Abstract
The cessation of physical activity in rodents and humans initiates obesogenic mechanisms. The overall purpose of the current study was to determine how the cessation of daily physical activity in rats at 49–56 days of age and at 70–77 days of age via wheel lock (WL) affects adipose tissue characteristics. Male Wistar rats began voluntary running at 28 days old and were either killed at 49–56 days old or at 70–77 days old. Two cohorts of rats always had wheel access (RUN), a second two cohorts of rats had wheel access restricted during the last 7 days (7d-WL), and a third two cohorts of rats did not have access to a voluntary running wheel after the first 6 days of (SED). We observed more robust changes with WL in the 70- to 77-day-old rats. Compared with RUN rats, 7d-WL rats exhibited greater rates of gain in fat mass and percent body fat, increased adipocyte number, higher percentage of small adipocytes, and greater cyclin A1 mRNA in epididymal and perirenal adipose tissue. In contrast, 49- to 56-day-old rats had no change in most of the same characteristics. There was no increase in inflammatory mRNA expression in either cohort with WL. These findings suggest that adipose tissue in 70- to 77-day-old rats is more protected from WL than 49- to 56-day-old rats and responds by expansion via hyperplasia.
Keywords: childhood obesity, inactivity, juvenile, adiposity
increased childhood physical inactivity is associated with the rise in childhood obesity. Specifically, an increased prevalence of physical inactivity occurs as children age to adolescents in the U.S. (6, 26). While over 70% of girls and over 95% of boys 6 to 8 yr old meet the recommend daily activity guidelines of 60 min of moderate to vigorous activity daily, <15% of girls and <43% of boys 12 to 14 yr old meet the same requirements. In addition, the U.S. prevalence of obesity increases from 12.1% among children aged 2 through 5 yr to 18.4% among adolescents aged 12 through 19 yr old (27). Taken together, these statistics highlight a growing crisis in public health as children become less active, which coincides with fat gain during decisive periods of growth and development that include various ages of prepubertal growth.
Adipose tissue is now well-recognized as an endocrine organ capable of demonstrating an inflammatory/immune component (12, 30). Further, the function of adipose tissue depends on its localization (2) with visceral fat more closely associated with an increased risk of developing a metabolic syndrome (16, 43). Adipose tissue is highly plastic and responds rapidly to environmental changes and can expand by either hypertrophy (increase in the size of an adipocyte) or hyperplasia (increase in the number of adipocytes). It is believed that adipose tissue expansion by hypertrophy, especially visceral adipose tissue expansion, leads to an unhealthy, proinflammatory phenotype that is concurrent with the development of metabolic complications and is a risk factor for type 2 diabetes and cardiovascular disease (5, 8, 34, 39). Conversely, adipose tissue expansion via hyperplasia retains a healthier, anti-inflammatory phenotype that is associated with positive metabolic consequences including maintained insulin sensitivity and favorable blood lipids (36) even in the presence of obesity (15). Dulloo (5) has described large decreases in body weight followed by weight recovery during infancy, childhood, or adulthood. In most cases, body fat, specifically visceral fat, is recovered via hypertrophy at a disproportionately faster rate than lean tissue (termed “catch-up fat”). These concepts regarding fat gain are important to the childhood obesity conversation because puberty is a critical time of adipose tissue expansion (17) where the number of adulthood adipocytes is generally set (37). Taken together, the current dogma is that environmental events early in life influence adipocyte number later in life, adipose tissue distribution, and adipose tissue plasticity later in life (41). Still, insufficient information is known about the acute mechanisms behind underlying adipose tissue expansion at various ages of prepubertal growth.
Animal models that allow study of adipose tissue plasticity within the abdominal cavity in an inactivity context are invaluable in gaining an understanding into potential negative consequences of early childhood inactivity and fat gain. Our laboratory has successfully employed a “rodent wheel lock model” (abbreviated WL) where we allow prepubertal rats access to a voluntary running wheel for a period of time (4–6 wk) and then “lock” the wheel for 1 wk to study the effects of transitioning from high levels of physical activity to low levels of physical activity on adipose tissue expansion (18–20, 33). In essence, the environmental factor of physical activity is “knocked down,” analogous to knock out of a gene to study gene-environment interaction. The notion is somewhat similar to the concept of catch-up fat (4), whereby refeeding occurs after caloric restriction. Distinctively though, the genesis of enhanced adipose tissue growth differs between the WL and catch-up fat models. We speculate that there are subtle mechanistic differences between the two processes. The WL model increases voluntary physical activity and then decreases physical activity, implying decreased caloric expenditure, which suggests that a physical activity-induced metabolic change influences adipose metabolism. The “classical” catch-up fat model that decreases caloric intake and then increases caloric intake suggests a net caloric balance influence on adipose metabolism. Importantly, both models induce the trajectory in the gain of adipose tissue mass to turn upward.
Previous work in our laboratory showed that WL resulted in the following adipose tissue changes: 1) in 49-day-old male rats, 2 days of WL induced an increase in epididymal fat mass via hypertrophy (18), while 2) in 70-day-old male rats, 1 wk of WL resulted in the epididymal adipose tissue depot expansion via hyperplasia (20). As these differences remain unresolved, we hypothesized that this incongruity could be related to the onset of WL occurring at 49 days of age vs. 70 days of age. Therefore, to further understand the dynamic adaptation of fat during WL, the overall purpose of this experiment was to determine what role prepubertal age (49–56 days of age vs. 70–77 days of age) plays in affecting catch-up growth of adipose tissue mass and adipocyte size in three visceral depots when physically active male rats undergo 7 days of WL (7d-WL). The two specific purposes were to determine if WL in 49–56 days of age and/or 70–77 days of age differentially altered the following: 1) adipose tissue characteristics (mass and/or adipocyte size and adipocyte number); and 2) the mRNA expression of proinflammatory, hypoxic, adipokine, and adipogenic genes. Of note, age-matched sedentary controls (SED) and rats that had continual running wheel access (RUN) were also assayed to stratify WL animals between these two control groups. We hypothesized that 7 days of WL would 1) increase the rate of percent body fat growth and adipose depot masses to levels seen in age-matched SED rats in both ages of rats; 2) result in a visceral adipose depot expansion via hypertrophy; and 3) increase inflammatory mRNAs in adipose tissue before other selected categories of mRNAs.
METHODS
Animals and Experimental Design
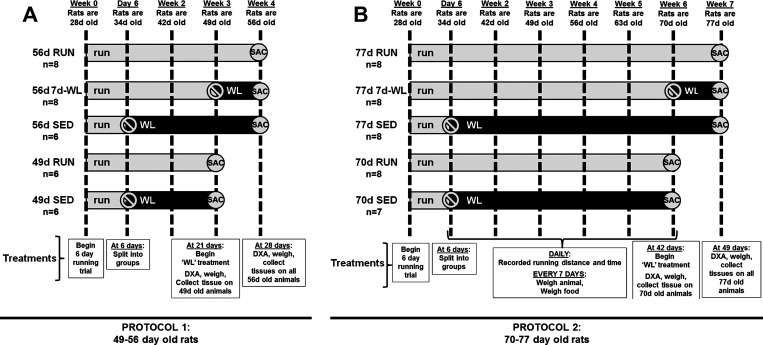
The University of Missouri Animal Care and Use Committee approved experimental protocols. Two similar protocols were employed at two distinct age groups: protocol 1, “49- to 56-day-old rats” and protocol 2, “70- to 77-day-old rats.” The rationale for this is that we have previously employed the WL model on two ages of rats with differing results on hyperplasia and hypertrophy of adipocytes (18, 20). In addition, these ages are considered prepubertal in rats (46) and were selected to mimic the rapid adipose growth phase observed in humans (17). Therefore, we performed WL on two ages, 49–56 days old and 70–77 days old to 1) match previous ages performed in our laboratory (18–20), and 2) determine if there was an age-related difference in characteristics of adipocyte enlargement in response to WL. In addition, a power test was performed (power = 0.9, alpha = 0.05) for major outcome variables (%body fat, body weight, fat depot weight, and adipocyte diameter), and a sample size of six was appropriate.
Protocol 1.
34 male, Wistar rats were randomly selected from a population of Wistar rats bred in our laboratory (Fig. 1A). These rats were selected from rats that run high distances from an ongoing experiment in our laboratory (32). The rats were weaned at 21 days of age and housed as pups in a single cage. At 28 days of age, rats were placed into individually housed cages with a voluntary running wheel equipped with a Sigma Sport BC 800 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA) to select the highest distance runners in an experiment whose primary outcome was selective breeding of high voluntary distances (44). After 6 days, 12 rats were randomly selected to be sedentary for the remainder of the experiment for 21 or 28 days (49d SED and 56d SED), were removed from the cages with voluntary running wheels, and were individually housed in standard rat cages without voluntary running wheels, respectively (note that the first number in the group name refers to age of rats). The remaining 22 rats were randomly assigned to running groups (49d RUN, 56d RUN, and 56d 7d-WL) and remained in cages with voluntary running wheels (note that 7d-WL in the group name refers to 7 days of wheels being locked for the final 7 days of the treatment period). At the end of week 3, eight rats were randomly selected to undergo WL, whereby a stainless steel rod was placed through the running wheel, thus prohibiting the running wheel from turning (56d 7d-WL). All rats were housed in the same temperature-controlled animal room (21°C) and maintained on a 12-h light (0700–1900), 12-h (1900–0700) dark cycle. All rats had ad libitum access to standard rodent chow (Formulab 5008; Purina Mills, St. Louis, MO) and water. Running activity, as determined by distance and time ran during the previous 24 h, was recorded daily. Animal cages were changed every 7 days at which time body weight and weekly food intake were recorded. This protocol had been used previously in our laboratory (18).
Fig. 1.
Experimental design. Experimental groups are named by the age at death and treatment. For example, 56d RUN was killed at 56 days of age and was part of the voluntary running group. Dashed lines separate each week and indicate time points that body weight and food intake were recorded. Light horizontal gray bars represent access to a voluntary running wheel. Black horizontal bars represent wheel-lock and/or no access to a voluntary running wheel. Rats in both protocols began treatment at the same age (28 days old). A: protocol 1 examining 49- to 56-day-old rats. 56d RUN: rats had access to a voluntary running wheel for 4 wk. 56d 7d-WL: rats had access to a voluntary running wheel for 3 wk then the wheel was locked for the final 7 days. 56d SED: rats had access to a voluntary running wheel for 6 days and then were housed in a cage without a voluntary running wheel through wk 2–4. 49d RUN: rats had access to a voluntary running wheel for 3 wk. 49d SED: rats had access to a voluntary running wheel for 6 days and then were housed in a cage without a voluntary running wheel for 2 wk through week 3. 49d RUN and 49d SED rats were killed after 3 wk of treatment (49 days old) and were immediately dual-energy X-ray absorptiometry (DXA) scanned and subsequently had fat pads removed for phenotyping. 56d RUN, 56d 7d-WL, and 56d SED were killed after 4 wk of treatment (56 days old) and were immediately DXA scanned and subsequently had fat pads removed for phenotyping. B: protocol 2, 70- to 77-day-old rats. 77d RUN: rats had access to a voluntary running wheel for 7 wk. 77d 7d-WL: rats had access to a voluntary running wheel for 6 wk then the wheel was locked for the remaining 7 days. 77d SED: rats had access to a voluntary running wheel for 6 days then were housed in a cage without a voluntary running wheel through weeks 2–7. 70d RUN: rats had access to a voluntary running wheel for 6 wk. 70d SED: rats had access to a voluntary running wheel for 6 days and then were housed in a cage without a voluntary running wheel for 5 additional weeks through weeks 2–6. 70d RUN and 70d SED rats were killed after 6 wk of treatment (70 days old) and were immediately DXA scanned and subsequently had fat pads removed for phenotyping. 77d RUN, 77d 7d-WL, and 77d SED were killed after 7 wk of treatment (77 days old) and were immediately DXA scanned and subsequently had fat pads removed for phenotyping.
Protocol 2.
40 male, Wistar rats were randomly selected from the same population of Wistar rats bred in our laboratory as in protocol 1 (Fig. 1B). The design is the same as protocol 1 except for duration of wheel running being 3 wk longer, the day of WL (day 70), and day of death (day 77) (see Fig. 1B for details). In short, rats in protocol 2 began treatment at the same age as rats in protocol 1, but were 3 wk older at the time of WL and death and thus were exposed to voluntary running wheels (70d RUN, 77d RUN, and 77d 7d-WL) or no voluntary running wheels (70d SED and 77d SED) 3 wk longer than the rats in protocol 1. During the final week, food intake for 77d 7d-WL was measured daily. This protocol had been used previously in our laboratory (18, 20).
Body Composition Analysis Using Dual-Energy X-Ray Absorptiometry
In both protocols, 7 days before death and on the day of death between 1000 and 1200, rats were weighed and whole body composition was measured by a dual-energy X-ray absorptiometry (DXA) machine calibrated for rats (QDR 4500A; Hologic, Bedford, MA). Seven days before death, rats not being killed that day were anesthetized with isofluorine before the DXA scan, weighed, and returned to their cages, while the remaining groups were killed before the DXA scan. All scans on the day of death were performed immediately after CO2 asphyxiation and immediately before tissue removal.
Animal Death
Immediately after CO2 asphyxiation between 1000 and 1200, rats were weighed and DXA scanned as mentioned previously. Four fat pads were collected in protocols 1 and 2: omental adipose tissue (OMAT), epididymal adipose tissue (EAT), perirenal adipose tissue (PRAT), and subcutaneous adipose tissue (SAT). The entire OMAT, EAT, and PRAT depots were removed and weighed, while a portion of umbilical/groin SAT was collected. A section of each fat depot was snap-frozen in liquid nitrogen and stored at −80°C for real-time RT-PCR mRNA analysis, and another section was saved for adipocyte sizing.
RT-PCR for Adipose Tissue mRNA Expression Patterns
Approximately 100 mg frozen AT were homogenized in 1 ml RNA lysis buffer (Tri Reagent; Sigma) using a high-speed shaking device with a stainless steel bead at 30 Hz for 1 min (Tissuelyser LT; Qiagen, Valencia CA). RNA was isolated using the method according to manufacturer's instructions (Tri Reagent; Sigma) and lack of RNA degradation was verified on a 1% agarose gel. 1 μg of RNA was DNase-treated using DNase I (Thermo Scientific, Glen Burnie, MD) and reverse transcribed using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA). Gene-specific primers were constructed (Table 1), and primer efficiency curves were produced for all genes. Primer efficiencies ranged between 90 and 110% for all genes. Twenty-five nanograms of cDNA were assayed (NanoDrop 1000; ThermoScientific) from each sample in duplicate for the targets listed in Table 1 using SYBR green chemistry (Power SYBR green Mastermix; Applied Biosystems). mRNA expression values are presented as 2ΔΔCT whereby ΔCT = B2M CT − gene of interest CT and were normalized to 49d RUN subcutaneous adipose tissue.
Table 1.
RT-PCR primers
| Target | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| Inflammatory | ||
| TNF-α | CCCAGAAAAGCAAGCAACCA | CCTCGGGCCAGTGTATGAGA |
| IL-6 | AGTGGCTAAGGACCAAGACCATCCA | GGCATAGCACACTAGGTTTGCCGAG |
| MCP-1 | CTGTCTCAGCCAGATGCAGTTAA | AGCCGACTCATTGGGATCAT |
| Hypoxia | ||
| HIF1-α | GCTGCCTCTTCGACAAGCTT | CGCTGGAGCTAGCAGAGTCA |
| VEGF | GGAGGATGTCCTCACTTGGA | CAAACAGACTTCGGCCTCTC |
| Adipokine | ||
| Leptin | CGCTTCCTGTGGCTTTGGTC | CCCACTGCGTGTGTGAAATG |
| Adiponectin | TCCCTCCACCCAAGGAAACT | GGCCCGGTATCCCATTG |
| Proliferation | ||
| Cyclin A1 | TGCCTGAGTGAGCTGCATAAA | TGCTTGCTGCGGTCGAT |
| CDK2 | ACTAAACCAGTGCCCCACCTT | ACCACAGGTGAAGAGGGCTTT |
| Differentiation | ||
| PPAR-γ | AAGTCCCAGTCGCTGACAAAGT | TGTCAGATTTTTTTCCCCTCAAA |
| C/EBPα | ATAAAGCCAAACAGCGCAAC | CGGTCATTGTCACTGGTCAA |
| Housekeeping | ||
| B2M | TGCTACGTGTCTCAGTTCCA | GCTCCTTCAGAGTGACGTGT |
TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; HIF-1α, hypoxia-inducible factor-1α subunit; VEGF, vascular endothelial growth factor; CDK2, cyclin-dependent kinase 2; PPAR-γ, peroxisome proliferator-activated receptor-γ; C/EBPα, CCAAT/enhancer binding protein-α.
Adipocyte Sizing and Number
Adipocyte sizing was performed on EAT in protocol 1 and on three visceral AT depots: OMAT, EAT, and PRAT in protocol 2 using techniques described previously (20). Briefly, three 60–80 mg adipose tissue fragments per depot per animal were placed in a glass scintillation tube containing 3 ml Krebs-Ringer-HEPES buffer (KRBH; 130 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 10 mM HEPES, 1 mM CaCL2, 1.2 mM MgSO4, and 0.25 mg/ml free fatty acid-free BSA, pH 7.4). Fragments were subsequently minced into ∼1-mm3 pieces, incubated with 0.920 Wunsch units of Liberase Blendzyme (Roche) in a 37°C incubator at 100 rpm for 1 h, and then transferred through a sterile 200-μm nylon mesh (Sefar America, Kansas City, MO) into a 15-ml conical tube. After centrifugation at 100 rpm for 1 min, the adipocytes were loosely packed at the top of the liquid and the bottom layer of liquid was removed using a syringe and needle. The adipocytes were washed with ∼5 ml KRBH then resuspended in 5 ml KRBH for every 1 ml of loosely packed adipocytes. A random sample of the adipocytes was viewed with a Nikon Eclipse E600 microscope and photographed by an Olympus DP72 camera. The diameters of >300 adipocytes were measured using DP2-BSW v2.1 software. The mean diameter and size distribution were calculated for each adipocyte depot.
The number of adipocytes per depot was calculated with the mass of the fat depot, a density conversion factor (0.915 ng·lipid−1·1 pl−1), and average diameter of adipocytes (24). Briefly, the average cell mass was calculated by: average cell size (pl) × 0.915 (ng lipid/1 pl) × g/109 ng. The number of cells per depot was calculated as: mass of adipose depot (g)/average mass of adipocyte (g). Reliability of this method is described in detail (24) and has been used by others (13).
Statistical Analysis
Outcome measures for between-group or within-group comparisons (adipose depot mass, adipocyte size, adipocyte size distribution, and RT-PCR) were analyzed with a one-way ANOVA. For outcome measures that had the same measures on the same animal (weekly body weight, daily distance run, and weekly food intake), a two-way ANOVAQ was performed (group × time). Significant main effects (P < 0.05) were followed up with Fisher's least significant difference post hoc comparisons. Sigmaplot 12.0 (San Jose, CA) was used for all statistical analyses. Values are reported as means ± SE, and significance for all tests was set at P < 0.05. Exceptions to these statistical methods are stated in figure legends.
RESULTS
Daily Running Distances
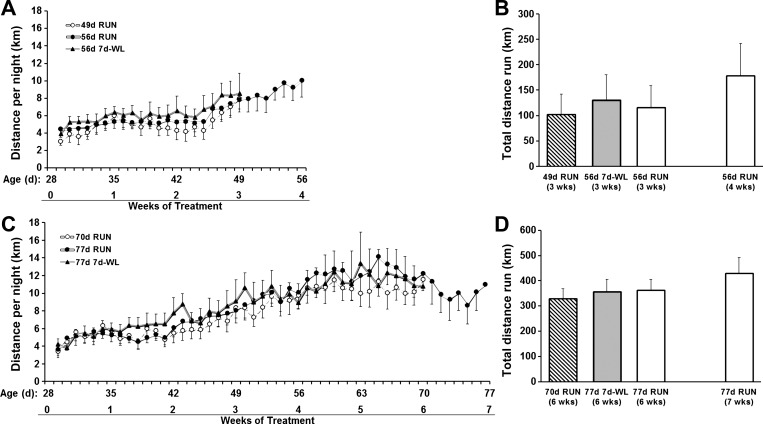
RUN only (77d RUN) and RUN followed by 7d-WL groups (hereafter referred to as 7d-WL) ran similar distances during the treatment period (Fig. 2). All 70- to 77-day-old groups (70d RUN, 77d 7d-WL, and 77d RUN) appeared to reach a plateau in nightly running distance by the 4th week of running.
Fig. 2.
Daily running distance. A and B: protocol 1, 24-h and total running distance in 49- to 56-day-old rats. C and D: protocol 2, 24-h and total running distance in 70- to 77-day-old rats. There were no differences in daily distances run between groups at any time point. B: there was no difference in total 3-wk distance run between groups, and there was no difference between total 4-wk distances run by the 56d RUN group compared with distances run at 3 wk of treatment. D: there was no difference in total 6-wk distance run among groups, and there was no difference between total 7-wk distances run by the 77d RUN group compared with distances run at 6 wk of treatment.
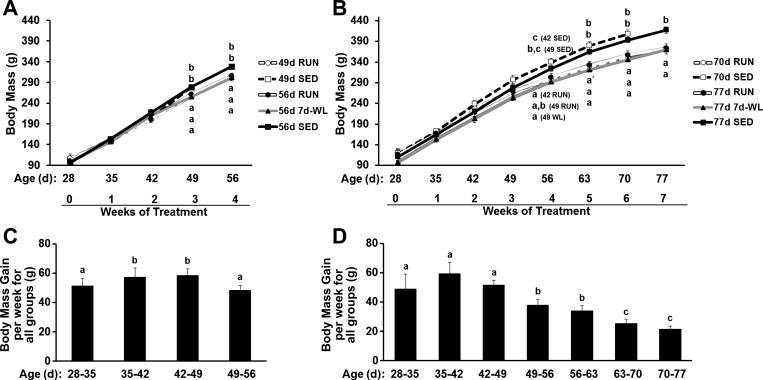
Weekly Body Weights
Gains in body weight expressed as grams gained per week for all groups are consistent through 49 days of age in both age group of rats then decrease through 77 days of age (Fig. 3, C and D). Importantly, in 49- to 56-day-old rats, WL was performed before any notable drop in grams gained per week. In contrast, in 70- to 77-day-old rats, WL was performed at an age where grams gained per week had significantly decreased. Body mass growth during WL (56d 7d-WL) between the ages of 49 and 56 days paralleled growth in the 56d RUN and 56d SED groups (Figs. 3A and 4A). However, in 3-wk older rats, body mass growth during WL (77d 7d-WL) no longer paralleled 77d RUN and 77d SED (Figs. 3B and 4E).
Fig. 3.
Weekly body mass. A and C: 49- to 56-day-old rats. B and D: 70- to 77-day-old rats. A and B: weekly body weights. C and D: each bar represents the mean weekly weight gain for all rats. All rats were collapsed into 1 group for each week, and changes in body weight for each week were calculated for each group. The lowercase letters “a” in A and B represent 49d RUN, 56d RUN, and 56d 7d-WL groups, while the lowercase letters “b” and “c” represent 49d SED and 56d SED. The SED groups were significantly heavier than the WL and RUN groups. Different letters indicate significant differences among groups at the same time point, P < 0.05.
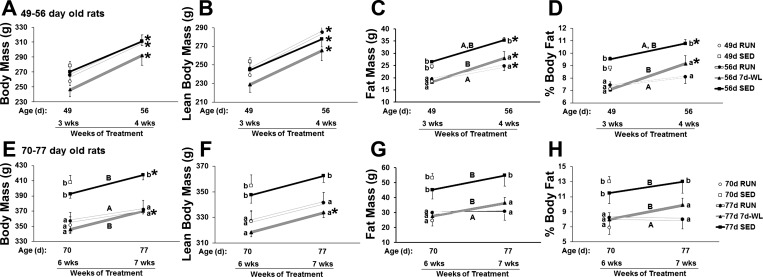
Fig. 4.
Body mass, DXA lean body mass, DXA fat mass, and DXA percent body fat. A-D: 49- to 56-day-old rats. E–H: 70- to 77-day-old rats. Different lowercase letters denote among group differences at the same time point, P < 0.05. Different uppercase letters denote differences in the change of weight gain (i.e., differences line slopes) among groups, P < 0.05. *P < 0.05, differences within groups from 3–4 wk of treatment (A-C) and from 6–7 wk of treatment (D–F).
Body Mass, DXA Lean Body Mass, DXA Fat Mass, and DXA Percent Body Fat During WL
WL rats showed an increased rate of fat mass gain and body fat percent gain compared with RUN rats in both 49- to 56 day-old rats (Fig. 4, C and D) and 70- to 77-day-old rats (Fig. 4, G and H), so that the slope of gain during WL was similar to SED. It is notable that the slope of percent body fat was essentially flat between both 49 and 56 days and 70 and 77 days in 56d RUN and 77d RUN vs. the respective upward slopes of WL (Fig. 4, D and H), and gains in percent body fat were attenuated in 56d RUN rats compared with 56d 7d-WL and 56d SED. WL did not affect lean body masses in either age group (Fig. 4, B and F).
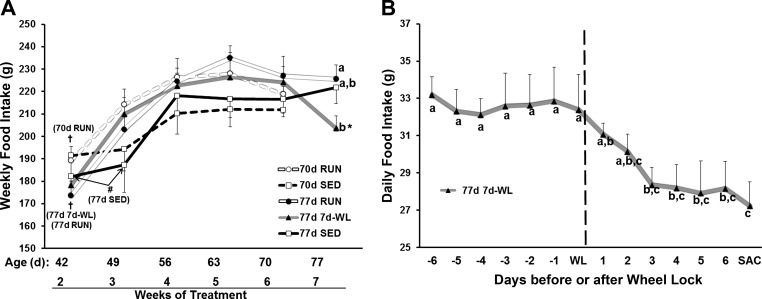
Weekly Food Intake in 70- to 77-Day-Old Rats
Apart from week 7, there was no activity effect in weekly food intake (Fig. 5A). All groups reached a relative steady-state in food intake by week 4 and, with the exception of 77d 7d-WL, remained constant through week 7. Three days after WL, 77d 7d-WL rats decreased food intake and maintained this level for the remaining 4 days (Fig. 5B), indicating a rapid response in appetite to decreased activity, as food intakes were tending to show progressive drops already on 1st and 2nd days of WL.
Fig. 5.
Weekly food intake in 70- to 77-day-old rats. A: weekly food intake for 70- to 77-day-old rats. There were no differences among groups at any time point except at week 7 in which the 77 7d-WL daily food intake decreased by the third day of WL. When within-group comparisons were performed, week 2 was lower than all other weeks in the 70d RUN, 77d RUN, and 77d 7d-WL groups (†P < 0.05). In 77d SED rats, weeks 2 and 3 were lower than all other weeks (#P < 0. 05). In 77d 7d-WL rats, week 7 was lower than weeks 4–6 (*P < 0.05). B: in 77d 7d-WL rats, daily food intake is plotted 7 days before and after WL (weeks 5–7). Vertical dashed line represents the day of WL. Different letters denote significance at P < 0.05.
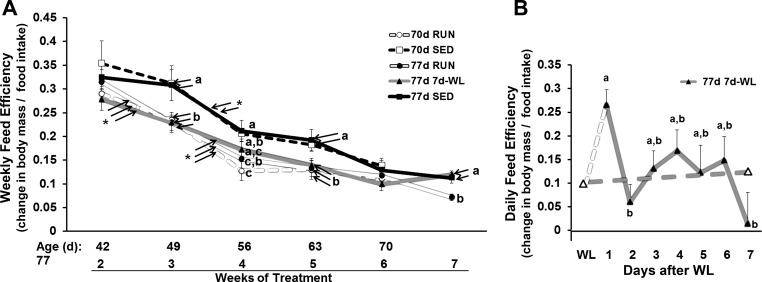
Feed Efficiency in 70- to 77-Day-Old Rats
The 77d SED rats had greater weekly feed efficiency during weeks 3, 5, and 7 compared with RUN rats, but WL increased weekly feed efficiency to SED levels (Fig. 6A), despite a rapid decrease in food intake (Fig. 5B). Day-to-day feed efficiency during WL showed a transient 1-day increase in feed efficiency at day 1 of WL followed by an abrupt decrease and plateau in feed efficiency to pre-WL levels from 2 to 7 days of WL (Fig. 6B), which highlights the impact a sudden decrease in activity has on energy homeostasis.
Fig. 6.
Feed efficiency in 70- to 77-day-old rats. Feed efficiency was calculated as: change in body weight (g)/food consumed (g). A: weekly feed efficiency is plotted for 70–77 day-old rats. For among-group comparisons (feed efficiency at each given week), different letters indicate significant differences, P < 0.05. For within group comparisons (changes in feed efficiency from 1 wk to the next), *P < 0.05 indicates significance. B: daily feed efficiency for 77d 7d-WL rats. Solid gray line represents daily feed efficiency. Dashed gray line between the open triangles represents weekly feed efficiency and is the same as the gray line (77d 7d-WL) in A between weeks 6 and 7. Dashed white line represents the estimated change in feed efficiency from the day of WL to 1d after WL. Daily food intake was not measured 1 day before WL (Δ), so a precise calculation of this time point is not available. Different letters represent differences among days, P < 0.05.
Adipose Tissue Depot Mass
The 49- to 56-day-old rat adipose tissue mass increased independent of voluntary running activity level (49d RUN < 56d RUN and 49d SED < 56d SED; Fig. 7, A–C), suggesting that these rats are “growing” in terms of adipose depot mass whether they are physically active or not. However, WL did not increase adipose depot mass (56d RUN = 56d 7d-WL) in any of the three depots (Fig. 7, A–C). In contrast, the 3-wk older 70- to 77-day-old rat adipose tissue mass reached a growth plateau and did not significantly increase between 70–77 days of age (70d RUN = 77d RUN and 70d SED = 77d SED; Fig. 7, E and F), indicating that these rats are not “growing” in terms of adipose depot mass like the 49- to 56-day-old rats are. Nonetheless, WL induced mass gains between 70 and 77 days (77 RUN < 7 WL) in EAT and PRAT (Fig. 7, D and E). Taken together, these data suggest to us that the 70- to 77-day-old rats adipose tissue growth curve had reached an asymptote and is increasing less rapidly. However, if physical activity levels suddenly drop (i.e., WL), adipose depot masses increase more than RUN in these 70- to 77-day-old rats.
Fig. 7.
Adipose tissue depot mass. A–C: 49- to 56-day-old rats. D–F: 70- to 77-day-old rats. Different letters indicate differences between groups within each adipose tissue depot, P < 0.05.
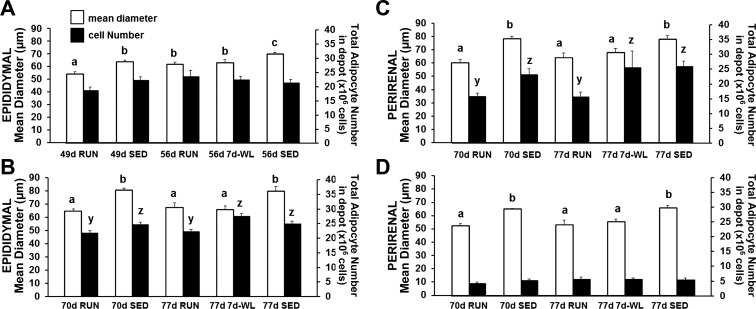
Adipocyte Diameter
Adipocyte diameter data trends follow depot mass trends. The 49- to 56-day-old rat EAT adipocytes appear to expanding via hypertrophy independent of activity level (49d RUN < 56d RUN and 49d SED < 56d SED; Fig. 8A), and WL did not affect this (56d RUN = 56d 7d-WL; Fig. 8A). In contrast, 70- to 77-day-old RUN and SED rat adipocyte diameter reached a statistical plateau (70d RUN = 77d RUN and 70d SED = 77d SED; Fig. 8, B–D). However, WL increased adipocyte diameter (77d RUN < 77d 7d-WL) in EAT and PRAT (Fig. 8, B and C).
Fig. 8.
Mean adipocyte diameter and total adipocyte number. White bars represent adipocyte diameter and dark bars represent total estimated cell number. A: 49- to 56-day-old rats. B–D: 70–77 day-old rats. Different letters indicate differences among groups within a given adipose tissue depot: a and b compare mean adipocyte diameter; y and z compare adipocyte number, P < 0.05.
Total Adipocyte Number in Depot
WL did not affect the normal increase in total adipocyte number in EAT in 49- to 56-day-old rats (56d RUN = 56d 7d-WL; Fig. 8A), emphasizing that the increased total number of adipocytes between 49d RUN and 56d 7d-WL was not due to WL, it was due to maturation. In 70- to 77-day-old rats, total adipocyte number reached a statistical plateau in all three adipose tissue depots (70 RUN = 77 RUN and 70 SED = 77 SED; Fig. 8, B–D). However, WL increased total adipocyte number in EAT and PRAT (77 RUN < 77 WL) in EAT and PRAT (Fig. 8, B and C).
Taken together, the adipocyte depot mass, adipocyte diameter, and total adipocyte number in an adipose tissue depot data suggest that 1) adipose tissue in 49- to 56-day-old rats is still expanding within this 7 day age range while it does not expand in 70- to 77-day-old rat age range; and 2) WL does not alter this adipose tissue expansion in 49- to 56-day-old rats, while WL does by increasing adipose tissue depot mass and total adipocyte number in 70- to 77-day-old rat age range.
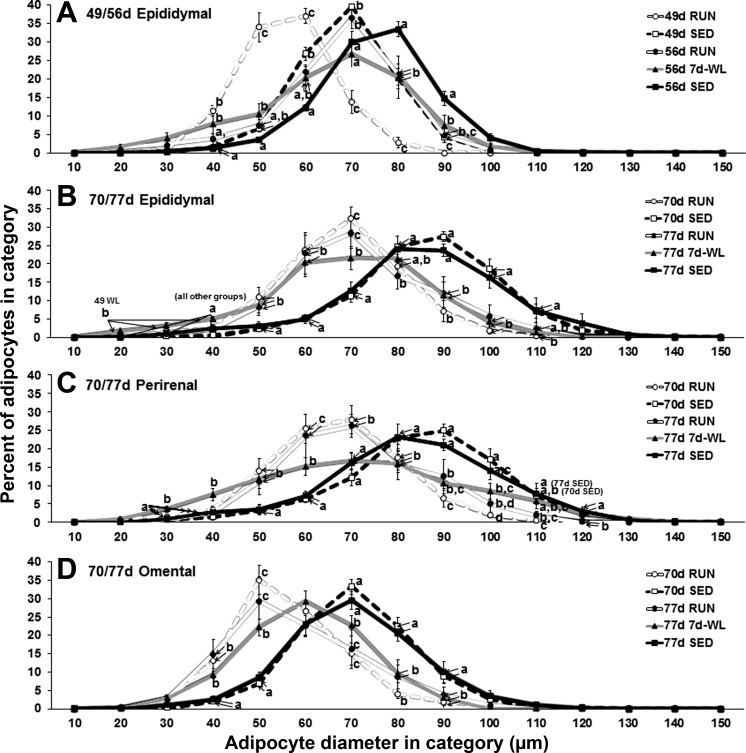
Adipocyte Size Distribution
Activity and age affect adipocyte size distribution, and WL alters the adipocyte size distribution in EAT and PRAT depots. In 49- to 56-day-old rat EAT, there is a rightward shift indicating a larger mean adipocyte value in the 56d 7d-WL group that parallels the shift seen in the 56 RUN group, but there are two distinct differences: 1) there is a “flattening out” of the 56d 7d-WL curve such that the peak is lower than 56 RUN, and 2) 56d 7d-WL has more adipocytes in the 40-μm category than 56d RUN (Fig. 9A), a finding that may indicate the formation of smaller adipocytes via hyperplasia is occurring. In contrast, the rightward shift in EAT is absent in 70- to 77-day-old rats within the 77d 7d-WL group, but there are more “small” adipocytes at 20-, 30-, and 40-μm categories (Fig. 9B); again, this being a finding that may indicate the formation of smaller adipocytes via hyperplasia is occurring. Similar to 70- to 77-day-old rat EAT, PRAT also shows a flattening out of the 77d 7d-WL curve with more smaller adipocytes at 30- and 40-μm categories (Fig. 9C). The 77d 7d-WL OMAT shows a rightward shift but little to no flattening out (Fig. 9D).
Fig. 9.
Adipocyte size distribution. Adipocyte diameters were classified in 10-μm categories (10: 0.0–9.9 μm, 20: 10.0–19.9 μm, 30: 20.0–29.9 μm, etc.) and the percentage of total adipocytes in a sample was plotted for each category. At least 300 adipocyte diameters were measured for each tissue sample. Different letters denote differences among groups at each category, P < 0.05.
Apart from the WL effect described above, there was a physical activity effect and age effect on adipocyte distribution. In both 49- to 56-day-old rats and the 70- to 77-day-old rats, SED rats showed a rightward shift in the peak percentage adipocyte diameter such that for all age-matched groups RUN < SED (Fig. 9). Objectively, the mean adipocyte diameter was greater in SED compared with RUN. Subjectively, the curve is visually shifted to the right in SED compared with RUN. In the 49- to 56-day-old rats, a rightward shift in the peak percentage from 49 days of age to 56 days of age was observed in both RUN and SED so that 49 RUN < 56 RUN and 49 SED < 56 SED (Fig. 9A), implying that at this age adipocytes are still in a growth phase, and this growth occurs independent of activity level. In contrast, this rightward shift is not present in the 70- to 77-day-old rats: 70d RUN = 77d RUN and 70d SED = 77d SED (Fig. 9, B–D) implying that the adipocyte growth has reached a steady-state at 70 to 77 days of age.
WL Increased Cyclin A1 mRNA Expression to SED Levels in 70- to 77-Day-Old Rats EAT, PRAT, and SAT But Not in OMAT
There was a physical activity effect on cyclin A1 mRNA levels in 70- to 77-day-old rats such that RUN expressed less cyclin A1 mRNA than SED and 77d 7d-WL in EAT, PRAT, and SAT, but not OMAT (Table 2). These data suggest that voluntary wheel running may act as a “brake” that attenuates expression of this proliferation gene in some, but not all, fat depots, and removing this brake via extraneous inactivity increases a marker of adipocyte hyperplasia, cyclin A1 (1). There were no differences in cyclin A1 mRNA in 49- to 56-day-old rats, suggesting that adipose tissue depots are still in the process of expanding and new adipocytes are still being formed regardless of physical activity level. In addition to cyclin A1 mRNA increases, WL increased hypoxia-inducible factor-1α mRNA to 77d SED levels in EAT and paradoxically decreased IL-6 mRNA of 70d RUN rats in PRAT.
Table 2.
RT-PCR results
| 70d RUN | 70d SED | 77d RUN | 77d 7d-WL | 77d SED | |
|---|---|---|---|---|---|
| Omental | |||||
| TNF-α | 1.00 ± 0.28 | 1.26 ± 0.51 | 0.75 ± 0.16 | 0.68 ± 0.06 | 0.95 ± 0.15 |
| IL-6 | 1.00 ± 0.41a | 2.49 ± 0.40b | 0.72 ± 0.15a | 0.64 ± 0.14a | 2.22 ± 0.40b |
| MCP-1 | 1.00 ± 0.19 | 1.08 ± 0.17 | 1.08 ± 0.16 | 1.48 ± 0.38 | 1.79 ± 0.43 |
| HIF-1α | 1.00 ± 0.14a | 1.53 ± 0.21a | 1.08 ± 0.12a | 0.93 ± 0.13a | 1.43 ± 0.27b |
| VEGF | 1.00 ± 0.25 | 0.88 ± 0.21 | 1.05 ± 0.15 | 0.69 ± 0.16 | 1.23 ± 0.26 |
| Leptin | 1.00 ± 0.22a,b | 1.53 ± 0.20a | 0.69 ± 0.19b | 0.75 ± 0.19b | 1.51 ± 0.25a |
| Adiponectin | 1.00 ± 0.15 | 0.61 ± 0.11 | 0.79 ± 0.16 | 0.66 ± 0.14 | 0.78 ± 0.27 |
| Cyclin A1 | 1.00 ± 0.15 | 0.85 ± 0.37 | 0.93 ± 0.34 | 0.63 ± 0.15 | 0.92 ± 0.30 |
| CDK2 | 1.00 ± 0.10 | 0.81 ± 0.10 | 1.06 ± 0.11 | 0.96 ± 0.09 | 1.15 ± 0.26 |
| PPAR-γ | 1.00 ± 0.22a | 0.53 ± 0.07b,c | 0.68 ± 0.12a,c | 0.43 ± 0.09b,c | 1.07 ± 0.18a |
| C/EBPα | 1.00 ± 0.22 | 0.82 ± 0.16 | 0.66 ± 0.11 | 0.69 ± 0.21 | 1.41 ± 0.39 |
| Epididymal | |||||
| TNF-α | 1.00 ± 0.11 | 1.83 ± 0.70 | 1.15 ± 0.22 | 1.88 ± 0.38 | 1.21 ± 0.26 |
| IL-6 | 1.00 ± 0.13a | 3.65 ± 0.68b | 2.03 ± 0.34a | 1.40 ± 0.33a | 2.58 ± 0.64a,b |
| MCP-1 | 1.00 ± 0.13a | 2.03 ± 0.41b | 1.21 ± 0.14a | 1.64 ± 0.21a,b | 1.45 ± 0.23a,b |
| HIF-1α | 1.00 ± 0.05a | 2.16 ± 0.19b | 0.98 ± 0.17a | 1.96 ± 0.27b | 1.72 ± 0.32b |
| VEGF | 1.00 ± 0.07 | 0.72 ± 0.10 | 1.43 ± 0.21 | 0.94 ± 0.17 | 0.89 ± 0.24 |
| Leptin | 1.00 ± 0.13 | 1.70 ± 0.37 | 0.76 ± 0.15 | 1.15 ± 0.11 | 1.38 ± 0.26 |
| Adiponectin | 1.00 ± 0.06 | 1.20 ± 0.17 | 0.85 ± 0.15 | 1.11 ± 0.09 | 1.19 ± 0.33 |
| Cyclin A1 | 1.00 ± 0.10a | 2.49 ± 0.26b | 1.10 ± 0.07a | 1.72 ± 0.16c | 2.24 ± 0.38b,c |
| CDK2 | 1.00 ± 0.08 | 1.15 ± 0.15 | 0.97 ± 0.16 | 1.14 ± 0.14 | 1.15 ± 0.15 |
| PPAR-γ | 1.00 ± 0.08 | 0.90 ± 0.26 | 1.04 ± 0.07 | 0.79 ± 0.08 | 1.09 ± 0.37 |
| C/EBPα | 1.00 ± 0.08 | 0.69 ± 0.22 | 1.08 ± 0.08 | 0.79 ± 0.13 | 0.86 ± 0.26 |
| Perirenal | |||||
| TNF-α | 1.00 ± 0.10 | 1.29 ± 0.14 | 0.99 ± 0.05 | 1.26 ± 0.17 | 1.19 ± 0.16 |
| IL-6 | 1.00 ± 0.18a | 2.84 ± 0.19b | 2.40 ± 0.18b | 1.35 ± 0.16a | 2.74 ± 0.52b |
| MCP-1 | 1.00 ± 0.18 | 1.62 ± 0.25 | 1.17 ± 0.24 | 1.42 ± 0.15 | 1.51 ± 0.31 |
| HIF-1α | 1.00 ± 0.06 | 1.07 ± 0.17 | 1.17 ± 0.12 | 1.22 ± 0.07a | 1.08 ± 0.19 |
| VEGF | 1.00 ± 0.08a,b | 0.88 ± 0.21b | 1.46 ± 0.20a | 1.15 ± 0.17a,b | 0.74 ± 0.15b |
| Leptin | 1.00 ± 0.16a,c | 1.69 ± 0.23b | 0.80 ± 0.19a | 0.85 ± 0.13a | 1.38 ± 0.21b,c |
| Adiponectin | 1.00 ± 0.05 | 0.96 ± 0.12 | 0.79 ± 0.08 | 0.86 ± 0.11 | 0.82 ± 0.12 |
| Cyclin A1 | 1.00 ± 0.08a | 2.16 ± 0.31b | 1.14 ± 0.22a | 1.78 ± 0.09b | 2.10 ± 0.30b |
| CDK2 | 1.00 ± 0.08 | 1.14 ± 0.38 | 0.91 ± 0.14 | 1.00 ± 0.10 | 0.88 ± 0.13 |
| PPAR-γ | 1.00 ± 0.10 | 1.08 ± 0.18 | 1.17 ± 0.08 | 0.93 ± 0.08 | 0.89 ± 0.20 |
| C/EBPα | 1.00 ± 0.08 | 0.62 ± 0.15 | 0.91 ± 0.12 | 0.92 ± 0.17 | 0.78 ± 0.19 |
| Subcutaneous | |||||
| TNF-α | 1.00 ± 0.28 | 1.06 ± 0.17 | 0.97 ± 0.24 | 0.87 ± 0.09 | 0.90 ± 0.11 |
| IL-6 | 1.00 ± 0.29 | 0.63 ± 0.30 | 1.27 ± 0.33 | 0.61 ± 0.13 | 1.16 ± 0.29 |
| MCP-1 | 1.00 ± 0.17 | 0.86 ± 0.33 | 1.24 ± 0.22 | 1.49 ± 0.25 | 1.35 ± 0.22 |
| HIF-1α | 1.00 ± 0.07 | 0.85 ± 0.12 | 0.94 ± 0.08 | 1.02 ± 0.20 | 0.85 ± 0.16 |
| VEGF | 1.00 ± 0.08 | 0.74 ± 0.14 | 1.21 ± 0.14 | 1.22 ± 0.34 | 0.84 ± 0.30 |
| Leptin | 1.00 ± 0.40 | 1.09 ± 0.47 | 1.07 ± 0.36 | 1.13 ± 0.30 | 1.03 ± 0.21 |
| Adiponectin | 1.00 ± 0.25 | 0.90 ± 0.17 | 0.97 ± 0.17 | 1.29 ± 0.35 | 0.89 ± 0.34 |
| Cyclin A1 | 1.00 ± 0.21a | 1.95 ± 0.35b | 0.98 ± 0.26a | 1.86 ± 0.19b | 2.08 ± 0.31b |
| CDK2 | 1.00 ± 0.14 | 0.95 ± 0.20 | 0.98 ± 0.13 | 1.06 ± 0.21 | 1.09 ± 0.26 |
| PPAR-γ | 1.00 ± 0.22 | 0.74 ± 0.13 | 1.02 ± 0.19 | 1.21 ± 0.33 | 0.78 ± 0.18 |
| C/EBPα | 1.00 ± 0.19 | 0.97 ± 0.11 | 0.76 ± 0.16 | 1.06 ± 0.34 | 0.74 ± 0.26 |
Values are means ± SE. mRNA expression in at-sexually mature rats. mRNA expression values are presented as 2ΔΔCT whereby ΔCT = B2M CT − gene of interest CT and were normalized to 1.0 for 70d RUN. The genes in italics highlight genes that showed a wheel lock (WL) effect (i.e., 77d 7d-WL was different than 77d RUN). Between-group differences are noted as by different letters, P < 0.05.
DISCUSSION
In the current study, two life phases were examined in rats whose ages were selected to mimic phases of human life where adipocyte number rapidly increases and then stabilizes (9, 17, 37, 41). The potential human relevance is that the prevalence of U.S. childhood obesity and physical inactivity both simultaneously increase at this age (see introduction for details and Refs. 6, 26, 33). We report 1) compared with 49–56 day-old rats, adipose tissue in 70- to 77-day-old rats was more protected from WL and respond by expansion via hyperplasia, as opposed to hypertrophy in 49- to 56-day-old rats; 2) in both ages, WL occurred without an increase in inflammatory mRNA expression; and 3) only in 70- to 77-day-old rats did 7d-WL increase cyclin A1 mRNA above RUN levels, reaching the higher sedentary levels in EAT, PRAT, and SAT (Table 2). We thus propose for the first time, to our knowledge, the notion that the type of adipocyte growth mechanism (i.e., hypertrophy or hyperplasia) is dependent on stage of growth during WL in rats.
Rate of Growth Decreases Between 49–56 and 70–77 Days of Age
Two ages of young rats exhibited different growth accretions, forming a novel, two-phase process, with 49- to 56-day-old rats showing no response to WL in many of our outcome measures (Table 3). We observed the beginning of a decrease in the increment of weekly gains in absolute body mass gains beginning around the 49- to 56-day age interval (Fig. 3). These data suggest that the more rapid growth phase of 49- to 56-day-old rats is near to an inflection point, preceding the next phase of slower growth. This is important because the progressively slower gain in body mass starting at ∼49 days of age may be associated with the previously reporting shutting down of adipocyte proliferation with maturation in sedentary rats (9). In summary, the 49- to 56-day-old rat data were collected at 49 and 56 days of age, essentially at the end of the rapid growth phase, while the 70- to 77-day-old group data were collected at 70 and 77 days of age, 3 wk into the progressively slower growth increments.
Table 3.
Summary of outcome measures
| Measure | GROUP |
|
|---|---|---|
| 49- to 56-day-old rats | 70- to 77-day-old rats | |
| Body mass | ||
| Absolute gain in last 7 days | No difference | WL gained mass |
| Rate of gain in last 7 days | No difference | WL > RUN |
| Lean mass | ||
| Absolute gain in last 7 days | No difference | WL gained mass |
| Rate of gain in last 7 days | No difference | No difference |
| Fat mass | ||
| Absolute gain in last 7 days | No difference | No difference |
| Rate of gain in last 7 days | WL > RUN | WL > RUN |
| %Body fat | ||
| Absolute gain in last 7 days | WL gained %BF | No difference |
| Rate of gain in last 7 days | WL > RUN | WL > RUN |
| Depot mass | ||
| EAT | No difference | 77d 7d-WL > 77d RUN |
| PRAT | No difference | 77d 7d-WL > 77d RUN |
| OAT | No difference | No difference |
| Mean adipocyte diameter | ||
| EAT | No difference | No difference |
| PRAT | N/A | No difference |
| OAT | N/A | No difference |
| Adipose tissue cellularity | ||
| EAT | No difference | 77d 7d-WL > 77d RUN, hyperplasia |
| PRAT | N/A | 77d 7d-WL > 77d RUN, hyperplasia |
| OAT | N/A | No difference |
| Adipocyte distribution | ||
| EAT | WL flattened curve and had more adipocytes in the 40-μm category | WL flattened curve and had more adipocytes in the 20-, 30-, and 40-μm categories |
| PRAT | N/A | WL flattened curve and had more adipocytes in the 30- and 40-μm categories |
| OAT | N/A | WL showed a rightward shift in distribution curve |
| Cyclin A1 mRNA | ||
| EAT | No difference | 77d 7d-WL > 77d RUN |
| PRAT | No difference | 77d 7d-WL > 77d RUN |
| OAT | No difference | No difference |
| SAT | No difference | 77d 7d-WL > 77d RUN |
Comparisons of 9 outcome measures (body mass, lean mass, %body fat, adipose tissue depot mass, adipocyte diameter, adipose tissue cellularity, adipocyte size distribution, and cyclin A1 mRNA expression) between 56d RUN and 56d 7d-WL rats in 49- to 56-day-old group and between 77d RUN and 77d 7d-WL in the 70- to 77-day-old group. EAT, epididymal adipose tissue; PRAT, perirenal adipose tissue' OAT, omental adipose tissue; SAT, subcutaneous adipose tissue.
Age of WL Affects Adipose Tissue Response
EAT in 49- to 56 day-old RUN rats showed increased adipocyte diameter between 49 and 56 days of age even though they continued running, suggesting that adipocytes were in a hypertrophic growth phase. Thus the effect of WL on adipocyte proliferation appears to be “lost” or “masked” due to the natural expansion occurring within the 7-day age range from 49–56 days of age. In contrast, WL in 70- to 77-day-old rats induces a hyperplasic response not seen rats 3 wk younger. Total adipocyte number in an adipose tissue depot was unchanged between 70 and 77 days of age in both RUN and SED rats in all examined adipose tissue depots. Unexpectedly, WL at 70–77 days of age induced an increase in total adipocyte number in EAT and PRAT. Age-matched run rats (77d RUN) did not exhibit this increase in total adipocyte number; thus the hyperplasic expansion in 70- to 77-day-old rats is due to WL, not age. Taken together, these novel findings suggest that the age in which a decrease in activity occurs influences the expansion characteristics of visceral adipose tissue.
WL Alters Adipocyte Diameter Distribution
In the 49- to 56 day-old rats, there is a clear rightward-shift in adipocyte diameter distribution curves with age (Fig. 9). Our usage of the term “rightward-shift” is described in more detail next. The 56d RUN and 56d SED curves are to the right of the 49d RUN and 49d SED curves, suggesting hypertrophic adipose tissue expansion. The 49d 7d-WL curve shows a rightward-shift compared with 49d RUN but also shows a flattening and increased number of adipocytes in the 40-μm category. In contrast, the 70- to 77-day-old rats no longer demonstrate the rightward-shift observed between the 70- to 77-day RUN groups or the 70- to 77-day SED groups in EAT and PRAT depots, suggesting that hypertrophic adipose expansion observed in the 49- to 56-day-old rats is no longer occurring. In addition, WL flattened the curve in both EAT and PRAT and showed more adipocytes in the 30- and 40-μm categories in the 77d 7d-WL rats. The emergence of small adipocytes has been observed by others: special examples being weight-regain after calorie-restricted weight loss in older rats (13, 23) and thiazolidinedione treatment in rodents (28); however, this phenomenon is the first time to our knowledge that this has been measured in inactivity-induced fat mass increase in young rats. These data would seem to support our supposition that 49- to 56-day-old rats are still expanding adipose tissue by hypertrophy, while 70- to 77-day-old rats are no longer expanding adipose tissue by hypertrophy.
In contrast to the other depots, the OMAT WL adipocyte diameter curve exhibited a rightward-shift with no increase in smaller adipocytes, suggesting that OMAT adipocytes continue to respond to WL by hypertrophy in 70- to 77-day-old rats. Supporting the previous observation, Tchkonia et al. (42) reported that human omental preadipocytes have lower potential for replication and differentiation than subcutaneous or mesenteric preadipocytes in morbidly obese adult humans. While our rats were younger and not obese, this lower proliferation and differentiation potential in OMAT may explain the different response to WL in OMAT compared with EAT and PRAT at 70–77 days of age. OMAT is noteworthy as high volumes of OMAT are associated with inflammation later in life.
There Was No Increase in Inflammatory mRNA Expression with WL
We measured three inflammatory markers, plus two hypoxic markers, and surprisingly found no increases in any of these markers with WL at either 49–56 or 70–77 days of age. Hypoxia markers were added with inflammatory markers because hypoxia is implicated in early adipose tissue expansion and is a precursor to inflammation (10–11, 45). We speculate that the lack of an inflammatory response may be due to hyperplasic adipose expansion, which is associated with a healthy adipose tissue adaptation (40). Another explanation is that the adipose tissue expansion in our model was not sufficient to evoke an inflammatory response. Many of the studies where an inflammatory response has been observed used either longer periods of observation and/or a more severe induction of adiposity, like a high-fat diet, (35, 38–39), or gene alteration (3, 14). While we can say that increases in adipose tissue mass seen with a sudden decrease in activity are not accompanied by a proinflammatory phenotype in these young rats, we are not certain of the extended effects of decreased activity if these animals were to age beyond 77 days.
WL Cyclin A1 mRNA Expression Equaled That of SED and Was Greater Than RUN
We found new evidence at the transcript level that suggests cell proliferation is increased EAT, PRAT, and SAT in WL rats. Cyclin A1 mRNA is a marker of proliferation, as its protein functions to promote cell proliferation and survival by its requirement for S phase and passage through G2 (7, 31). Cyclin A1 mRNA was greater in 77d 7d-WL than in 77d RUN group for three adipose tissue depots (EAT, PRAT, and SAT), without similar differences between 56d RUN and 56d 7d-WL. The emergence of a higher proportion of small adipocytes (<30 μm) in EAT and PRAT of 70- to 77-day-old rats after 1 wk of WL combined with the increase in cyclin A1 mRNA expression supports our notion of an inactivity-induced proliferation of preadipocytes and/or differentiation of preadipocytes present in 77-day-old 7d-WL depots. The above notion is supported by our previous report of the occurrence of hyperplasia in the epididymal depot with 7d-WL (20) and of others who have found adipocyte proliferation to occur with weight regain after calorie restriction in rats (13, 23, 47) and in weight-reduced humans (21). Surprisingly, peroxisome proliferator-activated receptor-γ (PPAR-γ) mRNA expression, a marker of cellular differentiation, did not differ among groups in the three depots that cyclin A1 did (EAT, PRAT, and SAT). In contrast, the one depot in which cyclin A1 mRNA did not show a change with WL (OMAT) was paralleled by no increase in small adipocytes. Further, PPAR-γ mRNA expression was lower OMAT in 77d WL rats compared with 70d RUN and 77d SED.
Taken together, we suggest the novel idea that physical activity acts as a brake on adipose tissue expansion via hyperplasia in at-sexual maturity rats, and once that brake is removed (i.e., WL), adipose tissue expands primarily by hyperplasia. We found new evidence for the potential importance of cyclin A1 mRNA in proliferation, as its protein functions to promote cell proliferation and survival by its requirement for S phase and passage through G2 (7, 31). The preferential increase in cyclin A1 mRNA before other mRNA changes in EAT, PRAT, and SAT for functional gene categories (proinflammatory, hypoxic, adipokine, and adipogenic) in only the 70- to 77-day-old WL group supports formation of a hypothesis that an upstream “activity”-dependent signal to the cell cycle, and not a proinflammatory adipokine, is an early signal to increase hyperplasia in the model employed here. The failure of OMAT to show increases in cyclin A1 mRNA and hyperplasia, which is in contrast to increases in other adipose tissue depots, offers additional evidence.
WL Rats Do Not Revert to SED Levels in Most Anthropometric Measures
The 56- and 77-day-old WL rats remained below SED levels in the following measures: body mass, lean mass, percent body fat, fat mass, depot mass (OMAT, EAT, and PRAT), and adipocyte diameter (OMAT, EAT, and PRAT). The 56d 7d-WL rats had fewer EAT adipocytes than 56d SED, but the 77d 7d-WL rats had a similar number of EAT and PRAT adipocytes as the 77d SED. These data indicate that 1 wk of WL is not adequate to completely revert to SED levels, suggesting that physical activity early in life has some short-term protective effect in terms of adiposity.
Resolutions of Previous Data Discrepancies
We previously reported that during WL adipocyte hypertrophy predominated during increased EAT mass in Fischer 344 Brown Norway rats aged 42–49 days of age in one study (18), but that hyperplasia was dominant in rats at the ages of 70–77 days in a second study (20). Our current results in Wistar rats showing these results in the same study, suggest a resolution to our previous two conflicting sets of data in that both of our previous studies are correct. The juvenile age of growth when WL takes place affects adipose tissue response. WL effects are essentially masked in younger rats, but we see increased cell size from 49 to 56 days regardless if the rats continue running (49d RUN < 56 d RUN) or undergo WL (49d RUN < 56d WL), suggesting that hypertrophy is occurring regardless of physical activity levels. However, in 70- to 77-day-old rats, WL increased adipocyte number, suggesting a hyperplasic response.
Limitations of the Study
While a 3-wk difference between groups raises questions on whether the groups were far enough apart in age to detect interpretable differences, the 3-wk difference in ages was sufficient in duration to detect different responses to WL between ages (49–56 vs. 70–77 days of age) in the following measures: body mass, lean mass, fat mass, body fat percent, EAT and PRAT depot mass, EAT and PRAT adipocyte tissue cellularity, and cyclin A1 mRNA. There are other iterations of WL that could be performed to further understand visceral adipose expansion with WL. For example, we did not specifically determine if age or duration of running is the primary factor inducing hyperplasic visceral expansion. Future studies could employ a group that gains access to a voluntary running wheel for 3 wk from 49 to 70 days of age and is wheel locked from 70 to 77 days of age. This group would be age matched with the 70- to 77-day-old rats that had 6 wk access to a voluntary running wheel. If the group with access for only 3 wk showed hyperplasic visceral expansion, then we gain more support to our conclusion. However, if the group with access for only 3 wk showed hypertrophic visceral expansion, then the duration of voluntary wheel running must be considered as a factor in the type of visceral adipose expansion. Another limitation in our interpretations is that changes could be occurring more rapidly than 7 days post-WL. Perhaps some of the mRNA, like PPAR-γ, could be upregulated within hours or days and then return back to baseline values by 7 days post-WL. A final limitation is that we were unable to measure food spillage in the cage, possibly suggesting that we overestimated daily food intake.
Perspectives and Significance
In the context of the current childhood obesity epidemic, there is an urgent need to understand underlying mechanisms of adipose tissue growth and investigate obesity-prevention strategies. Since no single tissue or factor can be fully responsible for weight regain, the WL model provides a unique environment to study how a sudden cessation of daily physical activity affects some characteristics of adipose tissue in juvenile rats. One alternating period of physical activity and inactivity had consequential effects on adipose cell number in the EAT and PRAT depots of 70- to 77-day-old rats. As human childhood usually consists of multiple intervals of activity-inactivity, the preclinical consequences of repeated periods of activity with inactivity should be further investigated with invasive approaches using said animal models.
A differential response in adipose tissue depots may have other clinical implications given that new, smaller adipocytes have greater capacity to preferentially accumulate fat compared with larger adipocytes and thereby buffer the onset of the metabolic syndrome. In addition, smaller adipocytes are less sensitive to antilipolytic effects of insulin (25), they exhibit lower rates of basal and catecholamine-stimulated lipolysis (22, 29), and they are more protective against metabolic disease compared with very large adipocytes (15). The emergence of small adipocytes has been observed by others: weight-regain after calorie-restricted weight loss in older rats (13, 23) and thiazolidinedione treatment in rodents (28). Therefore, we speculate that the failure of omental adipocytes to proliferate during WL in 70- to 77-day-old rats could play a contributing role to omental adipose tissue being an unhealthier adipose tissue depot.
GRANTS
All authors disclose no conflicts of interest. Funding was provided by American Heart Association Grant 11PRE7580074 (to J. M. Compnay), National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant T32-AR-048523 (to M. D. Roberts), and funds donated through the College of Veterinary Medicine's Development Office.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.C. and F.W.B. conception and design of research; J.M.C., R.G.T., and C.L.C. performed experiments; J.M.C., M.D.R., and F.W.B. analyzed data; J.M.C., M.D.R., and F.W.B. interpreted results of experiments; J.M.C. and M.D.R. prepared figures; J.M.C. drafted manuscript; J.M.C., M.D.R., and F.W.B. edited and revised manuscript; J.M.C., M.D.R., and F.W.B. approved final version of manuscript.
REFERENCES
- 1.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298: E1244–E1253, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Batra A, Siegmund B. The role of visceral fat. Dig Dis 30: 70–74, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shlomo S, Zvibel I, Varol C, Spektor L, Shlomai A, Santo EM, Halpern Z, Oren R, Fishman S. Role of glucose-dependent insulinotropic polypeptide in adipose tissue inflammation of dipeptidylpeptidase 4-deficient rats. Obesity (Silver Spring) 2013. February 14 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Dulloo AG. Adipose tissue plasticity in catch-up-growth trajectories to metabolic syndrome: hyperplastic versus hypertrophic catch-up fat. Diabetes 58: 1037–1039, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulloo AG. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract Res Clin Endocrinol Metab 22: 155–171, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Dumith SC, Gigante DP, Domingues MR, Kohl HW., 3rd Physical activity change during adolescence: a systematic review and a pooled analysis. Int J Epidemiol 40: 685–698, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Girard F, Strausfeld U, Fernandez A, Lamb NJ. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell 67: 1169–1179, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359: 61–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwood MR, Hirsch J. Postnatal development of adipocyte cellularity in the normal rat. J Lipid Res 15: 474–483, 1974 [PubMed] [Google Scholar]
- 10.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29: 4467–4483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Jackman MR, Steig A, Higgins JA, Johnson GC, Fleming-Elder BK, Bessesen DH, MacLean PS. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am J Physiol Regul Integr Comp Physiol 294: R1117–R1129, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Jenkins NT, Padilla J, Arce-Esquivel AA, Bayless DS, Martin JS, Leidy HJ, Booth FW, Rector RS, Laughlin MH. Effects of endurance exercise training, metformin, and their combination on adipose tissue leptin and IL-10 secretion in OLETF rats. J Appl Physiol 113: 1873–1883, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R. Waist Circumference and Cardiometabolic Risk: a Consensus Statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity (Silver Spring) 15: 1061–1067, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest 63: 239–246, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kump DS, Booth FW. Sustained rise in triacylglycerol synthesis and increased epididymal fat mass when rats cease voluntary wheel running. J Physiol 565: 911–925, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol 106: 161–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol 102: 1341–1347, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lofgren P, Andersson I, Adolfsson B, Leijonhufvud BM, Hertel K, Hoffstedt J, Arner P. Long-term prospective and controlled studies demonstrate adipose tissue hypercellularity and relative leptin deficiency in the postobese state. J Clin Endocrinol Metab 90: 6207–6213, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lofgren P, Hoffstedt J, Naslund E, Wiren M, Arner P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia 48: 2334–2342, 2005 [DOI] [PubMed] [Google Scholar]
- 23.MacLean PS, Higgins JA, Wyatt HR, Melanson EL, Johnson GC, Jackman MR, Giles ED, Brown IE, Hill JO. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol 297: R793–R802, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mize RR, Wu HH, Cork RJ, Scheiner CA. The role of nitric oxide in development of the patch-cluster system and retinocollicular pathways in the rodent superior colliculus. Prog Brain Res 118: 133–152, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Morimoto C, Tsujita T, Okuda H. Antilipolytic actions of insulin on basal and hormone-induced lipolysis in rat adipocytes. J Lipid Res 39: 957–962, 1998 [PubMed] [Google Scholar]
- 26.Nader PR, Bradley RH, Houts RM, McRitchie SL, O'Brien M. Moderate-to-vigorous physical activity from ages 9 to 15 years. JAMA 300: 295–305, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 307: 483–490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest 101: 1354–1361, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olefsky JM. Insensitivity of large rat adipocytes to the antilipolytic effects of insulin. J Lipid Res 18: 459–464, 1977 [PubMed] [Google Scholar]
- 30.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11: 85–97, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J 11: 961–971, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts MD, Brown JD, Company JM, Oberle LP, Heese AJ, Toedebusch RG, Wells KD, Cruthirds CL, Knouse JA, Ferreira JA, Childs TE, Brown M, Booth FW. Phenotypic and molecular differences between rats selectively bred to voluntarily run high vs. low nightly distances. Am J Physiol Regul Integr Comp Physiol 304: R1024–R1035, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts MD, Company JM, Brown JD, Toedebusch RG, Padilla J, Jenkins NT, Laughlin MH, Booth FW. Potential clinical translation of juvenile rodent inactivity models to study the onset of childhood obesity. Am J Physiol Regul Integr Comp Physiol 303: R247–R258, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J 276: 5738–5746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 19: 1109–1117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slawik M, Vidal-Puig A J. Adipose tissue expandability and the metabolic syndrome. Genes Nutr 2: 41–45, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature 453: 783–787, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Speretta GF, Rosante MC, Duarte FO, Leite RD, Lino AD, Andre RA, Silvestre JG, de Araujo HS, Duarte AC. The effects of exercise modalities on adiposity in obese rats. Clinics 67: 1469–1477, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56: 2910–2918, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 121: 2094–2101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Symonds ME, Budge H, Perkins AC, Lomax MA. Adipose tissue development–impact of the early life environment. Prog Biophys Mol Biol 106: 300–306, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, Kirkland JL. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes 55: 2571–2578, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Thomas EL, Frost G, Taylor-Robinson SD, Bell JD. Excess body fat in obese and normal-weight subjects. Nutr Res Rev 25: 150–161, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Toedebusch RG, Childs TE, Hamilton SR, Crowley JR, Booth FW, Roberts MD. Postprandial leucine and insulin responses and toxicological effects of a novel whey protein hydrolysate-based supplement in rats. J Int Soc Sports Nutr 9: 24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Zanato VF, Martins MP, Anselmo-Franci JA, Petenusci SO, Lamano-Carvalho TL. Sexual development of male Wistar rats Brazilian journal of medical and biological research. Braz J Med Biol Res 27: 1273–1280, 1994 [PubMed] [Google Scholar]
- 47.Zhu M, Lee GD, Ding L, Hu J, Qiu G, de Cabo R, Bernier M, Ingram DK, Zou S. Adipogenic signaling in rat white adipose tissue: modulation by aging and calorie restriction. Exp Gerontol 42: 733–744, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]