Abstract
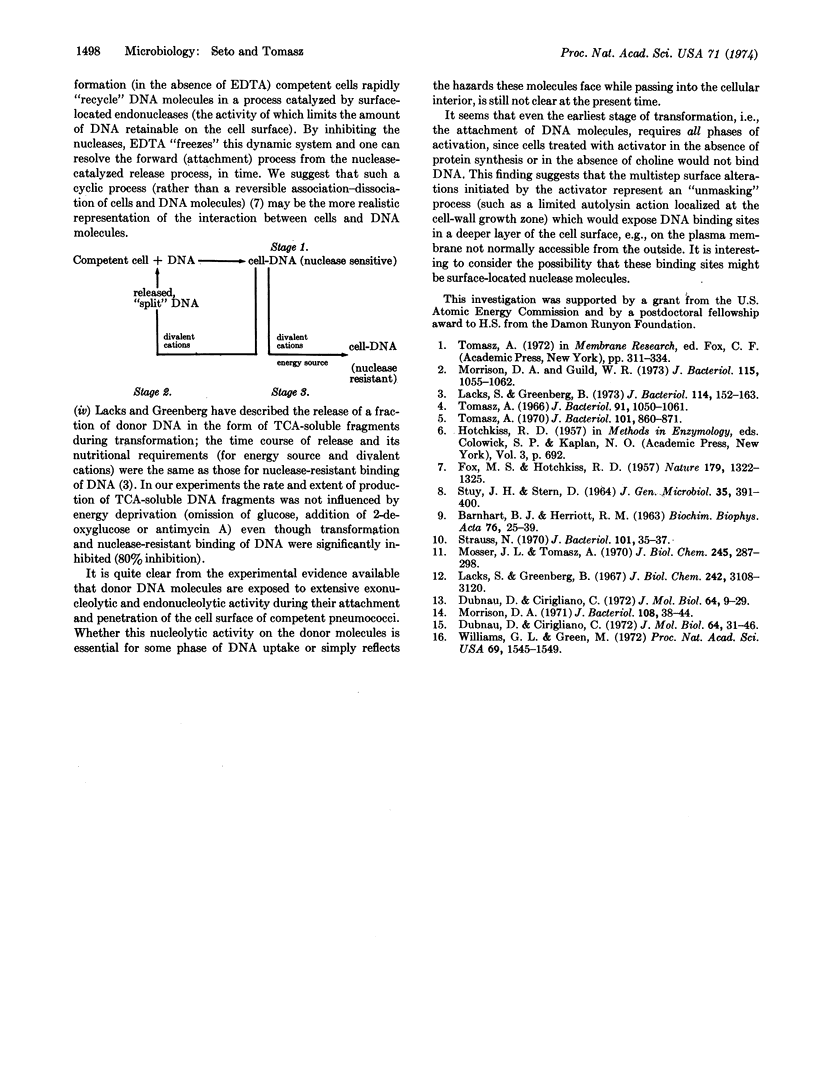
Ethylenediaminetetraacetate and other divalent-cation-complexing agents greatly stimulate the cellular binding of DNA molecules to competent pneumococci, while the appearance of genetic transformants and nuclease-resistant DNA binding are completely inhibited. Based on this finding, we developed an experimental system in which three early and consecutive stages of genetic transformation can be experimentally separated: (i) attachment of DNA molecules to cell surface sites that are only demonstrable in the competent state; (ii) a divalent-cation-dependent nucleolytic splitting and release of the adsorbed molecules to the medium; and (iii) emergence of potential transformants accompanied by an energy-requiring and divalent-cation-dependent process in which the cell-associated DNA molecules become inaccessible to shearing forces, nucleases, anti-DNA serum, and polycations.
Keywords: divalent cation complexing agents, cell surface sites, nucleolytic splitting
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNHART B. J., HERRIOTT R. M. PENETRATION OF DEOXYRIBONUCLEIC ACID INTO HEMOPHILUS INFLUENZAE. Biochim Biophys Acta. 1963 Sep 17;76:25–39. [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972 Feb 28;64(1):9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. IV. The endwise attachment and uptake of transforming DNA. J Mol Biol. 1972 Feb 28;64(1):31–46. doi: 10.1016/0022-2836(72)90319-1. [DOI] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Initiation of bacterial transformation. Nature. 1957 Jun 29;179(4574):1322–1325. doi: 10.1038/1791322a0. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Competence for deoxyribonucleic acid uptake and deoxyribonuclease action external to cells in the genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1973 Apr;114(1):152–163. doi: 10.1128/jb.114.1.152-163.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Deoxyribonucleases of Pneumococcus. J Biol Chem. 1967 Jul 10;242(13):3108–3120. [PubMed] [Google Scholar]
- Morrison D. A. Early intermediate state of transforming deoxyribonucleic acid during uptake by Bacillus subtilis. J Bacteriol. 1971 Oct;108(1):38–44. doi: 10.1128/jb.108.1.38-44.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Structure of deoxyribonucleic acid on the cell surface during uptake by pneumococcus. J Bacteriol. 1973 Sep;115(3):1055–1062. doi: 10.1128/jb.115.3.1055-1062.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- STUY J. H., STERN D. THE KINETICS OF DNA UPTAKE BY HAEMOPHILUS INFLUENZAE. J Gen Microbiol. 1964 Jun;35:391–400. doi: 10.1099/00221287-35-3-391. [DOI] [PubMed] [Google Scholar]
- Strauss N. Early energy-dependent step in the entry of transforming deoxyribonucleic acid. J Bacteriol. 1970 Jan;101(1):35–37. doi: 10.1128/jb.101.1.35-37.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Model for the mechanism controlling the expression of competent state in Pneumococcus cultures. J Bacteriol. 1966 Mar;91(3):1050–1061. doi: 10.1128/jb.91.3.1050-1061.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. L., Green D. M. Early extracellular events in infection of competent Bacillus subtilis by DNA of bacteriophage SP82G. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1545–1549. doi: 10.1073/pnas.69.6.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]


