Abstract
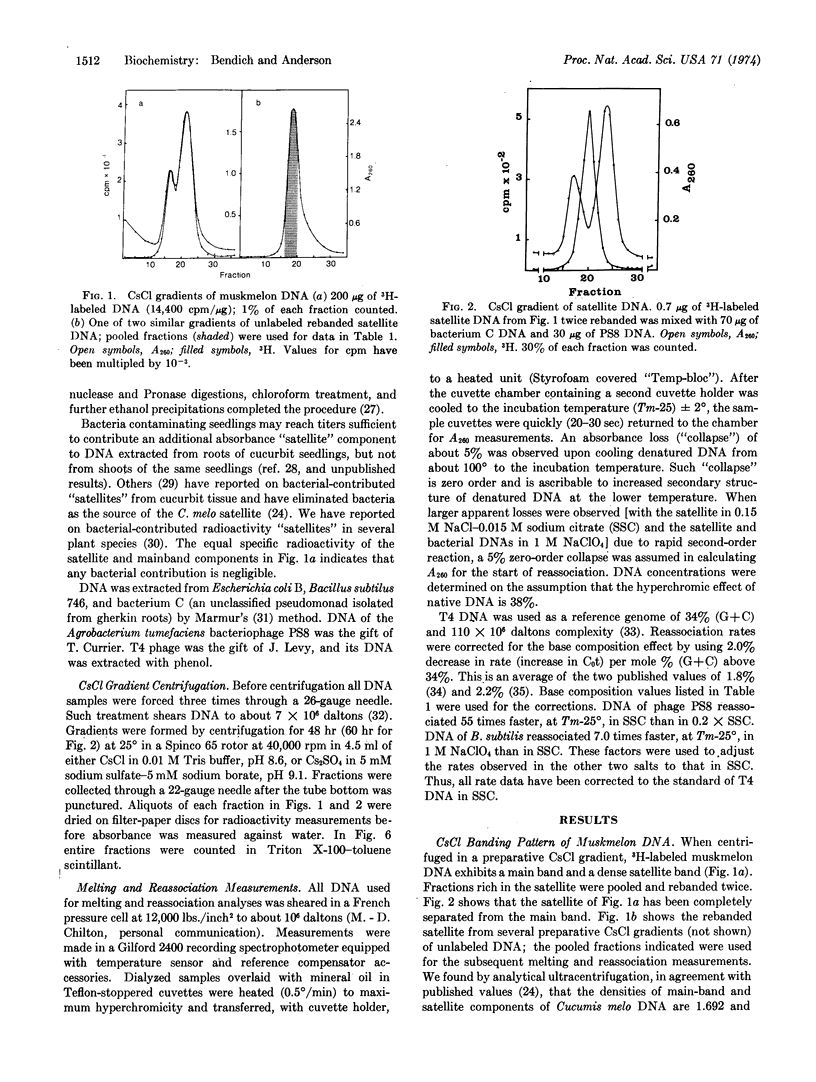
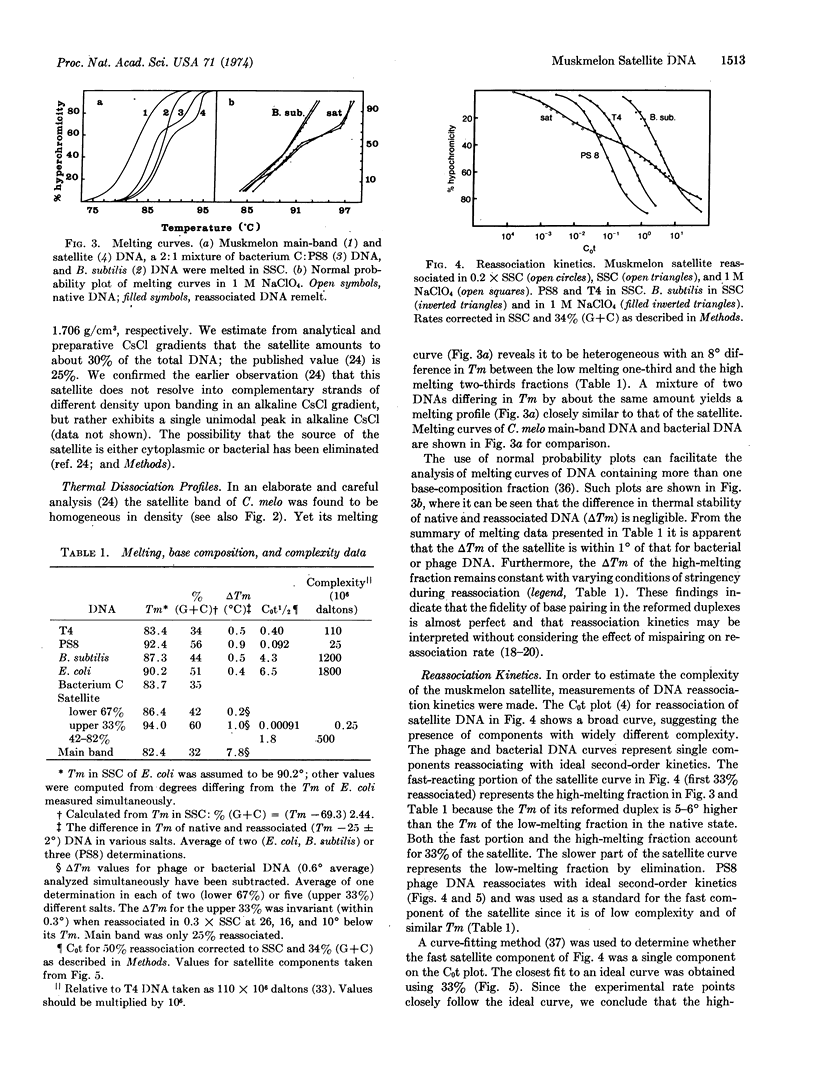
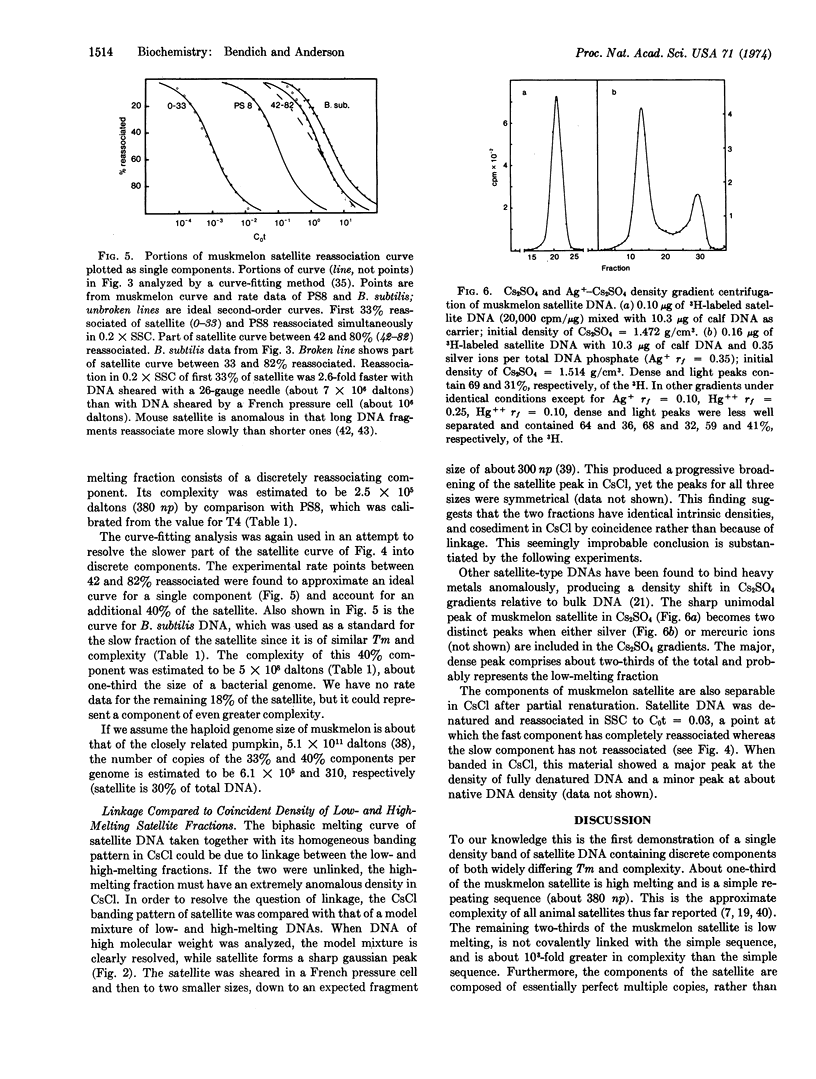
The purified dense satellite of muskmelon (Cucumis melo L.) represents 30% of the total DNA and forms a sharp, unimodal peak in both neutral and alkaline CsCl gradients. Spectrophotometric melting and DNA reassociation analyses revealed that one-third of the satellite is high melting and has a complexity of about 2.5 × 105 daltons, while the remaining two-thirds of the satellite melts 8° lower and has a complexity of about 5 × 108 daltons. The thermal stability of reassociated satellite DNA indicates that the multiple copies of the two melting fractions are essentially identical. The sharp, unimodal peak in Cs2SO4 becomes two distinct peaks when either silver or mercuric ions are included in the Cs2SO4 gradient
Keywords: melting curves, CsCl gradients, Ag+-Cs2SO4 gradients
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrighi F. E., Mandel M., Bergendahl J., Hsu T. C. Buoyant densities of DNA of mammals. Biochem Genet. 1970 Jun;4(3):367–376. doi: 10.1007/BF00485753. [DOI] [PubMed] [Google Scholar]
- Beattie W. G., Skinner D. M. The diversity of satellite DNAs of Crustacea. Biochim Biophys Acta. 1972 Oct 11;281(2):169–178. doi: 10.1016/0005-2787(72)90169-4. [DOI] [PubMed] [Google Scholar]
- Bendich A. J., Bolton E. T. Relatedness Among Plants as Measured by the DNA-Agar Technique. Plant Physiol. 1967 Jul;42(7):959–967. doi: 10.1104/pp.42.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendich A. J. Effect of contaminating bacteria on the radiolabeling of nucleic acids from seedlings: false DNA "satellites". Biochim Biophys Acta. 1972 Jul 31;272(4):494–503. doi: 10.1016/0005-2787(72)90504-7. [DOI] [PubMed] [Google Scholar]
- Bendich A. J., McCarthy B. J. DNA Comparisons among Barley, Oats, Rye, and Wheat. Genetics. 1970 Aug;65(4):545–565. doi: 10.1093/genetics/65.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendich A. J., McCarthy B. J. Ribosomal RNA homologies among distantly related organisms. Proc Natl Acad Sci U S A. 1970 Feb;65(2):349–356. doi: 10.1073/pnas.65.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Kram R., Schmid C. W., Hearst J. E. Isolation and chromosomal localization of highly repeated DNA sequences in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1125–1129. doi: 10.1073/pnas.68.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Weber C. S. Gene linkage by RNA-DNA hybridization. II. Arrangement of the redundant gene sequences for 28 s and 18 s ribosomal RNA. J Mol Biol. 1968 Jun 28;34(3):681–697. doi: 10.1016/0022-2836(68)90189-7. [DOI] [PubMed] [Google Scholar]
- Chilton M. D. Theoretical explanation of mouse satellite DNA renaturation kinetics. Nat New Biol. 1973 Nov 7;246(149):16–17. doi: 10.1038/newbio246016a0. [DOI] [PubMed] [Google Scholar]
- Corneo G., Ginelli E., Polli E. Different satellite deoxyribonucleic acids of guinea pig and ox. Biochemistry. 1970 Mar 31;9(7):1565–1571. doi: 10.1021/bi00809a014. [DOI] [PubMed] [Google Scholar]
- Corneo G., Ginelli E., Polli E. Repeated sequences in human DNA. J Mol Biol. 1970 Mar 14;48(2):319–327. doi: 10.1016/0022-2836(70)90163-4. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Amenson C. S., Britten R. J. General interspersion of repetitive with non-repetitive sequence elements in the DNA of Xenopus. J Mol Biol. 1973 Jun 15;77(1):1–23. doi: 10.1016/0022-2836(73)90359-8. [DOI] [PubMed] [Google Scholar]
- Duerksen J. D., McCarthy B. J. Distribution of deoxyribonucleic acid sequences in fractionated chromatin. Biochemistry. 1971 Apr 13;10(8):1471–1478. doi: 10.1021/bi00784a031. [DOI] [PubMed] [Google Scholar]
- Eckhardt R. A., Gall J. G. Satellite DNA associated with heterochromatin in Rhynchosciara. Chromosoma. 1971 Mar 16;32(4):407–427. doi: 10.1007/BF00285252. [DOI] [PubMed] [Google Scholar]
- Flamm W. G., McCallum M., Walker P. M. The isolation of complementary strands from a mouse DNA fraction. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1729–1734. doi: 10.1073/pnas.57.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Fry K., Poon R., Whitcome P., Idriss J., Salser W., Mazrimas J., Hatch F. Nucleotide sequence of HS-beta satellite DNA from kangaroo rat Dipodomys ordii. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2642–2646. doi: 10.1073/pnas.70.9.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G., Cohen E. H., Polan M. L. Reptitive DNA sequences in drosophila. Chromosoma. 1971;33(3):319–344. doi: 10.1007/BF00284948. [DOI] [PubMed] [Google Scholar]
- Graham D. E., Skinner D. M. Homologies of repetitive DNA sequences among Crustacea. Chromosoma. 1973;40(2):135–152. doi: 10.1007/BF00321459. [DOI] [PubMed] [Google Scholar]
- Hennig W., Walker P. M. Variations in the DNA from two rodent families (Cricetidae and Muridae). Nature. 1970 Mar 7;225(5236):915–919. doi: 10.1038/225915a0. [DOI] [PubMed] [Google Scholar]
- Hutton J. R., Wetmur J. G. Effect of chemical modification on the rate of renaturation of deoxyribonucleic acid. Deaminated and glyoxalated deoxyribonucleic acid. Biochemistry. 1973 Jan 30;12(3):558–563. doi: 10.1021/bi00727a032. [DOI] [PubMed] [Google Scholar]
- Hutton J. R., Wetmur J. G. Length dependence of the kinetic complexity of mouse satellite DNA. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1148–1155. doi: 10.1016/0006-291x(73)90620-7. [DOI] [PubMed] [Google Scholar]
- Ingle J., Pearson G. G., Sinclair J. Species distribution and properties of nuclear satellite DNA in higher plants. Nat New Biol. 1973 Apr 18;242(120):193–197. doi: 10.1038/newbio242193a0. [DOI] [PubMed] [Google Scholar]
- Jones K. W. Chromosomal and nuclear location of mouse satellite DNA in individual cells. Nature. 1970 Mar 7;225(5236):912–915. doi: 10.1038/225912a0. [DOI] [PubMed] [Google Scholar]
- Knittel M. D., Black C. H., Sandine W. E., Fraser D. K. Use of normal probability paper in determining thermal melting values of deoxyribonucleic acid. Can J Microbiol. 1968 Mar;14(3):239–245. doi: 10.1139/m68-040. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Physicochemical characterization of mitochondrial DNA from pea leaves. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1830–1834. doi: 10.1073/pnas.69.7.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird C. D. Chromatid structure: relationship between DNA content and nucleotide sequence diversity. Chromosoma. 1971 Mar 16;32(4):378–406. doi: 10.1007/BF00285251. [DOI] [PubMed] [Google Scholar]
- Laird C. D., McCarthy B. J. Molecular characterization of the Drosophila genome. Genetics. 1969 Dec;63(4):865–882. doi: 10.1093/genetics/63.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Siegel A. Hybridization of plant ribosomal RNA to DNA: the isolation of a DNA component rich in ribosomal RNA cistrons. Proc Natl Acad Sci U S A. 1967 Aug;58(2):673–680. doi: 10.1073/pnas.58.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Chromosomal localization of mouse satellite DNA. Science. 1970 Jun 12;168(3937):1356–1358. doi: 10.1126/science.168.3937.1356. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. L., Maio J. J. Studies on the intranuclear distribution and properties of mouse satellite DNA. Biochim Biophys Acta. 1968 Jun 18;161(1):76–93. doi: 10.1016/0005-2787(68)90296-7. [DOI] [PubMed] [Google Scholar]
- Seidler R. J., Mandel M. Quantitative aspects of deoxyribonucleic acid renaturation: base composition, state of chromosome replication, and polynucleotide homologies. J Bacteriol. 1971 May;106(2):608–614. doi: 10.1128/jb.106.2.608-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Base sequence and evolution of guinea-pig alpha-satellite DNA. Nature. 1970 Aug 22;227(5260):794–798. doi: 10.1038/227794a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Effects of sequence divergence on the reassociation properties of repetitive DNAs. Nat New Biol. 1971 Jul 21;232(29):82–83. doi: 10.1038/newbio232082a0. [DOI] [PubMed] [Google Scholar]
- Sutton W. D., McCallum M. Mismatching and the reassociation rate of mouse satellite DNA. Nat New Biol. 1971 Jul 21;232(29):83–85. doi: 10.1038/newbio232083a0. [DOI] [PubMed] [Google Scholar]
- Sutton W. D., McCallum M. Related satellite DNA's in the genus Mus. J Mol Biol. 1972 Nov 28;71(3):633–652. doi: 10.1016/s0022-2836(72)80028-7. [DOI] [PubMed] [Google Scholar]
- Thornburg W., Siegel A. Characteristics of the rapidly reassociating deoxyribonucleic acid of Cucurbita pepo L. and the sequences complementary to ribosomal and transfer ribonucleic acids. Biochemistry. 1973 Jul 3;12(14):2759–2765. doi: 10.1021/bi00738a032. [DOI] [PubMed] [Google Scholar]
- Walker P. M. "Repetitive" DNA in higher organisms. Prog Biophys Mol Biol. 1971;23:145–190. doi: 10.1016/0079-6107(71)90019-8. [DOI] [PubMed] [Google Scholar]
- Walker P. M. Origin of satellite DNA. Nature. 1971 Jan 29;229(5283):306–308. doi: 10.1038/229306a0. [DOI] [PubMed] [Google Scholar]
- Waring M., Britten R. J. Nucleotide sequence repetition: a rapidly reassociating fraction of mouse DNA. Science. 1966 Nov 11;154(3750):791–794. doi: 10.1126/science.154.3750.791. [DOI] [PubMed] [Google Scholar]
- Yasmineh W. G., Yunis J. J. Satellite DNA in calf heterochromatin. Exp Cell Res. 1971 Jan;64(1):41–48. doi: 10.1016/0014-4827(71)90190-x. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Yasmineh W. G. Satellite DNA in constitutive heterochromatin of the guinea pig. Science. 1970 Apr 10;168(3928):263–265. doi: 10.1126/science.168.3928.263. [DOI] [PubMed] [Google Scholar]