Abstract
Patients with obstructive sleep apnea, who experience episodic hypoxia and hypercapnia during sleep, often demonstrate increased inflammation, oxidative stress, and dyslipidemia. We hypothesized that sleep apnea patients would be predisposed to the development of atherosclerosis. To dissect the mechanisms involved, we developed an animal model in mice whereby we expose mice to intermittent hypoxia/hypercapnia (IHH) in normobaric environments. Two- to three-month-old low-density lipoprotein receptor deficient (Ldlr−/−) mice were fed a high-fat diet for 8 or 16 wk while being exposed to IHH for either 10 h/day or 24 h/day. Plasma lipid levels, pulmonary artery and aortic atherosclerotic lesions, and cardiac function were then assayed. Surprisingly, atherosclerosis in the aorta of IHH mice was similar compared with controls. However, in IHH mice, atherosclerosis was markedly increased in the trunk and proximal branches of the pulmonary artery of exposed mice; even though plasma cholesterol and triglycerides were lower than in controls. Hemodynamic analysis revealed that right ventricular maximum pressure and isovolumic relaxation constant were significantly increased in IHH exposed mice and left ventricular % fractional shortening was reduced. In conclusion, 1) Intermittent hypoxia/hypercapnia remarkably accelerated atherosclerotic lesions in the pulmonary artery of Ldlr−/− mice and 2) increased lesion formation in the pulmonary artery was associated with right and left ventricular dysfunction. These findings raise the possibility that patients with obstructive sleep apnea may be susceptible to atherosclerotic disease in the pulmonary vasculature, an observation that has not been previously recognized.
Keywords: obstructive sleep apnea, hypertension, hemodynamics, atherosclerosis, pulmonary artery, intermittent hypoxia and hypercapnia
obstructive sleep apnea (OSA), a disease known to affect up to 8% of adult males, 2% of adult females, and 1–3% of children, is also thought to promote cardiovascular disease (CVD) (70, 71). Intermittent pharyngeal collapse during sleep, the fundamental pathophysiologic mechanism behind OSA, leads to intermittent periods of apnea, significant hypoxemia (oxyhemoglobin desaturation to as low as 50%), but importantly, also to hypercapnia (29, 55), both of which, in turn, serve to induce pulmonary arteriolar vasoconstriction and pulmonary arterial hypertension. OSA also is recognized as a risk factor for systemic hypertension (53), cardiac arrhythmias (44), stroke, and coronary heart disease (52), all of which together cause increased CVD morbidity and mortality (41, 68). However, OSA is now considered an independent risk factor for arteriosclerotic vascular disease (33, 35, 43), but the mechanistic links between hypoxemia/hypercapnia and formation of atheroma remain unclear.
Atherosclerosis is, in part, an inflammatory disease (7, 40), and OSA has been associated with increased systemic cytokine markers of inflammation such as TNFα, C-reactive protein, interleukin-6 (IL-6), and IL-18 (17, 20, 27, 45, 50, 63, 69). In animal models, intermittent hypoxia (IH) has been shown to lead to oxidative stress, inflammation, and subsequent atherosclerosis (11, 12, 32, 58, 62). Repeated episodes of hypoxia and hypercapnia with intervening periods of normoxia and normocapnia may elicit distinct pathophysiological sequelae in sensitive tissues such as the heart and vasculature that induce an inflammatory response (33). However, very little is known about the adverse consequences of IH, intermittent hypercapnia and intermittent hypoxia/hypercapnia (IHH) on vascular integrity. We sought to test the hypothesis that IHH, which is characteristic of OSA patients (21, 43, 55, 67), will lead to accelerated atherosclerosis. In this study, we exposed low-density lipoprotein receptor deficient (Ldlr−/−) mice to a western diet (WD) and IHH for periods of 8 or 16 wk and examined the impact on atherogenesis. We decided to use this animal model since wild-type mice have difficulty developing lesions (22). Atherosclerosis was indeed accelerated in IHH-exposed mice. However, the increase was seen in the pulmonary artery and not the aorta.
MATERIALS AND METHODS
Animals.
The mice used in these studies were greater than 10th generation male Ldlr−/− mice on the C57BL/6J background (Stock Number 002207; The Jackson Laboratory, Bar Harbor, ME). Mice were weaned at 21 days of age and genotyped for the LDLR defect. Groups of mice were matched for age, body weight, and total cholesterol. Animals had ad libitum access to water and food and were housed in cages equipped with rodent enrichments (igloo and gnawing bone; Bio-Serv, Frenchtown, NJ). Care was exercised in the handling of these animals and the minimal number of animals that was absolutely required was used in this study. This study was conducted in conformity with the Guiding Principles for Research Involving Animals and Human Beings and was approved by the University of California, San Diego, Institutional Animal Care and Use Committee (Protocol number: S-5534). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Diet.
Ldlr−/− mice were fed with regular chow (RC) consisting of 0.01% cholesterol and 4.4% fat (TD8604; Harlan-Teklad, Madison, WI) until initiation of the dietary and environmental interventions. Starting at 2–3 mo of age, male mice were provided with a WD (1.25% cholesterol, 21% milk fat; 4.5 Kcal/g; TD96121; Harlan-Teklad) for 8 wk or 16 wk to induce lesion formation. A subset of mice was fed a regular chow diet during the IHH exposure for 8 wk, 10 h/day to function as a control.
Exposure to IHH: As we have previously described, a computer-controlled chamber system (OxyCycler, Reming Bioinstruments, Redfield, NY) was used for the induction and maintenance of IHH (16, 25). The chamber can hold up to four mouse cages simultaneously. Mice were exposed to short periods (∼4 min) of low fractional inspired concentration of O2 ([O2] = 8%) and moderate fractional inspired concentration of CO2 ([CO2] = 8%), with alternating periods (∼4 min) of normoxia ([O2] = 21%) and normocapnia ([CO2] = ∼0.1%) with ramp intervals of 1–2 min each for 24h/day and 10h/day during the light cycle. The relatively short periodicity of stress is planned to mimic the hypoxic periodicity that we see in clinical practice in diseases such as OSA. On the basis of previous experiments where we exposed mice to different levels of O2, we determined that the animals could tolerate 8% O2 very well (14, 15). We also performed experiments where we exposed mice to various levels of CO2 and found that mice tolerate moderate levels of CO2 well, with no significant loss in body weight gain (unpublished observations). The major impetus, however, that led us to use these levels of CO2 and O2 is that such levels can induce partial pressures seen in patients with OSA. A combination of nitrogen, oxygen (O2), and carbon dioxide (CO2) was injected into the chambers through a network of tubing to achieve selected concentrations of O2 and CO2. The flow of gases into the chambers is controlled by the Oxycycler hydraulic system (Model A84XOV, BioSpherix, Redfield, NY) which is supported by Ana-Win2 software, version 2.4.17 (Watlow Anafaze, CA). This software is designed to control the hydraulic component of the Oxycycler to achieve desired gas delivery as well as to continuously monitor gas tensions within the chambers. Therefore, this system allows the capture of real-time data and the storage of all information related to the experimental paradigm. Under this protocol, we imposed IHH on two groups of mice for 8 wk and 16 wk, respectively. We have previously demonstrated that mice tolerate combined IHH with 8% O2 nadirs and 8% CO2 peaks very well(15). Concurrently, Normoxic control groups of animals were maintained on the same WD but kept in normoxia in the same room and were exposed to the same level of noise and light during the duration of each experiment. Ambient temperature and relative humidity were maintained at 22–24°C and 40–50%, respectively.
Atherosclerosis analysis.
Mice exposed to WD as well as to IHH or room air for periods of 8 or 16 wk were euthanized using 100% CO2. Atherosclerosis was quantified by computer-assisted image analysis in Sudan-stained en face preparations of the entire aorta as previously described (36, 60). In a similar manner, the pulmonary root and left and right pulmonary arteries were dissected out, stained, and extent of lesion staining quantified using computer-assisted image analysis. Aortic root cross-sectional atherosclerosis was measured by cutting 10-μm paraffin sections from the origin of the aortic valve where the first leaflet was seen until the last leaflet, resulting in ∼58–78 sections. Modified van Gieson elastic stain was used to enhance the contrast between the intima and surrounding tissue. Quantitative analysis of lesion area was performed on every sixth section until a total of 7 to 10 sections were analyzed spanning 660 to 960 μm from the origin of the first visible leaflet. The results are presented as total lesion area in mm2 of all aortic cross sections analyzed. Quantification was performed by investigators blinded to treatment assignment using computer-assisted image analysis. Frozen sections of the PA were cut at 10 μm and stained with van Gieson stain.
Blood and plasma analyses.
At baseline and during the course of the experiment, facial vein blood was obtained via heparin-coated microtainer tubes (Becton-Dickinson, NJ) after 0, 4, 8, 12, and 16 wk of exposure. Total cholesterol and plasma triglycerides levels were determined using automated enzymatic assays (Roche Diagnostics, Indianapolis, IN, and Equal Diagnostics, Exton, PA). In addition, at the end of the experiment following euthanasia, 0.75–1.0 mL of blood was obtained from the portal vein. Lipoprotein profiling was performed using fast performance liquid chromatography (FPLC) equipped with a Superose 6 column, and cholesterol levels were determined in each fraction as described (36).
Echocardiography, angiography, and hemodynamics.
Echocardiography was performed, under isoflurane gas anesthesia, using a VisualSonics, Vevo 2100 (a division of SonoSite, Toronto, Ontario, Canada) with a 32–55 MHz linear transducer. Parasternal long and short axis views and apical four-chamber views were obtained in all animals. Two-dimensional guided M-mode tracings were recorded in the short axis plane for the internal diameter of the left ventricle (LV) and interventricular septum and posterior wall thicknesses just below the tips of the mitral valve leaflets. Pulsed wave Doppler tracings were obtained in the pulmonary artery. Screenings with color Doppler were performed for the assessment of tricuspid and pulmonic regurgitation.
For hemodynamic and angiographic analysis, following ketamine (100 mg/kg) and xylazine (10 mg/kg) ip, mice were intubated and placed on a ventilator (100–110 strokes/min, 0.4–0.5 mL stroke volume). The right carotid artery was then exposed. The femoral vein was cannulated with a stretched PE50 tubing for drug administration. A 1.4 French high-fidelity catheter-tip micromanometer (Millar Instruments, Houston, TX) was inserted retrogradely into the aorta via the right carotid artery and advanced into the left ventricle. Intracavitary pressure was visualized, recorded, and archived using an AD Instruments acquisition system (Colorado Springs, CO). After both sides of vagus nerve were cut, and following a period of pressure stabilization, baseline values were then recorded. In order to test contractile reserve, dobutamine was then given through the femoral vein at dosages of 0.75, 2, 4, 6, and 8 μg·kg−1·min−1, for 3 min each. Acquired data were analyzed at the end of 3 min for each dosage. Hemodynamic parameters determined included LV peak (+) and peak (−) dP/dt, LV peak and end-diastolic pressure, the time constant of isovolumic relaxation pressure decay (tau), and aortic pressure. The same micromanometer was then passed through the right jugular vein into the right atrium and right ventricle (RV). Pressure-derived indices obtained in the right heart included RV peak (+) and peak (−) dP/dt, RV peak and end-diastolic pressure, tau, and right atrial mean pressure. Angiography was performed after hemodynamic studies were completed. The contrast agent used was the iso-osmotic agent Optiray which was injected through the right internal jugular vein. The fluoroscopic image was acquired using a portable C-arm image intensifier and X-ray tube and archived on a VHF video tape recorder. These images were subsequently digitized frame-by-frame via computer (2, 13). Following euthanasia, mice were subsequently fixed in 4% paraformaldehyde, and tissues were collected for further analysis.
Statistical analysis.
Data are reported as mean ± standard deviation, except as noted. Results were analyzed using the Student's unpaired t-test, Mann Whitney test, or One-Way ANOVA with Bonferroni's posttest (GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego, CA) as appropriate. Differences in the means were considered statistically significant when P < 0.05.
RESULTS
Impact of IHH on weight and lipids/lipoprotein levels.
We initially set out to test the hypothesis that exposure of WD-fed mice to IHH would lead to acceleration of aortic atherosclerosis. The impact of diet and IHH on weight gain and lipids and lipoproteins are presented in detail in Tables 1 and 2. Control mice are denoted as “Normoxia” throughout the text. Normoxia mice appeared in good health and gained weight as expected over the 8 wk and 16 wk periods. In contrast, mice exposed to IHH demonstrated a generalized decrease rate of weight gain, which was particularly pronounced during the prolonged 16 wk period of IHH exposure, and especially in mice with 24 h/day exposure, where the decreases in weight gain compared with Normoxia mice were significantly different. Mice fed a regular chow diet showed a modest increase in body weight, but there were significant differences between Normoxic and IHH mice at 4 wk (P < 0.05) and 8 wk (P < 0.001) in that IHH weighed slightly less (data not shown).
Table 1.
Effect of IHH on body weight and lipid profiles
| 8 wk, 10 h/day |
16 wk, 10 h/day |
|||||||
|---|---|---|---|---|---|---|---|---|
| Weeks: | 0 | 4 | 8 | 0 | 4 | 8 | 12 | 16 |
| Body weight, g | ||||||||
| Normoxia | 25.24 ± 1.2 (8) | 29.88 ± 1.6 (8) | 33.21 ± 1.7 (8) | 26.13 ± 0.7 (20) | 31.13 ± 0.8 (20) | 35.22 ± 0.9 (20) | 36.97 ± 1.1 (20) | 36.57 ± 1.2 (17) |
| IHH | 24.76 ± 1.7 (8) | 26.38 ± 1.7 (8) | 27.74 ± 1.8 (7) | 24.39 ± 0.4 (20) | 24.68 ± 0.4 (20) | 26.98 ± 0.4 (20) | 27.49 ± 0.6 (19) | 26.81 ± 0.6 (14) |
| Total cholesterol, mg/dl | ||||||||
| Normoxia | 356.6 ± 36.1 (8) | 1,313 ± 110.7 (8) | 1,621 ± 120 (8) | 277.1 ± 12 (12) | 1,535 ± 104.5 (16) | 2,000 ± 138 (16) | 2,100 ± 121 (16) | 1,706 ± 133.1 (12) |
| IHH | 311.8 ± 23.3 (8) | 1,672 ± 88.8 (8) | 1,576 ± 118.9 (8) | 266.9 ± 7.9 (12) | 1,630 ± 66 (14) | 1,682 ± 59.7 (16) | 1,664 ± 52.7 (15) | 1,665 ± 109.3 (12) |
| Plasma triglycerides, mg/dl | ||||||||
| Normoxia | 128.5 ± 1.1 (8) | 121.4 ± 15.8 (8) | 144 ± 15 (8) | 139.7 ± 7.6 (12) | 271 ± 20.3 (16) | 335.8 ± 33.3 (16) | 371.8 ± 30.1 (16) | 340 ± 31.05 (12) |
| IHH | 116.3 ± 11.1 (8) | 122.9 ± 11.8 (8) | 126.6 ± 12.4 (8) | 135 ± 6.5 (12) | 202.2 ± 11 (14) | 162.1 ± 9.2 (16) | 183.4 ± 16.2 (15) | 313.8 ± 84.92 (12) |
Values are means ±SE. Numbers in parentheses indicate n values for each parameter.
Table 2.
Effect of IHH on body weight and lipid profiles
| 8 wk, 24 h/day |
16 wk, 24 h/day |
|||||||
|---|---|---|---|---|---|---|---|---|
| Weeks | 0 | 4 | 8 | 0 | 4 | 8 | 12 | 16 |
| Body weight, g | ||||||||
| Normoxia | 25.24 ± 1.2 (8) | 29.88 ± 1.6 (8) | 33.21 ± 1.7 (8) | 26.13 ± 0.7 (20) | 31.13 ± 0.8 (20) | 35.22 ± 0.9 (20) | 36.97 ± 1.1 (20) | 36.57 ± 1.2 (17) |
| IHH | 24.76 ± 1.7 (8) | 26.38 ± 1.7 (8) | 27.74 ± 1.8 (7) | 24.39 ± 0.4 (20) | 24.68 ± 0.4 (20) | 26.98 ± 0.4 (20) | 27.49 ± 0.6 (19) | 26.81 ± 0.6 (14) |
| Total cholesterol, mg/dl | ||||||||
| Normoxia | 356.6 ± 36.1 (8) | 1,313 ± 110.7 (8) | 1,621 ± 120 (8) | 277.1 ± 12 (12) | 1,535 ± 104.5 (16) | 2,000 ± 138 (16) | 2,100 ± 121 (16) | 1,706 ± 133.1 (12) |
| IHH | 311.8 ± 23.3 (8) | 1,672 ± 88.8 (8) | 1,576 ± 118.9 (8) | 266.9 ± 7.9 (12) | 1,630 ± 66 (14) | 1,682 ± 59.7 (16) | 1,664 ± 52.7 (15) | 1,665 ± 109.3 (12) |
| Plasma triglycerides, mg/dl | ||||||||
| Normoxia | 128.5 ± 1.1 (8) | 121.4 ± 15.8 (8) | 144 ± 15 (8) | 139.7 ± 7.6 (12) | 271 ± 20.3 (16) | 335.8 ± 33.3 (16) | 371.8 ± 30.1 (16) | 340 ± 31.05 (12) |
| IHH | 116.3 ± 11.1 (8) | 122.9 ± 11.8 (8) | 126.6 ± 12.4 (8) | 135 ± 6.5 (12) | 202.2 ± 11 (14) | 162.1 ± 9.2 (16) | 183.4 ± 16.2 (15) | 313.8 ± 84.92 (12) |
Values are means ±SE. Numbers in parentheses indicate n values for each parameter.
All mice had marked increases in plasma lipids in response to the WD, as expected. During the 8 wk dietary periods, there were no differences in total plasma cholesterol or triglyceride levels between IHH and Normoxia controls, but by 16 wk the IHH mice had lower plasma cholesterol and, particularly, lower plasma triglycerides during the final 8 wk of the IHH intervention. Mice fed a regular chow diet demonstrated lower total cholesterol and plasma triglycerides over the exposure period, but there were no significant differences between Normoxia and IHH mice (data not shown). Lipoprotein profiling indicated decreased content of all apoB containing lipoproteins, e.g., VLDL/IDL and LDL levels, while HDL was unchanged (data not shown).
Impact of IHH on aortic lesion formation.
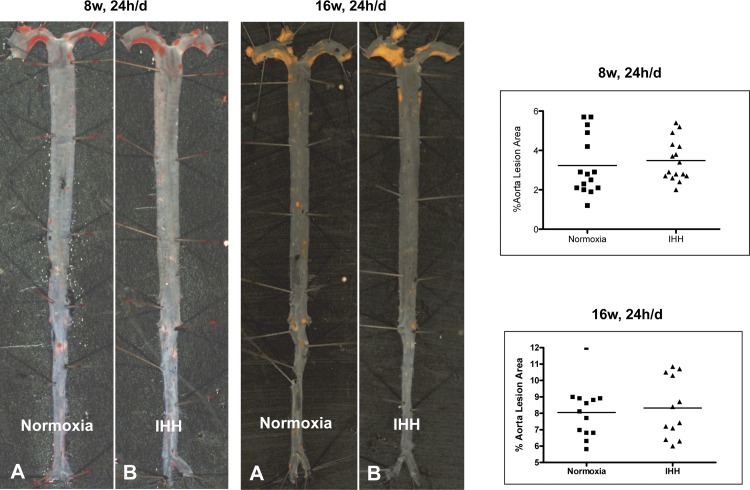
In an initial set of studies, we examined mice after 8 wk, 24 h/day of IHH exposure for the impact on aortic atherosclerosis. Contrary to our hypothesis, we were surprised to find that the extent of lesion formation at both the aortic root and the entire aorta in IHH mice was not different than that noted in the Normoxia mice (Fig. 1 and Table 3). Similarly, there was no difference at the level of the aortic valve. For mice exposed for 16 wk, 24 h/day, the absolute extent of en face lesion formation was increased in both groups (8.0 ± 1.6% vs. 8.3 ± 1.8%), but again there was no difference between the Normoxia and IHH groups (Fig. 1 and Table 3). One might argue that the decreased weight gain and lower plasma cholesterol levels might account for these findings in the 24 h/day IHH mice, but in a smaller cohort of mice exposed to IHH for 10 h/day for 16 wk, we also did not observe differences in lesion formation (Table 3).
Fig. 1.
Lesion development in Normoxia and intermittent hypoxia/hypercapnia (IHH) aortas. Mice were exposed to IHH for indicated times and en face atherosclerosis quantified as explained in methods. Representative aortas from mice exposed to IHH for 24 h/day for 8 wk and 16 wk are shown, as well as the values for all mice in these experimental protocols. Formation of lesions in control and IHH-induced mice: Ldlr−/− mice that were exposed to WD and either kept in room air (normoxia) or exposed to IHH for 8 wk and 16 wk. Sudan IV-stained aortas showed no difference in lesion area. Data are presented as mean ± SD.
Table 3.
Effect of IHH on lesion development
| 8 wk, 10 h/day |
16 wk, 10 h/day |
8 wk, 24 h/day |
16 wk, 24 h/day |
|||||
|---|---|---|---|---|---|---|---|---|
| Normoxia (4) | IHH (4) | Normoxia (3–4) | IHH (3) | Normoxia (15) | IHH (16) | Normoxia (12–13) | IHH (12) | |
| % PA trunk lesion area | 1.73 ± 0.52 | 22.8 ± 17.44* | 17.16 ± 2.91 | 26 ± 9.13 | 1.28 ± 0.49 (n = 7) | 28.4 ± 12.67*** (n = 7) | 27.19 ± 16.8 | 79.55 ± 17.46*** |
| % Aorta lesion area | 5.35 ± 0.4 | 5.7 ± 0.8 | 7.7 ± 0.82 | 4.73 ± 0.57** | 3.23 ± 1.51 | 3.49 ± 1.05 | 8.05 ± 1.61 | 8.31 ± 1.81 |
| % Aortic arch lesion Area | 17.03 ± 4.69 | 17.6 ± 5.56 | 24.95 ± 0.52 | 20.5 ± 3.2 | ND | ND | 26.52 ± 5.07 | 29.36 ± 5.51 |
| Aortic valve lesion area, mm2/section | 0.066 ± 0.01 | 0.151 ± 0.04 | 0.269 ± 0.06 | 0.153 ± 0.03 | 0.035 ± 0.03 (n = 7) | 0.033 ± 0.02 (n = 10) | 0.189 ± 0.08 | 0.186 ± 0.06 |
Values are means ±SD. Numbers in parentheses indicate n values for each parameter. *P = 0.05, **P = 0.001, and ***P = 0.0001.
Impact of IHH on pulmonary atherosclerosis.
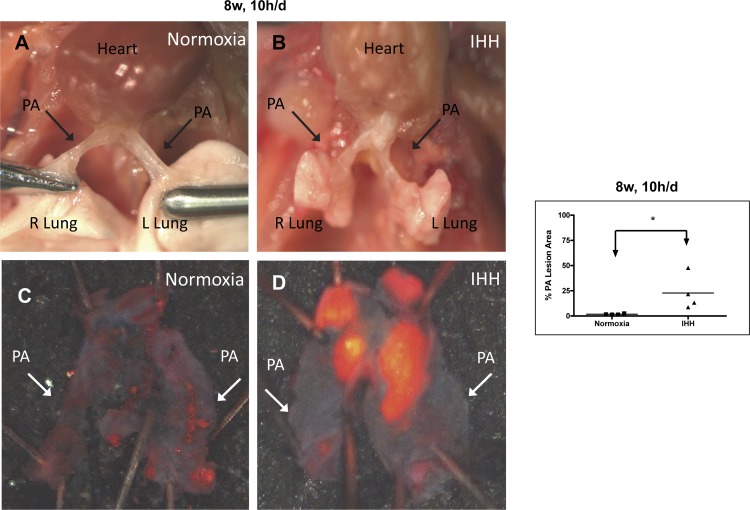
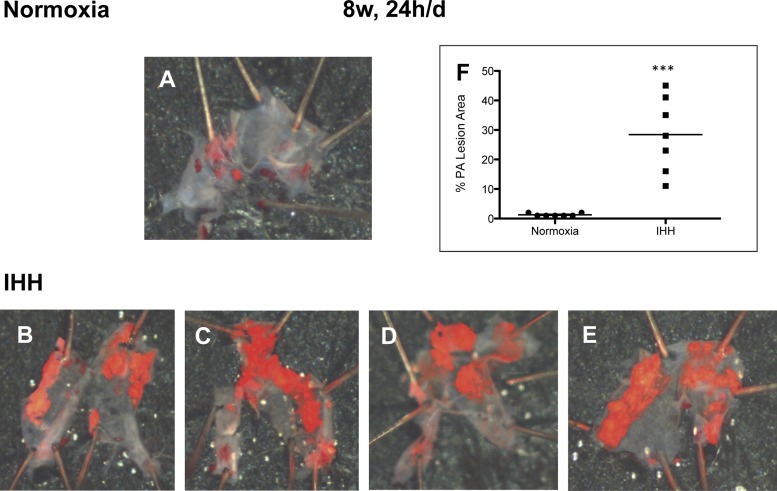
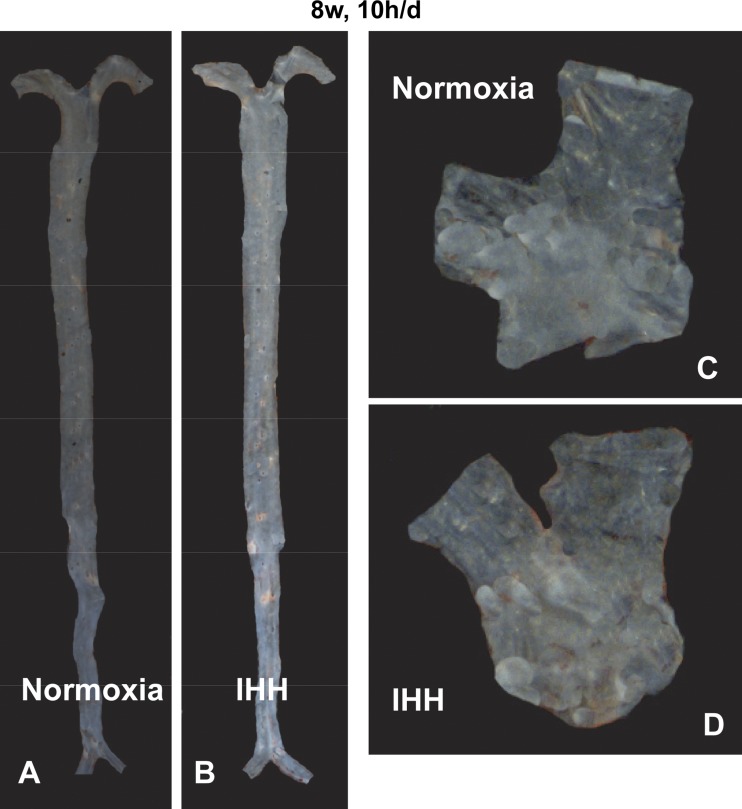
During the dissection of the heart and the aorta for the conventional analysis of atherosclerosis, two authors (K.B. and A.L.) noted an unusual and dramatic accumulation of lesion in the pulmonary root and arteries (designated PA) of the IHH mice. Because this was unexpected, it had not been looked for in the original set of mice. This was first noted in a small set of mice (n = 4) after 8 wk of 10 h/day IHH exposure, and in a subset of mice (n = 7) exposed to IHH for 8 wk, 24 h/day. In mice exposed for 8 wk, 10 h/day, there was a significant increase in PA lesion density compared with Normoxia mice (Fig. 2 and Table 3). Additionally, in the mice exposed to 8 wk, 24 h/day, lesions in the PA of the IHH mice were significantly larger than those in the Normoxia mice, which were almost undetectable, (28.4 ± 12.7% vs. 1.3 ± 0.5%, P < 0.0001) (Fig. 3 and Table 3).
Fig. 2.
Lesion development in the pulmonary arteries of Normoxia and IHH mice exposed to IHH for 8 wk, 10 h/day. A and B show the in situ appearance of pulmonary arteries (PAs), while C and D show the appearance of the arteries after dissection and Sudan IV staining. Figure shows the percent of Sudan IV staining of the entire PA for all IHH mice and Normoxia control. ***P = 0.06. Data are presented as mean ± SD.
Fig. 3.
Lesion development in the pulmonary arteries of Normoxia and IHH mice exposed to IHH for 8 wk, 24 h/day. A–E show the appearance of the pulmonary arteries (PAs) after dissection and Sudan IV staining. A shows the control PA, while B–E show the IHH Pas. F shows the percent of Sudan IV staining of the entire PA for all IHH mice and Normoxia control. P = 0.001. Data are presented as means ± SD.
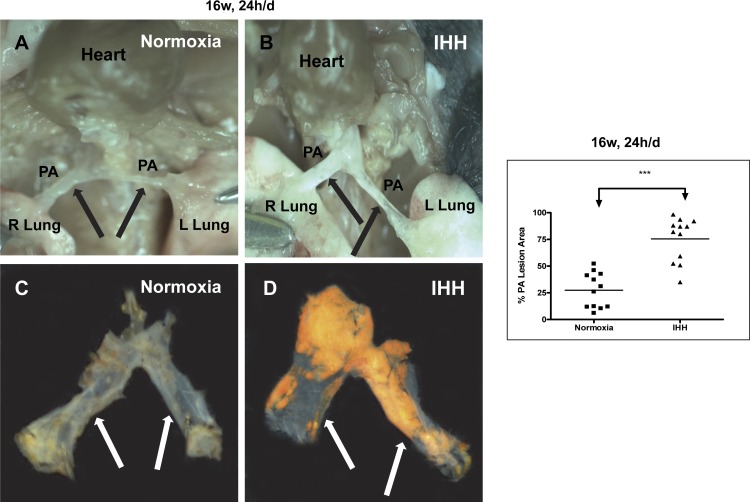
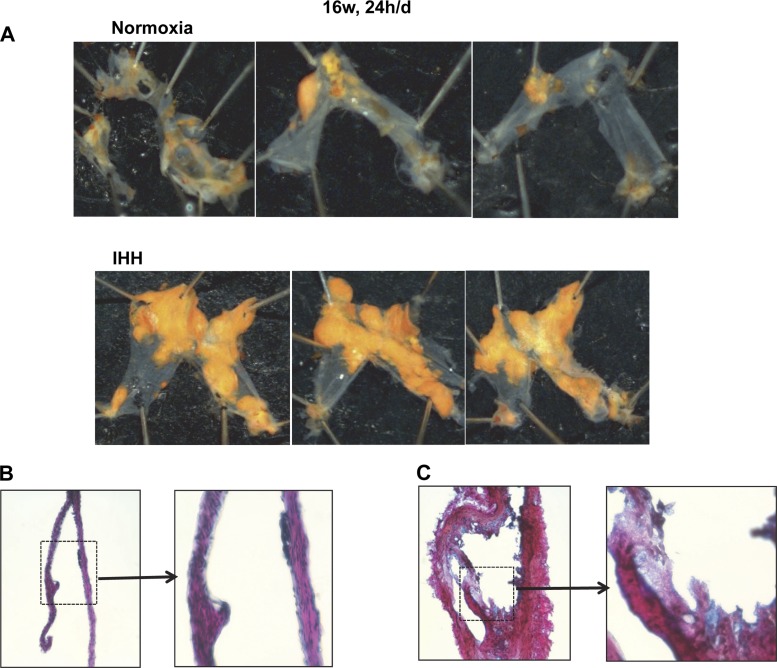
In mice exposed to IHH for 16 wk, 24 h/day, we examined prospectively for the extent of PA lesion formation (Fig. 4 and Table 3). These data confirmed the dramatic induction of lesion formation in the PA. Figure 4, A and B, displays the in situ appearance of the PA in mice after 16 wk of 24 h/day exposure. In both panels of Fig. 4, the associated fat has been removed and the heart has been reflected upwards to reveal the pulmonary trunk and arteries underneath. In the Normoxia mouse, the PA was very difficult to even detect, being very thin, and one was literally able to see through the vessel to structures underneath. In contrast, the PA vessels in the IHH mouse were readily visible, thickened, and opaque with grossly visible lesions involving most of the trunk and arteries. The PA vessels were dissected out of the chest and stained with Sudan IV and displayed in Panels C and D in the same orientation as found in situ. Lipid accumulation was dramatically enhanced in the mice exposed to IHH for 24 h/day (27 ± 19% vs. 79.6 ± 17.5%, P < 0.0001) (Fig. 4 and 5 for individual examples and Table 3). Remarkably, there was considerable measurable PA lesion formation even in the Normoxia mice at this time point, which was present in all of the mice examined (Fig. 5). Similarly, we also examined a small cohort of mice exposed to IHH for 16 wk but only 10 h/day, and even in these mice there was lesion formation in the PA in the Normoxia but much greater in the IHH mice (Table 3; data not shown). PA lesion formation was increased in going from 10 h/day to 24 h/day and in going from 8 wk to 16 wk. As a control, we exposed mice to IHH but fed them a regular chow diet. These mice did not demonstrate any lesions in either the aorta or pulmonary artery whether they were in room air or exposed to IHH (Fig. 6).
Fig. 4.
Lesion development in the pulmonary arteries of Normoxia and IHH mice exposed to IHH for 16 wk, 24 h/day. A and B show photographs of the in situ appearance of pulmonary arteries (PAs) of a Normoxia (A) and IHH (B) mouse, while C and D show the appearance of the arteries after dissection and Sudan IV staining. The graph shows the percent of the PA stained by Sudan IV for each of the mice examined. ***P < 0.0001. Data are presented as means ± SD.
Fig. 5.
Additional examples of pulmonary arteries dissected from Normoxia mice (A) and IHH mice (B) after 16 wk, 24 h/day exposure. Pulmonary arteries were stained with Sudan IV. B and C display photomicrographs of modified Gieson stained paraffin sections of PA from a Normoxia (B) or IHH (C) mouse (433 magnification), along with a blowup of the indicated portion of each artery section.
Fig. 6.
Lesion development in the aortas and pulmonary arteries of Normoxia and IHH mice exposed to IHH for 8 wk, 10 h/day but fed a regular chow diet. A and B show aortas that demonstrate no lesion development, while C and D show that pulmonary arteries do not develop lesions on a regular chow diet.
We also examined the morphology of frozen sections of the PA using a modified van Gieson stain. Because the PAs of the Normoxia were so small and thin, it was difficult to obtain good sections, but as shown in Fig. 5B, this mostly consisted of only occasional small lesions in the intima with no thickening of the media. In striking contrast, the PAs of the IHH were much larger (Fig. 5C); note that the magnification of the PAs in Fig. 5, B and C, is the same. The normal architecture was grossly distorted with abundant intimal lesions, markedly increased collagen deposition, and remarkably increased media compared with the PA of Normoxia mice. A higher magnification of indicated section of the lesion in the IHH PA (Fig. 5C) displays foam cells and necrotic areas, and some collagen deposition typical of advanced atherosclerotic lesion formation.
Impact of IHH on left and right ventricular function.
In order to understand if there were functional effects on the hearts of these mice, we performed echocardiography, angiography, and hemodynamic analysis to determine if functional changes occurred in the heart.
Right ventricle.
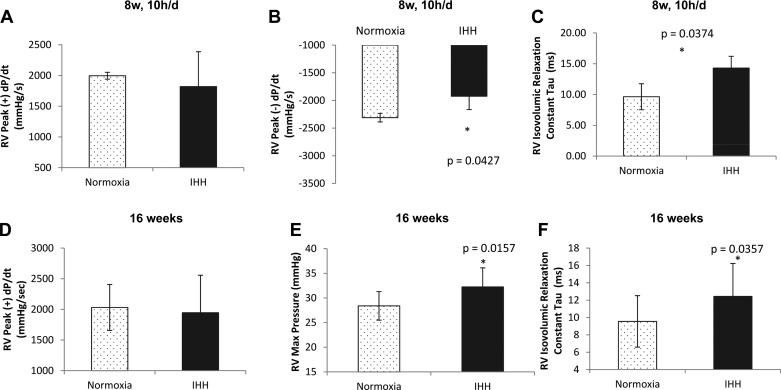
Since the PA demonstrated visible atherosclerotic lesion deposition, we hypothesized that there should be increased resistance to RV cardiac ejection, leading to a pressure overload. Indeed, hemodynamic analysis in the 8 wk, 10 h/day IHH group demonstrated that there was a decrease in right ventricular peak (−) dP/dt (−2310.4 ± 78 mmHg/s for Normoxia vs. −1924.3 ± 240.7 mmHg/s for IHH; P = 0.04) (Fig. 7B) while there was no difference in peak (+) dP/dt (1996.7 ± 56.1 vs. 1820 ± 567.9) (Fig. 7A). There was an increase in RV isovolumic relaxation constant, tau (9.6 ± 2.1 ms for Normoxia vs. 14.3 ± 1.9 ms for IHH; P = 0.04) (Fig. 7C) in mice exposed to IHH for 8 wk, 10 h/day. These data suggested that there was a delay in RV isovolumic relaxation. In mice exposed to IHH for 16 wk, there was an increase in RV max pressure (28.4 ± 0.9 mmHg vs. 32.3 ± 1.2 mmHg; P = 0.02) (Fig. 7E) with no change in RV peak dP/dt (Fig. 7D). RV isovolumic relaxation constant, tau was also increased in these mice (9.6 ± 0.9 ms vs. 12.4 ± 1.1 ms; P = 0.036), similar to the 8 wk, 10 h/day mice (Fig. 7F). Interestingly, there was an increase in PA max pressure prevagotomy, derived from the RV max pressure prevagotomy, in these mice (28.9 ± 1.3 mmHg vs. 32 ± 1.1 mmHg; P = 0.04) (data not shown). These increases in the RV max pressure are suggestive of an induced pulmonary hypertension secondary either to proximal vessel obstruction or smaller vessel changes in the more distal vasculature related to the prolonged exposure to hypoxemia. Hence, IHH and a WD compromise RV function in mice.
Fig. 7.
Hemodynamic properties of the right ventricle in mice exposed for 8 wk, 10 h/day and for 16 wk. Hemodynamic analysis of the right ventricle in mice exposed to IHH for 8 wk, 10 h/day revealed a decrease in peak (−) dP/dt (B), and an increase in the isovolumic relaxation constant, tau (C) while there was no difference in peak (+) dP/dt (A). In mice exposed to IHH for 16 wk, there was an increase in RV max pressure (E) and an increase the isovolumic relaxation constant, tau (F), while there was no difference in peak (+) dP/dt (D). Data are presented as means ± SD.
Left ventricle.
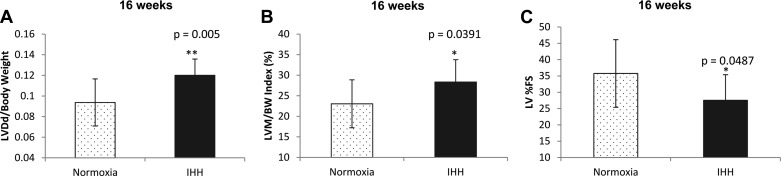
Overall, the response of the LV to IHH included changes in heart rate (HR) and % fractional shortening (%FS). In these analyses, we combined data from mice exposed to IHH for 16 wk, be it 10 h/day or 24 h/day due to the small number of animals assayed in the 16 wk, 10 h/day group. Echocardiographic analysis demonstrated that there was a decrease in %FS in IHH mice exposed for 16 wk compared with controls (27.6 ± 2.4% vs. 35.8 ± 3.1%; P = 0.05) (Fig. 8C). Heart rate at the time of echocardiographic assessment was decreased in IHH mice (499 ± 88 bpm for Normoxia vs. 445 ± 11.9 bpm for IHH; P = 0.007). LV dimension at end diastole/body weight (LVDd/BW) and LV mass/body weight index (LVM/BW) were increased (Fig. 8, A and B) but this is probably attributable to the lesser body weight of IHH mice. Hemodynamic analysis showed that HR was also decreased in IHH mice exposed for 8 wk, 10 h/day compared with Normoxia mice in response to the infusion of graded doses of dobutamine, a β-adrenergic agent (at baseline and at 2 μg·kg−1·mL−1; P < 0.05 for both measures) (data not shown). Mice fed a regular chow diet did not demonstrate any alterations in echo cardiographic, hemodynamic, or angiographic markers, and there were no significant differences between room air and IHH mice (data not shown).
Fig. 8.
Echocardiographic assessment of LV wall dimensions. A: LV end-diastolic dimension/body weight (LVDd/body weight). B: LV mass/body weight index (LVM/BW index). C: left ventricular percent fractional shortening (LV %FS). Data are presented as means ± SD.
DISCUSSION
This is one of the first reports that exposure of WD-fed Ldlr−/− mice to IHH led to an exaggerated development of atherosclerotic lesions in the pulmonary artery trunk and its proximal branches. There have been only rare reports of atherosclerosis in the PA trunk and pulmonary arteries, though clearly this was present even in the Normoxia mice especially after 16 wk of the WD. Most likely this was overlooked previously, as it is not apparent unless one looks for it prospectively.
We originally anticipated that IHH would accelerate atherosclerosis in the aorta, but surprisingly, we did not find differences between the Normoxia and IHH mice in either en face analysis of the aorta, or at the aortic root, at either the 8 wk or 16 wk time periods, irrespective of the extent of IHH exposure. Considering that the mice exposed to IHH had in general a lower rate of weight gain, and lower plasma cholesterol and triglyceride levels, it is remarkable that the extent of lesion formation was the same. This suggests that at the most extreme degrees of IHH over 16 wk of 24 h/day exposure, atherosclerosis was indeed accelerated for the degree of cholesterol exposure.
The marked induction of atherosclerosis in the pulmonary arteries induced by IHH was also associated with pathophysiological hemodynamic alterations and changes in both right and left ventricular function. These consisted of a modest increase in PA pressure (based upon the micromanometer RV pressure recordings under general anesthesia), a mild pressure overload on the RV, and a significant delay in myocardial relaxation (based upon the prolongation of the monoexponential decay of isovolumic pressure) in the RV. We were not able, however, to substantiate significant obstructive lesions in the main pulmonary artery, either by 2D echocardiography/Doppler or by contrast angiography. IH (without the hypercapnia) for 8 wk has been reported to increase right ventricular systolic pressure and thickness of the right ventricular anterior wall and to induce RV hypertrophy and pulmonary vascular remodeling in mice (49). Additionally, IH for a few weeks led to pulmonary arterial hypertension, pulmonary arterial remodeling, and right ventricular hypertrophy (10, 18, 47). It is also notable that our IHH mice manifested some depression of LV shortening, based upon the reduced %FS by M-mode echocardiography. These findings agree with previous clinical observations in humans that tie OSA to symptoms and signs of congestive heart failure. Presumably, these functional abnormalities of the heart relate to the effects of reduced oxygen delivery to the myocardium during hypoxemia, although this variable could not be measured directly in this experiment. In spite of the fact that IH alone has been reported to lead to sympatho-excitation (9, 54), we observed a decrease in HR when assessed by echocardiography and in response to dobutamine, a sympathomimetic, during our hemodynamic studies. Heart rate decrease and blood pressure rise were reported to be greater during IHH than IH alone (6). We hypothesize that HR was decreased during IHH because of decreased sensitivity of adrenergic receptors during dobutamine administration or that IHH alters vagal or peripheral chemoreceptor activity (42), thus changing the HR response.
Atherosclerosis.
The finding that IHH induced an increase in atherosclerotic lesion deposition in the PA but not in the aorta is unique and remarkable. To our knowledge, the only other report describing PA atherosclerotic lesions in a murine model is that of Langheinrich et al. (31), who described atherosclerotic PA lesions in Apoe−/−/Ldlr−/− double knockout mice. However, in that study, lesions did not develop until the age of 80 wk whereas our lesions were evident at ages of 16–20 wk and 24–28 wk. Additionally, PA atherosclerotic lesions have been described in only two other animal species, New Zealand and Japanese white rabbits, which were fed a high cholesterol diet (0.3 and 0.5% cholesterol) (24, 61) or lanolin (high cholesterol and oxysterols) plus pneumonectomy (28) and a single report in the African Grey parrot (59). In humans, there are a few reports of PA atherosclerosis. In a study of 25 chronic obstructive pulmonary disease (COPD) patients without pulmonary embolism, central PA lesions of the wall-adherent type were found in 12 COPD patients (48%) in the right PA (56). Some of these lesions were confirmed to be atherosclerotic by helical computed tomography and magnetic resonance angiography. There also have been reports of pulmonary atherosclerosis in a patient with atrial septal defect (46) and in 32% of 54 patients who had pulmonary thromboendarterectomies performed from 1990–2001 to treat chronic thromboembolic pulmonary hypertension (8).
Previous studies in mice have demonstrated that IH alone (no hypercapnia) with a WD increases aortic atherosclerosis (3, 23, 39, 57). In contrast, we found no difference in aortic lesion area between Normoxia and IHH mice fed a WD. It is therefore possible that the intermittent hypercapnia that we used along with IH might have had a mitigating effect on aortic lesion development while promoting lesion development in the PA. Hypercapnia has been reported to have both protective and deleterious effects (5). For example, hypercapnia has been reported to abrogate chronic hypoxia-induced pulmonary hypertension (26, 51). However, hypercapnia with normal pH injures alveolar epithelial cells, decreases the function of surfactant protein A in vitro, and impedes the clearance of lung edema (30, 64). CO2 is also known to interact with both reactive nitrogen species and reactive oxygen species (ROS) (65).
Effect of IHH on lipid profiles.
Plasma cholesterol and triglyceride levels were lower in IHH mice compared with Normoxia controls during prolonged periods of IHH exposure. In part, this may have been due to decreased food intake and decreased weight gain, particularly in those mice exposed to IHH for 24 h/day for 16 wk. Interestingly, others reported increased plasma cholesterol in studies utilizing IH alone and a high cholesterol diet (23, 39, 57); however, the changes in triglyceride levels were variable. Additionally, IH alone has been demonstrated to up-regulate genes involved in cholesterol and fatty acid synthesis as well as triglyceride and phospholipid biosynthesis (37). IH-induced hyperlipidemia may explain the increased aortic lesion seen in these studies while the decreased lipids seen in the present study may account for the lack of enhanced aortic lesion development. Indeed, IHH, as noted above, might target different signaling pathways than IH. For example, IHH might have an adverse effect on the liver, possibly by compromising the function of sterol regulatory element binding protein which is a transcription factor that regulates lipid biosynthesis in the liver and that is up-regulated by IH (38). These previous studies, however, do not give us potential explanations as to the difference between the aorta and the pulmonary artery. It is important to note here that it has been well known that different vessels in the body can be regulated differently by a stress such as hypoxia (66). Our current studies represent a major example where the PA behaves very differently from the aorta when the stress is that of IHH.
The mechanisms proposed for the development of atherosclerosis caused by IH and OSA are hypoxemia, production of ROS, inflammation, and endothelial dysfunction among other factors (4). The atherosclerotic lesions in the PA could have developed from ROS production and inflammation induced by IHH. OSA patients are dyslipidemic and develop carotid artery intima-media thickening and even plaque formation (19, 32, 48). Additionally, increased oxidative stress has been demonstrated in OSA patients in that production of ROS correlated with the severity of O2 desaturations and was reversed by nasal continuous positive airway pressure (32, 34). As such, these patients have demonstrated an enhanced ability to develop atherosclerosis. The possibility thus exists that they may be susceptible also to PA atherosclerosis. We wonder whether PA atherosclerosis could have been missed clinically or in previous experimental studies.
Our study does not address the issue of why the PA appears more susceptible to accelerated atherosclerosis than the aorta in response to IHH. There are, of course, major differences in the PA compared with the aorta. For example, the cellular elements in each type of vessel differ developmentally as the aorta arises from the fourth aortic arch, but the PA from the sixth arch (1). We speculate that this leads to profound differences in the cellular responses to hypoxia or CO2, and/or to the paradigm of hypoxia, i.e., whether constant or intermittent stress.
In summary, we present a model of IHH that mimics many of the pathophysiological effects of OSA. We found that this induced accelerated atherosclerosis in the PA, which was associated with hemodynamic effects consistent with early pulmonary hypertension. The model presented here should be of value in studies to define the mechanisms by which OSA produces atherosclerosis and consequently cardiac hemodynamic changes.
GRANTS
Funded by National Institutes of Health Grants HL086559 (J.L.W.), GM69338 (J.L.W.) and HL 088093 (A.C.L., J.L.W.), and HL 087391(A.C.L.), NIH grant 5 P01 32573 (G.G.H.), Perlman Fund for Cardiovascular Research and Education (K.L.P.) and support from the Leducq Foundation (J.L.W.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.M.D., J.L.W., G.G.H., and A.C.L. conception and design of research; R.M.D., K.B., J.P., A.B.P., J.J., N.D.D., Y.G., T.I., and A.C.L. performed experiments; R.M.D., K.B., J.P., A.B.P., J.J., N.D.D., Y.G., E.A., T.I., K.L.P., J.L.W., G.G.H., and A.C.L. analyzed data; R.M.D., N.D.D., E.A., T.I., K.L.P., J.L.W., G.G.H., and A.C.L. interpreted results of experiments; R.M.D., K.B., J.P., A.B.P., E.A., and A.C.L. prepared figures; R.M.D. drafted manuscript; R.M.D., K.L.P., J.L.W., G.G.H., and A.C.L. edited and revised manuscript; R.M.D., K.B., J.P., A.B.P., J.J., N.D.D., Y.G., E.A., T.I., K.L.P., J.L.W., G.G.H., and A.C.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Travis Smith and Orit Gavrialov for their excellent technical assistance.
REFERENCES
- 1.Anderson RH, Cook A, Brown NA, Henderson DJ, Chaudhry B, Mohun T. Development of the outflow tracts with reference to aortopulmonary windows and aortoventricular tunnels. Cardiol Young 20 Suppl 3: 92–99, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Arber S, Hunter JJ, Ross J, Jr, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell 88: 393–403, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Arnaud C, Poulain L, Levy P, Dematteis M. Inflammation contributes to the atherogenic role of intermittent hypoxia in apolipoprotein-E knock out mice. Atherosclerosis 219: 425–431, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Arter JL, Chi DS, MG, Fitzgerald SM, Guha B, Krishnaswamy G. Obstructive sleep apnea, inflammation, and cardiopulmonary disease. Front Biosci 9: 2892–2900, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Azzam ZS, Sharabi K, Guetta J, Bank EM, Gruenbaum Y. The physiological and molecular effects of elevated CO2 levels. Cell Cycle 9: 1528–1532 [DOI] [PubMed] [Google Scholar]
- 6.Bakehe M, Miramand JL, Chambille B, Gaultier C, Escourrou P. Cardiovascular changes during acute episodic repetitive hypoxic and hypercapnic breathing in rats. Eur Respir J 8: 1675–1680, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med 8: 1218–1226, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Blauwet LA, Edwards WD, Tazelaar HD, McGregor CG. Surgical pathology of pulmonary thromboendarterectomy: a study of 54 cases from 1990 to 2001. Hum Pathol 34: 1290–1298, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bosc LV, Resta T, Walker B, Kanagy NL. Mechanisms of intermittent hypoxia induced hypertension. J Cell Mol Med 14: 3–17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol 99: 2028–2035, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172: 915–920, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath 7: 105–110, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 8: 459–465, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Douglas RM, Miyasaka N, Takahashi K, Latuszek-Barrantes A, Haddad GG, Hetherington HP. Chronic intermittent but not constant hypoxia decreases NAA/Cr ratios in neonatal mouse hippocampus and thalamus. Am J Physiol Regul Integr Comp Physiol 292: R1254–R1259, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Douglas RM, Ryu J, Kanaan A, Del Carmen Rivero M, Dugan LL, Haddad GG, Ali SS. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction. Am J Physiol Cell Physiol 298: C1594–C1602, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douglas RM, Xue J, Chen JY, Haddad CG, Alper SL, Haddad GG. Chronic intermittent hypoxia decreases the expression of Na/H exchangers and HCO3− dependent transporters in mouse CNS. J Appl Physiol 95: 292–299, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 172: 613–618, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Fagan KA. Selected contribution: Pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol 90: 2502–2507, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Friedlander AH, Yueh R, Littner MR. The prevalence of calcified carotid artery atheromas in patients with obstructive sleep apnea syndrome. J Oral Maxillofac Surg 56: 950–954, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Goldbart AD, Krishna J, Li RC, Serpero LD, Gozal D. Inflammatory mediators in exhaled breath condensate of children with obstructive sleep apnea syndrome. Chest 130: 143–148, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Han F, Chen E, Wei H, He Q, Ding D, Strohl KP. Treatment effects on carbon dioxide retention in patients with obstructive sleep apnea-hypopnea syndrome. Chest 119: 1814–1819, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol 55: 503–517, 2004 [PubMed] [Google Scholar]
- 23.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 209: 381–386, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamimura R, Suzuki S, Miura N, Miyahara K. Pulmonary atherosclerosis and pulmonary arterial pressure in cholesterol-fed New Zealand white rabbits. J Vet Med Sci 63: 647–653, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol 290: R1105–R1114, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kantores C, McNamara PJ, Teixeira L, Engelberts D, Murthy P, Kavanagh BP, Jankov RP. Therapeutic hypercapnia prevents chronic hypoxia-induced pulmonary hypertension in the newborn rat. Am J Physiol Lung Cell Mol Physiol 291: L912–L922, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kasasbeh E, Chi DS, Krishnaswamy G. Inflammatory aspects of sleep apnea and their cardiovascular consequences. South Med J 99: 58–67; quiz 68–59, 81, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Komuro K, Rosenzweig DY. Experimental production of pulmonary hypertension and pulmonary atherosclerosis in the rabbit. Circ Res 20: 545–551, 1967 [DOI] [PubMed] [Google Scholar]
- 29.Krieger J, Sforza E, Apprill M, Lampert E, Weitzenblum E, Ratomaharo J. Pulmonary hypertension, hypoxemia, and hypercapnia in obstructive sleep apnea patients. Chest 96: 729–737, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Lang JD, Jr, Chumley P, Eiserich JP, Estevez A, Bamberg T, Adhami A, Crow J, Freeman BA. Hypercapnia induces injury to alveolar epithelial cells via a nitric oxide-dependent pathway. Am J Physiol Lung Cell Mol Physiol 279: L994–L1002, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Langheinrich AC, Michniewicz A, Bohle RM, Ritman EL. Vasa vasorum neovascularization and lesion distribution among different vascular beds in ApoE−/−/LDL−/− double knockout mice. Atherosclerosis 191: 73–81, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Lavie L. Obstructive sleep apnoea syndrome–an oxidative stress disorder. Sleep Med Rev 7: 35–51, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Lavie L, Dyugovskaya L, Lavie P. Sleep-apnea-related intermittent hypoxia and atherogenesis: adhesion molecules and monocytes/endothelial cells interactions. Atherosclerosis 183: 183–184, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 27: 123–128, 2004 [PubMed] [Google Scholar]
- 35.Lavie P, Lavie L. Cardiovascular morbidity and mortality in obstructive sleep apnea. Curr Pharm Des 14: 3466–3473, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest 106: 523–531, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Grigoryev DN, Ye SQ, Thorne L, Schwartz AR, Smith PL, O'Donnell CP, Polotsky VY. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol 99: 1643–1648, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 97: 698–706, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Li RC, Haribabu B, Mathis SP, Kim J, Gozal D. Leukotriene B4 receptor-1 mediates intermittent hypoxia-induced atherogenesis. Am J Respir Crit Care Med 184: 124–131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 54: 2129–2138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Masuyama S, Shinozaki T, Kohchiyama S, Okita S, Kimura H, Honda Y, Kuriyama T. Heart rate depression during sleep apnea depends on hypoxic chemosensitivity. A study at high altitude. Am Rev Respir Dis 141: 39–42, 1990 [DOI] [PubMed] [Google Scholar]
- 43.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29: 156–178, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 173: 910–916, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minoguchi K, Tazaki T, Yokoe T, Minoguchi H, Watanabe Y, Yamamoto M, Adachi M. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest 126: 1473–1479, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Nascimento D, Nunes L, Oliveira F, Vencio E, Teixeira V, Reis M. Pulmonary atherosclerosis associated with an atrial septal defect in old age: case report of an elderly autopsied patient. Pathol Res Pract 205: 137–141, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Nattie EE, Bartlett D, Jr, Johnson K. Pulmonary hypertension and right ventricular hypertrophy caused by intermittent hypoxia and hypercapnia in the rat. Am Rev Respir Dis 118: 653–658, 1978 [DOI] [PubMed] [Google Scholar]
- 48.Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, Quan SF. Relation of sleep-disordered breathing to cardiovascular disease risk factors: The sleep heart health study. Am J Epidemiol 154: 50–59, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol 94: 179–184, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Ooi H, Cadogan E, Sweeney M, Howell K, O'Regan RG, McLoughlin P. Chronic hypercapnia inhibits hypoxic pulmonary vascular remodeling. Am J Physiol Heart Circ Physiol 278: H331–H338, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Peker Y, Kraiczi H, Hedner J, Loth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J 14: 179–184, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: 1378–1384, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol 174: 156–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Resta O, Foschino Barbaro MP, Bonfitto P, Talamo S, Mastrosimone V, Stefano A, Giliberti T. Hypercapnia in obstructive sleep apnoea syndrome. Neth J Med 56: 215–222, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Russo A, De Luca M, Vigna C, De Rito V, Pacilli M, Lombardo A, Armillotta M, Fanelli R, Loperfido F. Central pulmonary artery lesions in chronic obstructive pulmonary disease: A transesophageal echocardiography study. Circulation 100: 1808–1815, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175: 1290–1297, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med 162: 566–570, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Sedacca CD, Campbell TW, Bright JM, Webb BT, Aboellail TA. Chronic cor pulmonale secondary to pulmonary atherosclerosis in an African Grey parrot. J Am Vet Med Assoc 234: 1055–1059, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Shaw PX, Horkko S, Tsimikas S, Chang MK, Palinski W, Silverman GJ, Chen PP, Witztum JL. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol 21: 1333–1339, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Sun YP, Zhu BQ, Sievers RE, Norkus EP, Parmley WW, Deedwania PC. Effects of antioxidant vitamins C and E on atherosclerosis in lipid-fed rabbits. Cardiology 89: 189–194, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Suzuki YJ, Jain V, Park AM, Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med 40: 1683–1692, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tauman R, O'Brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath 11: 77–84, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Toth PT, Lecuona E, Witters LA, Schumacker PT, Chandel NS, Seeger W, Sznajder JI. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest 118: 752–762, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vesela A, Wilhelm J. The role of carbon dioxide in free radical reactions of the organism. Physiol Res 51: 335–339, 2002 [PubMed] [Google Scholar]
- 66.Welsh DJ, Scott P, Plevin R, Wadsworth R, Peacock AJ. Hypoxia enhances cellular proliferation and inositol 1, 4, 5-triphosphate generation in fibroblasts from bovine pulmonary artery but not from mesenteric artery. Am J Respir Crit Care Med 158: 1757–1762, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Wiegand L, Zwillich CW. Obstructive sleep apnea. Dis Mon 40: 197–252, 1994 [DOI] [PubMed] [Google Scholar]
- 68.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353: 2034–2041, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107: 1129–1134, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]
- 71.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165: 1217–1239, 2002 [DOI] [PubMed] [Google Scholar]