Abstract
It is now established that airway smooth muscle (ASM) has roles in determining airway structure and function, well beyond that as the major contractile element. Indeed, changes in ASM function are central to the manifestation of allergic, inflammatory, and fibrotic airway diseases in both children and adults, as well as to airway responses to local and environmental exposures. Emerging evidence points to novel signaling mechanisms within ASM cells of different species that serve to control diverse features, including 1) [Ca2+]i contractility and relaxation, 2) cell proliferation and apoptosis, 3) production and modulation of extracellular components, and 4) release of pro- vs. anti-inflammatory mediators and factors that regulate immunity as well as the function of other airway cell types, such as epithelium, fibroblasts, and nerves. These diverse effects of ASM “activity” result in modulation of bronchoconstriction vs. bronchodilation relevant to airway hyperresponsiveness, airway thickening, and fibrosis that influence compliance. This perspective highlights recent discoveries that reveal the central role of ASM in this regard and helps set the stage for future research toward understanding the pathways regulating ASM and, in turn, the influence of ASM on airway structure and function. Such exploration is key to development of novel therapeutic strategies that influence the pathophysiology of diseases such as asthma, chronic obstructive pulmonary disease, and pulmonary fibrosis.
Keywords: lung, asthma, inflammation, calcium, bronchoconstriction, bronchodilation, proliferation, extracellular matrix, development
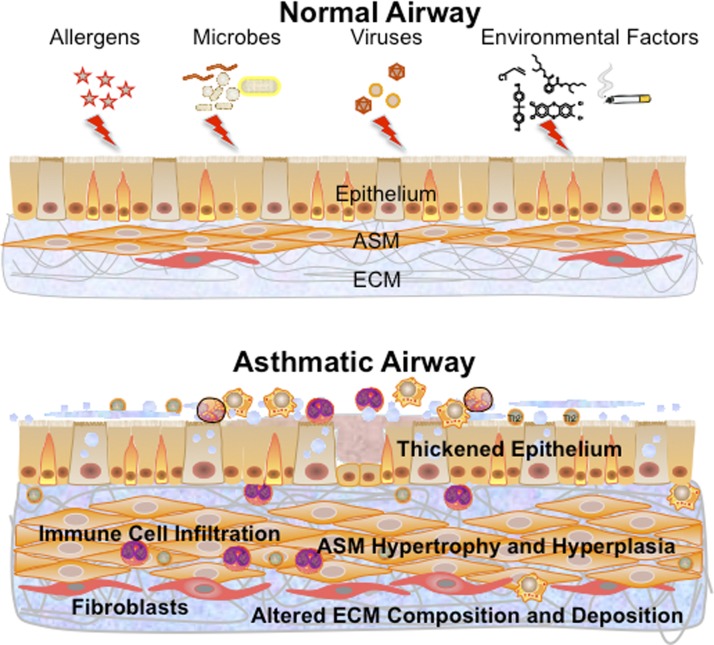
dysfunctional and excessive airway narrowing with impaired relaxation are hallmarks of diseases such as asthma (both in children and adults), bronchitis, and chronic obstructive pulmonary disease (COPD). Although structural changes to the diseased airway can involve a thickened (and also dysfunctional) epithelial layer, increased thickness of the airway smooth muscle (ASM) layer with varying levels of fibrosis are also key features in diseases of various etiologies, including allergy and infection, environmental exposures (e.g., cigarette smoke, toxins, and pollutants), and developmental abnormalities (Fig. 1). From a functional standpoint, the prime role of ASM is regulation of airway tone via a balance between the extent of contraction vs. dilation in response to local or circulating factors. Accordingly, factors that produce or enhance bronchoconstriction with concomitant impairment of dilatory mechanisms can result in increased airway tone that is typical in diseases such as asthma. Furthermore, structural changes induced by extrinsic factors can result in greater numbers (proliferation and hyperplasia) or size (hypertrophy) of ASM cells, contributing to reduced airway lumen, particularly in the face of ongoing airway hyperresponsiveness (AHR).
Fig. 1.
Transformations toward the asthmatic airway. Exposure of the normal airway to insults such as allergens, microbes, or viruses or to environmental factors such as pollutants, tobacco smoke, or nanoparticles results in changes throughout the epithelium, airway smooth muscle (ASM), and extracellular matrix (ECM). The asthmatic airway involves infiltration of a variety of immune cells, a thickened epithelium with goblet cell hyperplasia, increased mucus, a thickened, more fibrotic ASM layer with increased cell size (hypertrophy) and numbers (hyperplasia), along with altered ECM composition. Changes within the ASM layer can be a result of processes initiated or modulated by as well as involving ASM cells.
Despite the role of ASM in airway contractility per se being more “well established”, the mechanisms that regulate the “passive” response of ASM to extrinsic stimuli from airway innervation, other airway cell types (epithelium, fibroblasts, and immune cells), and/or circulating mediators are still being discovered, especially in the context of inflammation. It is now increasingly evident that, in diseases such as asthma and COPD and in environmental exposures, ASM are central to the induction and modulation of both structural and functional responses of the airway; i.e., ASM are active participants in airway responses to inflammation, infection, and injury. Here, ASM is now recognized to be a source of extracellular matrix (ECM) proteins that drive structural changes, as a producer of pro- and anti-inflammatory mediators that modulate the local immune environment and influence other resident cell types and even growth factors that affect cell proliferation, migration, and apoptosis. All of these “ASM-derived” factors can, in turn, influence ASM structure and function via a myriad of signaling pathways, as well as other cell types in the airway. Accordingly, it becomes important to understand the mechanisms by which ASM respond to extrinsic stimuli and how ASM reciprocally modulate the extracellular, local environment. Such understanding is critical to the development of novel strategies to target enhanced airway reactivity and structural changes (remodeling) that occur in important diseases such as asthma, COPD, and even fibrosis.
The current perspective highlights some recent discoveries that reveal the central role of ASM in this regard. It is important to emphasize that the work highlighted here is by no means all-encompassing of the substantial body of recent literature by several investigative teams using a wide variety of complementary approaches and a range of species. Indeed, the impressive findings of all of these many studies only underline the importance of ASM in health and disease. By summarizing some of these discoveries, the intent here is to help set the stage for future research toward exploring the pathways regulating ASM and, in turn, the influence of ASM on airway structure and function in the context of understanding disease pathophysiology and treatment.
ASM, [Ca2+]i, and Contractility
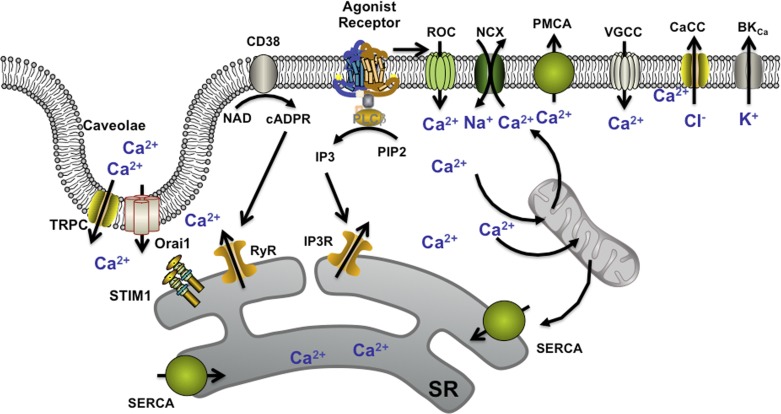
The major mechanisms by which elevation of [Ca2+]i occurs in ASM in response to agonist have been recently reviewed (17, 145, 186, 231, 261, 278, 285, 335). Although there are species differences in the contribution of specific pathways, some common mechanisms are well recognized. Activation of Gq-coupled receptors by a range of bronchoconstrictor agonists as well as a more complex and less-understood role for Gi-coupled receptors have been identified (20, 59, 227, 335). [Ca2+]i elevation involves voltage-gated, receptor-operated, store-operated, and nonspecific Ca2+ influx, as well as sarcoplasmic reticulum release through channels activated by the phospholipase C (PLC)/inositol trisphosphate (IP3) and CD38/cyclic ADP ribose (cADPR) pathways (IP3-sensitive and ryanodine receptor channels). Mechanisms such as the sarcoendoplasmic reticulum Ca2+ ATPase (SERCA), the bidirectional Na+/Ca2+ exchanger (NCX), and mitochondrial buffering help limit [Ca2+]i and restore levels following removal of agonist (Fig. 2). Beyond [Ca2+]i, the Ca2+-calmodulin-myosin light chain (MLC) kinase-MLC cascade regulates contractility mediated by actin-myosin interactions. Furthermore, Ca2+ sensitization for contraction involves activation of the RhoA/Rho kinase pathway, which serves to prevent desensitization of the contractile machinery from MLC phosphatases. Although such mechanisms have been extensively studied in different smooth muscle types, more recent work has revealed the complexity in the expression and function of these mechanisms and their finer regulation by plasma membrane, as well as intracellular signaling pathways, many of which are important in inflammatory signaling and in the pathophysiology of airway diseases. Thus the area of [Ca2+]i regulation in ASM continues to be a topic of substantial research interest in the respiratory and lung biology community.
Fig. 2.
Mechanisms of [Ca2+]i regulation in ASM. A number of regulatory mechanisms are well recognized, including agonist-induced G-protein coupled receptor (GPCR)-based production of inositol trisphosphate (IP3) and action of the ectoenzyme CD38 in producing the second messenger cyclic ADP ribose (cADPR), leading to sarcoplasmic reticulum (SR) Ca2+ release from IP3 receptor and ryanodine receptor (RyR) channels, respectively. Additionally, Ca2+ influx can occur through receptor-operated channels (ROC), voltage-gated Ca2+ channels (VGCCs), and perhaps through the bidirectional Na+/Ca2+ exchanger (NCX). Depletion of SR Ca2+ stores can lead to store-operated Ca2+ entry (SOCE) mediated by the SR Ca2+-sensing protein stromal interacting molecule (STIM1) and the plasma membrane influx channels Orai1 and canonical transient receptor potential (TRPC) (both being expressed with caveolae). Elevated [Ca2+]i can be sequestered by the SR via the Ca2+ ATPase (SERCA), by mitochondrial buffering, and plasma membrane efflux mechanisms such as the plasma membrane Ca2+ ATPase (PMCA) or by membrane hyperpolarization due to BKCa and Ca2+-activated chloride channels (CaCC). PLC, phospholipase C; cADPR, cyclic ADP ribose; PIP2, phosphatidylinositol 4,5-bisphosphate.
GPCRs.
G protein-coupled receptors (GPCRs) are a superfamily of plasma membrane proteins that transduce extracellular signals, resulting in intracellular cascades, leading to diverse cellular functions. The importance of GPCRs in the airway lies in the fact that many of the drugs currently in use for management of diseases such as asthma and COPD target these mechanisms, particularly in ASM. Traditional bronchoconstrictor agonist receptors for acetylcholine (ACh), histamine, and endothelin act via the well-studied Gq-coupled pathway, activating the PLC-IP3 pathway in elevating [Ca2+]i. Activation of the Gi-coupled pathway reduces cAMP and thus indirectly promotes contractility. Although much of the previous work in this area has focused on expression and function of different GPCRs (typically Gq, Gi, and Gs) in the context of ASM contractility/relaxation, there is increasing recognition that GPCRs acting alone, or in conjunction with other pathways such as receptor tyrosine kinases (18, 20, 59, 155, 162), can contribute to the “synthetic function” of ASM via cell proliferation/growth and secretion of growth factors and inflammatory mediators, thus influencing airway remodeling and the local inflammatory milieu (e.g., see Refs. 20 and 59 for review). However, it is important to first recognize some recently identified GPCR mechanisms that may help launch new avenues in bronchodilatory therapies and perhaps help target other aspects of ASM dysfunction as well. Furthermore, recent advances in understanding GPCR regulation per se in terms of desensitization, constitutional activation of receptors, and biased agonism (59, 227, 285) may help drive novel approaches to targeting these mechanisms and their downstream targets.
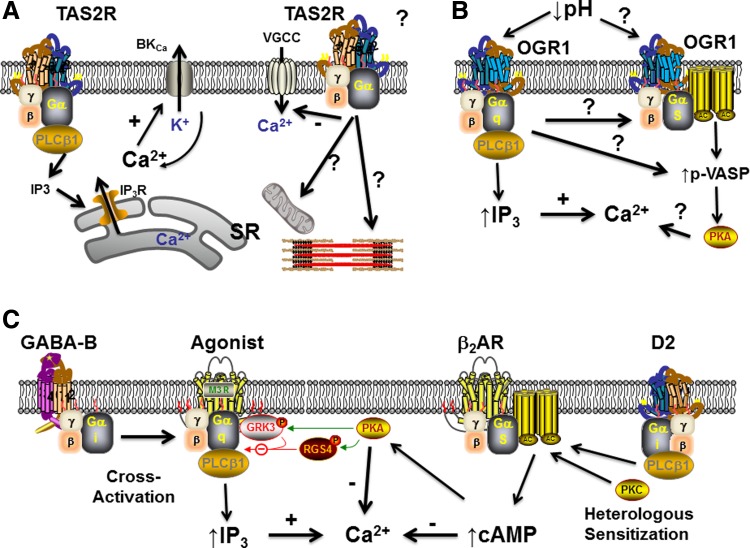
There has been considerable excitement regarding the identification of bitter taste receptors (BTRs) as novel bronchodilators (4, 43, 61, 82, 241, 262, 352). Of the 25 members in this family of GPCRs (type 2, bitter taste receptors, TAS2Rs; Fig. 3) activated by bitter tastants such as chloroquine and saccharin, six are prominently expressed and functional in human ASM, with three dominant TAS2Rs (subtypes 10, 14, and 31) (61). TAS2Rs differ from many other GPCRs in their ability to bind a range of bitter compounds with relatively low specificity and affinity. A fascinating aspect of TAS2Rs is the novel finding that their activation actually results in elevated [Ca2+]i levels in ASM [e.g., via IP3-mediated sarcoplasmic reticulum (SR) Ca2+ release] but leads to bronchodilation more efficaciously than β-agonists, making TAS2Rs highly appealing targets for enhancing bronchodilation (although their eventual clinical application may be determined by the concentrations required in disease and the problem of desensitization; 256). What is less clear are the mechanisms underlying the unexpected discrepancy between elevated [Ca2+]i and bronchodilation, especially when the dilatory effect does not appear to involve cyclic nucleotides. Alternative mechanisms have been proposed, including membrane hyperpolarization via the iberiotoxin-sensitive Ca2+-activated K (BKCa) channels (61, 241), especially in the context of compartmentalized signaling (superficial barriers). In this regard, recent studies in the guinea pig airway (241) suggest that different bitter tastants may work through differential mechanisms in inducing bronchodilation and may further be differently effective depending on the bronchoconstrictor in play. For example, denatonium (which activates the type 4 and 10 receptors) selectively inhibits constriction induced by muscarinic receptors, whereas chloroquine (which activates type 3 and 10) broadly inhibits contractions evoked by prostaglandin E2, thromboxane receptor activation, leukotriene D4 (LTD4), histamine, and antigen. Here, these TAS2R agonists may also differentially work through BKCa channels. Interestingly, none of PKA, PKC, or PKG appear to be involved. However, given that membrane potential is not as powerful a signal for contractility/relaxation in ASM as it is in vascular smooth muscle (but see below regarding novel findings), but that agonists such as histamine in fact depolarize the plasma membrane, and additional studies indicate a role for voltage-gated L-type channels (352), other pathways for TAS2R effect need to be considered. Certainly, given the variety of [Ca2+]i regulatory mechanisms within ASM (e.g., Fig. 2), a number of possibilities exist. Regardless, of which mechanisms are involved, the discordance between elevated [Ca2+]i and reduced contractility will need to be reconciled. If reduced contractility per se is the final outcome, inhibition of Ca2+ sensitivity and sensitization pathways need to be examined. Although these potential pathways are yet to be examined, the novelty and appeal of TAS2Rs is clear. Recent data showing that TAS2R activity is maintained even when β2 adrenoceptors (β2ARs) are desensitized are particularly interesting (4) because this lack of physiologically relevant cross desensitization may allow for use of bitter tastants as an adjunct or rescue therapy during ongoing β2AR desensitization as a result of chronic use of β2-agonists. Of course, more work is needed to determine the extent of TAS2R desensitization per se because limited data show a modest degree of desensitization (20–30%) in human ASM and monkey bronchus (256), which may be amplified in the diseased airway. Here, it may also be relevant to consider whether “standard” therapies such as glucocorticoids, which are thought to prevent β2AR desensitization, are equally effective in preventing TAS2R desensitization (43). Furthermore, given that a number of agonists and mediators may concurrently act on ASM, it may be helpful to determine whether TAS2Rs interact with other receptors (e.g., via heterodimerization). Finally, it will be important to establish the pattern of changes in TAS2Rs during the development and progression of diseases such as asthma or COPD, so the utility of bitter tastants as therapeutic agents could be further tested.
Fig. 3.
Novel GPCR-based signaling mechanisms in ASM. A: effect of bitter tastants such as chloroquine and saccharin on bitter taste receptor (TAS2R) GPCRs in ASM has been a topic of substantial recent interest. Working through a Gq protein-coupled mechanism (where the α-subunit may represent gustducin, transducin, or α1), TAS2R activation leads to increased SR Ca2+ release via IP3 receptor channels and elevated [Ca2+]i levels but intriguingly results in bronchodilation via mechanisms that remain to be established. Ca2+-activated K+ channels (BKCa) and inhibition of voltage-gated Ca2+ channels are thought to be involved, but pathways such as inhibition of other Ca2+ influx mechanisms, mitochondria, or contractile mechanisms downstream to Ca2+ may also be relevant. B: a novel, intriguing GPCR mechanism recently identified in ASM is an extracellular pH-sensitive pathway, ovarian cancer G protein-coupled receptor 1 (OGR1/GPR68), that on the one hand appears to work via Gq to increase [Ca2+]i via the IP3 mechanism, but on the other hand also activates the protein kinase A cascade either directly through Gq or perhaps through interactions with Gs. VASP, vasodilator-stimulated phosphoprotein. C: in contrast to the novel TAS2R and OGR1 pathways, there is now increasing evidence that the GABA and dopaminergic systems (well known in neuroscience) also have interesting effects in ASM. Activation of the metabotropic GABA-B receptor results in a Gi-coupled cross activation of Gq-coupled receptors of bronchoconstrictor agonists, leading to increased [Ca2+]i. On the other hand, prolonged dopaminergic signaling via a D2-like, Gi-coupled pathway leads to enhancement of the Gs-coupled β2-adrenoceptor (β2AR) system, thus increasing cAMP and promoting bronchodilation. GRK3, G protein-coupled receptor kinase; RGS4, G-protein signaling 4.
Another intriguing, recently identified signaling mechanism in ASM is the family of proton-sensing GPCRs that can respond to variations in extracellular pH (122, 196, 219, 269). Two of them (GPR4 and T cell death-associated gene 8 or GPR65) are Gs coupled, whereas the ovarian cancer G protein-coupled receptor 1 (OGR1 or GPR68; Fig. 3) is Gq coupled. The appealing aspect of these novel pathways is in the idea that airway reactivity could be modulated directly by extracellular pH beyond airway irritability induced by pH or acid effects on sensory neurons. Although the expression pattern and function of these novel receptors are still under investigation, OGR1 mRNA has been found in human ASM (122, 269) (while airway epithelium expresses multiple of these GPCRs; 269). The role of OGR1 activation has been characterized in vascular smooth muscles (304) and more recently in human ASM (304). Reductions in extracellular pH to 6.3 result in mobilization of [Ca2+]i although the mechanisms by which Ca2+ is elevated remain to be determined. Here, it may be interesting to explore a range of plasma membrane influx mechanisms, based on information from other lung cell types regarding pH or acid effects. For example, proton activation of vanilloid receptor subtype 1 (transient receptor potential vanilloid 1, TRPV1) on sensory neurons can induce membrane depolarization and stimulate local release of kinins (98, 253). In addition to expression in airway neurons (70, 176), there is limited evidence for TRPV1 in nonneuronal airway cells such as epithelium (298) and ASM (354) but considerable data on the expression of kinin receptors in ASM (173, 200, 252, 322). Thus it would be interesting to determine whether OGR1 can indirectly influence these mechanisms. There has been considerable interest in acid-sensing ion channels (ASICs) on sensory neurons (98, 99, 172), but the limited evidence in ASM suggests that ASICs may actually contribute to pH-induced ASM relaxation (70). In PC12 cells, changes in extracellular pH can influence store-operated Ca2+ influx that is dependent on the SR Ca2+ protein stromal interacting molecule 1 (STIM1) (299). Given the important role of STIM1/Orai1 in human ASM (80, 137, 225, 264, 292, 357), it is possible that OGR1 could modulate store-operated Ca2+ entry (SOCE) by enhancing STIM1 interactions with Orai1 or other channels that contribute to Ca2+ influx, particularly canonical transient receptor potentials (TRPCs) (80, 338). Furthermore, the limited data in human ASM suggest that OGR1 can further result in p42/44 MAPK phosphorylation (269) and IL-6 production (122) and therefore have more complex effects in the setting of disease. In this regard, an intriguing finding is that OGR1 also promotes phosphorylation of the PKA substrate vasodilator-stimulated phosphoprotein in response to reduced extracellular pH (269). This effect may reflect a duality of Gq/Gs activation by OGR1 or be independent of the expected Gq activation and thus direct coupling to Gs. Regardless of which mechanisms are at play, the emerging data on proton-sensing GPCRs are of considerable interest from the perspective of reactive airway diseases in several aspects. For example, acid fog is an environmental issue in developing countries and is associated with increased incidence of asthma (74). Gastroesophageal reflux disease and microaspiration of gastric contents can induce and exacerbate asthma (251–253). Even in asthma from other causes, and in COPD, airway inflammation is known to result in reduced local pH (157). Accordingly, developing drugs or other techniques to preferentially activate the Gs aspect of OGR1 signaling (or alternatively inhibiting Gq only) would be highly appealing.
Although concepts such as BTRs and proton-sensing receptors in the context of airway reactivity are emerging, there have been very interesting discoveries in the novel roles for an agonist very well known in the nervous system: γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the mammalian central nervous system. GABA is known to act at both ligand-gated ionotropic GABA-A receptors as well as G protein-linked metabotropic GABA-B receptors, the latter being typically linked to Gi. Both GABA and functional GABA-B receptors have been detected in a range of peripheral tissues (69, 84, 328). Initial clinical findings of worsened airway responses of asthmatics to methacholine in the presence of the GABA-B receptor agonist baclofen (62) raised the possibility of this mechanism within airways. Recent findings suggest the functional expression of GABA-B receptors in airway smooth muscle (221) as well as epithelial (206) cells. The interesting functionality of these receptors in ASM has been recently demonstrated in human and guinea pig ASM (76–78, 205, 207, 350) (Fig. 3). Although acting through Gi (blocked by pertussis toxin), both baclofen and GABA significantly increase the synthesis of IP3 and elevate [Ca2+]i (effects blocked by selective GABA-B antagonists, whereas GABA-A receptor agonists have no effect) (205). This unexpected finding appears to involve a Gβγ-mediated direct effect on PLC-β leading to IP3 production as well as a potentiation of Gq-mediated signaling (e.g., bradykinin), thus contributing to crosstalk between Gi and Gq pathways, leading to contraction. These novel data point to a very intriguing bronchoconstrictor mechanism that could in fact be targeted relatively easily given the availability of specific agonists and antagonists.
Akin to GABA, another atypical signaling pathway that has garnered recent interest is dopaminergic signaling in the airway (32, 208, 209). Dopamine effects are well known in several organ systems. “D1-like” dopamine receptors couple to Gs, whereas “D2-like” receptors (D2, D3, and D4) couple to Gi (204). Clinically, inhaled dopamine induces bronchodilation in patients with asthma, but the underlying mechanisms are not well understood. Indeed, functional expression of dopamine receptor subtypes in ASM have only recently been described (208). Although based on the physiological effects, a D1-like/Gi-linked pattern is to be expected, and the limited data actually suggest an intriguing functionality. In sensory neurons of the airway, a D2-like receptor has been identified (226). In human as well as guinea pig ASM, D2 receptor mRNA and/or protein has been reported (208, 209). Acute activation of D2 receptor (quinpirole) inhibits adenylyl cyclase activity (208), consistent with the classical function of Gi-coupled receptors that inhibit adenylyl cyclase (AC) activity and reduce cAMP (thus preventing bronchodilation). However, in ASM, chronic D2 receptor activation with quinpirole paradoxically enhances AC activity via heterologous sensitization (Fig. 3), which involves PLC and PKC, resulting in bronchodilation. Such sensitization is not unique to dopamine receptors, being observed in adenosine and adrenergic and opioid receptors (329, 330). Nonetheless, the ability of dopamine receptors to induce bronchodilation provides yet another novel avenue to target excessive bronchoconstriction in airway diseases.
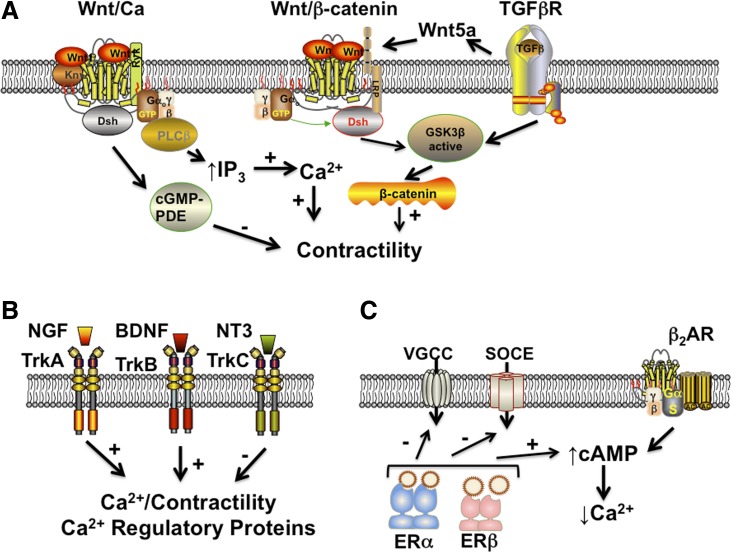
Beyond the usual and even unusual GPCRs, there has been a limited number of studies examining the novel role of Wnt signaling in ASM (8, 163, 325, 347, 358) (Fig. 4). Wnt signaling begins with one of the Wnt proteins binding the extracellular domain of a Frizzled (Fz) family receptor that spans the plasma membrane to constitute a distinct family of GPCRs (184). Such signaling may be facilitated by coreceptors typically of the receptor tyrosine kinase (RTK) family such as lipoprotein receptor-related protein (LRP)-5/6, Ryk, and ROR2. Thus Wnt represents a transmembrane signaling mechanism that may serve to integrate inputs with pleiotropic intracellular cascades via canonical β-catenin-dependent and -independent pathways and non-canonical Wnt/Ca2+ pathways. The emerging major theme of Wnt signaling in ASM appears to be related to airway remodeling, particularly in the context of TGF-β signaling (see below). However, limited data are now highlighting a role for both TGF-β itself elevating [Ca2+]i in ASM via enhanced Ca2+ influx (79) as well as SR Ca2+ release (154) and importantly the noncanonical Wnt/Ca2+ pathway (163). Here Wnt5A is markedly induced in response to TGF-β and is required for ECM production by ASM (163). However, Wnt5A also engages noncanonical WNT signaling pathways evidenced by lack of TGF-β effects when [Ca2+]i is inhibited (163). Here, the noncanonical Wnt/Ca2+ pathway can enhance SR Ca2+ release via PLC/IP3 or blunt [Ca2+]i via cGMP-specific phosphodiesterase (PDE). Certainly, it is possible that even the canonical Wnt pathways are at least indirectly involved in modulating ASM contractility via mechanisms involving β-catenin (347) (e.g., see below) or as yet unexplored increases in expression of [Ca2+]i or contractility proteins. The appeal of the Wnt signaling pathways lies in the potential for both biased agonism (19, 20, 193) (or antagonism) based on the desired effect of altered airway tone vs. remodeling.
Fig. 4.
Novel signaling cascades in ASM. A: activation of transforming growth factor (TGF)-β receptors in ASM leads to increased expression of Wnt5a that can in turn activate either the Wnt/GSK3β/β-catenin system in promoting contractility or alternatively the noncanonical Wnt/Ca system that increases [Ca2+]i via IP3. Conversely, the Wnt/Ca system could modulate contractility by activating cGMP-specific phosphodiesterases (PDE). B: although well recognized in the nervous system, there is now considerable evidence that the receptor tyrosine kinase family of neurotrophic receptors (tropomyosin-related kinases; Trks) are expressed and functional in ASM. The neurotrophin nerve growth factor (NGF) acts via TrkA, whereas brain-derived neurotrophic factor (BDNF) acts through TrkB to enhance [Ca2+]i and expression of Ca2+ regulatory proteins to promote contractility. Conversely, neurotrophin-3 (NT3) acts via TrkC to blunt Ca2+ and contractility. C: given sex differences in allergic airway diseases, the mechanisms by which sex steroids such as estrogens can influence ASM are being explored. Limited data suggest that estrogen receptors (ERs) can inhibit VGCC and SOCE to reduce [Ca2+]i while separately increasing cAMP that also aids in bronchodilation, perhaps by potentiating β2AR effects.
With regard to GPCRs as targets for bronchodilation, a limiting issue may be the concept of receptor desensitization, typified by β2ARs, but also present for TAS2R, for example (256). There is now increasing interest in techniques for preventing desensitization to promote and prolong the activity of bronchodilators (19, 21, 227). Here, the focus has been on mechanisms such as the G protein-coupled receptor kinase (GRKs) that normally limit GPCR activation and the β-arrestins involved in receptor internalization. For example, inhibiting GRK2/3 or blocking β-arrestin-2 can selectively enhance β2AR signaling in human ASM (60). Here, corticosteroids, a mainstay of asthma therapy, can also help restore β-agonist sensitivity and prevent desensitization although such effects may be specific to particular β-agonist agents (47). With TAS2R, recent but limited data based on prevention of desensitization by dynamin but lack of effect of PKA or PKC antagonists (256) also suggest a role for GRK/β-arrestins. Certainly, much work is needed to identify techniques or complementary drugs to target specific GRK isoforms or β-arrestins to enhance or maintain the functionality of particular bronchodilator therapies, be it β2-agonists or bitter tastants and other novel therapies in the future. Along the lines of GRKs and β-arrestins, is the increasing recognition that regulators of G-protein signaling (RGS) may play a role in airway contractility (as well as remodeling) (49, 64, 289, 290, 340, 343). A specific RGS usually prevents the continued activation of multiple G proteins, and thus upregulation or downregulation of RGS can have broad effects. Here, one study identified increased RGS4 as being inhibitory to ASM contractility in severe, recalcitrant asthma but interestingly promoting a proliferative phenotype of ASM (49). Separately, RGS2, an inhibitor of multiple Gq-coupled receptors expressed in human and murine ASM, is downregulated in ovalbumin-sensitized/challenged mice as well as in the lungs of asthmatic humans (340). These emerging data point to multiple novel strategies to reduce bronchoconstriction while enhancing bronchodilation. Here a key limitation may be the widespread expression and different functions of GRK, β-arrestin, and RGS isoforms within different cell types of the airway, necessitating targeting of the most relevant pathways within ASM per se.
In addition to preventing desensitization of specific receptors, there is increasing interest in identifying ligands with biased agonism toward different GPCRs (19, 20, 193). Certainly, partial vs. full agonists acting at the same receptor are known to produce effects of different magnitude. However, different agonists, or stereoisomers of a single agonist, can work through the same receptor to activate different downstream pathways to different extents, i.e., it is the ligand that determines the direction of signaling, rather than the GPCR, presumably by differential stabilization of the activation state of the receptor. Such biased agonism has been demonstrated in the context of asthma and ASM for β2AR, which can activate Gs to enhance cAMP (and thus be of benefit by enhancing bronchodilation for example) vs. their ability to work via the β-arrestin-mediated MAPK pathway, which may be detrimental in terms of contractility, promoting cell proliferation, and remodeling (60, 326). Thus the potential to direct β2AR signaling toward a beneficial pathway while avoiding activation of detrimental mechanisms is a highly appealing goal for drug development. In this regard, further beneficial cAMP signaling may be produced by better understanding of how cAMP itself is compartmentalized and regulated in ASM cells, especially via A-kinase anchoring proteins (AKAPs). A recent study identified AKAPs in human ASM (118) and found that they regulate compartmentalized cAMP accumulation in response to β2AR stimulation, raising the possibility that the combination of an agonist biased toward greater cAMP signaling with another that promotes local cAMP accumulation to synergetically enhance bronchodilation may be even more beneficial.
Non-GPCR-based mechanisms.
In addition to bronchoconstrictors or bronchodilators that work through GPCR mechanisms, there is now considerable evidence for signaling in ASM that involves nuclear hormone receptors (a classical example being the glucocorticoid receptor), tyrosine kinases, and serine/threonine kinases.
In terms of serine/threonine kinases, there has been much focus on the receptor for TGF-β and its interactions with other receptors (e.g., Wnt as above; Fig. 4) (9, 79, 121, 163, 201, 216, 217, 347). For example, expression of contractile proteins is enhanced in human ASM cells exposed to both methacholine and TGF-β (216, 217). However, it appears that, consistent with its role in other cell types, TGF-β largely affects ASM in the context of remodeling (121, 216, 217, 273) (see below).
Previous work on families of receptor tyrosine kinases such as endothelial growth factor (EGF) receptors (171, 214, 270, 284), insulin receptors (54, 55, 90, 128, 224, 271), or platelet-derived growth factor (PDGF) receptors (123) has shown a role for these receptors in airway contractility and/or remodeling. However, a number of recent studies have found that the Trk family of receptors (RTK class VII; Fig. 4) that are responsive to the neurotrophin family of growth factors (238) has novel, diverse roles in ASM, including enhancing [Ca2+]i and contractility, particularly in the setting of inflammation (120, 200, 223, 236, 239, 248, 268, 344). Neurotrophins such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) are well recognized in the nervous system for both long-term genomic effects on neuronal growth, differentiation, and survival (181), as well as nongenomic, rapid effects of enhancing [Ca2+]i and neurotransmitter release (24, 159). There is now convincing and increasing evidence for a number of neurotrophins being expressed in peripheral tissues including lung, with increased expression in asthma, COPD, and even lung cancer (see Refs. 119, 238, 257, and 345 for review). However, the functional role of neurotrophins in the airway are still under investigation. Here, recent data suggest that NGF is released by airway nerves in response to irritants [e.g., ozone (120, 322) or cigarette smoke (336)], allergens (346), and infectious agents (38) contributing to irritability and enhanced contractility. NGF can also be derived from airway epithelial cells, for example following viral or bacterial infections (38, 222). The presumed target of the released NGF is ASM, given expression of the cognate TrkA receptors in ASM (75, 213, 222, 238). However, there are now considerable data that ASM also expresses the TrkB and TrkC receptors for BDNF/neurotrophin 4 and neurotrophin 3, respectively (200, 213, 236, 239, 268, 291, 345). Here, it appears that ASM itself can release BDNF (200, 324) both at baseline and in response to inflammatory stimuli. Importantly, BDNF can nongenomically enhance ASM [Ca2+]i (236) and potentiate the effects of receptors for neurally derived factors such as the neurokinins (200). BDNF further appears to play a role in airway remodeling (5) (see below). The relevance of these novel pathways is the highlighting of ASM as both a source of growth factors and a recipient of such factors secreted by ASM, as well as surrounding cell types, resulting in pleiotropic effects that are also involved in asthma and other airway diseases. Whether this concept is also valid for other secreted growth factors such as vascular endothelial growth factor (VEGF) (or even TGF-β) is not known and is certainly worthy of exploration in the context of establishing ASM as more than a passive recipient in airway structure and function.
There is now increasing interest in the role of sex differences and sex steroids in the context of lung diseases, particularly asthma and COPD, given clinical evidence for peripubertal and perimenopausal switches in the incidence of asthma and increased asthma in the elderly male (25, 87, 277, 308, 323). However, understanding of the mechanisms by which sex steroids influence ASM is still very much in its infancy. As nuclear receptors, estrogens, progesterone, and/or testosterone may be expected to alter gene regulation, and the clinical evidence for altered airway reactivity or differences in asthma especially with changing estrogen levels is consistent with this concept. However, recent studies show that, akin to their role in vascular smooth muscle, estrogens can rapidly and nongenomically reduce [Ca2+]i in human ASM, largely via blunting of Ca2+ influx via L-type Ca2+ channels and SOCE (but surprisingly not via the expected BKCa channels) (311) (Fig. 4). Here, estrogens appear to act via ER-α and ER-β to enhance cAMP and potentiate adrenoceptor action (309). Whether ERs also influence SR Ca2+ release in ASM is not entirely clear, but, given that nonnuclear ERs (potentially in the plasma membrane) are involved, estrogen effects on PLC or CD38 may be relevant. Furthermore, estrogens can indirectly influence airway contractility by enhancing epithelial NO release (306, 307). Overall, these interesting data demonstrate that estrogens may play an important role in bronchodilation, and it may be possible to modulate ER signaling in the context of asthma, particularly with an eye on β2-agonist-sparing therapies. What remains to be established is whether estrogens, as steroid receptors of the same family as for glucocorticoids, could potentially also alter the response of ASM to inflammation. Here, the differential roles of ER-α and ER-β in the context of inflammation remain to be elucidated. Furthermore, it is important to consider whether and how progesterone influences ASM, given limited data of its effects on airway function and asthma (104, 109, 126, 295). A similar question can be raised regarding a “protective” effect for testosterone acting via androgen receptors (33, 36, 37, 158).
Ca2+ Regulatory Pathways
Second messengers.
While the importance of the PLC/IP3 and CD38/cADPR pathways in ASM is now well recognized (17, 145, 186, 231, 261, 285), several recent studies have explored how other mechanisms may indirectly regulate either second-messenger pathway, resulting in either elevated [Ca2+]i or in aiding bronchodilation. Certainly, both OGR1 and TAS2R can increase IP3 and consequent SR Ca2+ release. Conversely, quercetin, a naturally occurring PDE4 blocker has been shown to prevent methacholine-induced increases in airway resistance in mice as well as reduce [Ca2+]i in human ASM cells by inhibiting PLC-β and reducing IP3 production (305). Along the line of natural bronchodilators, constituents of ginger such as 6-gingerol, 8-gingerol, or 6-shogaol inhibit PDE4D, whereas 8-gingerol and 6-shogaol also inhibit PLC-β activity, both of which may contribute to reduced IP3 levels (310, 312). In terms of CD38, several previous studies have demonstrated that inflammation can substantially increase CD38 levels and thus enhance cADPR-mediated [Ca2+]i and contractility (101, 125, 141–144, 147, 300, 301), and more recent data show that altered CD38/cADPR signaling contributes to the asthma phenotype (141). Here, cytokines such as TNF-α are thought to produce greater increases in CD38 in asthmatic ASM via increased transcriptional regulation involving ERK and p38 MAPK activation [but not NF-κB or activated protein (AP)-1, which do regulate CD38 in nonasthmatics] (144). Furthermore, in human asthmatic ASM, TNF-α attenuates expression of the microRNA miR-140–3p that targets the CD38 3′-UTR and influences CD38 stability (142). Interestingly, the same miRNA also modulates TNF-α effects on p38 MAPK and NF-κB, both of which are relevant to CD38 regulation. Given the relevance of these pathways to a number of inflammatory cytokines, elevated CD38 in airway inflammation likely plays a large role in the observed increases in contractility in the inflamed/asthmatic airway.
Influx/efflux mechanisms.
Previous studies have established the existence of voltage-gated Ca2+ influx in ASM (145, 231, 261, 285). Indeed, there is recent evidence that P2X-mediated ligand-gated ion channels can enhance influx via L-type Ca2+ channels (102). However, unlike its clear role in cardiac muscle or even vascular smooth muscle, a continuing question is the relative importance of membrane potential and voltage changes in ASM contractility or relaxation (115, 131–133, 179). Here, it may be inappropriate to ignore the contribution of membrane potential because other regulatory mechanisms may be at play that are not completely understood. For example, blockade of K+ channels does cause contraction (131), whereas voltage-gated T-type calcium channels can increase force (115, 132). Additionally, membrane potential can regulate calcium sensitization via Rho kinase activity (179). Furthermore, the role of chloride regulation in ASM is not well understood and may be important in ASM contractility vs. relaxation (130). For example, a recent study showed that GABA, acting via the ionotropic GABA-A receptors can potentiate β2AR-mediated relaxation but in fact causes membrane depolarization involving chloride-mediated currents (78). Although elevated [Ca2+]i can activate very small-conductance Ca2+-dependent Cl− channels, opening of such channels should cause depolarization and thus contraction. For example, recent data in ovalbumin sensitized/challenged mouse airways showed a role for transmembrane protein 16A (TMEM16A) and less so for TMEM16B Ca2+-activated Cl− channels in enhancing airway contractility (351). Furthermore, previous studies have shown that agonists such as ACh can induce repetitive [Ca2+]i oscillations and waves (148, 237), which can be regulated by Ca2+-activated Cl− channels (215). Accordingly, understanding the role of Cl− movement during ASM contraction vs. relaxation may be relevant (130). In terms of bronchodilation, activation of K+ channels to hyperpolarize the membrane may be important as well, as recently demonstrated for Kv7 channels in ASM (29). Furthermore, there is now convincing evidence for the bidirectional NCX in ASM (1, 2, 116, 178, 243, 266). Although the role of NCX as a largely efflux pathway (with variable influx contribution when reversed) is established in cardiac muscle, the conditions under which NCX contributes to [Ca2+]i regulation in ASM are less clear. Certainly, NCX1 expression is robust in ASM of different species (116, 266) and is upregulated with inflammation (266). Both robust efflux and influx modes can be demonstrated, suggesting that elevation of [Ca2+]i may occur under certain conditions (e.g., to act as yet another source for store refilling), whereas removal of [Ca2+]i may contribute to bronchodilation, especially following agonist stimulation (116, 266). The relevance of increased NCX expression in airway inflammation is not clear because it may contribute to increased reactivity, as is thought to be the case for store-operated influx mechanisms, such as Orai1 and STIM1 (178). Overall, these emerging data show that, beyond intracellular Ca2+ release/reuptake, plasma membrane voltage-dependent mechanisms may be important in ASM function, which should generate substantial interest in the idea of developing drugs that target Ca2+ influx/efflux, Cl−, or K+ channels in enhancing bronchodilation.
In ASM, sequestration of elevated [Ca2+]i is largely mediated by SERCA. There is now substantial interest in the idea that reduced SERCA expression/function occurs in the asthmatic ASM and with inflammation (185, 186, 203, 267), allowing for [Ca2+]i to, not only remain elevated, but also contribute to the remodeling process. Surprisingly, there are little to no data on the calmodulin-dependent plasma membrane Ca2+ ATPase (PMCA; a major efflux mechanism in other cell types) beyond its localization to caveolae (52). Unpublished evidence (personal communication, E. E. Strehler) suggests that inflammatory cytokines such as TNF-α can downregulate PMCA expression and thus allow for elevated [Ca2+]i. Given the plasma membrane location of this mechanism, PMCA may be a prime target for intervention in diseases such as asthma.
Beyond production of ATP, there is now abundant information regarding the Ca2+ buffering role of mitochondria (6, 46, 255). Indeed, cytosolic Ca2+ serves as a stimulus and regulator for mitochondrial energy production to match demand, and, in turn, mitochondria can regulate Ca2+ mechanisms including the endoplasmic reticulum, influx pathways such as SOCE, and cell proliferation and survival. Emerging novel evidence in ASM demonstrates the existence of such roles for mitochondria (40, 48, 56, 108, 165, 192, 201, 314). For example, depletion of mitochondrial DNA (without reducing energy production) eliminates the plateau phase of the [Ca2+]i responses to bronchoconstrictor agonist in rat ASM cells, an effect involving H2O2 (40). Conversely, TGF-β can enhance mitochondrial reactive oxygen species (ROS) generation, leading to altered ASM secretion of cytokines (201). Inflammation has been recently shown to enhance [Ca2+]i via impairment of mitochondrial Ca2+ buffering (56). In addition to ROS generated as part of mitochondrial activity, there appears to be a further role for mitochondrial networks, fission/fusion pathways, and mitochondrial mobility (6). Although there are currently no data on these mechanisms in the context of ASM [Ca2+]i or contractility, in developing ASM cells, mitochondria do respond to altered oxygen levels by becoming more fragmented (108), which could lead to reduced cellular stability and a tendency to proliferate while dismantling the SR/ER network. These data point to a potentially novel avenue for addressing increases in [Ca2+]i or even downstream effects such as ER stress and function, proliferation/migration, and cellular metabolism in the context of ASM structure and function.
Structural aspects.
Beyond [Ca2+]i and Ca2+ sensitivity, there is now interest in the idea that “remodeling” of intracellular and extracellular structures may also contribute to ASM tone. One example is the emerging role of caveolae and their regulatory caveolin and cavin proteins (7, 52, 89, 91, 105, 240, 264, 265, 272, 280). Caveolae in human ASM have been found to harbor agonist receptors, Ca2+ influx channels (TRPC isoforms, Orai1, NCX; Fig. 2), and proteins for Ca2+ sensitization (RhoA) (52, 240). Here, of the three isoforms, caveolin-1 appears to be important in that reduced caveolin-1 expression or function blunts agonist-induced ASM [Ca2+]i and contractile responses (52, 92, 240). Such effects appear to involve reduced [Ca2+]i influx, impaired SR Ca2+ release, as well as blunted Ca2+ sensitivity for force generation via RhoA (91, 240, 264). Here, proinflammatory cytokines such as TNF-α can enhance caveolin-1 expression and function, and conversely caveolin-1 facilitates enhancement of [Ca2+]i and contractile responses to inflammation (265). In this regard, it appears that caveolae may differentially modulate ASM responses to cytokines such as TNF-α vs. IL-13, at least in part due to caveolar vs. noncaveolar expression of their respective receptors (265). In contrast to these [Ca2+]i/contraction data, reduced caveolin-1 expression has been reported to facilitate ASM proliferation (89, 91, 93). Thus it could be reasoned that caveolin-1 contributes to the balance between contractile vs. proliferative phenotype of ASM in the context of inflammation but in either fashion enhance airway changes in asthma for example. Unfortunately, this distinction may not be as clear cut, as evidenced by a recent report that airways of caveolin-1 knockout mice are actually more responsive to methacholine challenge, a feature exacerbated with ovalbumin sensitization and challenge (7). However, airway thickness as well as markers of cell proliferation are increased in caveolin-1 KO mice, consistent with other in vitro data on cell proliferation. Although the reasons for discrepancies between in vitro [Ca2+]i/contractility and in vivo airway reactivity remain to be determined, the potential contribution of airway epithelial caveolin-1 itself should be considered (7, 103, 161, 281). Certainly, caveolin-1 is associated with endothelial NOS, and, if epithelial NO is important in regulation of airway tone, then dysfunction due to lack of caveolin-1 may be contributory. Alternatively, other epithelial mechanisms such as cyclooxygenase 2 signaling may be altered, as suggested by a recent study (281). In addition to Ca2+/contractility per se, the relevance of caveolae may further lie in the increasing recognition that ASM can modulate ECM production. Here, by virtue of their ability to interact with intracellular cytoskeletal elements, caveolae may form a nexus or tether between intracellular and extracellular structures and facilitate signaling. Overall, these data highlight the importance of plasma membrane mechanisms such as caveolae in regulation of airway structure and function, aspects that could be examined in the context of inflammation, asthma, fibrosis, and other conditions.
Structural aspects of ASM may modulate both passive airway stiffness as well as active responses to agonist stimulation (41, 279, 353). Furthermore, long-term changes in these pathways could contribute to more established structural changes within the airway. The physiological relevance of these parameters lies in the recognition that ASM is extremely “plastic” in that it rapidly adapts and regains its ability for force generation following a decline in force induced by repetitive length changes, as occurs during tidal volume breathing (26, 57). Thus, even in the setting of a range of active forces, passive stiffness can be maintained due to adaptations in the airway. Accordingly, the reduced airway lumen in asthma due to increased contractility and remodeling would cause baseline ASM length to decrease and thus influence the adaptability of ASM. Here, increases in passive stiffness could contribute to increased airway reactivity by attenuating the extent of ASM length fluctuations during tidal breathing (i.e., offer greater passive resistance to stretch and opening of the airway lumen). A recent study showed that such adaptations are enhanced at shorter lengths of ASM cells/tissue, an effect mediated by increased cross-linking of cytoskeletal filaments (but not actin polymerization or contractile actin-myosin interactions reflected by lack of effect of latrunculin B or Rho kinase inhibition) (26). Other cytoskeletal elements such as intermediate filaments or even ECM components may be involved. For example, dense bodies, one of the most prominent structures in smooth muscle cells, can serve as anchors for actin filaments (akin to Z-disks in striated muscle). Such dense bodies and intermediate filaments can form cable-like structures and adjust their cable length according to cell length and tension via connections to the ECM, thus helping to maintain passive stress (353). Furthermore, in vitro studies using dissolution of different ECM proteins suggest that elastase exerts its functional effects by reducing parenchymal tethering, whereas collagenase reduces airway wall stiffness, thus differentially influencing the load on ASM during contraction (153). On the other hand, passive load on ASM can enhance expression of smooth muscle myosin heavy chain and decrease Akt signaling via integrins (58), whereas stretching of ASM results in increased TGF-β expression (mediated via multiple signaling mechanisms) (210) and induces ASM cell hypertrophy (211, 212). Such stretch-induced hypertrophy may be mediated via miRNAs (e.g., miR-26a) (212), but Akt may also be important (183). Separately, the β-catenin signaling cascade appears to be important for stabilizing adherens junctions and thus enhancing cell-cell contacts in ASM, resulting in enhanced contractility (88, 128). Accordingly, ECM-cell or cell-cell mechanotransduction events in ASM become particularly relevant to the idea of detrimental ASM adaptations in the setting of airway diseases and point to novel avenues for targeting excessive airway contractility and remodeling. Furthermore, in the present era of organ bioengineering, understanding the mechanisms that regulate ASM mechanical properties is key to building biocompatible artificial airways (334).
Neural Control
In airway diseases such as asthma and bronchitis, airway hyperresponsiveness can be modulated by increased cholinergic outflow from the parasympathetic nervous system (34, 276, 315). Design for reflex protection of the airway via bidirectional central nervous system (CNS)-airway pathways, repeated exposures to environmental pollutants, inflammatory stimuli, and other noxious agents may modulate afferent airway sensory pathways (150, 315, 316), leading to increased excitability of airway-related vagal preganglionic neurons and airway hyperresponsiveness. The extensive and exquisite neural control of airways has been previously reviewed (150, 177, 317, 318). CNS control of the airway involves integrated networks along the central neural axis, particularly the vagal preganglionic neurons within the medulla, which form the final common pathway from the brain to the airways. Signals are transmitted to intrinsic tracheobronchial ganglia in close proximity to effector systems of the postganglionic axons, which are distributed within the airways. In addition to the cholinergic mechanisms, intrinsic airway neurons release a variety of neuromodulators, such as vasoactive intestinal peptide and nitric oxide (NO) that form the nonadrenergic, noncholinergic system that aids bronchodilation. Vagal afferent fibers of sensory ganglia are present throughout the bronchopulmonary tree and innervate sensory receptors that detect changes in chemical, mechanical, or thermal stimuli. Different sensory receptors and afferent fibers are expressed with different functions. For example, C-fiber receptors contain the neuropeptides such as substance P and neurokinin A that can induce bronchoconstriction and further cause a local, neurogenic inflammation, whereas other mechanosensitive reflex systems control breathing and parasympathetic outflow (and thus airway tone).
The relevance of the complex neural pathways lies in their enhanced contribution in airway diseases, for example in allergen-induced changes in ASM tone and mucous secretion due to increased activation of preganglionic airway parasympathetic nerves (neurogenic asthma) (34, 276, 315), and the fact that commonly used drugs such as ipratropium target cholinergic innervation in preventing excessive bronchoconstriction. However, new mechanisms by which neural control of ASM is dynamically modulated are continuing to be identified, highlighting novel pathways for pharmacological intervention. Furthermore, beyond anatomical and immunohistochemical localization of nerve pathways, their functional aspects are also being explored. For example, one study (331) found that the level of integration by ganglionic parasympathetic neurons in the airway differs between species, an important point to consider when using transgenic and other models that may have a neurogenic component. Separately, in guinea pigs, a subset of esophageal neurons that express calretinin and ACh have been found to provide excitatory input to tracheal cholinergic ganglia and thus indirectly regulate airway tone (197). Finally, the effects of inflammation (321, 332), infection (124), pollutants (252), and other stimuli on airway nerves are being explored, again with the intent of suppressing excessive bronchoconstriction, as well as cough reflexes. Here, an emerging theme is again neurotrophins such as NGF, BDNF, and glial-derived neurotrophic factor that serve to influence airway reactivity (175, 176, 200) as well as enhance immunity and reactivity in the airway following bacterial infections (38), exposure to house dust mite (and important aspect of allergic asthma) (346), allergen challenge (176), or ozone (322). Furthermore, it may be relevant to consider whether novel mechanisms such as TAS2R and OGR1 are present and functional in neural pathways involved in airway tone.
ASM in Airway Remodeling
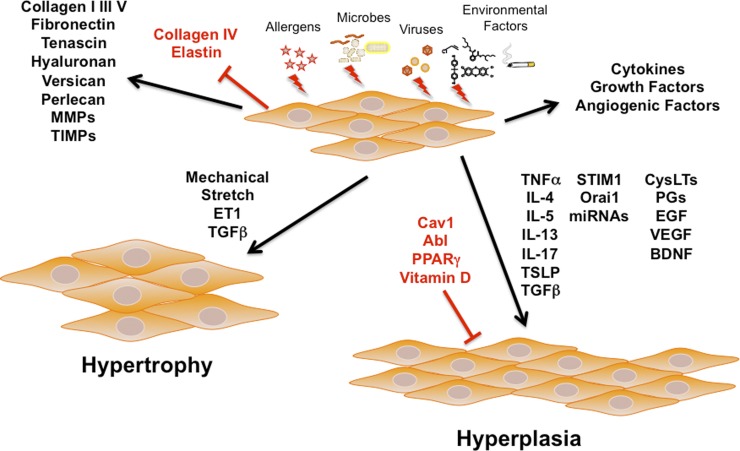
It is now well established that there is extensive airway remodeling that occurs in diseases such as asthma (11, 22, 113, 169, 190, 285, 302, 333, 337, 348). Such remodeling is characterized by structural changes including a thickened abnormal epithelium with mucus gland hypertrophy, subepithelial membrane thickening, and fibrosis with altered ECM composition and deposition, angiogenesis, and importantly increased ASM mass (Fig. 1). Indeed, increased ASM mass may be a key feature of airway remodeling, being associated with severe asthma and decreased lung function (228) and likely contributing to enhanced airway contractility and a narrowed lumen. In this regard, the mechanisms by which increased ASM mass occurs are still under investigation, with the key question of whether ASM cell hyperplasia or hypertrophy is more contributory (11, 13–15, 66, 127). It is possible that such changes are not homogeneous in different sized airways, wherein large bronchi demonstrate hyperplasia in some cases, but not others, whereas hypertrophy is present in different parts of the airway (13, 66). Certainly, the increased deposition of ECM proteins and associated fibrosis is also likely to be contributory. Regardless, the mechanisms underlying hypertrophy have been examined to a certain extent (e.g., see Ref. 15 for review; Fig. 5). For example, as mentioned above, mechanical stretch can induce hypertrophy, potentially via miRNA, glycogen synthase kinase (GSK)-3β, or Akt (14, 183, 212). Activation of mammalian target of rapamycin (mTOR) can also induce hypertrophy via multiple pathways (356), and this pathway may be stimulated by factors such as TGF-β or endothelin (85, 199, 355). In asthmatic ASM, hypertrophy is associated with increased myosin light chain kinase (13).
Fig. 5.
Role of ASM in remodeling. Besides its role in modulating contractility, there is now substantial evidence that insults such as allergens, infection, and environmental factors can modulate the “synthetic” aspects of ASM. Here, ASM can produce a range of ECM proteins including collagen, fibronectin, the matrix metalloproteinases (MMPs), and their tissue inhibitors (TIMPs), as well as a number of pro- and anti-inflammatory cytokines, growth factors (e.g., BDNF), and angiogenic factors (e.g., vascular endothelial growth factor, VEGF). Furthermore, in response to insults, autocrine/paracrine effects of locally produced factors (e.g., cytokines and growth factors) and intracellular mechanisms (e.g., STIM1, Orai1, miRNAs), ASM cells can increase in size (hypertrophy) or number (hyperplasia), thus contributing to increased ASM mass noted in asthma. PPAR, peroxisome proliferator-activated receptor; TSLP, thymic stromal lymphopoietin; ET1, endothelin 1; PGs, prostaglandins.
There are now a number of studies on ASM hyperplasia and the mechanisms that regulate it in the context of airway remodeling (11, 16, 22, 113, 160, 188, 190, 259, 285, 333, 348). In general, an upregulation of proproliferative pathways or, alternatively, blunting of endogenous mechanisms to prevent proliferation as well of apoptotic pathways have been described. Furthermore, some studies have reported that ASM cells of asthmatic patients have greater proliferation at baseline (140, 314) although the underlying mechanisms are not completely understood (the role of increased mitochondria biogenesis and impaired Ca2+ reuptake have been proposed) (314). However, it should also be emphasized that the evidence for such increased ASM proliferation in asthmatics is not conclusive with some ex vivo studies (140, 314) showing proliferation but others showing little (149, 327). Nonetheless, a plethora of signaling pathways has been found to be involved in enhancing ASM proliferation, including p38 and p42/44 MAP kinases, PI3/Akt, NF-κB, and TGF-β-associated kinase-1 (5, 39, 49, 92, 229, 230). Conversely, antiproliferative mechanisms such as the transcription factor CCAAT/enhancer binding protein-α have been found to be downregulated (258).
In terms of “stimuli” for enhanced ASM proliferation, several proinflammatory mediators are thought to be important (Fig. 5), including TNF-α, IL-4, IL-5, IL-13 (but see Ref, 254), TGF-β, thymic stromal lymphopoietin (TSLP), and those of the Th17 family (11, 39, 152, 190, 232, 245, 246). However, there is also some suggestion that “normal” stimuli such as bronchoconstrictor agonists (114, 233, 341) and other locally produced factors (5, 53, 75) could trigger increased proliferation under certain conditions, leading to the asthmatic phenotype. In this regard, interactions between GPCRs and RTKs may be important (18, 67, 68, 155, 162, 303). For example, M2 muscarinic receptors can potentiate TGF-β-induced enhancement of proliferation (216). EGF receptor signaling (particularly that from epithelium) is thought to be a potent player in airway remodeling in models of chronic allergic asthma (171, 284) and can in turn mediate the effects of cysteinyl leukotrienes (cysLTs; LTC4, LTD 4), which are synthesized from arachidonic acid and can themselves increase ASM mass (198, 296). However, one study found that prostaglandins are more effective in inducing ASM remodeling compared with leukotrienes (319). Mechanical stress in airways can also induce EGF release from epithelial cells and thus contribute to remodeling (283). Emerging data suggest a role for the nonreceptor tyrosine kinase Abl, which appears to inhibit ERK1 phosphorylation, modulate actin dynamics, and further prevent the mitogenic effects of factors such as endothelin-1 and PDGF (138, 139), the latter well known to be mitogenic in ASM (286). Other growth factors such as VEGF (187) and even the neurotrophin BDNF (5) can enhance ASM proliferation. These latter data highlight the ability of local factors to modulate ASM and the need to target such mechanisms beyond inflammation. In addition to these factors, within ASM cells, microRNAs are also thought to play a role in orchestrating proliferative and migratory responses (45, 168), a theme better examined in pulmonary artery (95, 96, 263, 342) but an emerging topic in ASM. Finally, consistent with the idea that sex steroids could influence airway structure and function (308), it is important to consider whether estrogens could potentially influence ASM proliferation, particularly given their mitogenic role in breast cancer and other malignancies. Here, the data are not as clear cut in that estrogens may in fact be antiproliferative in the airway in some settings (308). Nonetheless, estrogen signaling in the airway be further relevant to the fascinating but rare progressive disease of young women, lymphangioleiomyomatosis (LAM) (110), which involves smooth muscle-like LAM cells within the lung. LAM may represent abnormal ASM or vascular smooth muscle, and exacerbation of this disease during pregnancy would suggest a role for sex steroids. For example, interference with estrogen signaling appears to potentiate the effects of inhibitors of mTOR in alleviating LAM (100).
Overall, the information to date points to multiple, potentially interacting mechanisms that can contribute to airway remodeling represented by ASM proliferation or migration. Although increased cell proliferation can occur in the presence of inflammation via different pathways, there are less data on what prevents or blunts ongoing proliferation from continuing indefinitely. One study suggests that ASM modifies the extracellular environment (e.g., collagen fibril density), resulting in limited proliferation (274). Furthermore, signaling mechanisms within ASM itself could be involved. For example, caveolin-1 can inhibit proliferation (7, 89, 92), whereas the SOCE mechanisms STIM1 and Orai1 can enhance TGF-β1-induced proliferation (79) (Fig. 5). Certainly, therapeutic agents such as corticosteroids and even β2-agonists can reduce proliferation (19, 86). Furthermore, peroxisome proliferator-activated receptor (PPAR)-γ ligands can blunt ASM proliferation, migration, and ECM formation (63, 72, 73, 250, 293), and this mechanism may be of interest, given its emerging role in lung development and injury (180, 220, 247, 260). Finally, there is increasing interest in atypical anti-inflammatory stimuli such as vitamin D (44, 50). These emerging and exciting data point to novel avenues by which airway remodeling could be curtailed if not reversed.
Overall increases in ASM cell numbers in the context of remodeling could reflect both increased cell proliferation as well as reduced apoptosis. Although it is commonly assumed that the two processes reflect opposite sides of the same coin, their regulatory mechanisms are known to be substantially different and are not just inhibition or reduction in specific pathways that result in proliferation vs. apoptosis. Here, in contrast to the substantial data on increased ASM cell proliferation, there is much less information on apoptosis per se. Some studies have reported reduced rates of ASM apoptosis following exposure to Th17-associated cytokines (39), IL-8, eotaxin, and monocyte inflammatory protein-1a (107), as well as with TRPV1 agonist (354), extracellular laminin (313), and γ-glutamyl transpeptidase-1 (GGT1) antagonism (83). However, several other studies have found that mechanisms such as phosphatase and tensin homolog deleted on chromosome 10 (182), PPAR-γ (73), collagens (274), and vitamin D (50) modulate ASM cell proliferation but do not influence apoptosis. Much of the data in favor of or against apoptosis are derived from in vitro work in cultured ASM cells. Furthermore, it remains to be determined whether there are temporal patterns, dose responsiveness, and other modulatory factors that influence the extent of proliferation vs. apoptosis following inflammatory or other insults, particularly in vivo.
Regardless of whether significant apoptosis is a key element of ASM, an emerging topic in the area of airway biology is the concept of autophagy (or autophagocytosis), which is now recognized to be necessary for cellular homeostasis and assurance of cell survival during times of stress or altered cellular energy levels. Autophagy can be seen as an adaptive response to survival but could also promote cell death in the context of disease. There is presently limited information regarding how autophagy may play a role in asthma, or of the cell types involved. Because autophagy plays a major role in the immune responses to various pathogens relevant to asthma induction or exacerbation (particularly viruses), it is possible that autophagic responses in airway epithelia or smooth muscle occur in the setting of infection or alternatively contribute to worsening of inflammatory effects when infection is also present (146). Whether autophagy in ASM is important has been examined only to a limited extent. For example, pharmacological inhibition of GGT1 induces p53-dependent autophagy in human ASM cells (83). Furthermore, excessive ROS, as may occur in airway inflammation, could induce autophagy and thus contribute to asthma pathophysiology (234).
In addition to increased proliferation, given the “synthetic” aspect of ASM, there is now much interest in the concept of ASM-generated factors that can have autocrine/paracrine effects in terms of remodeling. Here, generation of ECM proteins by ASM (23, 31, 53) (in addition to the well-known role of fibroblasts) is particularly important and relevant, considering the fact that the fractional area of the matrix is increased in smooth muscle layers in fatal asthma (10). In this regard, ASM cells derived from asthmatics may produce a different profile of ECM proteins or respond differently to the inflammatory milieu. The importance of the ECM composition further lies in responsiveness of the airway to therapy (particularly resistance to corticosteroids and β2AR agonists; Refs. 27, 274) and the very question of whether airway remodeling is reversible or controlled by current therapies.
In the airway, a network of collagenous and noncollagenous ECM protein structures surrounds cells, and thus the density and composition of the ECM influence cellular behaviors such as proliferation, migration, differentiation, and survival. Particularly relevant ECM elements include collagens and fibronectin (30, 117) and members of the matrix metalloproteinase (MMP) family (MMP-1, MMP-2, MMP-9, and MMP-12) as well as their tissue inhibitors of metalloproteinase, (TIMP)-1 and TIMP-2 (42, 151, 160, 167, 218, 294) (Fig. 5). Furthermore, signaling moieties on ECM proteins can in turn regulate epithelial, ASM, and other cells. Here, ECM can itself regulate formation and release of growth factors and MMPs (which cleave a number of extracellular proteins), thus creating a complex network that can regulate the extent of remodeling. Alterations in ECM in the asthmatic airway include enhanced deposition of collagens I, III, and V, fibronectin, tenascin, hyaluronan, versican, and perlecan but a decrease in other proteins such as collagen IV and elastin (30). Altered ECM production can be modulated by inflammatory mediators produced by surrounding cells as well as growth factors. Here, a number of signaling cascades have been identified (30, 160), and new ones are frequently reported. Conversely novel mechanisms that blunt ASM-derived ECM production are also identified. For example, IL-1β alone or in combination with TNF-α increases ASM production of MMP-12 (339), as well as MMP-9 (174), which, by degrading elastin, collagen, etc., can promote cell migration, tissue repair, and remodeling. Noncanonical Wnt5a and β-catenin regulate TGF-β effects on ASM production of ECM proteins (9, 160), whereas TGF-β alone can enhance deposition of perlecan in ASM from patients with COPD (121). Loss of caveolin-1 enhances ECM levels in chronic inflammation within the airway (7), whereas Rho kinase inhibition prevents ECM remodeling (235). Decorin, an ECM proteoglycan, can bind TGF-β and blunt ECM production within airways (191). Even infections can alter ECM production by ASM, exemplified by rhinovirus-induced increases in fibronectin and perlecan, particularly in asthmatic ASM (164). These data, by no means comprehensive, underline the complex involvement of ASM in matrix remodeling. Here, it is important to emphasize that the effects of the matrix per se on ASM structure and function form an even more complex layer that is yet to be well established.
Like many other cell types within the airway, ASM not only respond to inflammatory mediators but are themselves a source of a wide variety of pro- and anti-inflammatory factors, granting ASM an immunomodulatory function as well. Indeed, such an immunomodulatory function itself can be triggered by inflammation, infection, and microbial products (160). For example, anti-CD3-stimulated peripheral blood T cells as well as bronchoalveolar lavage-derived T-cells from challenged atopic donors can adhere to ASM, upregulating intercellular adhesion molecule-1 expression and inducing ASM surface expression of MHC class II (170). The relevance of such interesting functions of ASM lies in the ability of secreted factors to have both autocrine effects on ASM responses to inflammation as well as in the recruitment of other cell types in the inflammatory response of the airway. For example, exposure of ASM cells can present bacterial superantigen to CD4+ T cells via such MHC class II to enhance production of proasthmatic cytokine such as IL-13 (320). Indeed, IL-13 is just one of the many factors produced by ASM, with the list ever increasing (160, 287). Examples include IL-1β, IL-5, IL-6, IL-8, IL-17, PDGF, and TGF-β although their autocrine/paracrine function within the airway in the context of preexisting inflammation is still being explored. For example, IL-6, a pleiotropic cytokine that can induce ASM hyperplasia and modulate immune cell function, is secreted by ASM cells when they are stimulated by other cytokines such as IL-1β or TNF-α. Furthermore, TNF-α can mediate its effects on ASM by enhancing IFN-β secretion (195) or via other receptors [including those for microbes such as Toll-like receptor 2 (189)]. Similarly, TGF-β can regulate IL-6 release from ASM cells (201). Recent data also demonstrate that ASM is a source as well as target of the early cytokine TSLP that can recruit dendritic cells and mediate a host of airway responses (244, 245, 288). The mechanisms by which regulation of cytokine release by ASM occurs are plentiful and include recognized players in inflammation such as NF-κB and AP-1, but new pathways such as Nox4, ROS (201), GSK-3β (9) are being identified. There is also emerging evidence that ASM may be a major source of chemokines such as RANTES, eotaxin, monocyte chemoattractant protein-1, and CXCL10 (51). The mechanisms by which such release occurs are also under investigation and include players such as MAPK, JAK/Stat, JNK, and even [Ca2+]i (3, 297). Although it is not clear whether ASM-derived chemokines serve any other function beyond the expected recruitment of inflammatory cells, it is nonetheless clear that ASM per se plays an important role in the inflamed airway. In addition to these typical inflammatory factors, there is also emerging evidence that growth factors such as VEGF and BDNF can be secreted by ASM cells (5, 160, 200, 324), which may have autocrine effects in terms of cell proliferation as well as contractility. The overall relevance of ASM-derived factors from a clinical perspective lies in the ability of therapies such as glucocorticoids in blunting the secretion of these factors and thus inflammation and its effects as a whole. Here, limited data suggest that the profile of ASM-derived factors may drive steroid insensitivity and limit the ability of this line of therapy in blunting airway inflammation.
Airway Development
Although much of the above discussion has focused on the importance of ASM in adult airways and diseases such as asthma or COPD, it is also increasingly evident that factors that detrimentally influence airway development in both the fetal and early postnatal periods can have longstanding effects through childhood and beyond (12, 65, 282). Certainly, our understanding of the processes that regulate embryonic lung development is now vastly advanced with the introduction of lineage studies, transgenic animals, and a variety of sophisticated imaging techniques. Unlike alveoli that continue to form and grow postnatally for several years, the number of airways is fixed at birth. Accordingly, disruption of airway branching, elongation, and growth in the late fetal period can have substantial postnatal effects. Added to this is the frequent complication of premature birth requiring exposure of immature airways to high levels of oxygen with or without mechanical ventilation in the neonatal intensive care unit. A well-recognized sequelae is bronchopulmonary dysplasia, represented by alveolar simplification and vascular dysmorphogenesis. However, a cardinal feature of survivors of the premature period is wheezing and asthma that extends into childhood and sometimes adulthood (194, 249, 345). Further complications may occur due to environmental exposures in the neonate such as second-hand cigarette smoke and respiratory infections. What is not well established is what role the developing ASM plays in these scenarios and what effects insults such as hyperoxia (or even hypoxia) and environmental triggers play in altering airway structure and function.
One relevant issue during fetal development is the role of mechanical stretch and the concept of airway peristalsis (134, 135) and cyclical Ca2+ waves within fetal ASM cells that can promote airway branching and elongation. Such spontaneous peristaltic Ca2+ waves and contractions appear to be coupled and arise from specific “pacemaker”-like ASM cells and intercellular communications via gap junctions (71, 136). Although the mechanisms underlying these waves are still under investigation, membrane potential, L-type Ca2+ channels, and chloride currents appear to play a role (which makes the developing ASM different from the adult). Given the reemergence of chloride pathways in ASM (see above), it may be relevant to examine this issue as well as the role of membrane potential pathways in the developing ASM in the context of potential intervention in the face of lung or airway hypoplasia as occurs with diseases such as congenital diaphragmatic hernia or diaphragm paralysis, intrauterine growth retardation, infection, etc. Regardless, these novel findings have helped identify ASM as being more than a vestigial cell type and an active player in airway development. Indeed, it would be important to imagine the immature ASM with a high proliferative rate as being akin to the synthetic, proliferative ASM of in adult asthma where intracellular Ca2+ regulatory structures are deconstructed, making the cells more dependent on influx pathways. Conversely, the concept of pacemakers and their interruption as being key to dysfunctional airway growth (136) could be leveraged toward understanding why emerging technologies such as focal bronchial thermoplasty are effective in blunting airway remodeling in asthma. Furthermore, the emerging role of stretch in modulating expression of genes and proteins in adult tissues should serve as a model to examine how mechanical forces within the developing airway induced by ASM cells could influence airway and lung growth. The relevance of stretch may conversely lie in determining how injurious mechanical forces, especially in the setting of prematurity could influence lung development (111, 112).
As with other lung regions, a number of growth factors and transcription factors are being identified as being important for ASM development per se, and their upregulation or downregulation with insults may affect overall airway development as well as postnatal responses to insults and their sequelae. However, given the complex and coordinated processes that define lung development, it is difficult to target specific, single mechanisms that may be alleviating. Nonetheless, some targets such as fibroblast growth factor 10 (94, 135, 275), the neurotrophins [particularly BDNF/TrkB; (81, 242, 291, 344, 345)], and Runt-related transcription factors (106) are being examined. Changes in airway development and growth following inflammation/injury (28, 35, 111, 112, 166), diaphragm paralysis (349), oxygen exposure (97, 108, 156), and cigarette smoke (106) are also now being characterized. Further development in this area is dependent on appropriate in vitro and in vivo models that reflect human lung development and growth, not an easy task to accomplish given differences between immature and adult ASM (108). Furthermore, from a practical perspective, it is important to consider whether and how pharmacological or other interventions targeting ASM influence other cell types in the growing lung.
What Have We Learned?
As with other cell types in the lung, indeed any organ, developments in biochemical, molecular and cell biology, imaging, and in vivo techniques have resulted in an explosion of new information on what makes ASM “tick”. Here, a major leap over the past several years has been the clear identification of novel synthetic roles for ASM, well beyond its recognized function as the determinant of airway tone via contractility. In this regard, what is truly impressive is the wide variety of factors produced by ASM as well as those that ASM responds to in myriad ways in a highly context-sensitive fashion (be it for inflammation, growth, survival, differentiation, etc.). This certainly makes it difficult to discuss airway reactivity without considering both the contractile and synthetic/remodeling aspects of ASM. Conversely, from a practical perspective of “interference” with detrimental aspects of ASM structure or function to alleviate disease, or enhancement of beneficial aspects, the task becomes even more difficult when one considers that many of the relevant factors, mechanisms, pathways, genes, etc. are extensively and intricately interlinked. Indeed, the idea that a single gene, mechanism, or pathway, even if perfectly targeted, would alleviate disease should be considered blasé. Therefore, it becomes all the more important, not only to understand the different mechanisms that contribute to ASM structure and function in health and disease, but also to develop sophisticated, integrative biological imaging, informatics, and other techniques to be able to analyze pathways and interactions so that the placement and importance of potential targets for intervention can be understood. Here, it is important to consider the relevance and validity of both in vitro and in vivo models with questions such as the following: 1) “How representative are cultured ASM cells of tissues in vivo, and should work in cells be verified in tissues?”; 2) “What is the equivalency of ASM cells or tissues derived from different species?”; 3) “What is the role of in vivo models such as the mouse in examination of ASM per se, and here should there be greater attention on development and validation of an ASM-specific transgenic technique (akin to the more global smooth-muscle specific transgenics)/?”; and 4) “Should there be more attention on delivery and targeting mechanisms for ASM that overcome the epithelial or vascular barrier without disruptions in either?”. However, from a functional perspective, in the final analysis, the questions to consider are as follows: 1) “How does ASM fit into the normal and abnormal structure and function of the airway as a whole, i.e., what is the importance of ASM and its interactions with ECM, epithelium, and other permanent or transient (immune cell) residents of the airway?”; 2) “In diseases such as asthma and COPD, what aspect of the ASM is practical to target using our pharmacological or biological armamentarium, i.e., should there be greater focus on novel bronchodilatory mechanisms or the recalcitrant problem of airway remodeling that is thought to be largely irreversible?”. The above discussion of just a sliver of the exciting research relating to all of these themes provides much confidence that the central role of ASM in normal and abnormal airway structure and function at different life stages and conditions will continue to be a major focus of translational research.
GRANTS
This work was supported by NIH grants HL088029 and HL056470 (Y. Prakash).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
REFERENCES
- 1.Algara-Suarez P, Mejia-Elizondo R, Sims SM, Saavedra-Alanis VM, Espinosa-Tanguma R. The 13 isoform of Na+-Ca2+ exchanger expressed in guinea pig tracheal smooth muscle is less sensitive to KB-R7943. J Physiol Biochem 66: 117–125, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Algara-Suarez P, Romero-Mendez C, Chrones T, Sanchez-Armass S, Meza U, Sims SM, Espinosa-Tanguma R. Functional coupling between the Na+/Ca2+ exchanger and nonselective cation channels during histamine stimulation in guinea pig tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L191–L198, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Alrashdan YA, Alkhouri H, Chen E, Lalor DJ, Poniris M, Henness S, Brightling CE, Burgess JK, Armour CL, Ammit AJ, Hughes JM. Asthmatic airway smooth muscle CXCL10 production: mitogen-activated protein kinase JNK involvement. Am J Physiol Lung Cell Mol Physiol 302: L1118–L1127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An SS, Wang WC, Koziol-White CJ, Ahn K, Lee DY, Kurten RC, Panettieri RA, Jr, Liggett SB. TAS2R activation promotes airway smooth muscle relaxation despite β(2)-adrenergic receptor tachyphylaxis. Am J Physiol Lung Cell Mol Physiol 303: L304–L311, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med 16: 812–823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aravamudan B, Thompson MA, Pabelick CM, Prakash YS. Mitochondria in lung diseases. Expert Rev Respir Med. 2013, August 27, Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aravamudan B, VanOosten SK, Meuchel LW, Vohra P, Thompson M, Sieck GC, Prakash YS, Pabelick CM. Caveolin-1 knockout mice exhibit airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol 303: L669–L681, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baarsma HA, Konigshoff M, Gosens R. The WNT signaling pathway from ligand secretion to gene transcription: molecular mechanisms and pharmacological targets. Pharmacol Ther 138: 66–83, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Baarsma HA, Menzen MH, Halayko AJ, Meurs H, Kerstjens HA, Gosens R. β-Catenin signaling is required for TGF-β1-induced extracellular matrix production by airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 301: L956–L965, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Bai TR, Cooper J, Koelmeyer T, Pare PD, Weir TD. The effect of age and duration of disease on airway structure in fatal asthma. Am J Respir Crit Care Med 162: 663–669, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Bara I, Ozier A, Tunon de Lara JM, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J 36: 1174–1184, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Beers MF, Morrisey EE. The three R's of lung health and disease: repair, remodeling, and regeneration. J Clin Invest 121: 2065–2073, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 167: 1360–1368, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Bentley JK, Deng H, Linn MJ, Lei J, Dokshin GA, Fingar DC, Bitar KN, Henderson WR, Jr, Hershenson MB. Airway smooth muscle hyperplasia and hypertrophy correlate with glycogen synthase kinase-3β phosphorylation in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol 296: L176–L184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentley JK, Hershenson MB. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc Am Thorac Soc 5: 89–96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berair R, Saunders R, Brightling CE. Origins of increased airway smooth muscle mass in asthma. BMC Med 11: 145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol 586: 5047–5061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billington CK, Kong KC, Bhattacharyya R, Wedegaertner PB, Panettieri RA, Jr, Chan TO, Penn RB. Cooperative regulation of p70S6 kinase by receptor tyrosine kinases and G protein-coupled receptors augments airway smooth muscle growth. Biochemistry 44: 14595–14605, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Billington CK, Ojo OO, Penn RB, Ito S. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther 26: 112–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res 4: 2, 2003 [PMC free article] [PubMed] [Google Scholar]
- 21.Black JL, Oliver BG, Roth M. Molecular mechanisms of combination therapy with inhaled corticosteroids and long-acting beta-agonists. Chest 136: 1095–1100, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Black JL, Panettieri RA, Jr, Banerjee A, Berger P. Airway smooth muscle in asthma: just a target for bronchodilation? Clin Chest Med 33: 543–558, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black JL, Roth M. Intrinsic asthma: is it intrinsic to the smooth muscle? Clin Exp Allergy 39: 962–965, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda) 20: 70–78, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol 13: 92–99, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosse Y, Solomon D, Chin LY, Lian K, Pare PD, Seow CY. Increase in passive stiffness at reduced airway smooth muscle length: potential impact on airway responsiveness. Am J Physiol Lung Cell Mol Physiol 298: L277–L287, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Bourke JE, Li X, Foster SR, Wee E, Dagher H, Ziogas J, Harris T, Bonacci JV, Stewart AG. Collagen remodelling by airway smooth muscle is resistant to steroids and beta(2)-agonists. Eur Respir J 37: 173–182, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Brew N, Hooper SB, Allison BJ, Wallace MJ, Harding R. Injury and repair in the very immature lung following brief mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 301: L917–L926, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Brueggemann LI, Kakad PP, Love RB, Solway J, Dowell ML, Cribbs LL, Byron KL. Kv7 potassium channels in airway smooth muscle cells: signal transduction intermediates and pharmacological targets for bronchodilator therapy. Am J Physiol Lung Cell Mol Physiol 302: L120–L132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess JK. The role of the extracellular matrix and specific growth factors in the regulation of inflammation and remodelling in asthma. Pharmacol Ther 122: 19–29, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Burgess JK, Ceresa C, Johnson SR, Kanabar V, Moir LM, Nguyen TT, Oliver BG, Schuliga M, Ward J. Tissue and matrix influences on airway smooth muscle function. Pulm Pharmacol Ther 22: 379–387, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Cabezas GA, Velasco M. DA1 receptors modulation in rat isolated trachea. Am J Ther 17: 301–305, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Canguven O, Albayrak S. Do low testosterone levels contribute to the pathogenesis of asthma? Med Hypotheses 76: 585–588, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Canning BJ, Spina D. Sensory nerves and airway irritability. Handb Exp Pharmacol: 139–183, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao L, Wang J, Zhu Y, Tseu I, Post M. Maternal endotoxin exposure attenuates allergic airway disease in infant rats. Am J Physiol Lung Cell Mol Physiol 298: L670–L677, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol 177: 621–630, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Card JW, Voltz JW, Ferguson CD, Carey MA, DeGraff LM, Peddada SD, Morgan DL, Zeldin DC. Male sex hormones promote vagally mediated reflex airway responsiveness to cholinergic stimulation. Am J Physiol Lung Cell Mol Physiol 292: L908–L914, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardenas S, Scuri M, Samsell L, Ducatman B, Bejarano P, Auais A, Doud M, Mathee K, Piedimonte G. Neurotrophic and neuroimmune responses to early-life Pseudomonas aeruginosa infection in rat lungs. Am J Physiol Lung Cell Mol Physiol 299: L334–L344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang Y, Al-Alwan L, Risse PA, Halayko AJ, Martin JG, Baglole CJ, Eidelman DH, Hamid Q. Th17-associated cytokines promote human airway smooth muscle cell proliferation. FASEB J 26: 5152–5160, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Chen T, Zhu L, Wang T, Ye H, Huang K, Hu Q. Mitochondria depletion abolishes agonist-induced Ca2+ plateau in airway smooth muscle cells: potential role of H2O2. Am J Physiol Lung Cell Mol Physiol 298: L178–L188, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Chin LY, Bosse Y, Pascoe C, Hackett TL, Seow CY, Pare PD. Mechanical properties of asthmatic airway smooth muscle. Eur Respir J 40: 45–54, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Churg A, Zhou S, Wright JL. Series “matrix metalloproteinases in lung health and disease”: Matrix metalloproteinases in COPD. Eur Respir J 39: 197–209, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Clifford RL, Knox AJ. Future bronchodilator therapy: a bitter pill to swallow? Am J Physiol Lung Cell Mol Physiol 303: L953–L955, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Clifford RL, Knox AJ. Vitamin D—a new treatment for airway remodelling in asthma? Br J Pharmacol 158: 1426–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clifford RL, Singer CA, John AE. Epigenetics and miRNA emerge as key regulators of smooth muscle cell phenotype and function. Pulm Pharmacol Ther 26: 75–85, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta 1797: 607–618, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Cooper PR, Kurten RC, Zhang J, Nicholls DJ, Dainty IA, Panettieri RA. Formoterol and salmeterol induce a similar degree of beta2-adrenoceptor tolerance in human small airways but via different mechanisms. Br J Pharmacol 163: 521–532, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai J, Kuo KH, Leo JM, van Breemen C, Lee CH. Rearrangement of the close contact between the mitochondria and the sarcoplasmic reticulum in airway smooth muscle. Cell Calcium 37: 333–340, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Damera G, Druey KM, Cooper PR, Krymskaya VP, Soberman RJ, Amrani Y, Hoshi T, Brightling CE, Panettieri RA., Jr An RGS4-mediated phenotypic switch of bronchial smooth muscle cells promotes fixed airway obstruction in asthma. PloS One 7: e28504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA., Jr Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol 158: 1429–1441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damera G, Tliba O, Panettieri RA., Jr Airway smooth muscle as an immunomodulatory cell. Pulm Pharmacol Ther 22: 353–359, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darby PJ, Kwan CY, Daniel EE. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca2+ handling. Br J Pharmacol 279: L1226–L1235, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Dekkers BG, Maarsingh H, Meurs H, Gosens R. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thorac Soc 6: 683–692, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Dekkers BG, Pehlic A, Mariani R, Bos IS, Meurs H, Zaagsma J. Glucocorticosteroids and beta(2)-adrenoceptor agonists synergize to inhibit airway smooth muscle remodeling. J Pharmacol Exp Ther 342: 780–787, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol 41: 494–504, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Delmotte P, Yang B, Thompson MA, Pabelick CM, Prakash YS, Sieck GC. Inflammation alters regional mitochondrial Ca(2)+ in human airway smooth muscle cells. Am J Physiol Cell Physiol 303: C244–C256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng L, Bosse Y, Brown N, Chin LY, Connolly SC, Fairbank NJ, King GG, Maksym GN, Pare PD, Seow CY, Stephen NL. Stress and strain in the contractile and cytoskeletal filaments of airway smooth muscle. Pulm Pharmacol Ther 22: 407–416, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Desai LP, Wu Y, Tepper RS, Gunst SJ. Mechanical stimuli and IL-13 interact at integrin adhesion complexes to regulate expression of smooth muscle myosin heavy chain in airway smooth muscle tissue. Am J Physiol Lung Cell Mol Physiol 301: L275–L284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deshpande DA, Penn RB. Targeting G protein-coupled receptor signaling in asthma. Cell Signal 18: 2105–2120, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Deshpande DA, Theriot BS, Penn RB, Walker JK. Beta-arrestins specifically constrain β2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J 22: 2134–2141, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dicpinigaitis PV. Effect of the GABA-agonist baclofen on bronchial responsiveness in asthmatics. Pulm Pharmacol Ther 12: 257–260, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Donovan C, Tan X, Bourke JE. PPARgamma ligands regulate noncontractile and contractile functions of airway smooth muscle: implications for asthma therapy. PPAR Res 2012: 809164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Druey KM. Regulators of G protein signalling: potential targets for treatment of allergic inflammatory diseases such as asthma. Expert Opin Ther Targets 7: 475–484, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Duijts L. Fetal and infant origins of asthma. Eur J Epidemiol 27: 5–14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis 148: 720–726, 1993 [DOI] [PubMed] [Google Scholar]
- 67.Ediger TL, Danforth BL, Toews ML. Lysophosphatidic acid upregulates the epidermal growth factor receptor in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 282: L91–L98, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Ediger TL, Schulte NA, Murphy TJ, Toews ML. Transcription factor activation and mitogenic synergism in airway smooth muscle cells. Eur Respir J 21: 759–769, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Enna SJ, Bowery NG. GABA(B) receptor alterations as indicators of physiological and pharmacological function. Biochem Pharmacol 68: 1541–1548, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Faisy C, Planquette B, Naline E, Risse PA, Frossard N, Fagon JY, Advenier C, Devillier P. Acid-induced modulation of airway basal tone and contractility: role of acid-sensing ion channels (ASICs) and TRPV1 receptor. Life Sci 81: 1094–1102, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Featherstone NC, Jesudason EC, Connell MG, Fernig DG, Wray S, Losty PD, Burdyga TV. Spontaneous propagating calcium waves underpin airway peristalsis in embryonic rat lung. Am J Respir Cell Mol Biol 33: 153–160, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Fogli S, Pellegrini S, Adinolfi B, Mariotti V, Melissari E, Betti L, Fabbrini L, Giannaccini G, Lucacchini A, Bardelli C, Stefanelli F, Brunelleschi S, Breschi MC. Rosiglitazone reverses salbutamol-induced beta(2) -adrenoceptor tolerance in airway smooth muscle. Br J Pharmacol 162: 378–391, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fogli S, Stefanelli F, Picchianti L, Del Re M, Mey V, Bardelli C, Danesi R, Breschi MC. Synergistic interaction between PPAR ligands and salbutamol on human bronchial smooth muscle cell proliferation. Br J Pharmacol 168: 266–275, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Folinsbee LJ. Human health effects of exposure to airborne acid. Environ Health Perspect 79: 195–199, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Freund-Michel V, Bertrand C, Frossard N. TrkA signalling pathways in human airway smooth muscle cell proliferation. Cell Signal 18: 621–627, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Gallos G, Gleason NR, Zhang Y, Pak SW, Sonett JR, Yang J, Emala CW. Activation of endogenous GABAA channels on airway smooth muscle potentiates isoproterenol-mediated relaxation. Am J Physiol Lung Cell Mol Physiol 295: L1040–L1047, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gallos G, Townsend E, Yim P, Virag L, Zhang Y, Xu D, Bacchetta M, Emala CW. Airway epithelium is a predominant source of endogenous airway GABA and contributes to relaxation of airway smooth muscle tone. Am J Physiol Lung Cell Mol Physiol 304: L191–L197, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallos G, Yim P, Chang S, Zhang Y, Xu D, Cook JM, Gerthoffer WT, Emala CW., Sr Targeting the restricted α-subunit repertoire of airway smooth muscle GABAA receptors augments airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol 302: L248–L256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao YD, Zheng JW, Li P, Cheng M, Yang J. Store-operated Ca2+ entry is involved in transforming growth factor-beta1 facilitated proliferation of rat airway smooth muscle cells. J Asthma 50: 439–448, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Gao YD, Zou JJ, Zheng JW, Shang M, Chen X, Geng S, Yang J. Promoting effects of IL-13 on Ca2+ release and store-operated Ca2+ entry in airway smooth muscle cells. Pulm Pharmacol Ther 23: 182–189, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Garcia-Suarez O, Perez-Pinera P, Laura R, Germana A, Esteban I, Cabo R, Silos-Santiago I, Cobo JL, Vega JA. TrkB is necessary for the normal development of the lung. Respir Physiol Neurobiol 167: 281–291, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Gerthoffer WT, Solway J, Camoretti-Mercado B. Emerging targets for novel therapy of asthma. Curr Opin Pharmacol 13: 324–330, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghavami S, Mutawe MM, Schaafsma D, Yeganeh B, Unruh H, Klonisch T, Halayko AJ. Geranylgeranyl transferase 1 modulates autophagy and apoptosis in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 302: L420–L428, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Gladkevich A, Korf J, Hakobyan VP, Melkonyan KV. The peripheral GABAergic system as a target in endocrine disorders. Auton Neurosci 124: 1–8, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Goldsmith AM, Bentley JK, Zhou L, Jia Y, Bitar KN, Fingar DC, Hershenson MB. Transforming growth factor-beta induces airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol 34: 247–254, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goncharova EA, Goncharov DA, Zhao H, Penn RB, Krymskaya VP, Panettieri RA., Jr beta2-adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein. Am J Respir Cell Mol Biol 46: 48–54, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez-Arenas A, Agramonte-Hevia J. Sex steroid hormone effects in normal and pathologic conditions in lung physiology. Mini Rev Med Chem 12: 1055–1062, 2012 [DOI] [PubMed] [Google Scholar]
- 88.Gosens R, Baarsma HA, Heijink IH, Oenema TA, Halayko AJ, Meurs H, Schmidt M. De novo synthesis of β-catenin via H-Ras and MEK regulates airway smooth muscle growth. FASEB J 24: 757–768, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Gosens R, Mutawe M, Martin S, Basu S, Bos ST, Tran T, Halayko AJ. Caveolae and caveolins in the respiratory system. Curr Mol Med 8: 741–753, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Gosens R, Nelemans SA, Hiemstra M, Grootte Bromhaar MM, Meurs H, Zaagsma J. Insulin induces a hypercontractile airway smooth muscle phenotype. Eur J Pharmacol 481: 125–131, 2003 [DOI] [PubMed] [Google Scholar]
- 91.Gosens R, Stelmack GL, Bos ST, Dueck G, Mutawe MM, Schaafsma D, Unruh H, Gerthoffer WT, Zaagsma J, Meurs H, Halayko AJ. Caveolin-1 is required for contractile phenotype expression by airway smooth muscle cells. J Cell Mol Med 15: 2430–2442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L523–L534, 2006 [DOI] [PubMed] [Google Scholar]
- 93.Gosens R, Stelmack GL, Dueck G, Mutawe MM, Hinton M, McNeill KD, Paulson A, Dakshinamurti S, Gerthoffer WT, Thliveris JA, Unruh H, Zaagsma J, Halayko AJ. Caveolae facilitate muscarinic receptor-mediated intracellular Ca2+ mobilization and contraction in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1406–L1418, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Goss AM, Tian Y, Cheng L, Yang J, Zhou D, Cohen ED, Morrisey EE. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev Biol 356: 541–552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gou D, Ramchandran R, Peng X, Yao L, Kang K, Sarkar J, Wang Z, Zhou G, Raj JU. miR-210 has an antiapoptotic effect in pulmonary artery smooth muscle cells during hypoxia. Am J Physiol Lung Cell Mol Physiol 303: L682–L691, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grant JS, White K, Maclean MR, Baker AH. MicroRNAs in pulmonary arterial remodeling. Cell Mol Life Sci. 2013, Jun 6, Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Greenwood KK, Proper SP, Saini Y, Bramble LA, Jackson-Humbles DN, Wagner JG, Harkema JR, LaPres JJ. Neonatal epithelial hypoxia inducible factor-1α expression regulates the response of the lung to experimental asthma. Am J Physiol Lung Cell Mol Physiol 302: L455–L462, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 291: L58–L65, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gu Q, Lee LY. Regulation of acid signaling in rat pulmonary sensory neurons by protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol 298: L454–L461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gu X, Yu JJ, Ilter D, Blenis N, Henske EP, Blenis J. Integration of mTOR and estrogen-ERK2 signaling in lymphangioleiomyomatosis pathogenesis. Proc Natl Acad Sci USA 110: 14960–14965, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guedes AG, Jude JA, Paulin J, Kita H, Lund FE, Kannan MS. Role of CD38 in TNF-α-induced airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol 294: L290–L299, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Gui Y, Wang Z, Sun X, Walsh MP, Li JJ, Gao J, Zheng XL. Uridine adenosine tetraphosphate induces contraction of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 301: L789–L794, 2011 [DOI] [PubMed] [Google Scholar]
- 103.Hackett TL, de Bruin HG, Shaheen F, van den Berge M, van Oosterhout AJ, Postma DS, Heijink IH. Caveolin-1 controls airway epithelial barrier function: implications for asthma. Am J Respir Cell Mol Biol 49: 662–671, 2013 [DOI] [PubMed] [Google Scholar]
- 104.Haggerty CL, Ness RB, Kelsey S, Waterer GW. The impact of estrogen and progesterone on asthma. Ann Allergy Asthma Immunol 90: 284–291; quiz 291–283, 347, 2003 [DOI] [PubMed] [Google Scholar]
- 105.Halayko AJ, Tran T, Gosens R. Phenotype and functional plasticity of airway smooth muscle: role of caveolae and caveolins. Proc Am Thorac Soc 5: 80–88, 2008 [DOI] [PubMed] [Google Scholar]
- 106.Haley KJ, Lasky-Su J, Manoli SE, Smith LA, Shahsafaei A, Weiss ST, Tantisira K. RUNX transcription factors: association with pediatric asthma and modulated by maternal smoking. Am J Physiol Lung Cell Mol Physiol 301: L693–L701, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Halwani R, Al-Abri J, Beland M, Al-Jahdali H, Halayko AJ, Lee TH, Al-Muhsen S, Hamid Q. CC and CXC chemokines induce airway smooth muscle proliferation and survival. J Immunol 186: 4156–4163, 2011 [DOI] [PubMed] [Google Scholar]
- 108.Hartman WR, Smelter DF, Sathish V, Karass M, Kim S, Aravamudan B, Thompson MA, Amrani Y, Pandya HC, Martin RJ, Prakash YS, Pabelick CM. Oxygen dose responsiveness of human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303: L711–L719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hellings PW, Vandekerckhove P, Claeys R, Billen J, Kasran A, Ceuppens JL. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy 33: 1457–1463, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Henske EP, McCormack FX. Lymphangioleiomyomatosis—a wolf in sheep's clothing. J Clin Invest 122: 3807–3816, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hillman NH, Nitsos I, Berry C, Pillow JJ, Kallapur SG, Jobe AH. Positive end-expiratory pressure and surfactant decrease lung injury during initiation of ventilation in fetal sheep. Am J Physiol Lung Cell Mol Physiol 301: L712–L720, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hillman NH, Polglase GR, Pillow JJ, Saito M, Kallapur SG, Jobe AH. Inflammation and lung maturation from stretch injury in preterm fetal sheep. Am J Physiol Lung Cell Mol Physiol 300: L232–L241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest 144: 1026–1032, 2013 [DOI] [PubMed] [Google Scholar]
- 114.Hirota N, Risse PA, Novali M, McGovern T, Al-Alwan L, McCuaig S, Proud D, Hayden P, Hamid Q, Martin JG. Histamine may induce airway remodeling through release of epidermal growth factor receptor ligands from bronchial epithelial cells. FASEB J 26: 1704–1716, 2012 [DOI] [PubMed] [Google Scholar]
- 115.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J 30: 114–133, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Hirota S, Janssen LJ. Store-refilling involves both L-type calcium channels and reverse-mode sodium-calcium exchange in airway smooth muscle. Eur Respir J 30: 269–278, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Hocking DC. Fibronectin matrix deposition and cell contractility: implications for airway remodeling in asthma. Chest 122: 275S–278S, 2002 [DOI] [PubMed] [Google Scholar]
- 118.Horvat SJ, Deshpande DA, Yan H, Panettieri RA, Codina J, DuBose TD, Jr, Xin W, Rich TC, Penn RB. A-kinase anchoring proteins regulate compartmentalized cAMP signaling in airway smooth muscle. FASEB J 26: 3670–3679, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoyle GW. Neurotrophins and lung disease. Cytokine Growth Factor Rev 14: 551–558, 2003 [DOI] [PubMed] [Google Scholar]
- 120.Hunter DD, Carrell-Jacks LA, Batchelor TP, Dey RD. Role of nerve growth factor in ozone-induced neural responses in early postnatal airway development. Am J Respir Cell Mol Biol 45: 359–365, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ichimaru Y, Krimmer DI, Burgess JK, Black JL, Oliver BG. TGF-β enhances deposition of perlecan from COPD airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 302: L325–L333, 2012 [DOI] [PubMed] [Google Scholar]
- 122.Ichimonji I, Tomura H, Mogi C, Sato K, Aoki H, Hisada T, Dobashi K, Ishizuka T, Mori M, Okajima F. Extracellular acidification stimulates IL-6 production and Ca(2+) mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 299: L567–L577, 2010 [DOI] [PubMed] [Google Scholar]
- 123.Ingram JL, Bonner JC. EGF and PDGF receptor tyrosine kinases as therapeutic targets for chronic lung diseases. Curr Mol Med 6: 409–421, 2006 [DOI] [PubMed] [Google Scholar]
- 124.Jacoby DB. Pathophysiology of airway viral infections. Pulm Pharmacol Ther 17: 333–336, 2004 [DOI] [PubMed] [Google Scholar]
- 125.Jain D, Keslacy S, Tliba O, Cao Y, Kierstein S, Amin K, Panettieri RA, Jr, Haczku A, Amrani Y. Essential role of IFNbeta and CD38 in TNFalpha-induced airway smooth muscle hyper-responsiveness. Immunobiology 213: 499–509, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jain R, Ray JM, Pan JH, Brody SL. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol 46: 446–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.James A, Mauad T, Abramson M, Green F. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med 186: 568; author reply 569, 2012 [DOI] [PubMed] [Google Scholar]
- 128.Jansen SR, Van Ziel AM, Baarsma HA, Gosens R. β-Catenin regulates airway smooth muscle contraction. Am J Physiol Lung Cell Mol Physiol 299: L204–L214, 2010 [DOI] [PubMed] [Google Scholar]
- 130.Janssen LJ. Airway smooth muscle electrophysiology in a state of flux? Am J Physiol Lung Cell Mol Physiol 302: L730–L732, 2012 [DOI] [PubMed] [Google Scholar]
- 131.Janssen LJ. Ionic mechanisms and Ca(2+) regulation in airway smooth muscle contraction: do the data contradict dogma? Am J Physiol Lung Cell Mol Physiol 282: L1161–L1178, 2002 [DOI] [PubMed] [Google Scholar]
- 132.Janssen LJ. T-type and L-type Ca2+ currents in canine bronchial smooth muscle: characterization and physiological roles. Am J Physiol Cell Physiol 272: C1757–C1765, 1997 [DOI] [PubMed] [Google Scholar]
- 133.Janssen LJ, Hague C, Nana R. Ionic mechanisms underlying electrical slow waves in canine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 275: L516–L523, 1998 [DOI] [PubMed] [Google Scholar]
- 134.Jesudason EC. Airway smooth muscle: an architect of the lung? Thorax 64: 541–545, 2009 [DOI] [PubMed] [Google Scholar]
- 135.Jesudason EC, Keshet E, Warburton D. Entrained pulmonary clocks: epithelium and vasculature keeping pace. Am J Physiol Lung Cell Mol Physiol 299: L453–L454, 2010 [DOI] [PubMed] [Google Scholar]
- 136.Jesudason EC, Smith NP, Connell MG, Spiller DG, White MR, Fernig DG, Losty PD. Peristalsis of airway smooth muscle is developmentally regulated and uncoupled from hypoplastic lung growth. Am J Physiol Lung Cell Mol Physiol 291: L559–L565, 2006 [DOI] [PubMed] [Google Scholar]
- 137.Jia L, Delmotte P, Aravamudan B, Pabelick CM, Prakash YS, Sieck GC. The effect of the inflammatory cytokines TNFalpha and IL-13 on STIM1 aggregation in human airway smooth muscle [Ca] regulation. Am J Respir Cell Mol Biol 49: 601–608, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jia L, Tang DD. Abl activation regulates the dissociation of CAS from cytoskeletal vimentin by modulating CAS phosphorylation in smooth muscle. Am J Physiol Cell Physiol 299: C630–C637, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jia L, Wang R, Tang DD. Abl regulates smooth muscle cell proliferation by modulating actin dynamics and ERK1/2 activation. Am J Physiol Cell Physiol 302: C1026–C1034, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, Burgess JK, Black JL. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med 164: 474–477, 2001 [DOI] [PubMed] [Google Scholar]
- 141.Jude JA, Dileepan M, Panettieri RA, Jr, Walseth TF, Kannan MS. Altered CD38/cyclic ADP-ribose signaling contributes to the asthmatic phenotype. J Allergy 2012: 289468, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jude JA, Dileepan M, Subramanian S, Solway J, Panettieri RA, Jr, Walseth TF, Kannan MS. miR-140–3p regulation of TNF-α-induced CD38 expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303: L460–L468, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jude JA, Panettieri RA, Jr, Walseth TF, Kannan MS. TNF-alpha regulation of CD38 expression in human airwaysmooth muscle: role of MAP kinases and NF-kappaB. Adv Exp Med Biol 691: 449–459, 2011 [DOI] [PubMed] [Google Scholar]
- 144.Jude JA, Solway J, Panettieri RA, Jr, Walseth TF, Kannan MS. Differential induction of CD38 expression by TNF-α in asthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 299: L879–L890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jude JA, Wylam ME, Walseth TF, Kannan MS. Calcium signaling in airway smooth muscle. Proc Am Thorac Soc 5: 15–22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jyothula SS, Eissa NT. Autophagy and role in asthma. Curr Opin Pulm Med 19: 30–35, 2013 [DOI] [PubMed] [Google Scholar]
- 147.Kang BN, Tirumurugaan KG, Deshpande DA, Amrani Y, Panettieri RA, Walseth TF, Kannan MS. Transcriptional regulation of CD38 expression by tumor necrosis factor-α in human airway smooth muscle cells: role of NF-κB and sensitivity to glucocorticoids. FASEB J 20: 1000–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 148.Kannan MS, Prakash YS, Brenner T, Mickelson JR, Sieck GC. Role of ryanodine receptor channels in Ca2+ oscillations of porcine tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol 272: L659–L664, 1997 [DOI] [PubMed] [Google Scholar]
- 149.Kaur D, Hollins F, Saunders R, Woodman L, Sutcliffe A, Cruse G, Bradding P, Brightling C. Airway smooth muscle proliferation and survival is not modulated by mast cells. Clin Exp Allergy 40: 279–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kc P, Martin RJ. Role of central neurotransmission and chemoreception on airway control. Respir Physiol Neurobiol 173: 213–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kelly EA, Jarjour NN. Role of matrix metalloproteinases in asthma. Curr Opin Pulm Med 9: 28–33, 2003 [DOI] [PubMed] [Google Scholar]
- 152.Khan MA. Inflammation signals airway smooth muscle cell proliferation in asthma pathogenesis. Multidiscip Respir Med 8: 11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khan MA, Ellis R, Inman MD, Bates JH, Sanderson MJ, Janssen LJ. Influence of airway wall stiffness and parenchymal tethering on the dynamics of bronchoconstriction. Am J Physiol Lung Cell Mol Physiol 299: L98–L108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim JH, Jain D, Tliba O, Yang B, Jester WF, Jr, Panettieri RA, Jr, Amrani Y, Pure E. TGF-β potentiates airway smooth muscle responsiveness to bradykinin. Am J Physiol Lung Cell Mol Physiol 289: L511–L520, 2005 [DOI] [PubMed] [Google Scholar]
- 155.Kong KC, Billington CK, Gandhi U, Panettieri RA, Jr, Penn RB. Cooperative mitogenic signaling by G protein-coupled receptors and growth factors is dependent on G(q/11). FASEB J 20: 1558–1560, 2006 [DOI] [PubMed] [Google Scholar]
- 156.Konsavage WM, Zhang L, Wu Y, Shenberger JS. Hyperoxia-induced activation of the integrated stress response in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol 302: L27–L35, 2012 [DOI] [PubMed] [Google Scholar]
- 157.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med 165: 1364–1370, 2002 [DOI] [PubMed] [Google Scholar]
- 158.Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. Non-genomic effect of testosterone on airway smooth muscle. Br J Pharmacol 149: 1083–1091, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kovalchuk Y, Holthoff K, Konnerth A. Neurotrophin action on a rapid timescale. Curr Opin Neurobiol 14: 558–563, 2004 [DOI] [PubMed] [Google Scholar]
- 160.Koziol-White CJ, Panettieri RA., Jr Airway smooth muscle and immunomodulation in acute exacerbations of airway disease. Immunol Rev 242: 178–185, 2011 [DOI] [PubMed] [Google Scholar]
- 161.Krasteva G, Pfeil U, Drab M, Kummer W, Konig P. Caveolin-1 and -2 in airway epithelium: expression and in situ association as detected by FRET-CLSM. Respir Res 7: 108, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krymskaya VP, Orsini MJ, Eszterhas AJ, Brodbeck KC, Benovic JL, Panettieri RA, Jr, Penn RB. Mechanisms of proliferation synergy by receptor tyrosine kinase and G protein-coupled receptor activation in human airway smooth muscle. Am J Respir Cell Mol Biol 23: 546–554, 2000 [DOI] [PubMed] [Google Scholar]
- 163.Kumawat K, Menzen MH, Bos IS, Baarsma HA, Borger P, Roth M, Tamm M, Halayko AJ, Simoons M, Prins A, Postma DS, Schmidt M, Gosens R. Noncanonical WNT-5A signaling regulates TGF-β-induced extracellular matrix production by airway smooth muscle cells. FASEB J 27: 1631–1643, 2013 [DOI] [PubMed] [Google Scholar]
- 164.Kuo C, Lim S, King NJ, Johnston SL, Burgess JK, Black JL, Oliver BG. Rhinovirus infection induces extracellular matrix protein deposition in asthmatic and nonasthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L951–L957, 2011 [DOI] [PubMed] [Google Scholar]
- 165.Kuo KH, Herrera AM, Seow CY. Ultrastructure of airway smooth muscle. Respir Physiol Neurobiol 137: 197–208, 2003 [DOI] [PubMed] [Google Scholar]
- 166.Kuypers E, Collins JJ, Kramer BW, Ofman G, Nitsos I, Pillow JJ, Polglase GR, Kemp MW, Newnham JP, Gavilanes AW, Nowacki R, Ikegami M, Jobe AH, Kallapur SG. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol 302: L380–L389, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lagente V, Boichot E. Role of matrix metalloproteinases in the inflammatory process of respiratory diseases. J Mol Cell Cardiol 48: 440–444, 2010 [DOI] [PubMed] [Google Scholar]
- 168.Larner-Svensson HM, Williams AE, Tsitsiou E, Perry MM, Jiang X, Chung KF, Lindsay MA. Pharmacological studies of the mechanism and function of interleukin-1beta-induced miRNA-146a expression in primary human airway smooth muscle. Respir Res 11: 68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lazaar AL, Panettieri RA., Jr Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol 116: 488–495; quiz 496, 2005 [DOI] [PubMed] [Google Scholar]
- 170.Lazaar AL, Reitz HE, Panettieri RA, Jr, Peters SP, Pure E. Antigen receptor-stimulated peripheral blood and bronchoalveolar lavage-derived T cells induce MHC class II and ICAM-1 expression on human airway smooth muscle. Am J Respir Cell Mol Biol 16: 38–45, 1997 [DOI] [PubMed] [Google Scholar]
- 171.Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U, Ericksen M, Gibson AM, Holtzman MJ, Whitsett JA, Hershey GK. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol 300: L414–L421, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee LY, Gu Q, Xu F, Hong JL. Acid-sensing by airway afferent nerves. Pulm Pharmacol Ther 26: 491–497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li M, Shang YX, Wei B, Yang YG. The effect of substance P on asthmatic rat airway smooth muscle cell proliferation, migration, and cytoplasmic calcium concentration in vitro. J Inflamm 8: 18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, Yang CM. Interleukin-1beta induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-kappaB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol 211: 759–770, 2007 [DOI] [PubMed] [Google Scholar]
- 175.Lieu T, Kollarik M, Myers AC, Undem BJ. Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol 300: L790–L798, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lieu TM, Myers AC, Meeker S, Undem BJ. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol 302: L941–L948, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Linden A. NANC neural control of airway smooth muscle tone. Gen Pharmacol 27: 1109–1121, 1996 [DOI] [PubMed] [Google Scholar]
- 178.Liu B, Peel SE, Fox J, Hall IP. Reverse mode Na+/Ca2+ exchange mediated by STIM1 contributes to Ca2+ influx in airway smooth muscle following agonist stimulation. Respir Res 11: 168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu C, Zuo J, Pertens E, Helli PB, Janssen LJ. Regulation of Rho/ROCK signaling in airway smooth muscle by membrane potential and [Ca2+]i. Am J Physiol Lung Cell Mol Physiol 289: L574–L582, 2005 [DOI] [PubMed] [Google Scholar]
- 180.Liu J, Sakurai R, O'Roark EM, Kenyon NJ, Torday JS, Rehan VK. PPARγ agonist rosiglitazone prevents perinatal nicotine exposure-induced asthma in rat offspring. Am J Physiol Lung Cell Mol Physiol 300: L710–L717, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Longo FM, Massa SM. Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat Rev Drug Discov 12: 507–525, 2013 [DOI] [PubMed] [Google Scholar]
- 182.Luo L, Gong YQ, Qi X, Lai W, Lan H, Luo Y. Effect of tumor suppressor PTEN gene on apoptosis and cell cycle of human airway smooth muscle cells. Mol Cell Biochem 375: 1–9, 2013 [DOI] [PubMed] [Google Scholar]
- 183.Ma L, Brown M, Kogut P, Serban K, Li X, McConville J, Chen B, Bentley JK, Hershenson MB, Dulin N, Solway J, Camoretti-Mercado B. Akt activation induces hypertrophy without contractile phenotypic maturation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 300: L701–L709, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, Snetkov VA, O'Connor BJ, Karner C, Cousins DJ, Macedo P, Chung KF, Corrigan CJ, Ward JP, Lee TH. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci USA 106: 10775–10780, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mahn K, Ojo OO, Chadwick G, Aaronson PI, Ward JP, Lee TH. Ca(2+) homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax 65: 547–552, 2010 [DOI] [PubMed] [Google Scholar]
- 187.Makinde T, Murphy RF, Agrawal DK. Immunomodulatory role of vascular endothelial growth factor and angiopoietin-1 in airway remodeling. Curr Mol Med 6: 831–841, 2006 [DOI] [PubMed] [Google Scholar]
- 188.Malmstrom K, Pelkonen AS, Makela MJ. Remodeling, inflammation and airway responsiveness in early childhood asthma. Curr Opin Allergy Clin Immunol 13: 203–210, 2013 [DOI] [PubMed] [Google Scholar]
- 189.Manetsch M, Seidel P, Heintz U, Che W, Hughes JM, Ge Q, Sukkar MB, Ammit AJ. TLR2 ligand engagement upregulates airway smooth muscle TNFα-induced cytokine production. Am J Physiol Lung Cell Mol Physiol 302: L838–L845, 2012 [DOI] [PubMed] [Google Scholar]
- 190.Manuyakorn W, Howarth PH, Holgate ST. Airway remodelling in asthma and novel therapy. Asian Pac J Allergy Immunol 31: 3–10, 2013 [PubMed] [Google Scholar]
- 191.Marchica CL, Pinelli V, Borges M, Zummer J, Narayanan V, Iozzo RV, Ludwig MS. A role for decorin in a murine model of allergen-induced asthma. Am J Physiol Lung Cell Mol Physiol 300: L863–L873, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Marhl M, Noble D, Roux E. Modeling of molecular and cellular mechanisms involved in Ca2+ signal encoding in airway myocytes. Cell Biochem Biophys 46: 285–302, 2006 [DOI] [PubMed] [Google Scholar]
- 193.Marti-Solano M, Guixa-Gonzalez R, Sanz F, Pastor M, Selent J. Novel insights into biased agonism at G protein-coupled receptors and their potential for drug design. Curr Pharm Des 19: 5156–5166, 2013 [DOI] [PubMed] [Google Scholar]
- 194.Martin RJ, Walsh MC. Pre- and postnatal factors in chronic lung disease: implications for management. Paediatr Respir Rev 5, Suppl A: S235–S240, 2004 [DOI] [PubMed] [Google Scholar]
- 195.Matera MG, Calzetta L, Cazzola M. TNF-alpha inhibitors in asthma and COPD: we must not throw the baby out with the bath water. Pulm Pharmacol Ther 23: 121–128, 2010 [DOI] [PubMed] [Google Scholar]
- 196.Matsuzaki S, Ishizuka T, Yamada H, Kamide Y, Hisada T, Ichimonji I, Aoki H, Yatomi M, Komachi M, Tsurumaki H, Ono A, Koga Y, Dobashi K, Mogi C, Sato K, Tomura H, Mori M, Okajima F. Extracellular acidification induces connective tissue growth factor production through proton-sensing receptor OGR1 in human airway smooth muscle cells. Biochem Biophys Res Commun 413: 499–503, 2011 [DOI] [PubMed] [Google Scholar]
- 197.Mazzone SB, McGovern AE. Innervation of tracheal parasympathetic ganglia by esophageal cholinergic neurons: evidence from anatomic and functional studies in guinea pigs. Am J Physiol Lung Cell Mol Physiol 298: L404–L416, 2010 [DOI] [PubMed] [Google Scholar]
- 198.McGovern T, Risse PA, Tsuchiya K, Hassan M, Frigola G, Martin JG. LTD(4) induces HB-EGF-dependent CXCL8 release through EGFR activation in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 299: L808–L815, 2010 [DOI] [PubMed] [Google Scholar]
- 199.McWhinnie R, Pechkovsky DV, Zhou D, Lane D, Halayko AJ, Knight DA, Bai TR. Endothelin-1 induces hypertrophy and inhibits apoptosis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L278–L286, 2007 [DOI] [PubMed] [Google Scholar]
- 200.Meuchel LW, Stewart A, Smelter DF, Abcejo AJ, Thompson MA, Zaidi SI, Martin RJ, Prakash YS. Neurokinin-neurotrophin interactions in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 301: L91–L98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF. TGF-β regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L295–L304, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Miller M, Tam AB, Cho JY, Doherty TA, Pham A, Khorram N, Rosenthal P, Mueller JL, Hoffman HM, Suzukawa M, Niwa M, Broide DH. ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc Natl Acad Sci USA 109: 16648–16653, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998 [DOI] [PubMed] [Google Scholar]
- 205.Mizuta K, Mizuta F, Xu D, Masaki E, Panettieri RA, Jr, Emala CW. Gi-coupled gamma-aminobutyric acid-B receptors cross-regulate phospholipase C and calcium in airway smooth muscle. Am J Respir Cell Mol Biol 45: 1232–1238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mizuta K, Osawa Y, Mizuta F, Xu D, Emala CW. Functional expression of GABAB receptors in airway epithelium. Am J Respir Cell Mol Biol 39: 296–304, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mizuta K, Xu D, Pan Y, Comas G, Sonett JR, Zhang Y, Panettieri RA, Jr, Yang J, Emala CW., Sr GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 294: L1206–L1216, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mizuta K, Zhang Y, Xu D, Masaki E, Panettieri RA, Jr, Emala CW. The dopamine D(2) receptor is expressed and sensitizes adenylyl cyclase activity in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 302: L316–L324, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mizuta K, Zhang Y, Xu D, Mizuta F, FDO, Masaki E, Emala CW. The dopamine D1 receptor is expressed and facilitates relaxation in airway smooth muscle. Respir Res 14: 89, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mohamed JS, Boriek AM. Stretch augments TGF-β1 expression through RhoA/ROCK1/2, PTK, and PI3K in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 299: L413–L424, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mohamed JS, Hajira A, Li Z, Paulin D, Boriek AM. Desmin regulates airway smooth muscle hypertrophy through early growth-responsive protein-1 and microRNA-26a. J Biol Chem 286: 43394–43404, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mohamed JS, Lopez MA, Boriek AM. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3beta. J Biol Chem 285: 29336–29347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nassenstein C, Dawbarn D, Pollock K, Allen SJ, Erpenbeck VJ, Spies E, Krug N, Braun A. Pulmonary distribution, regulation, and functional role of Trk receptors in a murine model of asthma. J Allergy Clin Immunol 118: 597–605, 2006 [DOI] [PubMed] [Google Scholar]
- 214.Nasuhara Y, Munakata M, Sato A, Amishima M, Homma Y, Kawakami Y. Mechanisms of epidermal growth factor-induced contraction of guinea pig airways. Eur J Pharmacol 296: 161–168, 1996 [DOI] [PubMed] [Google Scholar]
- 215.Nuttle LC, Farley JM. Frequency modulation of acetylcholine-induced oscillations in Ca++ and Ca(++)-activated Cl- current by cAMP in tracheal smooth muscle. J Pharmacol Exp Ther 277: 753–760, 1996 [PubMed] [Google Scholar]
- 216.Oenema TA, Mensink G, Smedinga L, Halayko AJ, Zaagsma J, Meurs H, Gosens R, Dekkers BG. Cross-talk between transforming growth factor-beta(1) and muscarinic M(2) receptors augments airway smooth muscle proliferation. Am J Respir Cell Mol Biol 49: 18–27, 2013 [DOI] [PubMed] [Google Scholar]
- 217.Oenema TA, Smit M, Smedinga L, Racke K, Halayko AJ, Meurs H, Gosens R. Muscarinic receptor stimulation augments TGF-β1-induced contractile protein expression by airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303: L589–L597, 2012 [DOI] [PubMed] [Google Scholar]
- 218.Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Targets Inflamm Allergy 4: 177–181, 2005 [DOI] [PubMed] [Google Scholar]
- 219.Okajima F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal 25: 2263–2271, 2013 [DOI] [PubMed] [Google Scholar]
- 220.Oldenburg PJ, Poole JA, Sisson JH. Alcohol reduces airway hyperresponsiveness (AHR) and allergic airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol 302: L308–L315, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Osawa Y, Xu D, Sternberg D, Sonett JR, D'Armiento J, Panettieri RA, Emala CW. Functional expression of the GABAB receptor in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L923–L931, 2006 [DOI] [PubMed] [Google Scholar]
- 222.Othumpangat S, Regier M, Piedimonte G. Nerve growth factor modulates human rhinovirus infection in airway epithelial cells by controlling ICAM-1 expression. Am J Physiol Lung Cell Mol Physiol 302: L1057–L1066, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pan J, Rhode HK, Undem BJ, Myers AC. Neurotransmitters in airway parasympathetic neurons altered by neurotrophin-3 and repeated allergen challenge. Am J Respir Cell Mol Biol 43: 452–457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Papagianni M, Hatziefthimiou A, Chachami G, Gourgoulianis K, Molyvdas PA, Paraskeva E. Insulin causes a transient induction of proliferation via activation of the PI3-kinase pathway in airway smooth muscle cells. Exp Clin Endocrinol Diabetes 115: 118–123, 2007 [DOI] [PubMed] [Google Scholar]
- 225.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol 38: 744–749, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Peiser C, Trevisani M, Groneberg DA, Dinh QT, Lencer D, Amadesi S, Maggiore B, Harrison S, Geppetti P, Fischer A. Dopamine type 2 receptor expression and function in rodent sensory neurons projecting to the airways. Am J Physiol Lung Cell Mol Physiol 289: L153–L158, 2005 [DOI] [PubMed] [Google Scholar]
- 227.Penn RB. Embracing emerging paradigms of G protein-coupled receptor agonism and signaling to address airway smooth muscle pathobiology in asthma. Naunyn Schmiedebergs Arch Pharmacol 378: 149–169, 2008 [DOI] [PubMed] [Google Scholar]
- 228.Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, Ludwig MS, Martin JG, Hamid Q. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol 116: 544–549, 2005 [DOI] [PubMed] [Google Scholar]
- 229.Pera T, Gosens R, Lesterhuis AH, Sami R, van der Toorn M, Zaagsma J, Meurs H. Cigarette smoke and lipopolysaccharide induce a proliferative airway smooth muscle phenotype. Respir Res 11: 48, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pera T, Sami R, Zaagsma J, Meurs H. TAK1 plays a major role in growth factor-induced phenotypic modulation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 301: L822–L828, 2011 [DOI] [PubMed] [Google Scholar]
- 231.Perez-Zoghbi JF, Karner C, Ito S, Shepherd M, Alrashdan Y, Sanderson MJ. Ion channel regulation of intracellular calcium and airway smooth muscle function. Pulm Pharmacol Ther 22: 388–397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Perkins C, Yanase N, Smulian G, Gildea L, Orekov T, Potter C, Brombacher F, Aronow B, Wills-Karp M, Finkelman FD. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J Exp Med 208: 853–867, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pieper MP, Chaudhary NI, Park JE. Acetylcholine-induced proliferation of fibroblasts and myofibroblasts in vitro is inhibited by tiotropium bromide. Life Sci 80: 2270–2273, 2007 [DOI] [PubMed] [Google Scholar]
- 234.Poon A, Eidelman D, Laprise C, Hamid Q. ATG5, autophagy and lung function in asthma. Autophagy 8: 694–695, 2012 [DOI] [PubMed] [Google Scholar]
- 235.Possa SS, Charafeddine HT, Righetti RF, da Silva PA, Almeida-Reis R, Saraiva-Romanholo BM, Perini A, Prado CM, Leick-Maldonado EA, Martins MA, Tiberio Ide F. Rho-kinase inhibition attenuates airway responsiveness, inflammation, matrix remodeling, and oxidative stress activation induced by chronic inflammation. Am J Physiol Lung Cell Mol Physiol 303: L939–L952, 2012 [DOI] [PubMed] [Google Scholar]
- 236.Prakash YS, Iyanoye A, Ay B, Mantilla CB, Pabelick CM. Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L447–L456, 2006 [DOI] [PubMed] [Google Scholar]
- 237.Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol Cell Physiol 272: C966–C975, 1997 [DOI] [PubMed] [Google Scholar]
- 238.Prakash YS, Thompson MA, Meuchel L, Pabelick CM, Mantilla CB, Zaidi S, Martin RJ. Neurotrophins in lung health and disease. Expert Rev Respir Med 4: 395–411, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Prakash YS, Thompson MA, Pabelick CM. Brain-derived neurotrophic factor in TNF-alpha modulation of Ca2+ in human airway smooth muscle. Am J Respir Cell Mol Biol 41: 603–611, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1118–L1126, 2007 [DOI] [PubMed] [Google Scholar]
- 241.Pulkkinen V, Manson ML, Safholm J, Adner M, Dahlen SE. The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am J Physiol Lung Cell Mol Physiol 303: L956–L966, 2012 [DOI] [PubMed] [Google Scholar]
- 242.Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, Lu J, Fine A, Ai X. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci 31: 15407–15415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rahman M, Inman M, Kiss L, Janssen LJ. Reverse-mode NCX current in mouse airway smooth muscle: Na(+) and voltage dependence, contributions to Ca(2+) influx and contraction, and altered expression in a model of allergen-induced hyperresponsiveness. Acta Physiol (Oxf) 205: 279–291, 2012 [DOI] [PubMed] [Google Scholar]
- 244.Redhu NS, Gounni AS. Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin Exp Allergy 42: 994–1005, 2012 [DOI] [PubMed] [Google Scholar]
- 245.Redhu NS, Saleh A, Halayko AJ, Ali AS, Gounni AS. Essential role of NF-κB and AP-1 transcription factors in TNF-α-induced TSLP expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L479–L485, 2011 [DOI] [PubMed] [Google Scholar]
- 246.Redhu NS, Shan L, Movassagh H, Gounni AS. Thymic stromal lymphopoietin induces migration in human airway smooth muscle cells. Sci Rep 3: 2301, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rehan VK, Sakurai R, Torday JS. Thirdhand smoke: a new dimension to the effects of cigarette smoke on the developing lung. Am J Physiol Lung Cell Mol Physiol 301: L1–L8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Renz H, Kerzel S, Nockher WA. The role of neurotrophins in bronchial asthma: contribution of the pan-neurotrophin receptor p75. Prog Brain Res 146: 325–333, 2004 [DOI] [PubMed] [Google Scholar]
- 249.Reyburn B, Martin RJ, Prakash YS, MacFarlane PM. Mechanisms of injury to the preterm lung and airway: implications for long-term pulmonary outcome. Neonatology 101: 345–352, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rhee CK, Lee SY, Kang JY, Kim SJ, Kwon SS, Kim YK, Park SH. Effect of peroxisome proliferator-activated receptor-gamma on airway smooth muscle thickening in a murine model of chronic asthma. Int Arch Allergy Immunol 148: 289–296, 2009 [DOI] [PubMed] [Google Scholar]
- 251.Ricciardolo FL. Mechanisms of citric acid-induced bronchoconstriction. Am J Med 111, Suppl 8A: 18S–24S, 2001 [DOI] [PubMed] [Google Scholar]
- 252.Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol 113: 610–619, 2004 [DOI] [PubMed] [Google Scholar]
- 253.Ricciardolo FL, Rado V, Fabbri LM, Sterk PJ, Di Maria GU, Geppetti P. Bronchoconstriction induced by citric acid inhalation in guinea pigs: role of tachykinins, bradykinin, and nitric oxide. Am J Respir Crit Care Med 159: 557–562, 1999 [DOI] [PubMed] [Google Scholar]
- 254.Risse PA, Jo T, Suarez F, Hirota N, Tolloczko B, Ferraro P, Grutter P, Martin JG. Interleukin-13 inhibits proliferation and enhances contractility of human airway smooth muscle cells without change in contractile phenotype. Am J Physiol Lung Cell Mol Physiol 300: L958–L966, 2011 [DOI] [PubMed] [Google Scholar]
- 255.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13: 566–578, 2012 [DOI] [PubMed] [Google Scholar]
- 256.Robinett KS, Deshpande DA, Malone MM, Liggett SB. Agonist-promoted homologous desensitization of human airway smooth muscle bitter taste receptors. Am J Respir Cell Mol Biol 45: 1069–1074, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Rochlitzer S, Nassenstein C, Braun A. The contribution of neurotrophins to the pathogenesis of allergic asthma. Biochem Soc Trans 34: 594–599, 2006 [DOI] [PubMed] [Google Scholar]
- 258.Roth M, Black JL. An imbalance in C/EBPs and increased mitochondrial activity in asthmatic airway smooth muscle cells: novel targets in asthma therapy? Br J Pharmacol 157: 334–341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rydell-Tormanen K, Risse PA, Kanabar V, Bagchi R, Czubryt MP, Johnson JR. Smooth muscle in tissue remodeling and hyper-reactivity: airways and arteries. Pulm Pharmacol Ther 26: 13–23, 2013 [DOI] [PubMed] [Google Scholar]
- 260.Sakurai R, Li Y, Torday JS, Rehan VK. Curcumin augments lung maturation, preventing neonatal lung injury by inhibiting TGF-β signaling. Am J Physiol Lung Cell Mol Physiol 301: L721–L730, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc 5: 23–31, 2008 [DOI] [PubMed] [Google Scholar]
- 262.Sanderson MJ, Madison JM. Bitter treats for better breathing. Nat Med 16: 1190–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 263.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol 299: L861–L871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sathish V, Abcejo AJ, Thompson MA, Sieck GC, Prakash YS, Pabelick CM. Caveolin-1 regulation of store-operated Ca(2+) influx in human airway smooth muscle. Eur Respir J 40: 470–478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sathish V, Abcejo AJ, VanOosten SK, Thompson MA, Prakash YS, Pabelick CM. Caveolin-1 in cytokine-induced enhancement of intracellular Ca(2+) in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 301: L607–L614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sathish V, Delmotte PF, Thompson MA, Pabelick CM, Sieck GC, Prakash YS. Sodium-calcium exchange in intracellular calcium handling of human airway smooth muscle. PLoS One 6: e23662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 297: L26–L34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sathish V, Vanoosten SK, Miller BS, Aravamudan B, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Brain-derived neurotrophic factor in cigarette smoke-induced airway hyperreactivity. Am J Respir Cell Mol Biol 48: 431–438, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Saxena H, Deshpande DA, Tiegs BC, Yan H, Battafarano RJ, Burrows WM, Damera G, Panettieri RA, Dubose TD, Jr, An SS, Penn RB. The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br J Pharmacol 166: 981–990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Schaafsma D, Gosens R, Bos IS, Meurs H, Zaagsma J, Nelemans SA. Role of contractile prostaglandins and Rho-kinase in growth factor-induced airway smooth muscle contraction. Respir Res 6: 85, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schaafsma D, Gosens R, Ris JM, Zaagsma J, Meurs H, Nelemans SA. Insulin induces airway smooth muscle contraction. Br J Pharmacol 150: 136–142, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Schlenz H, Kummer W, Jositsch G, Wess J, Krasteva G. Muscarinic receptor-mediated bronchoconstriction is coupled to caveolae in murine airways. Am J Physiol Lung Cell Mol Physiol 298: L626–L636, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schuliga M, Javeed A, Harris T, Xia Y, Qin C, Wang Z, Zhang X, Lee PV, Camoretti-Mercado B, Stewart AG. Transforming growth factor-beta-induced differentiation of airway smooth muscle cells is inhibited by fibroblast growth factor-2. Am J Respir Cell Mol Biol 48: 346–353, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schuliga M, Ong SC, Soon L, Zal F, Harris T, Stewart AG. Airway smooth muscle remodels pericellular collagen fibrils: implications for proliferation. Am J Physiol Lung Cell Mol Physiol 298: L584–L592, 2010 [DOI] [PubMed] [Google Scholar]
- 275.Scott CL, Walker DJ, Cwiklinski E, Tait C, Tee AR, Land SC. Control of HIF-1α and vascular signaling in fetal lung involves cross talk between mTORC1 and the FGF-10/FGFR2b/Spry2 airway branching periodicity clock. Am J Physiol Lung Cell Mol Physiol 299: L455–L471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Scott GD, Fryer AD. Role of parasympathetic nerves and muscarinic receptors in allergy and asthma. Chem Immunol Allergy 98: 48–69, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab 21: 729–738, 2010 [DOI] [PubMed] [Google Scholar]
- 278.Seow CY. Passive stiffness of airway smooth muscle: the next target for improving airway distensibility and treatment for asthma? Pulm Pharmacol Ther 26: 37–41, 2013 [DOI] [PubMed] [Google Scholar]
- 279.Seow CY. Passive stiffness of airway smooth muscle: the next target for improving airway distensibility and treatment for asthma? Pulm Pharmacol Ther 26: 37–41, 2013 [DOI] [PubMed] [Google Scholar]
- 280.Sharma P, Ghavami S, Stelmack GL, McNeill KD, Mutawe MM, Klonisch T, Unruh H, Halayko AJ. beta-Dystroglycan binds caveolin-1 in smooth muscle: a functional role in caveolae distribution and Ca2+ release. J Cell Sci 123: 3061–3070, 2010 [DOI] [PubMed] [Google Scholar]
- 281.Sharma P, Ryu MH, Basu S, Maltby SA, Yeganeh B, Mutawe MM, Mitchell RW, Halayko AJ. Epithelium-dependent modulation of responsiveness of airways from caveolin-1 knockout mice is mediated through cyclooxygenase-2 and 5-lipoxygenase. Br J Pharmacol 167: 548–560, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest 132: 651–656, 2007 [DOI] [PubMed] [Google Scholar]
- 283.Shiomi T, Tschumperlin DJ, Park JA, Sunnarborg SW, Horiuchi K, Blobel CP, Drazen JM. TNF-alpha-converting enzyme/a disintegrin and metalloprotease-17 mediates mechanotransduction in murine tracheal epithelial cells. Am J Respir Cell Mol Biol 45: 376–385, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Siddiqui S, Novali M, Tsuchiya K, Hirota N, Geller BJ, McGovern TK, Risse PA, Jo T, Zeroual MA, Martin JG. The modulation of large airway smooth muscle phenotype and effects of epidermal growth factor receptor inhibition in the repeatedly allergen-challenged rat. Am J Physiol Lung Cell Mol Physiol 304: L853–L862, 2013 [DOI] [PubMed] [Google Scholar]
- 285.Siddiqui S, Redhu NS, Ojo OO, Liu B, Irechukwu N, Billington C, Janssen L, Moir LM. Emerging airway smooth muscle targets to treat asthma. Pulm Pharmacol Ther 26: 132–144, 2013 [DOI] [PubMed] [Google Scholar]
- 286.Simeone-Penney MC, Severgnini M, Rozo L, Takahashi S, Cochran BH, Simon AR. PDGF-induced human airway smooth muscle cell proliferation requires STAT3 and the small GTPase Rac1. Am J Physiol Lung Cell Mol Physiol 294: L698–L704, 2008 [DOI] [PubMed] [Google Scholar]
- 287.Singer CA. T-bet is induced by interferon-gamma to mediate chemokine secretion and migration in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L633–L641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol 185: 3035–3040, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Song KS, Choi YH, Kim JM, Lee H, Lee TJ, Yoon JH. Suppression of prostaglandin E2-induced MUC5AC overproduction by RGS4 in the airway. Am J Physiol Lung Cell Mol Physiol 296: L684–L692, 2009 [DOI] [PubMed] [Google Scholar]
- 290.Song KS, Kim HJ, Kim K, Lee JG, Yoon JH. Regulator of G-protein signaling 4 suppresses LPS-induced MUC5AC overproduction in the airway. Am J Respir Cell Mol Biol 41: 40–49, 2009 [DOI] [PubMed] [Google Scholar]
- 291.Sopi RB, Martin RJ, Haxhiu MA, Dreshaj IA, Yao Q, Jafri A, Zaidi SI. Role of brain-derived neurotrophic factor in hyperoxia-induced enhancement of contractility and impairment of relaxation in lung parenchyma. Am J Physiol Lung Cell Mol Physiol 295: L348–L355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Spinelli AM, Gonzalez-Cobos JC, Zhang X, Motiani RK, Rowan S, Zhang W, Garrett J, Vincent PA, Matrougui K, Singer HA, Trebak M. Airway smooth muscle STIM1 and Orai1 are upregulated in asthmatic mice and mediate PDGF-activated SOCE, CRAC currents, proliferation, and migration. Pflügers Arch 464: 481–492, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Stephen J, Delvecchio C, Spitale N, Giesler A, Radford K, Bilan P, Cox PG, Capone JP, Nair P. PPAR ligands decrease human airway smooth muscle cell migration and extracellular matrix synthesis. Eur Respir J 41: 425–432, 2013 [DOI] [PubMed] [Google Scholar]
- 294.Suzuki R, Miyazaki Y, Takagi K, Torii K, Taniguchi H. Matrix metalloproteinases in the pathogenesis of asthma and COPD: implications for therapy. Treat Respir Med 3: 17–27, 2004 [DOI] [PubMed] [Google Scholar]
- 295.Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health 11: 24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tamaoka M, Hassan M, McGovern T, Ramos-Barbon D, Jo T, Yoshizawa Y, Tolloczko B, Hamid Q, Martin JG. The epidermal growth factor receptor mediates allergic airway remodelling in the rat. Eur Respir J 32: 1213–1223, 2008 [DOI] [PubMed] [Google Scholar]
- 297.Tan X, Alrashdan YA, Alkhouri H, Oliver BG, Armour CL, Hughes JM. Airway smooth muscle CXCR3 ligand production: regulation by JAK-STAT1 and intracellular Ca2+. Am J Physiol Lung Cell Mol Physiol 304: L790–L802, 2013 [DOI] [PubMed] [Google Scholar]
- 298.Thomas KC, Roberts JK, Deering-Rice CE, Romero EG, Dull RO, Lee J, Yost GS, Reilly CA. Contributions of TRPV1, endovanilloids, and endoplasmic reticulum stress in lung cell death in vitro and lung injury. Am J Physiol Lung Cell Mol Physiol 302: L111–L119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Thompson MA, Pabelick CM, Prakash YS. Role of STIM1 in regulation of store-operated Ca2+ influx in pheochromocytoma cells. Cell Mol Neurobiol 29: 193–202, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tirumurugaan KG, Jude JA, Kang BN, Panettieri RA, Walseth TF, Kannan MS. TNF-α induced CD38 expression in human airway smooth muscle cells: role of MAP kinases and transcription factors NF-κB and AP-1. Am J Physiol Lung Cell Mol Physiol 292: L1385–L1395, 2007 [DOI] [PubMed] [Google Scholar]
- 301.Tirumurugaan KG, Kang BN, Panettieri RA, Foster DN, Walseth TF, Kannan MS. Regulation of the cd38 promoter in human airway smooth muscle cells by TNF-alpha and dexamethasone. Respir Res 9: 26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tliba O, Panettieri RA., Jr Noncontractile functions of airway smooth muscle cells in asthma. Annu Rev Physiol 71: 509–535, 2009 [DOI] [PubMed] [Google Scholar]
- 303.Toews ML, Ediger TL, Romberger DJ, Rennard SI. Lysophosphatidic acid in airway function and disease. Biochim Biophys Acta 1582: 240–250, 2002 [DOI] [PubMed] [Google Scholar]
- 304.Tomura H, Wang JQ, Komachi M, Damirin A, Mogi C, Tobo M, Kon J, Misawa N, Sato K, Okajima F. Prostaglandin I(2) production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J Biol Chem 280: 34458–34464, 2005 [DOI] [PubMed] [Google Scholar]
- 305.Townsend EA, Emala CW., Sr Quercetin acutely relaxes airway smooth muscle and potentiates β-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. Am J Physiol Lung Cell Mol Physiol 305: L396–L403, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Townsend EA, Meuchel LW, Thompson MA, Pabelick CM, Prakash YS. Estrogen increases nitric-oxide production in human bronchial epithelium. J Pharmacol Exp Ther 339: 815–824, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Townsend EA, Meuchel LW, Thompson MA, Pabelick CM, Prakash YS. Estrogen modulation of nitric oxide signaling in the airway. J Cell Physiol 228: 688, 2013 [DOI] [PubMed] [Google Scholar]
- 308.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 33: 1–47, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Townsend EA, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol 303: L923–L928, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Townsend EA, Siviski ME, Zhang Y, Xu C, Hoonjan B, Emala CW. Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation. Am J Respir Cell Mol Biol 48: 157–163, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Townsend EA, Thompson MA, Pabelick CM, Prakash YS. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 298: L521–L530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Townsend EA, Zhang Y, Xu C, Wakita R, Emala CW. Active components of ginger potentiate beta-agonist induced relaxation of airway smooth muscle by modulating cytoskeletal regulatory proteins. Am J Respir Cell Mol Biol. 2013, August 20, Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Tran T, Teoh CM, Tam JK, Qiao Y, Chin CY, Chong OK, Stewart AG, Harris T, Wong WS, Guan SP, Leung BP, Gerthoffer WT, Unruh H, Halayko AJ. Laminin drives survival signals to promote a contractile smooth muscle phenotype and airway hyperreactivity. FASEB J 27: 3991–4003, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, Ghosh D, Ousova O, Vernejoux JM, Marthan R, Tunon-de-Lara JM, Berger P. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med 204: 3173–3181, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Undem BJ, Kajekar R, Hunter DD, Myers AC. Neural integration and allergic disease. J Allergy Clin Immunol 106: S213–S220, 2000 [DOI] [PubMed] [Google Scholar]
- 316.Undem BJ, Nassenstein C. Airway nerves and dyspnea associated with inflammatory airway disease. Respir Physiol Neurobiol 167: 36–44, 2009 [DOI] [PubMed] [Google Scholar]
- 317.Undem BJ, Potenzieri C. Autonomic neural control of intrathoracic airways. Compr Physiol 2: 1241–1267, 2012 [DOI] [PubMed] [Google Scholar]
- 318.van der Velden VH, Hulsmann AR. Autonomic innervation of human airways: structure, function, and pathophysiology in asthma. Neuroimmunomodulation 6: 145–159, 1999 [DOI] [PubMed] [Google Scholar]
- 319.Van Ly D, Burgess JK, Brock TG, Lee TH, Black JL, Oliver BG. Prostaglandins but not leukotrienes alter extracellular matrix protein deposition and cytokine release in primary human airway smooth muscle cells and fibroblasts. Am J Physiol Lung Cell Mol Physiol 303: L239–L250, 2012 [DOI] [PubMed] [Google Scholar]
- 320.Veler H, Hu A, Fatma S, Grunstein JS, DeStephan CM, Campbell D, Orange JS, Grunstein MM. Superantigen presentation by airway smooth muscle to CD4+ T lymphocytes elicits reciprocal proasthmatic changes in airway function. J Immunol 178: 3627–3636, 2007 [DOI] [PubMed] [Google Scholar]
- 321.Verhein KC, Fryer AD, Jacoby DB. Neural control of airway inflammation. Curr Allergy Asthma Rep 9: 484–490, 2009 [DOI] [PubMed] [Google Scholar]
- 322.Verhein KC, Hazari MS, Moulton BC, Jacoby IW, Jacoby DB, Fryer AD. Three days after a single exposure to ozone, the mechanism of airway hyperreactivity is dependent on substance P and nerve growth factor. Am J Physiol Lung Cell Mol Physiol 300: L176–L184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Verma MK, Miki Y, Sasano H. Sex steroid receptors in human lung diseases. J Steroid Biochem Mol Biol 127: 216–222, 2011 [DOI] [PubMed] [Google Scholar]
- 324.Vohra PK, Thompson MA, Sathish V, Kiel A, Jerde C, Pabelick CM, Singh BB, Prakash YS. TRPC3 regulates release of brain-derived neurotrophic factor from human airway smooth muscle. Biochim Biophys Acta 1833: 2953–2960, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest 121: 4409–4419, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wang WC, Schillinger RM, Malone MM, Liggett SB. Paradoxical attenuation of β2-AR function in airway smooth muscle by Gi-mediated counterregulation in transgenic mice overexpressing type 5 adenylyl cyclase. Am J Physiol Lung Cell Mol Physiol 300: L472–L478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ward JE, Harris T, Bamford T, Mast A, Pain MC, Robertson C, Smallwood D, Tran T, Wilson J, Stewart AG. Proliferation is not increased in airway myofibroblasts isolated from asthmatics. Eur Respir J 32: 362–371, 2008 [DOI] [PubMed] [Google Scholar]
- 328.Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol 213: 1–47, 2002 [DOI] [PubMed] [Google Scholar]
- 329.Watts VJ. Molecular mechanisms for heterologous sensitization of adenylate cyclase. J Pharmacol Exp Ther 302: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
- 330.Watts VJ, Neve KA. Sensitization of adenylate cyclase by Galpha i/o-coupled receptors. Pharmacol Ther 106: 405–421, 2005 [DOI] [PubMed] [Google Scholar]
- 331.Weigand LA, Myers AC. Synaptic and membrane properties of parasympathetic ganglionic neurons innervating mouse trachea and bronchi. Am J Physiol Lung Cell Mol Physiol 298: L593–L599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Weigand LA, Undem BJ. Allergen-induced neuromodulation in the respiratory tract. Chem Immunol Allergy 98: 142–162, 2012 [DOI] [PubMed] [Google Scholar]
- 333.West AR, Syyong HT, Siddiqui S, Pascoe CD, Murphy TM, Maarsingh H, Deng L, Maksym GN, Bosse Y. Airway contractility and remodeling: links to asthma symptoms. Pulm Pharmacol Ther 26: 3–12, 2013 [DOI] [PubMed] [Google Scholar]
- 334.West AR, Zaman N, Cole DJ, Walker MJ, Legant WR, Boudou T, Chen CS, Favreau JT, Gaudette GR, Cowley EA, Maksym GN. Development and characterization of a 3D multicell microtissue culture model of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 304: L4–L16, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wright DB, Tripathi S, Sikarwar A, Santosh KT, Perez-Zoghbi J, Ojo OO, Irechukwu N, Ward JP, Schaafsma D. Regulation of GPCR-mediated smooth muscle contraction: implications for asthma and pulmonary hypertension. Pulm Pharmacol Ther 26: 121–131, 2013 [DOI] [PubMed] [Google Scholar]
- 336.Wu ZX, Benders KB, Hunter DD, Dey RD. Early postnatal exposure of mice to side-steam tobacco smoke increases neuropeptide Y in lung. Am J Physiol Lung Cell Mol Physiol 302: L152–L159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Xia YC, Redhu NS, Moir LM, Koziol-White C, Ammit AJ, Al-Alwan L, Camoretti-Mercado B, Clifford RL. Pro-inflammatory and immunomodulatory functions of airway smooth muscle: emerging concepts. Pulm Pharmacol Ther 26: 64–74, 2013 [DOI] [PubMed] [Google Scholar]
- 338.Xiao JH, Zheng YM, Liao B, Wang YX. Functional role of canonical transient receptor potential 1 and canonical transient receptor potential 3 in normal and asthmatic airway smooth muscle cells. Am J Respir Cell Mol Biol 43: 17–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Xie S, Issa R, Sukkar MB, Oltmanns U, Bhavsar PK, Papi A, Caramori G, Adcock I, Chung KF. Induction and regulation of matrix metalloproteinase-12 in human airway smooth muscle cells. Respir Res 6: 148, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Xie Y, Jiang H, Nguyen H, Jia S, Berro A, Panettieri RA, Jr, Wolff DW, Abel PW, Casale TB, Tu Y. Regulator of G protein signaling 2 is a key modulator of airway hyperresponsiveness. J Allergy Clin Immunol 130: 968–976; e963, 2012 [DOI] [PubMed] [Google Scholar]
- 341.Yahiaoui L, Villeneuve A, Valderrama-Carvajal H, Burke F, Fixman ED. Endothelin-1 regulates proliferative responses, both alone and synergistically with PDGF, in rat tracheal smooth muscle cells. Cell Physiol Biochem 17: 37–46, 2006 [DOI] [PubMed] [Google Scholar]
- 342.Yang S, Banerjee S, Freitas A, Cui H, Xie N, Abraham E, Liu G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 302: L521–L529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yang Z, Cooper PR, Damera G, Mukhopadhyay I, Cho H, Kehrl JH, Panettieri RA, Jr, Druey KM. Beta-agonist-associated reduction in RGS5 expression promotes airway smooth muscle hyper-responsiveness. J Biol Chem 286: 11444–11455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yao Q, Haxhiu MA, Zaidi SI, Liu S, Jafri A, Martin RJ. Hyperoxia enhances brain-derived neurotrophic factor and tyrosine kinase B receptor expression in peribronchial smooth muscle of neonatal rats. Am J Physiol Lung Cell Mol Physiol 289: L307–L314, 2005 [DOI] [PubMed] [Google Scholar]
- 345.Yao Q, Zaidi SI, Haxhiu MA, Martin RJ. Neonatal lung and airway injury: a role for neurotrophins. Semin Perinatol 30: 156–162, 2006 [DOI] [PubMed] [Google Scholar]
- 346.Ye YL, Wu HT, Lin CF, Hsieh CY, Wang JY, Liu FH, Ma CT, Bei CH, Cheng YL, Chen CC, Chiang BL, Tsao CW. Dermatophagoides pteronyssinus 2 regulates nerve growth factor release to induce airway inflammation via a reactive oxygen species-dependent pathway. Am J Physiol Lung Cell Mol Physiol 300: L216–L224, 2011 [DOI] [PubMed] [Google Scholar]
- 347.Yeganeh B, Mukherjee S, Moir LM, Kumawat K, Kashani HH, Bagchi RA, Baarsma HA, Gosens R, Ghavami S. Novel non-canonical TGF-beta signaling networks: emerging roles in airway smooth muscle phenotype and function. Pulm Pharmacol Ther 26: 50–63, 2013 [DOI] [PubMed] [Google Scholar]
- 348.Yeganeh B, Xia C, Movassagh H, Koziol-White C, Chang Y, Al-Alwan L, Bourke JE, Oliver BG. Emerging mediators of airway smooth muscle dysfunction in asthma. Pulm Pharmacol Ther 26: 105–111, 2013 [DOI] [PubMed] [Google Scholar]
- 349.Ysasi AB, Belle JM, Gibney BC, Fedulov AV, Wagner W, Tsuda A, Konerding MA, Mentzer SJ. Effect of unilateral diaphragmatic paralysis on post-pneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol 305: L439–L445, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zaidi S, Gallos G, Yim PD, Xu D, Sonett JR, Panettieri RA, Jr, Gerthoffer W, Emala CW. Functional expression of gamma-amino butyric acid transporter 2 in human and guinea pig airway epithelium and smooth muscle. Am J Respir Cell Mol Biol 45: 332–339, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Zhang CH, Li Y, Zhao W, Lifshitz LM, Li H, Harfe BD, Zhu MS, ZhuGe R. The transmembrane protein 16A Ca(2+)-activated Cl- channel in airway smooth muscle contributes to airway hyperresponsiveness. Am J Respir Crit Care Med 187: 374–381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol 11: e1001501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Zhang J, Herrera AM, Pare PD, Seow CY. Dense-body aggregates as plastic structures supporting tension in smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 299: L631–L638, 2010 [DOI] [PubMed] [Google Scholar]
- 354.Zhao L, Zhang X, Kuang H, Wu J, Guo Y, Ma L. Effect of TRPV1 channel on the proliferation and apoptosis in asthmatic rat airway smooth muscle cells. Exp Lung Res 39: 283–294, 2013 [DOI] [PubMed] [Google Scholar]
- 355.Zhou D, Zheng X, Wang L, Stelmack G, Halayko AJ, Dorscheid D, Bai TR. Expression and effects of cardiotrophin-1 (CT-1) in human airway smooth muscle cells. Br J Pharmacol 140: 1237–1244, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Zhou L, Goldsmith AM, Bentley JK, Jia Y, Rodriguez ML, Abe MK, Fingar DC, Hershenson MB. 4E-binding protein phosphorylation and eukaryotic initiation factor-4E release are required for airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol 33: 195–202, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Zou JJ, Gao YD, Geng S, Yang J. Role of STIM1/Orai1-mediated store-operated Ca2+ entry in airway smooth muscle cell proliferation. J Appl Physiol 110: 1256–1263, 2011 [DOI] [PubMed] [Google Scholar]
- 358.Zou W, Zou Y, Zhao Z, Li B, Ran P. Nicotine-induced epithelial-mesenchymal transition via Wnt/β-catenin signaling in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 304: L199–L209, 2013 [DOI] [PubMed] [Google Scholar]