Abstract
The Anaphase-Promoting Complex/Cyclosome (APC/C) is an ubiquitin ligase that functions during mitosis. Here we identify the transcriptional regulator, Transcriptional Intermediary Factor 1γ, TIF1γ as an APC/C-interacting protein that regulates APC/C function. TIF1γ is not a substrate for APC/C-dependent ubiquitylation but instead, associates specifically with the APC/C holoenzyme and Cdc20 to affect APC/C activity and progression through mitosis. RNA interference studies indicate that TIF1γ knockdown results in a specific reduction in APC/C ubiquitin ligase activity, the stabilization of APC/C substrates, and an increase in the time taken for cells to progress through mitosis from nuclear envelope breakdown (NEBD) to anaphase. TIF1γ knockdown cells are also characterized by the inappropriate presence of cyclin A at metaphase, and an increase in the number of cells that fail to undergo metaphase-to-anaphase transition. Expression of a siRNA-resistant TIF1γ species relieves the mitotic phenotype imposed by TIF1γ knockdown and allows for mitotic progression. Binding studies indicate that TIF1γ is also a component of the APC/C-Mitotic Checkpoint Complex (MCC), but is not required for MCC dissociation from the APC/C once the Spindle Assembly Checkpoint (SAC) is satisfied. TIF1γ inactivation also results in chromosome misalignment at metaphase, and SAC activation; inactivation of the SAC relieves the mitotic block imposed by TIF1γ knockdown. Together these data define novel functions for TIF1γ during mitosis and suggest that a reduction in APC/C ubiquitin ligase activity promotes SAC activation.
INTRODUCTION
The APC/C is a multiprotein E3 ubiquitin ligase complex that coordinates mitotic progression and exit through targeting substrates such as Securin and cyclin B1 for proteasomal-mediated degradation (1, 2). APC/C activity is controlled by the cell cycle-dependent recruitment of one of two activators, Cdc20 or Cdh1, to specific APC/C proteins (1, 2). Cdc20 and Cdh1 also serve in conjunction with particular APC/C subunits to bind substrates (1, 2). APC/C-Cdc20 regulates metaphase-to-anaphase transition primarily by targeting the Separase inhibitor, Securin, for degradation (1). APC/C-Cdc20 activity is tightly controlled by the SAC which monitors microtubule attachment to kinetochores, and ensures the fidelity of sister chromatid segregation at anaphase (2, 3). When the SAC is activated by the presence of unattached kinetochores, SAC components MAD2, BubR1 and Bub3 all serve to inhibit APC/C-Cdc20 activity, and metaphase-to-anaphase transition (2, 3). APC/C-Cdc20 and APC/C-Cdh1 are also regulated by the transcriptional co-activators CBP and p300, which bind to APC/C subunits APC5 and APC7, through interaction domains conserved in adenovirus E1A (4, 5). The DNA damage response protein, MDC1 also regulates APC/C-Cdc20 activity during mitosis and functions independently of SAC and DNA damage response pathways, to facilitate Cdc20 association with the APC/C (6).
TIF1γ also known as TRIM33 and hEctodermin is a member of the Tripartite Motif/RING finger, B-boxes, and a coiled coil domain (TRIM/RBCC) family of proteins (7). It was initially identified as a transcriptional repressor and along with TIF1α has been shown to be fused to the RET receptor tyrosine kinase in childhood papillary thyroid carcinomas (8, 9). The zebra fish TIF1γ ortholog, mon, is required for differentiation of erythroid progenitor cells and posterior mesenchymal cells (10). Recent work suggests that the TGF-β dependent differentiation of hematopoietic, mesenchymal, and epithelial cells is dependent upon the ability of TIF1γ to compete with SMAD4 for binding to activated SMAD2/SMAD3 transcription factor complexes (11). Other workers suggest that TIF1γ serves as a SMAD4 monoubiquitin ligase and inhibits TGF-β signalling pathways by dissociating SMAD2/3-SMAD4 complexes (12, 13).
To explore further the complexity of APC/C function we undertook mass spectrometric analyses to identify novel APC/C-binding partners. Here, we detail the characterization of TIF1γ as an APC/C-interacting protein that affects APC/C activity in mitosis. We show that TIF1γ interacts specifically with APC3 and Cdc20, and regulates cell cycle progression through mitosis; TIF1γ knockdown reduces APC/C-Cdc20 ligase activity, stabilizes APC/C substrate levels and leads to an accumulation of cells in metaphase. Consistent with these observations, the time taken for cells to pass from NEBD to anaphase increases significantly following TIF1γ knockdown. Indeed, a significant proportion of TIF1γ knockdown cells fail to undergo metaphase-to-anaphase transition. TIF1γ knockdown cells are also characterized by the inappropriate expression of APC/C substrate cyclin A at metaphase and metaphases with misaligned chromosomes. Consistent with a role for TIF1γ in mitosis, a small interfering (si)RNA-resistant TIF1γ relieves the mitotic block imposed by TIF1γ knockdown and allows for cell cycle progression. The mitotic phenotype exhibited by TIF1γ knockdown cells is also relieved by inactivation of the SAC, suggesting that TIF1γ activity in mitosis is monitored by the SAC, such that TIF1γ inactivation results in SAC activation. Taken together, this work defines a new role for TIF1γ in regulating progression through mitosis.
RESULTS
TIF1γ associates with the APC/C and Cdc20 but it is not a substrate for APC/C-directed ubiquitylation
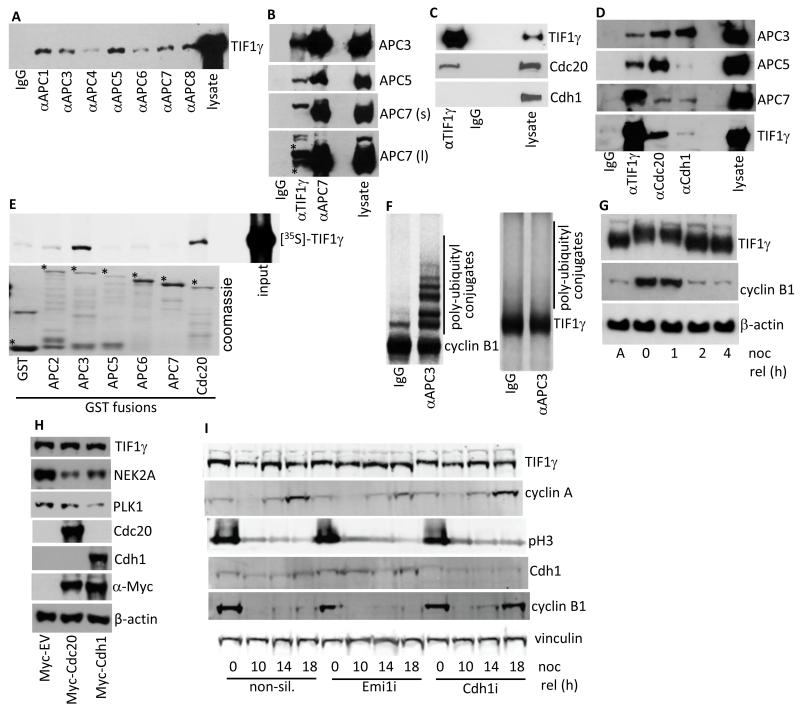
In order to identify novel APC/C-interacting proteins we performed immunoprecipitation from an asynchronous population of HeLa cells, with anti-APC7 antibodies. We subsequently isolated protein bands following SDS-PAGE and analyzed tryptic peptides using a Thermo Fisher Scientific LCQ DECA XP Plus ion trap as described previously (14). In addition to identifying peptides specific for APC7, a number of APC/C subunits and the APC/C E2, UbcH10, we also isolated peptides specific for TIF1γ (amino acid residues 394-407, KLLQQQNDITGLSR and 482-504, PAPGYTPNVVVGQVPPGTNHISK). To verify these findings we made an antibody that was specific for TIF1γ (Fig. 1A; Fig. 3A) and then assessed the ability of the APC/C holoenzyme to interact with TIF1γ. Reciprocal immunoprecipitation-Western blot analyses revealed that TIF1γ co-immunopreciptated with a number of distinct APC/C subunits, and that APC/C components co-immunoprecipitated with TIF1γ (Fig. 1A and 1B). These analyses revealed that TIF1γ associated preferentially with a higher molecular weight form of APC7, though it also bound the major APC7 form as well (Fig. 1B). Further analyses revealed that TIF1γ associated specifically with the APC/C activator Cdc20, but did not associate with Cdh1 (Fig. 1C; Fig 1D). To assess the stoichometry of TIF1γ association with the APC/C we next determined the ability of TIF1γ, relative to APC/C activators, Cdc20 and Cdh1, to bind to APC/C subunits. This experiment revealed that TIF1γ associated with a smaller pool of APC3, relative to Cdc20 and Cdh1, and a smaller pool of APC5, relative to Cdc20 (Fig. 1D). These analyses also revealed that TIF1γ associated more efficiently with the higher molecular weight form of APC7 than Cdc20 or Cdh1, though TIF1γ, Cdc20 and Cdh1, all bound the major form of APC7 with equal avidity (Fig. 1D). GST pull-downs revealed that TIF1γ associated preferentially with APC3 and Cdc20 (Fig. 1E). As TIF1γ binds the APC/C, and possesses three potential RxxL destruction box motifs within its primary sequence (residues 440-443, 968-971 and 1114-1117) we next investigated whether TIF1γ is a target for APC/C ubiquitin ligase activity. First we performed in vitro ubiquitin ligase assays with anti-APC3 immunoprecipitates using [35S]-labelled TIF1γ or [35S]-labelled cyclin B1 as substrates. Consistent with previous findings cyclin B1 was efficiently polyubiquitylated in an APC/C-dependent manner, whereas TIF1γ was not a target for APC/C-directed ubiquitin ligase activity in this assay (Fig. 1F). Next, we assessed TIF1γ protein levels in vivo, following the release of cells from a nocodazole-induced mitotic block. In accordance with the in vitro APC/C ligase assays, cyclin B1 levels were reduced significantly following the passage of cells through mitosis and into the successive G1 phase, whilst levels of the TIF1γ protein were not altered following release of the cells from the mitotic block (Fig. 1G). It appeared however, that TIF1γ was subject to post-translational modification in nocodazole-treated cells, as gauged by reduced mobility upon SDS-PAGE (Fig. 1G). To corroborate our findings that TIF1γ is not targeted for degradation by the APC/C we next assessed TIF1γ protein levels following the exogenous expression of Myc-tagged Cdc20 and Cdh1 (Fig. 1H). TIF1γ levels remained unaffected following the expression of Cdc20 or Cdh1, whereas the levels of APC/C-Cdc20 substrate, NEK2A, were reduced following Myc-tagged Cdc20 expression, and levels of APC/C-Cdh1 substrate, PLK1, were reduced following the expression of Myc-tagged Cdh1 (Fig. 1H). In agreement with these findings TIF1γ protein levels were not altered following the ablation of Cdc20, or Cdh1, expression by RNAi (Fig. 3A). To substantiate these findings we next determined whether knockdown of the APC/C inhibitor, Emi1 (15, 16), or knockdown of Cdh1, affected TIF1γ protein levels following release from a mitotic block (Fig 1I). This experiment revealed that although Emi1 knockdown, relative to non-silencing controls, prevented the re-accumulation of cyclin A 18 hours after nocodazole release, it did not affect appreciably the levels of TIF1γ (cf lane 4 and lane 8; Fig 1I). Similarly, Cdh1 knockdown allowed for the precocious accumulation of cyclin B1 at 18 hours after nocodazole release, but did not enhance TIF1γ levels (cf lane 4 and lane 12; Fig. 1I). Taken together these data indicate that TIF1γ associates preferentially with APC/C-Cdc20, but that TIF1γ is not a substrate for APC/C-Cdc20-, or APC/C-Cdh1-, directed ubiquitylation.
Figure 1. TIF1γ associates with the APC/C but is not a substrate for APC/C ligase activity.
(A-D) APC/C components, Cdc20, Cdh1 and TIF1γ were immunoprecipitated from asynchronous whole cell HeLa extracts, separated by SDS-PAGE, and Western blotted for coprecipitating proteins. APC7 (s), short exposure; APC7 (l), long exposure. (E) TIF1γ binds to APC3 and Cdc20 in vitro. GST and GST-APC2, APC3, APC5, APC6, APC7 and Cdc20 fusion proteins were incubated with L-α-[35S]-methionine labeled TIF1γ GST-fusion, and binding proteins were purified upon glutathione-agarose beads and separated by SDS-PAGE. The binding of TIF1γ to GST proteins was assessed following autoradiography of the dried gel. *indicates full-length GST fusion protein; upper band in GST lane is GST dimer (F) APC/C complexes were immunoprecipitaed from asynchronous whole cell HeLa extracts using an anti-APC3 monoclonal antibody. APC/C immune complexes were assayed for E3 ligase activity in the presence of L-α-[35S]-methionine labeled cyclin B1 or TIF1γ. After 1 h incubation samples were separated by SDS-PAGE. Polyubiquitylation of cyclin B1 and TIF1γ was assessed following autoradiography of the dried gel. (G) TIF1γ is post-translationally modified in response to nocodazole treatment but it is not degraded following nocodazole release (noc rel). HeLa cells were treated with 200ng/ml nocodazole for 20h. Mitotic cells were then isolated by shake-off and either harvested, or released back into cycle following the removal of nocodazole and then harvested at later times. Proteins were separated by SDS-PAGE and then Western blotted for TIF1γ, cyclin B1 and β-actin. Lane 1: asynchronous cells (A); lane 2: mitotic shake-off following nocodazole treatment (0); lanes 3-5: 1, 2 and 4h post nocodazole release. (H) TIF1γ is not targeted for degradation by Cdc20 or Cdh1. HeLa cells were transfected with mammalian expression plasmids expressing either Myc-tag alone (Myc-EV), Myc-tagged Cdc20, or Myc-tagged Cdh1. Samples were harvested 24h post-transfection and subject to SDS-PAGE and Western blotting. (I) Emi1 knockdown does not promote TIF1γ degradation. HeLa cells were treated with non-silencing siRNA, Emi1, and Cdh1 siRNAs. Forty-Eight h post-knockdown cells were treated with 200 ng/ml nocodazole for 20h. Mitotic shake-off cells were then released from the nocodazole block (noc rel) and harvested at the times indicated and subject to SDS-PAGE and Western blotting.
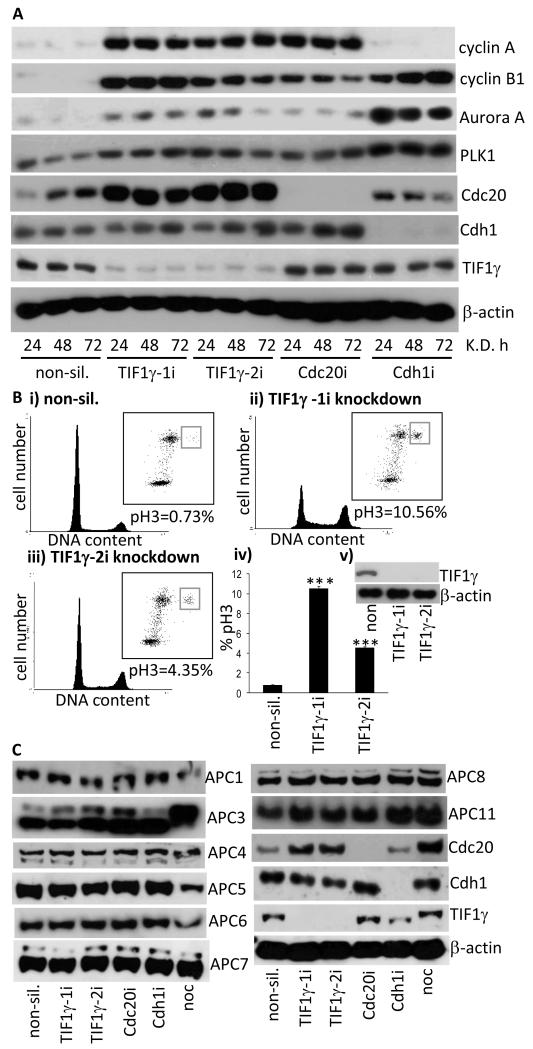
Figure 3. Ablation of TIF1γ expression by RNA interference stabilizes APC/C substrates and increases the number of cells in mitosis.
(A) Mitotic APC/C substrates are stabilized following TIF1γ knockdown. HeLa cells were treated with either non-silencing siRNA, or siRNAs specific for TIF1γ. Cell lysates were prepared at the times indicated and levels of APC/C substrates determined by Western blotting. TIF1γ levels were determined to monitor knockdown efficiency and α-actin levels were determined as a loading control. (B) TIF1γ knockdown increases the mitotic index. HeLa cells were treated with either non-silencing siRNA, or siRNAs specific for TIF1γ Cells were fixed in 70% (v/v) ice-cold ethanol 72h post-transfection and subsequently subjected to FACS analysis, following co-staining with propidium iodide, to gauge cell cycle distribution, and an anti-phospho Ser10-Histone H3 antibody to gauge the numbers of cells specifically in mitosis. ***P<0.001; data taken from three independent experiments. Error bars represent standard deviation. (C) TIF1γ knockdown does not affect APC/C subunit expression. HeLa cells were treated with either non-silencing siRNA, or siRNAs specific for TIF1γ Cdc20 and Cdh1. Forty-Eight h post-knockdown cells were harvested and subject to SDS-PAGE and Western blotting.
TIF1γ associates preferentially with the APC/C in mitosis and S phase
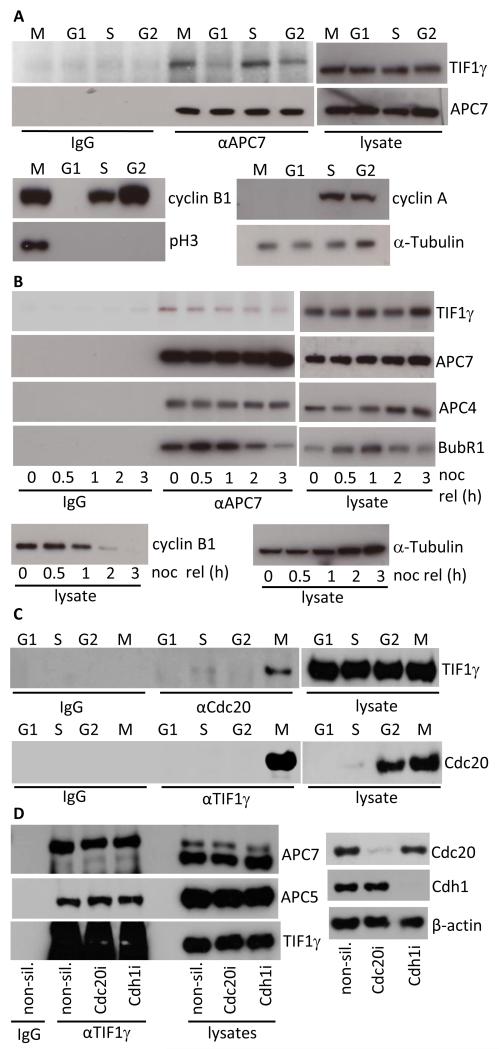
As Cdc20 binding to the APC/C is cell cycle-regulated and TIF1γ binds preferentially to APC/C-Cdc20, we thought it appropriate to examine the ability of TIF1γ to bind to the APC/C during each stage of the cell cycle. To do this we collected highly enriched populations of mitotic, G1, S and G2 HeLa cells and subjected them to immunoprecipitation with anti-APC7 antibodies, followed by Western blotting for TIF1γ. Consistent with the ability of TIF1γ to bind APC/C-Cdc20, we determined that TIF1γ had a greater capacity to bind the APC/C in mitosis than in G1 or G2 phases, despite TIF1γ levels not changing appreciably during the cell cycle (Fig. 2A). TIF1γ also had a greater ability to bind the APC/C in S-phase, relative to its ability to bind the APC/C in G1 and G2 phases (Fig. 2A). Cell cycle status was verified by blotting for cell cycle-regulated proteins (Fig. 2A). Given that TIF1γ associates preferentially with Cdc20, and not Cdh1 (Fig. 1), and that TIF1γ interaction with the APC/C is cell cycle-dependent (Fig. 2A), we hypothesized that TIF1γ might associate with the APC/C only in discrete phases of mitosis. To investigate this possibility we immunoprecipitated APC7 complexes from nocodazole-treated cells, and those cells released back into the cell cycle following nocodazole withdrawal, and subsequently performed Western blots for TIF1γ. Interestingly, the ability of TIF1γ to bind the APC/C was maximal in SAC-activated cells, but was reduced appreciably once the SAC was satisfied and cells were allowed to progress through mitosis (Fig. 2B). To establish whether TIF1γ also bound Cdc20 in a cell cycle-dependent manner we next immunoprecipitated Cdc20 and TIF1γ from mitotic, G1, S and G2 populations of HeLa cells and performed the corresponding TIF1γ and Cdc20 Western blots (Fig. 2C). These analyses revealed that TIF1γ associated with Cdc20, exclusively in mitosis, suggesting a role for TIF1γ-APC/C-Cdc20 complexes, specifically in mitosis (Fig. 2C). Given these findings and those that suggest that TIF1γ associates with APC3 and Cdc20 independently (Fig. 1D), we next determined whether Cdc20, or Cdh1, knockdown affected the ability of TIF1γ to interact with the APC/C holoenzyme (Fig. 2D). Significantly, the ability of TIF1γ to associate with the APC/C was not affected by Cdc20, or Cdh1 knockdown, suggesting that TIF1γ might also affect APC/C function, independent of its association with Cdc20. These data suggest that TIF1γ associates directly with the APC/C. Whether TIF1γ associates with Cdc20 directly, in vivo, remains unknown.
Figure 2. TIF1γ association with the APC/C and Cdc20 is cell-cycle dependent.
(A) TIF1γ binds the APC/C preferentially in mitosis and S phase. HeLa whole cell extracts were prepared from G1, S, G2 and M phase enriched cell populations. APC7 was then immunoprecipitated from each fraction, isolated upon Protein G Sepharose, and the relative amount of TIF1γ associated with APC7 immune complexes was determined by Western blot. Cell cycle status was verified by Western blotting for cyclins A, B, phospho Ser10-Histone H3 (pH3) and α-tubulin. (B) The ability of TIF1γ to bind the APC/C decreases once the SAC is satisfied and cells progress through mitosis. HeLa cells were treated with 200ng/ml nocodazole for 20h. Mitotic cells were then isolated by shake-off and either harvested, or released back into cycle following the removal of nocodazole and then harvested at later times. APC7 was then immunoprecipitated from cell lystaes, isolated upon Protein G Sepharose and TIF1γ binding to the APC/C was assessed by Western blot. Levels of BubR1, cyclin B1, APC4, APC7, TIF1γ and α-tubulin were also assessed by Western blot. (C) TIF1γ binds Cdc20 preferentially in mitosis. HeLa whole cell extracts were prepared from G1, S, G2 and M phase enriched cell populations. Cdc20 and TIF1γ was then immunoprecipitated from each fraction, isolated upon Protein G Sepharose, and the relative amount of TIF1γ associated with Cdc20 was determined by Western blot. (D) Cdc20 knockdown does not affect APC/C association with TIF1γ HeLa cells were treated with non-silencing siRNA, Cdc20, and Cdh1 siRNAs. Forty-Eight h post-knockdown cells were harvested and subject to immunoprecipitation with anti-TIF1γ antibodies Following SDS-PAGE TIF1γ association with the APC/C was assessed by Western blotting.
TIF1γ knockdown stabilizes the levels of APC/C substrates and blocks cells in mitosis
To address the functional significance of the TIF1γ-APC/C interaction we first investigated whether TIF1γ expression affects the levels of known APC/C substrates. We therefore ablated TIF1γ expression in HeLa cells using two different siRNAs and then determined the protein levels of different APC/C substrates by Western blotting. As positive controls we also determined the effects of Cdc20 and Cdh1 knockdown upon APC/C substrate levels. These analyses revealed that the knockdown of TIF1γ, Cdc20 and Cdh1 was highly efficient; knockdown was essentially complete after 24h and protein levels remained low for the duration of the experiment (Fig. 3A). Consistent with the notion that TIF1γ might regulate APC/C ligase activity, the protein levels of APC/C substrates cyclin A, cyclin B1, Aurora A, PLK1 and Cdc20 were all elevated in cells treated with siRNAs against TIF1γ, relative to non-silencing controls (Fig. 3A). Cdc20 and Cdh1 knockdown affected the protein levels of APC/C substrates as expected (Fig. 3A). Given these findings, we next investigated whether TIF1γ knockdown affected cell cycle distribution. We therefore treated asynchronous HeLa cells with the appropriate siRNAs and then determined cell cycle status 72h post treatment (Fig 3B). Propidium iodide staining indicated that there were a greater proportion of TIF1γ knockdown cells in G2/M, relative to non-silencing controls (Fig 3B). In accordance with the established role of the APC/C in mitosis and the affect of TIF1γ knockdown on the levels of APC/C substrates, phospho-serine 10 histone H3 staining revealed that there was also a dramatic increase in the mitotic index following TIF1γ knockdown, relative to non-silencing controls (Fig 3B). Treatment of cells with the TIF1γ-1i siRNA caused a greater than 14-fold increase in the mitotic index relative to non-silencing siRNA, whilst TIF1γ-2i siRNA increased the number of cells in mitosis approximately 6-fold (Fig. 3B). As TIF1γ also regulates transcription we next investigated whether TIF1γ knockdown affected the protein levels of APC/C subunits (Fig. 3C). Significantly, ablation of TIF1γ expression by RNAi did not down-regulate the protein levels of APC/C subunits (Fig. 3C), suggesting that TIF1γ transcriptional activity does not account for its affects upon the APC/C. Taken together, these data are important in establishing a potential role for TIF1γ in modulating APC/C activity and regulating cell cycle progression.
TIF1γ knockdown prolongs time taken to progress through mitosis
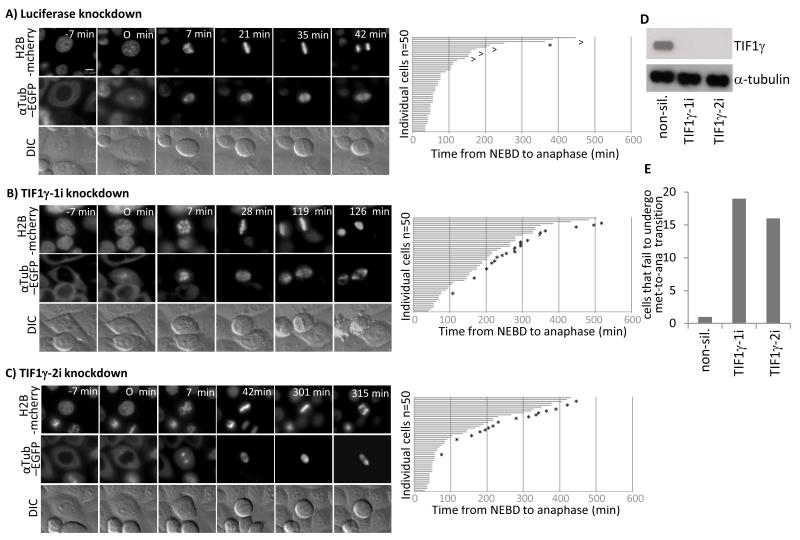
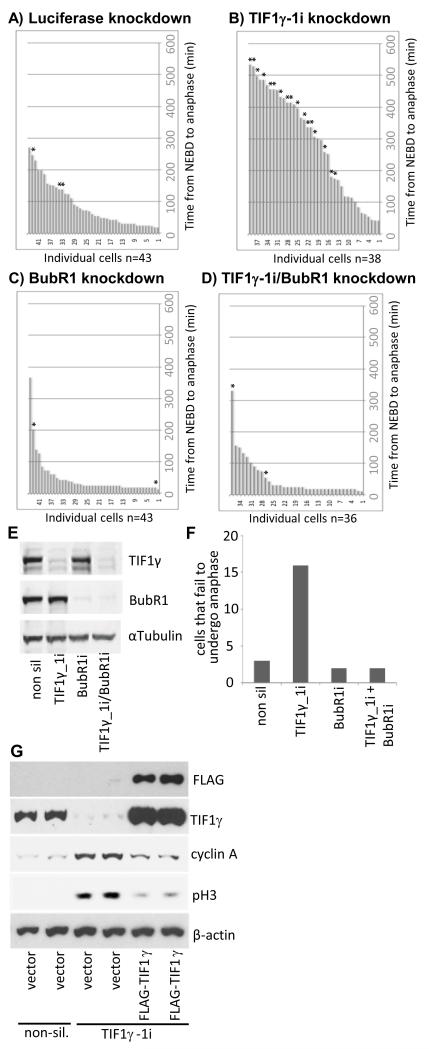
Given these findings we next addressed whether TIF1γ regulates progression through mitosis. We therefore performed live-cell Differential Interference Contrast (DIC), and immunofluorescent, video microscopy with HeLa cells expressing EGFP-labelled α-tubulin and mCherry-labelled histone H2B and calculated the time taken for cells, treated with non-silencing siRNA or siRNAs targeted against TIF1γ, to progress from the beginning of NEBD to anaphase (Fig. 4). Consistent with a role for TIF1γ in mitosis, cells treated with non-silencing siRNA took on average 105.2 min to pass from NEBD to anaphase, whilst cells treated with siRNAs targeted against TIF1γ, TIF1γ-1i and TIF1γ-2i, took 199.04 min and 142.2 min, respectively (cf Fig 4A-C). Interestingly, a large proportion of the cells treated with siRNAs targeted against TIF1γ appeared to have prolonged metaphases that were characterized by a failure to undergo metaphase-to-anaphase transition (Fig. 4E). Indeed, image analysis revealed that those TIF1γ knockdown cells that failed to undergo metaphase-to-anaphase transition were characterized at late times by plasma membrane blebbing, compacted chromatin and aberrant mitotic spindle formation. Close inspection of the data revealed that only three cells of fifty analyzed took over 300 min to pass from NEBD to anaphase following treatment with non-silencing siRNA (Fig. 4A), whilst there was a 3-fold increase in the number of cells taking over 300 min to pass from NEBD to anaphase following treatment with TIF1γ siRNAs (Fig. 4). Taken together these data are supportive of the notion that TIF1γ functions to regulate progression through mitosis.
Figure 4. TIF1γ knockdown delays progression through mitosis from NEBD to anaphase.
HeLa cells expressing Histone H2B mCherry and α-tubulin-EGFP were treated with either an siRNA specific for luciferase or siRNAs specific for TIF1γ 48h post-knockdown cells were subjected to a thymidine block for 16 h, whereupon cells were released back into cycle and video imaging began 9 h later. Video images representative of treatments with (A) luciferase siRNA (B) TIF1γ-1i siRNA and (C) TIF1γ-2i siRNA are presented. Corresponding bar charts illustrating the time taken for cells to progress from NEBD to anaphase are also presented. ^ represents cells that failed to exit mitosis during filming. * represents cells that failed to undergo successful metaphase-to-anaphase transition during filming (depicted in Fig. 4E). DIC and immunofluorescent imaging revealed that those cells that failed to undergo metaphase-to-anaphase transition were characterized at late times by plasma membrane blebbing, compacted chromatin and aberrant mitotic spindle formation. Video imaging was therefore terminated at this time, and the time taken from NEBD to this point was recorded. The mean values calculated for time taken from NEBD to anaphase are therefore, underestimated for those cells that failed to undergo metaphase-to-anaphase transition. (D) Western blot for TIF1γ and α-tubulin in cells treated with luciferase siRNA, TIF1γ-1i or TIF1γ-2i siRNAs.
TIF1γ knockdown leads to an accumulation of cells in metaphase
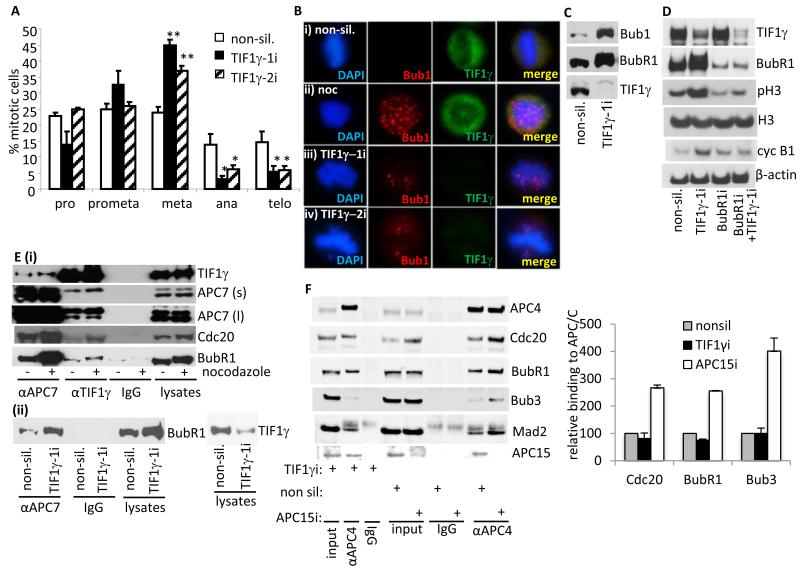
To substantiate these findings further we next investigated whether TIF1γ knockdown affects mitotic distribution. To do this we treated asynchronous HeLa cells with either non-silencing siRNAs or siRNAs targeted against TIF1γ and determined the number of cells in prophase, prometaphase, metaphase, anaphase and telophase by immunofluorescent microscopy. Consistent with the observation that TIF1γ knockdown affects progression from NEBD to anaphase, it was notable that TIF1γ knockdown with either TIF1γ siRNA caused a significant increase in the number of metaphase cells and a corresponding, significant decrease in the number of anaphase and telophase cells (Fig. 5A). These data are supportive of the idea that TIF1γ regulates metaphase-to anaphase transition.
Figure 5. Ablation of TIF1γ expression by RNA interference delays progression through mitosis and activates the SAC.
(A and B) TIF1γ knockdown increases the number of cells with misaligned chromosomes at metaphase. HeLa cells were treated with either non-silencing siRNA or siRNAs specific for TIF1γ and seeded onto glass slides. 72h post-transfection cells were fixed and permeabilized and then co-stained with either α-tubulin, TIF1γ and DAPI, TIF1γ and DAPI, or Bub1, TIF1γ and DAPI, to gauge mitotic index, mitotic distribution and SAC activation. Nocodazole-treated cells were included as a control. (A) pro, prophase; prometa, prometaphase; meta, metaphase; ana, anaphase; telo, telophase. TIF1γ knockdown cells exhibiting a bipolar spindle and misaligned chromosomes at metaphase, were scored as metaphase cells. The bar chart represents results from three independent experiments (At least 300 mitotic cells were counted per experiment). Error bars denote standard deviation. *P<0.05; **P<0.01. (B) Immunofluorescent visualization of Bub1 associated with unattached kinetochores and attached kinetochores not under full tension in TIF1γ knockdown cells (276 out of 300 TIF1γ-1 metaphase knockdown cells had misaligned chromosomes and positive Bub1 staining whilst 253 out of 300 TIF1γ-2 metaphase knockdown cells had misaligned chromosomes and positive Bub1 staining compared to 17 out of 300 for non-silencing controls). (C) TIF1γ knockdown activates the SAC. HeLa cells were treated with either non-silencing siRNA, or siRNAs specific to TIF1γ. The levels of TIF1γ and the SAC proteins, BubR1 and Bub1 were determined by Western blotting 72h post knockdown. (D) BubR1 knockdown relieves SAC activation imposed by TIF1γ knockdown. HeLa cells were treated with either non-silencing siRNA, or siRNAs specific for TIF1γ BubR1, or TIF1γ and BubR1. Cell lysates were harvested after 72h and levels ofTIF1γ, BubR1, phospho Ser10-histone H3 (pH3), histone H3 (H3), cyclin B1 and β-actin were all determined by Western blotting. (Ei) TIF1γ associates with the APC/C-MCC in SAC-activated cells. HeLa cells were treated with nocodazole (200 ng/ml) for 20h. Cell lysates were then prepared and APC7 and TIF1γ were immunoprecipitated. The relative amounts of TIF1γ, APC7, Cdc20 and BubR1 associated with APC7 and TIF1γ were determined by Western blot. APC7 (s), short exposure; APC7 (l), long exposure. (Eii) HeLa cells were treated with either non-silencing siRNA, or siRNAs specific for TIF1γ APC7 and TIF1γ were immunoprecipitated from cell lysates prepared 72h post-transfection. The relative amount of BubR1 associated with APC7 was determined by Western blot. (F) HeLa cells were treated with either non-silencing siRNA, or siRNAs specific for TIF1γ or APC15 and 48h later, treated with nocodazole (200 ng/ml) for 20h. The association of Cdc20, BubR1 and Bub3 with the APC/C was assessed by Western blot following immunoprecipitation with an anti-APC4 antibody.
Loss of TIF1γ expression results in SAC activation
It was evident whilst analyzing the effect of TIF1γ knockdown upon mitotic distribution that although all TIF1γ knockdown cells in metaphase had a characteristic bipolar spindle, the majority of these cells (>90%) had misaligned chromosomes. We reasoned therefore that TIF1γ depletion might result in the activation of the SAC. To address this possibility, we treated HeLa cells with either non-silencing siRNAs or siRNAs targeted against TIF1γ and assessed Bub1 association with kinetochores. Consistent with the idea that the SAC is activated following TIF1γ knockdown, microscopic studies revealed that Bub1 was associated with the kinetochores of misaligned chromosomes at metaphase in TIF1γ knockdown cells (Fig. 5B). Furthermore, Western blotting revealed that TIF1γ knockdown promoted the upregulation, and phosphorylation, of SAC regulators Bub1 and BubR1 (Fig. 5C). To substantiate these findings we next attempted to override the SAC phenotype displayed by TIF1γ knockdown cells, by coordinately ablating the expression of both TIF1γ and BubR1 (Fig. 5D). Consistent with the hypothesis that TIF1γ knockdown activates the SAC, we determined that cells depleted of TIF1γ alone had elevated levels of cyclin B1, phospho-serine 10 histone H3 and phosphorylated BubR1 (Fig. 5D), whilst cells depleted of both TIF1γ and BubR1 had substantially reduced levels of cyclin B1 and phospho-serine 10 histone H3, relative to cells treated with non-silencing siRNA or TIF1γ siRNA (Fig. 5D). These results indicate that knockdown of BubR1 relieves SAC activation induced by TIF1γ knockdown, and allows for metaphase-to anaphase transition and mitotic exit. Taken together these data indicate that the SAC is activated following TIF1γ knockdown, and that TIF1γ regulates APC/C function prior to anaphase.
TIF1γ is also associated with the APC/C-MCC in SAC-activated cells
To examine the relationship between TIF1γ and the SAC in more detail we investigated the ability of TIF1γ to associate with BubR1, the APC/C and Cdc20 upon SAC activation. Initially, we determined the relative abilities of TIF1γ and the APC/C to associate with Cdc20 and BubR1 in asynchronously growing HeLa cells, and HeLa cells collected by mitotic shake-off where the SAC was activated by nocodazole treatment (Fig. 5E). In line with our studies examining TIF1γ association with the APC/C, both the APC/C and TIF1γ had a greater propensity to bind to Cdc20 and BubR1 in mitosis following SAC activation (Fig. 5E). Consistent with these findings, the APC/C also had a greater propensity to bind BubR1 when TIF1γ levels were depleted by siRNA (Fig. 5E). There was however, only a modest increase in TIF1γ-APC7 association following SAC activation, suggesting that TIF1γ association with the APC/C holoenzyme is largely SAC-independent (Fig. 5E). As APC15 has recently been implicated in controlling the SAC by regulating the APC/C-dependent turnover of MCC components (17) we investigated whether TIF1γ was also involved in regulating MCC turnover. To do this we activated the SAC in HeLa cells and monitored MCC attachment to the APC/C in cells where TIF1γ or APC15 had been depleted by siRNA. Consistent with its known function, APC15 depletion stabilized MCC association with the APC/C, whilst TIF1γ did not (Fig. 5F). These data indicate that although TIF1γ is associated with the APC/C-MCC in SAC-activated cells it is not required to inactivate the SAC.
Role for TIF1γ in APC/C-mediated degradation of cyclin A and cyclin B1 in mitosis
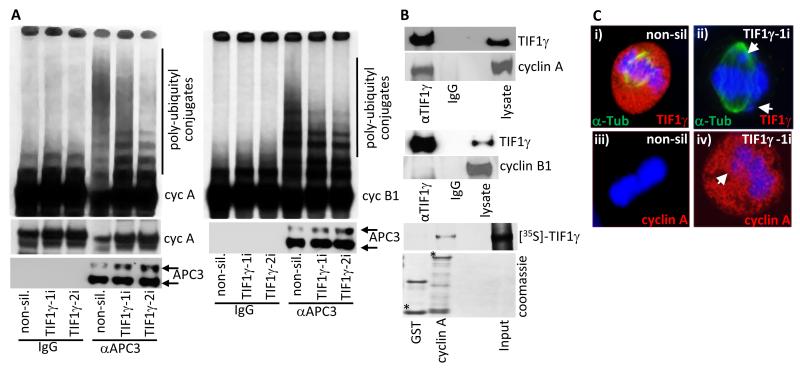
To determine a direct role for TIF1γ in the APC/C-mediated degradation of APC/C substrates in mitosis, we next performed in vitro APC/C ubiquitin ligase assays upon anti-APC3 immunoprecipitates from HeLa cells treated with either non-silencing siRNA, or siRNA against TIF1γ. These experiments revealed that TIF1γ knockdown, relative to non-silencing controls, reduced substantially the ability of the APC/C to polyubiquitylate [35S]-labelled cyclin A by about 80% (Fig. 6A). As cyclin A degradation is typically SAC-independent (18, 19), these data are supportive of a model whereby TIF1γ modulates APC/C-Cdc20 activity directly to regulate the polyubiquitylation of cyclin A. To determine whether the impairment of APC/C ubiquitin ligase activity in TIF1γ knockdown cells also extends to SAC-dependent substrates, we assessed the ability of the APC/C immunoprecipitated from HeLa cells, treated with either non-silencing siRNA, or siRNA against TIF1γ, to ubiquitylate cyclin B1 (Fig. 6A). Consistent with a more general role for TIF1γ in the regulation of APC/C activity, TIF1γ knockdown, relative to non-silencing controls, reduced the ability of the APC/C to polyubiquitylate [35S]-labelled cyclin B1 by approximately 60% (Fig. 6A).
Figure 6. Cyclin A is stabilized in mitotic TIF1γ knockdown cells and is degraded upon expression of an siRNA resistant TIF1γ species.
(A) APC/C ligase activity directed towards cyclin A and cyclin B1 is reduced following TIF1γ knockdown. HeLa cells were treated with either non-silencing siRNA, or siRNAs specific for TIF1γ. The APC/C holoenzyme was immunoprecipitated from cell lysates 72h post-treatment using an antibody to APC3. APC/C activity was then assayed by incubating anti-APC3 immunoprecipitates with L-α-[35S]-methionine labeled cyclin A or cyclin B1. After 1 h incubation samples were separated by SDS-PAGE and polyubiquitylation of cyclin A and cyclin B1 was assessed following autoradiography of the dried gel. (B) TIF1γ associates with cyclin A but not cyclin B1. HeLa cells were subject to immunoprecipitation with TIF1γ, and Western blotted for cyclin A or cyclin B1; GST and GST-cyclin A were incubated with L-α-[35S]-methionine labelled TIF1γ GST- fusion, and binding proteins were purified upon glutathione-agarose beads and separated by SDS-PAGE. The binding of TIF1γ to GST-cyclin A was assessed following autoradiography of the dried gel. * indicates full-length GST fusion protein; upper band in GST lane is GST dimer. (C) Cyclin A is inappropriately present at metaphase in TIF1γ knockdown cells. HeLa cells were treated with either non-silencing siRNA or siRNAs specific for TIF1γ and seeded onto glass slides. 72h post-transfection cells were fixed and permeabilized and then co-stained with either α-tubulin, TIF1γ and DAPI (i) or cyclin A and DAPI (ii)
Since the APC/C and Cdc20 cooperate in binding substrates (20, 21), and data presented here has determined that TIF1γ associates with APC/C-Cdc20, and affects APC/C-Cdc20 activity in mitosis, we next investigated whether TIF1γ was also able to associate with the APC/C substrates, cyclin A and cyclin B1. Consistent with the idea that TIF1γ modulates APC/C activity during mitosis, TIF1γ was able to bind cyclin A in vivo (Fig. 6B); it was not however, able to bind cyclin B1 (Fig. 6B). Consistent with this finding [35S]-labelled TIF1γ also associated specifically with GST-cyclin A in vitro (Fig. 6B). Taken together these data support the notion that TIF1γ promotes the APC/C-Cdc20-dependent degradation of cyclin A in mitosis potentially though interaction with the APC/C holoenzyme, Cdc20, and cyclin A itself. These data also suggest that TIF1γ can affect APC/C E3 ligase activity directed towards cyclin B1, independently of binding to cyclin B1. As the APC/C-mediated degradation of cyclin A normally begins at the onset of NEBD in mitosis (22), we next investigated whether cyclin A expression was elevated in metaphase cells where TIF1γ expression had been ablated by RNAi. As before, TIF1γ knockdown resulted in metaphase cells with misaligned chromosomes (Fig 6C). Consistent with the Western blot data (Fig. 3A), confocal microscopy revealed that cyclin A levels were higher in TIF1γ knockdown cells at metaphase, relative to cells treated with non-silencing siRNA (Fig 6C); TIF1γ cells expressing elevated levels of cyclin A at metaphase had characteristic misaligned chromosomes (Fig 6C).
TIF1γ mitotic effects are dependent upon the SAC
As overexpression of exogenous cyclin A has previously been reported to result in chromosome misalignment and anaphase delay in a SAC-independent manner (22), we wanted to discern whether TIF1γ knockdown affected cell cycle progression through mitosis independently of the SAC through the modulation of endogenous cyclin A levels, or, alternatively affected mitotic progression in a SAC-dependent manner. To do this, we investigated by DIC, and immunofluorescent, video microscopy the effect of dual TIF1γ and BubR1 knockdown in HeLa cells upon the time taken for cells to progress from NEBD to anaphase (Fig. 7). As we previously demonstrated, TIF1γ knockdown alone resulted in a significant delay in the ability of cells to progress through mitosis and undergo metaphase-to-anaphase transition (Figs. cf 7A and 7B; Fig. 7F), whereas BubR1 knockdown alone resulted in more rapid progress from NEBD to anaphase, relative to control cells, presumably as the SAC had been inactivated (cf Figs. 7A and 7C). Cells that were depleted of both TIF1γ and BubR1 progressed through mitosis with similar kinetics to those cells that had been treated with BubR1 siRNA alone (cf Fig. 7C and 7D). Taken together these data suggest that the ability of TIF1γ to affect cell cycle progression through mitosis is mediated exclusively through the SAC.
Figure 7. Inactivation of the SAC allows TIF1γ knockdown cells to pass through mitosis from NEBD to anaphase.
HeLa cells expressing Histone H2B mCherry and α-tubulin-EGFP were treated with either an siRNA specific for luciferase (A) or siRNAs specific for TIF1γ (B), BubR1 (C) or TIF1γ and BubR1 (D). 48h post-knockdown cells were subjected to a thymidine block for 16 h, whereupon cells were released back into cycle and video imaging began 9 h later. Bar charts depicting time taken to pass from NEBD to anaphase are presented. * represents cells that failed to undergo successful metaphase-to-anaphase transition during filming (depicted graphically in Fig. 7F). (E) Western blots for TIF1γ BubR1 and α-tubulin in cells treated with luciferase, TIF1γ BubR1, or γ and BubR1 siRNAs. (G) Expression of a siRNA-resisitant TIF1γ species allows for cyclin A degradation and mitotic progression. HeLa cells were treated with either non-silencing siRNA, or siRNAs specific for TIF1γ for 48 h. Cells were then transfected with vector alone or a construct expressing FLAG-TIF1γ and cultured for a further 24h. Cell lysates were prepared, and the levels of FLAG-TIF1γ, endogenous TIF1γ, cyclin A, phospho Ser10-histone H3 (pH3) and β-actin were all determined by Western blot.
Cellular expression of a siRNA-resistant TIF1γ facilitates mitotic progression and mitotic exit
As TIF1γ knockdown leads to the accumulation of APC/C substrates and SAC activation, we reasoned that expression of a siRNA-resistant TIF1γ might restore APC/C activity against mitotic substrates and allow cells to progress through, and exit mitosis. To investigate this possibility we first ablated TIF1γ expression by siRNA and, 48h later, transfected knockdown cells with a siRNA-resistant FLAG-tagged TIF1γ species. We harvested cells 24h post-transfection and performed Western blot analyses to determine the consequences of TIF1γ expression on APC/C function (Fig. 7G). Importantly, these analyses revealed that expression of the siRNA-resistant FLAG-tagged TIF1γ species reversed TIF1γ knockdown effects, and led to a significant reduction in the levels of the APC/C substrate, cyclin A, and the mitotic indicator, phospho-serine 10 histone H3 (Fig. 7G). Taken together these data support the notion that TIF1γ regulates APC/C activity directly and allows for the temporally coordinated, APC/C-dependent progression of cells through mitosis.
DISCUSSION
In this report we have identified TIF1γ as an APC3 and Cdc20 binding protein that associates specifically in vivo with the APC/C holoenzyme, APC/C-Cdc20 and APC/C-MCC complexes and promotes mitotic progression (Fig. 1-7). In this regard TIF1γ associated with a number of APC/C subunits in vivo (Fig. 1). Interestingly, TIF1γ associated with two forms of APC7, a major 66 kDa species, and a minor 75 kDa species; TIF1γ associated preferentially with the minor species (Figs. 1B and 1D). It is not clear at present whether this minor species of APC7 represents an APC7 isoform, or whether it is a post-translationally modified form of the major APC7 species. It will be important to characterize the minor form of APC7 as it might give new insight into APC/C function. We also presented considerable evidence to indicate that TIF1γ is not a substrate for APC/C-dependent ubiquitylation (Fig. 1; Fig 3C), and that it associated preferentially with Cdc20 and not Cdh1 (Fig.1). Perhaps surprisingly, TIF1γ associated with the APC/C in S phase as well G2 and mitosis (Fig. 2). Previous reports have suggested that Emi1 acts as a pseudosubstrate inhibitor of the APC/C to prevent premature APC/C activation (15, 16); it will be of interest to determine whether TIF1γ functions in a similar manner to regulate APC/C activity during S phase. Our data also revealed that TIF1γ associated with Cdc20 preferentially in mitosis (Fig. 2), and that Cdc20 knockdown did not appreciably affect TIF1γ association with the APC/C (Fig. 2). It will be of interest in the future therefore, to dissect the relative contributions of TIF1γ-APC/C- and TIF1γ-Cdc20 interactions in controlling mitotic progression.
It is now well established that APC/C-Cdc20 promotes the degradation of early mitotic substrates such as Nek2A and cyclin A (22, 23) and initiates metaphase-to-anaphase transition by promoting the polybiquitylation and proteasomal-mediated degradation of the Separase inhibitor, Securin (1, 2). We determined that TIF1γ knockdown reduces APC/C ligase activity, stabilizes APC/C substrates and increases the number of cells in metaphase, relative to control-treated cells (Figs. 3, 5 and 6). Our studies also revealed that TIF1γ knockdown prolonged the time taken for cells to pass from NEBD to anaphase, and that a significant proportion of TIF1γ knockdown cells failed to undergo metaphase-to-anaphase transition (Figs. 4 and 5A). Consistent with these findings, we established that TIF1γ knockdown promotes SAC activation (Fig. 5). The notion that TIF1γ inactivation promotes SAC activation is supported by the observation that knockdown of the SAC component, BubR1, can overcome SAC activation imposed by TIF1γ knockdown (Fig. 5D; Fig 7). However, unlike APC15 which regulates SAC by coordinating MCC dissociation from the APC/C, TIF1γ does not play a role in regulating SAC inactivation (Fig. 5F). A direct role for TIF1γ in mitotic progression was given credence by the observation that siRNA-resistant TIF1γ relieves SAC activation, promotes cyclin A degradation, and facilitates cell cycle progression through mitosis (Fig. 7G). Collectively, these data indicate that, not only does TIF1γ modulate APC/C-Cdc20 activity and the progression of cells through mitosis, it also associates with the APC/C-MCC.
The APC/C-Cdc20-mediated degradation of early mitotic substrates such as cyclin A occurs despite SAC activation (19, 24). It has been suggested that cyclin A outcompetes SAC proteins for binding to Cdc20, and that cyclin A-Cdc20 complexes are subsequently recruited to the APC/C by the cyclin-dependent kinase cofactor, Cks (19, 24). As TIF1γ binds specifically to APC/C-Cdc20 complexes and cyclin A in vivo, and has the capability to bind to Cdc20, APC3 and cyclin A independently in vitro (Fig. 1; Fig. 6B), it is possible that TIF1γ cooperates with Cdc20 and Cks in the binding and recruitment of cyclin A to the APC/C. Given that TIF1γ knockdown also affects the ability of the APC/C to polyubiquitylate cyclin A (Fig. 6A), TIF1γ might facilitate the degradation of cyclin A by modulating APC/C-Cdc20 ligase activity directly. The notion that TIF1γ functions directly to regulate APC/C activity is supported by the observations that the APC/C-Cdc20-dependent degradation of cyclin A is SAC-independent, and that significantly more APC/C ligase activity against cyclin A can be immunoprecipiated from asynchronous cells relative to TIF1γ knockdown cells, even though there are between 5- and 10-fold more cells in mitosis following TIF1γ knockdown (cf Fig. 5A and Fig. 6A). A more general role for TIF1γ in modulating APC/C ubiquitin ligase activity is given credence by the observation that TIF1γ knockdown also affects the ability of the APC/C to polyubiquitylate cyclin B1 (Fig. 6A). It is possible therefore that TIF1γ might function more generally to affect APC/C ligase activity directed towards its substrates.
Pertinently, it is has previously been demonstrated that cyclin A is targeted for degradation by APC/C-Cdc20 at the commencement of NEBD, and that cyclin A degradation is required for proper chromosome alignment at metaphase; cyclin A overexpression delays chromosome alignment and anaphase onset (22). As TIF1γ knockdown leads to an increase in metaphase cells, a concomitant reduction in the number of anaphase and telophase cells, and the stabilization of cyclin A at metaphase (Fig. 3; Fig. 6B), it could be reasoned that increased cyclin A expression in TIF1γ knockdown cells is responsible, at least in part, for the mitotic phenotype observed in these cells (Fig. 3; Fig. 4). It has been suggested that cyclin A overexpression delays metaphase-to-anaphase transition independently of SAC as MAD2 does not appear to associate with kinetochores in this instance, though whether it promotes BubR1 association with the APC/C, akin to TIF1γ knockdown, has not been investigated (22). As SAC inactivation relieves completely the mitotic phenotype imposed by TIF1γ knockdown (Fig. 5D; Fig. 7), our data indicates that SAC inactivation overcomes any cell cycle restrictions imposed by the inappropriate presence of endogenous cyclin A at metaphase in TIF1γ knockdown cells, such that chromosome misalignment at metaphase in TIF1γ knockdown cells is subject to control by the SAC (Fig. 7). It is perhaps not surprising that the TIF1γ knockdown phenotype shares only some characteristics of the cyclin A overexpression phenotype, as it might be expected that TIF1γ knockdown reduces APC/C ligase activity directed against other substrates that modulate progression through mitosis (Fig 3A; Fig. 6A). In this regard, it is possible that the reduction of APC/C activity per se in TIF1γ knockdown cells, leads to the accumulation of APC/C substrates that activate and/or sustain the SAC. It is also possible that TIF1γ has other activities in mitosis, unrelated to its ability to affect APC/C ligase activity that regulates chromosome attachment. For instance, TIF1γ might ubiquitylate components of the kinetochore or MCC to coordinate chromosome attachment. TIF1γ association with chromatin might also promote mitotic progression. These possibilities however, await investigation.
Indeed, it has recently been reported that the cell permeable, prodrug form of the small molecule inhibitor of the APC/C, tosyl-L-arginine methyl ester (TAME), proTAME, induces a SAC-dependent metaphase arrest in the absence of spindle damage (25). As the proteasome inhibitor, MG132 also promotes a SAC-dependent mitotic arrest, the authors of this article proposed that APC/C-dependent proteolysis was required to inactivate the SAC, and that APC/C inhibition activated the SAC. Interestingly, some laboratories have proposed that the APC/C-mediated ubiquitylation, but not degradation, of Cdc20 releases the APC/C from SAC inhibition (26, 27), though we and others suggest that APC/C-mediated ubiquitylation and degradation of Cdc20 sustains the SAC (28, 29). As pro-TAME activates the SAC in an APC/C-dependent manner, without chromosome misalignment at metaphase, it could be reasoned that the reduction in APC/C ligase activity in TIF1γ knockdown cells similarly activates the SAC through the stabilization of multiple APC/C substrates. In accordance with this hypothesis, Cdc20, cyclin B1, Aurora A and PLK1 are all elevated in TIF1γ knockdown cells (Fig. 3A). As cyclin-dependent kinase activity has previously been shown to sustain the SAC (30, 31), it is possible that elevated cyclin expression, and presumably therefore, cyclin-dependent kinase activity in TIF1γ knockdown cells also sustains activation of the SAC and prevents metaphase-to-anaphase transition. Alternatively, other APC/C substrates elevated in TIF1γ knockdown cells might similarly promote chromosome misalignment and activate and/or sustain the SAC by targeting SAC components through post-translational modification or protein-protein interaction. Indeed, Bub1 and BubR1 are both APC/C substrates (32, 33) and are activated and elevated following TIF1γ knockdown (Figs. 5B and 5C). Pertinent to this idea, transgenic overexpression of Bub1 in mice gives rise to metaphases with misaligned chromosomes, aneuploidy and tumour formation (34) whilst expression of an acetylated BubR1 mimetic mutant inhibits the APC/C-mediated degradation of BubR1 and leads to a delay in anaphase onset (33).
Consistent with our observations that TIF1γ modulates APC/C-Cdc20 activity, TIF1γ binds to the APC/C preferentially in mitosis and S-phase, and binds to Cdc20 exclusively in mitosis (Fig. 2). Interestingly TIF1γ binding to the APC/C was unaffected by Cdc20 knockdown, suggesting that TIF1γ, binds proportionally to more APC/C holoenzyme than Cdc20 (Fig. 2D). Further analyses revealed that TIF1γ is associated with the APC/C-MCC in SAC-activated cells, but loses the ability to bind the APC/C once the SAC had been satisfied (Fig. 2B). This observation suggests that TIF1γ might participate actively in SAC inactivation by promoting the APC/C-mediated degradation of substrates prior to anaphase that maintain SAC activation. Taken together the data presented herein suggests that TIF1γ associates with the APC/C holoenzyme, active APC/C-Cdc20 complexes, and the APC/C-MCC, and as such participates in the regulation of APC/C-Cdc20 complexes during mitosis.
It has been reported that the TGF-β antagonist, SnoN is a critical target for APC/C-Cdh1-mediated degradation in TGF-β–stimulated cells (35, 36). As we have determined that TIF1γ associates specifically with the Cdc20-bound form of the APC/C, and not the Cdh1-bound form (Fig. 1), these data suggest that TIF1γ does not promote the APC/C-dependent degradation of SnoN in TGF-β-stimulated cells. However, as APC/C-Cdc20 has recently been implicated in regulating presynaptic axonal differentiation programmes (37) it is possible that TIF1γ-APC/C-Cdc20 complexes could participate in TGF-β signaling pathways that coordinate cell cycle exit and cellular differentiation (38).
In conclusion data presented here demonstrates a role for TIF1γ in regulating APC/C function in mitosis. We propose that TIF1γ associates specifically with the APC/C in order to promote the temporally-coordinated destruction of APC/C substrates and progression of cells through mitosis.
MATERIALS AND METHODS
Antibodies
The anti- Cdc20, APC1, APC4, APC6, APC8, cyclin A, cyclin B1, PLK1 anti-Myc and phospho-Histone H3 (serine-10) antibodies were from Santa Cruz. The anti-Cdh1 antibody was from Neomarkers, the anti-β-actin, anti-α-tubulin and vinculin antibodies were from Sigma, the anti-histone H3 antibody was from Millipore, the anti-Bub1 and anti-Aurora A antibodies were from Abcam, the anti-Bub3, anti-BubR1 and NEK2 antibodies were from Becton Dickinson, the anti-APC15 antibody was from Sigma, and the anti-APC3 antibodies were from Becton Dickinson or CR-UK. Anti-APC5, APC4, APC7 and APC11 antibodies have been described previously (4, 29). The rabbit polyclonal against TIF1γ was raised against an N-terminal protein fragment (residues 1-392). Anti-mouse and anti-rabbit HRP antibodies were from Dako. Alexa - 488 and -555 fluorophores were from Invitrogen.
Plasmids
APC/C subunits, UbcH4, UbcH5, UbcH10, Cdc20, cyclin A and TIF1γ were cloned into pGEX4T-1 for bacterial expression. pcDNA3-cyclin A and pcDNA3-cyclin B1 were used for in vitro transcription/translation. pCS2-FLAG-TIF1γ construct was used for in vitro and mammalian expression. Myc-tagged Cdc20 and Cdh1 constructs were from addgene.
Cells and treatments
HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma) supplemented with 8% foetal calf serum and 2 mM L-glutamine (Invitrogen) and cultured at 37°C in humidified incubators supplied with 5% CO2. Cells were arrested in prometaphase by treatment with 200ng/ml nocodazole for 20 hours and collected by shake off. G1 cells were collected 3 hours after nocodazole release, and remaining mitotic cells were removed by shake-off. Cells were synchronized at the G1-S border by double thymidine block (2.5mM). G2 cells were obtained by release from double thymidine block for 8 hours followed by mitotic shake-off (39, 40).
Immunoprecipitation and GST pull-down
Cells were lysed using immunoprecipitation buffer (50 mM Tris-HCl, pH 7.4, 0.825 M NaCl, 1% (v/v) NP-40) and clarified by sonication and centrifugation. Immunoprecipitation was then performed as described previously (41). L-α-[35S]-methionine-labelled TIF1γ was expressed in vitro using a TNT T7 Coupled Reticulocyte Lysate System (Promega) according to the manufacturer’s guidelines. In vitro glutathione-S-transferase (GST) pulldown assays using GST, GST-APC/C and GST-Cdc20 proteins were then performed using established protocols (42).
SDS-PAGE and Western blotting
Whole-cell protein extracts were prepared in denaturing buffer (9 M urea, 150 mM β-mercaptoethanol, 50 mM Tris-HCl, pH 7.4). Extracts were clarified by sonication, and protein concentrations determined by Bradford assay (Bio-Rad). Proteins were separated by SDS-PAGE in the presence of 100 mM Tris, 100 mM Bicine and 0.1% (w/v) SDS. Proteins were then transferred onto nitrocellulose membranes (Pall), before being blocked in Tris-buffered saline containing 5% (w/v) dried milk powder and 0.1% (v/v) Tween 80 and incubation with the appropriate antibodies. Antigens were visualized by enhanced chemiluminescence.
RNA interference and transfections
The sequences of the siRNA oligonucleotides, purchased from Ambion, were CCUGCAUCUAGAAAGUGAAdTdT (TIF1γ-1i), GCGACUGAUUACUUUCCAGdTdT (TIF1γ-2i), GGAAAUAGCCGAGAGGUAAdTdT (APC3), GUCUGGUCUAAGUUUCUUUdTdT (APC15) and CGUACGCGGAAUACUUCGAdTdT (control). The BubR1 siRNA used was a Dharmacon on target plus SMARTpool. SiRNAs were transfected using Oligofectamine (Invitrogen) as described previously (41). HeLa cells were transfected with a siRNA resistant FLAG-tagged TIF1γ expression construct using Lipofectamine 2000 (Invitrogen). FLAG-TIF1γ was rendered insensitive to TIF1γ-1i siRNA by the introduction of two silent mutations: CCUGCAUCUGGAGAGUGAAdTdT
Mass spectrometry
Immunoprecipitates isolated on Protein G Sepharose beads were separated by SDS-PAGE and stained with Coomassie Brilliant Blue (Sigma). Protein bands were excised and subject to treatment with 10 mM DTT and then 50 mM iodoacetamide. Bands were dried then subject to treatment with trypsin (Promega). Tryptic peptides were analyzed by positive electrospray ionisation/mass spectrometry, using a Thermo Fisher Scientific LCQ DECA XP Plus ion trap as described previously (14). Mass spectrometry/mass spectrometry raw data were searched against a non-redundant human protein database using TurboSEQUEST, as part of the BioworksBrowser 3.1 software suite.
Video microscopy
Live cell imaging was performed using a Deltavision core microscope (Applied precision) equipped with an incubation chamber maintained at 37°C in an atmosphere of 5% CO2. Videos were acquired at seven-minute intervals using a magnification objective of 40 and Softworx software.
APC/C ligase assays
APC/C complexes were immunoprecipitated from cells using anti-APC3 mAb and protein G sepharose. APC/C ubiquitin ligase activity was assayed as described (4) and resolved by SDS–PAGE. Gels were then dried and visualized by autoradiography.
Confocal microscopy
Cells were seeded onto poly-L-lysine-coated glass slides and fixed and permeabilized with methanol cooled to −20 °C. Slides were then incubated for 1 h at room temperature in blocking buffer (2 % (v/v) foetal calf serum in PBS), and then incubated for 2 h with the primary antibody diluted in blocking buffer. Slides were then washed in PBS, and incubated for 2 h with secondary antibody diluted in blocking buffer. After further washing in PBS, slides were mounted in Vectashield containing DAPI (Vectorlabs). Cells were observed and images were acquired using a Zeiss LSM510-Meta laser scanning confocal microscope.
FACS analysis
Cells were fixed in 70% (v/v) ethanol for 1 h, and then washed with PBS prior to permeabilisation in PBS containing 0.25 % (v/v) Triton X-100. After washing with 1 % (w/v) BSA in PBS, cells were incubated with an anti-phosphohistone H3 (Ser10) polyclonal antibody in 1 % (w/v) BSA in PBS for 1.5 h at room temperature. Samples were then washed twice with 1% (w/v) BSA in PBS, and incubated for 1 h with a fluorescein isothiocyanate (FITC)-conjugated-anti-Rabbit antibody diluted in 1 % w/v BSA in PBS. After washing with PBS alone, cells were resuspended in PBS containing propidium iodide (25 μg/ml) and 0.1 mg/ml of RNAse A, and left at room temperature for a further 1 h. Cell cycle analysis was then carried out using a Coulter XL-MCL flow cytometer (Beckman-Coulter).
ACKNOWLEDGEMENTS
We would like to thank Francis Barr, Andrew Fry, Stefano Piccolo, Frank Rauscher III, Hughes de Thé and Hiroyuki Yamano for reagents. This work was funded by CR-UK (C10000/A7542) and The University of Birmingham, College of Medical and Dental Sciences Research Development Fund. JN is supported by The Danish Cancer Society and the Lundbeck Foundation.
CONFLICT OF INTEREST
The authors declare they have no conflict of interest.
REFERENCES
- 1.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 2.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 3.Yu H. Cdc20: A WD40 Activator for a Cell Cycle Degradation Machine. Mol. Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Turnell AS, Stewart GS, Grand RJ, Rookes SM, Martin A, Yamano H, et al. The APC/C and CBP/p300 cooperate to regulate transcription and cell-cycle progression. Nature. 2005;438:690–695. doi: 10.1038/nature04151. [DOI] [PubMed] [Google Scholar]
- 5.Turnell AS, Mymryk JS. Roles for the coactivators CBP and p300 and the APC/C E3 ubiquitin ligase in E1A-dependent cell transformation. Br. J. Cancer. 2006;95:555–560. doi: 10.1038/sj.bjc.6603304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townsend K, Mason H, Blackford AN, Miller ES, Chapman JR, Sedgwick GG, et al. Mediator of DNA damage checkpoint 1 regulates mitotic progression. J. Biol. Chem. 2009;284:33939–33948. doi: 10.1074/jbc.M109.009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venturini L, You J, Stadler M, Galien R, Lallemand V, Koken MH, et al. TIF1gamma, a novel member of the transcriptional intermediary factor 1 family. Oncogene. 1999;18:1209–1217. doi: 10.1038/sj.onc.1202655. [DOI] [PubMed] [Google Scholar]
- 8.Peng H, Feldman I, Rauscher FJ., 3rd. Hetero-oligomerization among the TIF family of RBCC/TRIM domain-containing nuclear cofactors: a potential mechanism for regulating the switch between coactivation and corepression. J. Mol. Biol. 2002;320:629–644. doi: 10.1016/S0022-2836(02)00477-1. [DOI] [PubMed] [Google Scholar]
- 9.Klugbauer S, Rabes HM. The transcription coactivator HTIF1 and a related protein are fused to the RET receptor tyrosine kinase in childhood papillary thyroid carcinomas. Oncogene. 1999;18:4388–4393. doi: 10.1038/sj.onc.1202824. [DOI] [PubMed] [Google Scholar]
- 10.Ransom DG, Bahary N, Niss K, Traver D, Burns C, Trede NS, et al. The zebrafish moonshine gene encodes transcriptional intermediary factor 1 gamma, an essential regulator of hematopoiesis. PLoS Biol. 2004;2:E237, 1188–1196. doi: 10.1371/journal.pbio.0020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massague J. Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell. 2006;125:929–941. doi: 10.1016/j.cell.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 12.Dupont S, Zacchigna L, Cordenonsi M, Soligo S, Adorno M, Rugge M, et al. Germ-layer specification and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase. Cell. 2005;121:87–99. doi: 10.1016/j.cell.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Dupont S, Mamidi A, Cordenonsi M, Montagner M, Zacchigna L, Adorno M, et al. FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell. 2009;136:123–135. doi: 10.1016/j.cell.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 14.Shimwell NJ, Martin A, Bruton RK, Blackford AN, Gallimore PH, Turnell AS, et al. Adenovirus 5 early region 1A associates with insulin receptor substrates. Oncogene. 2009;28:686–697. doi: 10.1038/onc.2008.417. [DOI] [PubMed] [Google Scholar]
- 15.Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001;105:645–655. doi: 10.1016/s0092-8674(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 16.Miller JJ, Summers MK, Hansen DV, Nachury MV, Lehman NL, Loktev A, Jackson PK. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 2006;20:2410–20. doi: 10.1101/gad.1454006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansfeld J, Collin P, Collins MO, Choudhary JS, Pines J. APC15 drives the turnover of MCC-CDC20 to make the spindle assembly checkpoint responsive to kinetochore attachment. Nat. Cell Biol. 2011;13:1234–1243. doi: 10.1038/ncb2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt T, Luca FC, Ruderman JV. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J. Cell Biol. 1992;116:707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Fiore B, Pines J. How cyclin A destruction escapes the spindle assembly checkpoint. J. Cell Biol. 2010;190:501–509. doi: 10.1083/jcb.201001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matyskiela ME, Morgan DO. Analysis of activator-binding sites on the APC/C supports a cooperative substrate-binding mechanism. Mol. Cell. 2009;34:68–80. doi: 10.1016/j.molcel.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izawa D, Pines J. How APC/C-Cdc20 changes its substrate specificity in mitosis. Nat. Cell Biol. 2011;13:223–233. doi: 10.1038/ncb2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.den Elzen N, Pines J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 2001;153:121–136. doi: 10.1083/jcb.153.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, et al. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat. Cell Biol. 2006;8:607–614. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- 24.Wolthuis R, Clay-Farrace L, van Zon W, Yekezare M, Koop L, Ogink J, et al. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol. Cell. 2008;30:290–302. doi: 10.1016/j.molcel.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 25.Zeng X, Sigoillot F, Gaur S, Choi S, Pfaff KL, Oh DC, et al. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell. 2010;18:382–395. doi: 10.1016/j.ccr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 27.Stegmeier F, Rape M, Draviam VM, Nalepa G, Sowa ME, Ang XL, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- 28.Ge S, Skaar JR, Pagano M. APC/C- and Mad2-mediated degradation of Cdc20 during spindle checkpoint activation. Cell Cycle. 2009;8:167–171. doi: 10.4161/cc.8.1.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson J, Yekezare M, Minshull J, Pines J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat. Cell Biol. 2008;10:1411–1420. doi: 10.1038/ncb1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung E, Chen RH. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat Cell Biol. 2003;5:748–753. doi: 10.1038/ncb1022. [DOI] [PubMed] [Google Scholar]
- 31.D’Angiolella V, Mari C, Nocera D, Rametti L, Grieco D. The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 2003;17:2520–2525. doi: 10.1101/gad.267603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi W, Yu H. KEN-box-dependent degradation of the Bub1 spindle checkpoint kinase by the anaphase-promoting complex/cyclosome. J. Biol. Chem. 2007;282:3672–3679. doi: 10.1074/jbc.M609376200. [DOI] [PubMed] [Google Scholar]
- 33.Choi E, Choe H, Min J, Choi JY, Kim J, Lee H. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. EMBO J. 2009;28:2077–2089. doi: 10.1038/emboj.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricke RM, Jeganathan KB, van Deursen JM. Bub1 overexpression induces aneuploidy and tumor formation through Aurora B kinase hyperactivation. J. Cell Biol. 2011;193:1049–1064. doi: 10.1083/jcb.201012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan Y, Liu X, Kirschner MW. The anaphase-promoting complex mediates TGF-β signaling by targeting SnoN for destruction. Mol. Cell. 2001;8:1027–1039. doi: 10.1016/s1097-2765(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Fujita T, Wu G, Xiao X, Wan Y. Phosphorylation of APC/Cdc27 is involved in TGF-β signaling. J. Biol. Chem. 2011;286:10041–10050. doi: 10.1074/jbc.M110.205518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Kim AH, Yamada T, Wu B, Bilimoria PM, Ikeuchi Y, et al. A Cdc20-APC ubiquitin signaling pathway regulates presynaptic differentiation. Science. 2009;326:575–578. doi: 10.1126/science.1177087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massagué J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhry MA, Chodosh LA, McKenna WG, Muschel RJ. Gene expression profiling of HeLa cells in G1 or G2 phases. Oncogene. 2002;21:1934–1942. doi: 10.1038/sj.onc.1205264. [DOI] [PubMed] [Google Scholar]
- 40.Xu YX, Manley JL. New insights into mitotic chromosome condensation: a role for the prolyl isomerase Pin1. Cell Cycle. 2007;23:2896–2901. doi: 10.4161/cc.6.23.4977. [DOI] [PubMed] [Google Scholar]
- 41.Blackford AN, Patel RN, Forrester NA, Theil K, Groitl P, Stewart GS, et al. Adenovirus E4orf6 inhibits ATR activation by promoting TOPBP1 degradation. Proc. Natl. Acad. Sci. U S A. 2010;107:12251–12256. doi: 10.1073/pnas.0914605107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasti M, Grand RJA, Yousef YF, Shuen M, Mymryk JS, Gallimore PH, et al. Roles for APIS and the 20S proteasome in adenovirus E1A-dependent transcription. EMBO J. 2006;25:2710–2722. doi: 10.1038/sj.emboj.7601169. [DOI] [PMC free article] [PubMed] [Google Scholar]