of dendritic spines from cultured rat hippocampal neurons.
This work was supported by funds from the Netherlands Organization for Scientific Research (NWO-FOM) within the program “Barriers in the Brain: the Molecular Physics of Learning and Memory” (No. FOM-E1012M).
Supporting Material
References
- 1.Jackson A.C., Nicoll R.A. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Straub C., Tomita S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr. Opin. Neurobiol. 2012;22:488–495. doi: 10.1016/j.conb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepherd J.D., Huganir R.L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 4.Bredt D.S., Nicoll R.A. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy M.J., Ehlers M.D. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69:856–875. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triller A., Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Sheng M., Hoogenraad C.C. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu. Rev. Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 8.Opazo P., Choquet D. A three-step model for the synaptic recruitment of AMPA receptors. Mol. Cell. Neurosci. 2011;46:1–8. doi: 10.1016/j.mcn.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Czöndör K., Thoumine O. Biophysical mechanisms regulating AMPA receptor accumulation at synapses. Brain Res. Bull. 2013;93:57–68. doi: 10.1016/j.brainresbull.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Peters A., Kaiserman-Abramof I.R. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am. J. Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi Y., Majewska A.K. Dendritic spine geometry: functional implication and regulation. Neuron. 2005;46:529–532. doi: 10.1016/j.neuron.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Bourne J.N., Harris K.M. Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtmaat A., Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 14.Richards D.A., De Paola V., McKinney R.A. AMPA-receptor activation regulates the diffusion of a membrane marker in parallel with dendritic spine motility in the mouse hippocampus. J. Physiol. 2004;558:503–512. doi: 10.1113/jphysiol.2004.062091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashby M.C., Maier S.R., Henley J.M. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J. Neurosci. 2006;26:7046–7055. doi: 10.1523/JNEUROSCI.1235-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arellano J.I., Benavides-Piccione R., Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front. Neurosci. 2007;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newpher T.M., Ehlers M.D. Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol. 2009;19:218–227. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Bloodgood B.L., Sabatini B.L. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. doi: 10.1126/science.1114816. [DOI] [PubMed] [Google Scholar]
- 19.Grunditz A., Holbro N., Oertner T.G. Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization. J. Neurosci. 2008;28:13457–13466. doi: 10.1523/JNEUROSCI.2702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuste R. Electrical compartmentalization in dendritic spines. Annu. Rev. Neurosci. 2013;36:429–449. doi: 10.1146/annurev-neuro-062111-150455. [DOI] [PubMed] [Google Scholar]
- 21.Holcman D., Triller A. Modeling synaptic dynamics driven by receptor lateral diffusion. Biophys. J. 2006;91:2405–2415. doi: 10.1529/biophysj.106.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holcman D., Schuss Z. Diffusion laws in dendritic spines. J. Math. Neurosci. 2011;1:10. doi: 10.1186/2190-8567-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shouval H.Z. Clusters of interacting receptors can stabilize synaptic efficacies. Proc. Natl. Acad. Sci. USA. 2005;102:14440–14445. doi: 10.1073/pnas.0506934102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earnshaw B.A., Bressloff P.C. Biophysical model of AMPA receptor trafficking and its regulation during long-term potentiation/long-term depression. J. Neurosci. 2006;26:12362–12373. doi: 10.1523/JNEUROSCI.3601-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolle D.P., Le Novère N. Brownian diffusion of AMPA receptors is sufficient to explain fast onset of LTP. BMC Syst. Biol. 2010;4:25. doi: 10.1186/1752-0509-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czöndör K., Mondin M., Thoumine O.R. Unified quantitative model of AMPA receptor trafficking at synapses. Proc. Natl. Acad. Sci. USA. 2012;109:3522–3527. doi: 10.1073/pnas.1109818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris K.M. Springer; New York: 2008. Diversity in Synapse Structure and Composition. [Google Scholar]
- 28.Christensen M. How to simulate anisotropic diffusion processes on curved surfaces. J. Comput. Phys. 2004;201:421–438. [Google Scholar]
- 29.Berg H.C., Purcell E.M. Physics of chemoreception. Biophys. J. 1977;20:193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linderman J.J., Lauffenburger D.A. Analysis of intracellular receptor/ligand sorting in endosomes. J. Theor. Biol. 1988;132:203–245. doi: 10.1016/s0022-5193(88)80157-7. [DOI] [PubMed] [Google Scholar]
- 31.Holcman D., Schuss Z. Escape through a small opening: receptor trafficking in a synaptic membrane. J. Stat. Phys. 2004;117:975–1014. [Google Scholar]
- 32.Yuste R., Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 33.Hotulainen P., Hoogenraad C.C. Actin in dendritic spines: connecting dynamics to function. J. Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newpher T.M., Ehlers M.D. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacGillavry H.D., Cornelis J., van Kesteren R.E. Genome-wide gene expression and promoter binding analysis identifies NFIL3 as a repressor of C/EBP target genes in neuronal outgrowth. Mol. Cell. Neurosci. 2011;46:460–468. doi: 10.1016/j.mcn.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Opazo P., Sainlos M., Choquet D. Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr. Opin. Neurobiol. 2012;22:453–460. doi: 10.1016/j.conb.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Makino H., Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domanov Y.A., Aimon S., Bassereau P. Mobility in geometrically confined membranes. Proc. Natl. Acad. Sci. USA. 2011;108:12605–12610. doi: 10.1073/pnas.1102646108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renner M., Domanov Y., Triller A. Lateral diffusion on tubular membranes: quantification of measurements bias. PLoS ONE. 2011;6:e25731. doi: 10.1371/journal.pone.0025731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


