Abstract
The natural plant alkaloids caffeine and theophylline were the first adenosine receptor (AR) antagonists described in the literature. They exhibit micromolar affinities and are non-selective. A large number of derivatives and analogs have subsequently been synthesized and evaluated as AR antagonists. Very potent antagonists have thus been developed with selectivity for each of the four AR subtypes.
Keywords: adenosine receptors, A1 receptor antagonists, A2A receptor antagonists, A2B receptor antagonists, A3 receptor antagonists, caffeine, deazaxanthines, molecular probes, paraxanthine, theobromine, theophylline, tricyclic xanthine derivatives, xanthines
1. Caffeine and theophylline - historical aspects and early structural modification
1.1. Naturally occuring xanthines
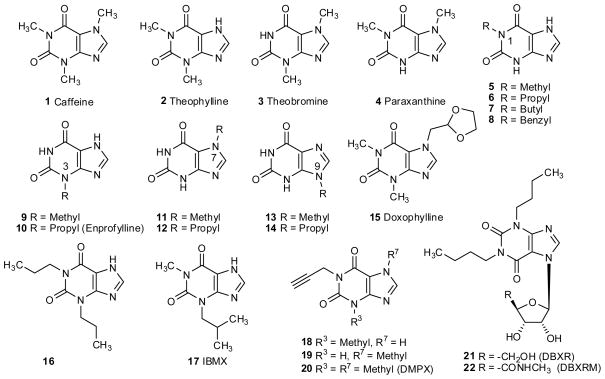
The earliest adenosine receptor (AR) antagonists identified were the naturally-occurring alkylxanthines, most notably among these being caffeine (1,3,7-trimethylxanthine, 1) and theophylline (1,3-dimethylxanthine, 2) (see Fig. 1) (Daly 1982; Fredholm 1999; Stefanovich 1989). Another simple natural xanthine, theobromine (3) was shown to have only weak activity as AR antagonist (Müller et al. 1993a). The major caffeine metabolites in humans, paraxanthine (4) and 1-methylxanthine (5) (Krämer and Testa 2008), the latter being also the major metabolite of theophylline, are similarly potent as caffeine and theophylline and may contribute their activity (Daly et al. 1986a; Müller et al. 1993b). These simple alkylxanthines are of micromolar affinity, at best, at the ARs, and variation of affinity between species has been documented (see table 1). This affinity range was later shown to apply generally to all human AR subtypes, A1, A2A, A2B and A3, but only to three of the AR subtypes of the rat (A1, A2A, and A2B). At the rat A3AR, the simple alkylxanthines were shown to have much higher Ki values of ~ 10−4 M or higher (Fredholm et al. 1994; van Galen et al. 1994).
Fig. 1.
8-Unsubstituted xanthine derivatives
Table 1.
Adenosine receptor affinities of 8-unsubstituted xanthine derivatives
| Ki (nM)a | |||||
|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | ||
| Natural xanthine (X) derivatives | |||||
| 1 | Caffeine (1,3,7-TrimethylX) | 10,700 (h)1 44,900 (h)2 41,000 (r)3 44,000 (r)4 47,000 (gp)5 44,000 (c)5 |
23,400 (h)2 9,560 (h)1 45,000 (r)4 32,500 (r)6 48,000 (r)1 |
33,800 (h)7 10,400 (h)8 20,500 (h)9 30,000 (r)10 13,000 (m)10 |
13,300 (h)1 >100,000 (r)11 |
| 2 | Theophylline (1,3-DimethylX) | 6,770 (h)12 14,000 (r)13 8,740 (r)1 7,060 (gp)14 4,710 (rb)14 9,050 (s)14 6,330 (c)14 |
1,710 (h)12 6,700 (h)1 22,000 (r)13 25,300 (r)1 |
9,070 (h)8 74,000 (h)9 15,100 (r)8 5,630 (m)15 11,000 (gp)16 17,700 (rb)15 38,700 (d)15 |
22,300 (h)1 86,400 (h)12 >100,000 (r)11 85,000 (r)17 >100,000 (d)18 |
| 3 | Theobromine (3,7-DimethylX) | 105,000 (r)13 83,400 (r)19 |
>250,000 (r)13 187,000 (r)19 |
130,000 (h)19 | >100,000 (r)11 |
| 4 | Paraxanthine (1,7-DimethylX) | 21,000 (r)13 | 32,000 (r)13 | 4,500 (h)20 | >100,000 (r)11 |
| Mono-substituted xanthine derivatives | |||||
| 5 | 1-MethylX | 36,000 (r)13 11,400 (r)19 |
47,000 (r) 13 36,200 (r)19 |
6,600 (h)19 | >100,000 (r)11 |
| 6 | 1-PropylX | 13,000 (r) 13 | 33,000 (r) 13 | 360 (h)8 1,880 (r)8 |
2,370 (h)8 |
| 7 | 1-ButylX | 9,000 (r) 13 | 61,000 (r) 13 | 421 (h)8 | 4,610 (h)8 |
| 8 | 1-BenzylX | 2,800 (r) 13 | 22,000 (r) 13 | nd | nd |
| 9 | 3-MethylX | >100,000 (r) 13 35,000 (r) 21 |
59,000 (r) 13 | 87,000 (h)20 | >100,000 (r)11 |
| 10 | Enprofylline (3-PropylX) | 156,000 (h)22 42,000 (h)1 32,000 (r)13 29,100 (r)19 >100,000 (d)18 |
32,000 (h)22 81,300 (h)1 137,000 (r)13 103,000 (r)19 |
7,000 (h)22 4,730 (h)8 19,800 (h)23 26,000 (r)24 5,630 (m)15 5,840 (rb)15 6,960 (d)15 |
92,600 (h)1 65,000 (h)22 93,000 (r)1 >100,000 (d)18 |
| 11 | 7-MethylX | 33,000 (r) 13 | 59,000 (r) 13 | 97,000 (h)20 | >100,000 (r)11 |
| 12 | 7-PropylX | 18,000 (r) 13 | >200,000 (r) 13 | nd | nd |
| 13 | 9-MethylX | >250,000 (r) 13 | >250,000 (r) 13 | >1,000,000 (h)20 | >100,000 (r)11 |
| 14 | 9-PropylX | >250,000 (r) 13 | >250,000 (r) 13 | nd | nd |
| Di- and trisubstituted xanthine derivatives | |||||
| 15 | Doxofylline | ca. 100,00025 | ca. 100,00025 | nd | nd |
| 16 | 1,3-DipropylX | 700 (r)13 450 (r)19 1,310 (gp)5 340 (c)5 |
6,600 (r)13 5,160 (r)19 |
1,110 (h)8 680 (h)20 |
1,940 (h)1 |
| 17 IBMX | 3-Isobutyl-1-methylX | 7,000 (r)13 2,460 (r)19 8,600 (gp)5 4,400 (c)5 |
16,000 (r)13 13,800 (r)19 |
3,500 (h)19 | nd |
| 18 | 3-Methyl-1-propargylX | 820 (r) 13 5,830 (r)8 |
4,800 (r) 13 33,600 (r)8 |
511 (h)8 2,150 (r)8 |
10,900 (h)8 |
| 19 | 7-Methyl-1-propargylX | 22,000 (r) 13 | 16,000 (r) 13 | nd | nd |
| 20 DMPX | 3,7-Dimethyl-1-propargylX | 45,000 (r)4 11,000 (r)5 25,800 (gp)5 16,400 (c)5 |
16,000 (r)4 5,600 (r)6 |
4,130 (h)8 | >10,000 (r)26 |
| 21 | DBXR | 4,190 (r)11 | 19,500(r)11 | nd | 6,030 (r)11,b |
| 22 | DBXRM | 37,300 (r)27 | >100,000 (r)27 | nd | 229 (r)27,c |
h = human; c = cow; d = dog; gp = guinea pig; m= mouse; r = rat; rb = rabbit; a few A2B data are from functional (cAMP) studies; nd = no data available
partial agonist;
full agonist
1.1. Early modification of 8-unsubstituted xanthine derivatives
The first xanthine analogues with enhanced affinity at the ARs were modified from caffeine and theophylline (Daly 2000; Dlay 2007; Müller et al. 1993a; Fredholm et al. 2009). In the early 1980s, one particular type of modification of the xanthine structure proved to be especially useful in enhancing affinity: the elongation of the 1,3-dimethyl groups to propyl or larger alkyl groups (Bruns 1981; Ukena et al. 1986b). For example, substitution of the 1,3-dimethyl groups with 1,3-dipropyl groups in 16 increased affinity at the rat A1AR by ~ 20-fold (table 1). Substitutions at the 1-, 3-, or 7-positions, particularly small hydrophobic groups, were generally much better tolerated in AR binding than substitution at the 9-position (Daly 1982; Müller et al. 1993a). Evaluation of a series of mono-substituted xanthines (5–14) showed that substitution at N1 was most important for all AR subtypes (Müller et al., 1993b). While a 1-propyl residue was best for A2B and A3 receptors, a 1-benzyl residue was optimal for the A1 and A2A AR subtypes (see table 1). 1-Propylxanthine (6) shows the highest affinity of the small, simple xanthine derivatives for the human A2B receptor (Ki 360 nM) along with some selectivity (at least 7-fold vs. the other subtypes) (Kim et al. 2002), while 3-propylxanthine (enprofylline, 10) is less potent (Ki human A2B 4,730 nM), but even more selective (at least 14-fold).
3,7-Dimethyl-1-propargylxanthine (DMPX, 20) was the first A2-selective AR antagonist described in the literature (Ukena et al. 1986b; Seale et al. 1988). It is similarly potent at A2A and A2B receptors, but the degree of selectivity versus A1 receptors is low (Jacobson et al. 1992a). A comparison with the 7-unmethylated derivative 3-methyl-1-propargylxanthine (18) indicated that a 7-methyl group led to a large decrease in A1 affinity and thus increased selectivity for A2A or A2B (Müller et al. 1993b; Kim et al. 2002). The theophylline derivative doxofylline 15 bearing a 1,3-dioxolan-2-ylmethyl residue in the 7-position is virtually inactive at A1 and A2AAR and is believed to exert its antiasthmatic activity via inhibition of phosphodiesterases (Cirillo et al. 1988; Shukla et al. 2009).
The branched analogue IBMX 17 shows potency as a phosphodiesterase (PDE) inhibitor in the same concentration range as is required to block ARs (Ukena et al. 1993).
1,3-Dibutylxanthine-7-ribosides (21,22) were found to bind effectively at A3 receptors indicating that the ribose group – as in adenosine – can act as a secondary anchor or recognition moiety in the receptor binding site (van Galen et al. 1994; Kim et al. 1994b; Park et al. 1998). This series of xanthine-7-ribosides also provided an early indication of modes of ligand binding at ARs, i.e. the overlay of receptor-bound positions of xanthine and adenine moieties. The 5′-uronamide modification of the CH2OH group of the ribose moiety greatly enhanced A3AR affinity as was shown for adenosine agonists, such that DBXRM was 143-fold selective (Kim et al. 1994b). DBXRM (22) was found to be a full agonist at the A3AR, unlike other xanthine derivatives. It is proposed that the ribose moiety contains the essential structure and required flexibility to effect the conformational change of the receptor needed for activation (Gao et al. 2002).
1.2. Progression to xanthines with subtype selectivity
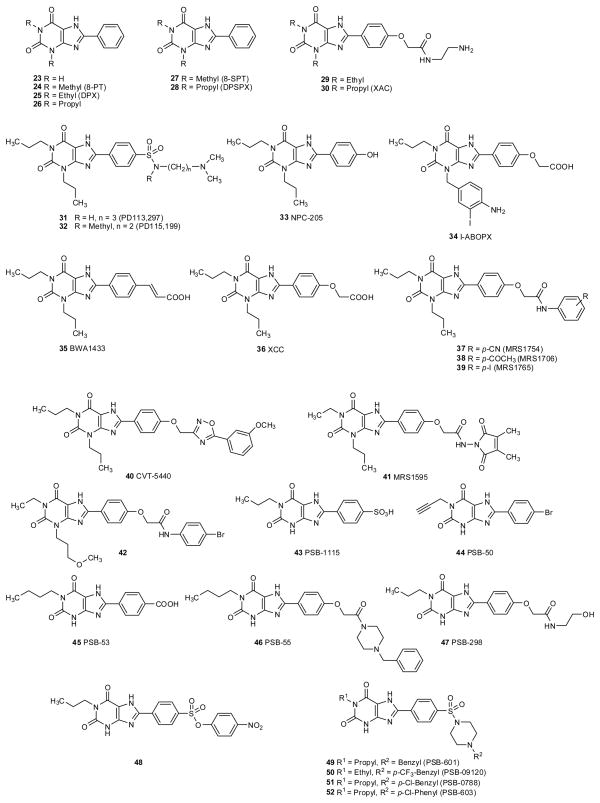
In addition to substitution of the 1,3-dimethyl groups with larger 1,3-dialkyl groups, a means of increasing affinity at the rat A1AR was found to be the introduction of 8-aryl substituents (fig. 2 and table 2) (Bruns 1981; Daly et al. 1986b; Jacobson et al. 1988; Jacobson et al. 1992a; Müller et al. 1996). For example, placement of a phenyl group at the 8-position, generally increased A1AR affinity by at least an order of magnitude (table 2). The first analogue having both 1,3-dialkyl and 8-phenyl modifications to be studied in detail was 1,3-diethyl-8-phenylxanthine (DPX, 25), which diplayed a Ki value of 44 nM at the rat A1AR and was beginning to show selectivity for that subtype (Bruns et al. 1987a). [3H]DPX was demonstrated as the first AR antagonist radioligand, but its hydrophobicity limited its use (Bruns et al. 1980). Homologation to the 1,3-propyl groups in the 8-aryl analogue 26 provided a desired boost in affinity, however, the unintended consequence of rapidly diminishing aqueous solubility made this series unable to be used in typical pharmacological studies (Ukena et al. 1986b; Bruns and Fergus 1989). The 8-p-hydroxyphenyl-substituted derivative 33 (NPC-205) was slightly more potent than 26 (Shamim et al. 1988). The low aqueous solubility, which is both a function of lipophilic groups present on the xanthine and the tendency of 8-arylxanthine derivatives to form highly stable crystal lattices, resulted in low bioavailability of 26 and similar compounds (Müller et al. 1996; Frédérick et al. 2005).
Fig. 2.
8-Phenyl- and 8-phenylalkyl-substituted xanthines and heteroaromatically substituted derivatives
Table 2.
Adenosine receptor affinities of 8-phenyl- and 8-phenylalkyl-substituted xanthines and heteroaromatically substituted derivatives
| Ki (nM)a | |||||
|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | ||
| First-generation 8-phenylxanthine derivatives | |||||
| 23 | 8-PhenylX | 2,500 (r)1 | 21,000 (r)1 | 810 (h)2 | nd |
| 24 | 8-Phenyl-theophylline (8-PT) | 1,340 (h)3 115 (h)4 86 (r)5 76 (r)6 1,540 (gp)6 7.6 (c)6 |
454 (h)3 850 (r)5 |
415 (h)7 436 (m)8 249 (rb)8 371 (d)8 |
1,250 (h)3 >100,000 (r)9 |
| 25 | 1,3-Diethyl-8-phenylX (DPX) | 44 (r)10 | 860 (r)10 190 (h)11 |
62.0 (h)7 | nd |
| 26 | 1,3-Dipropyl-8-phenylX | 10 (r)6 0.22 (c)6 20.9 (gp)6 |
180 (r)12 2100 (h)13 |
18.9 (h)7 | nd |
| 27 | SPT | 1,000 (h)3 4,500 (r)5 1,000 (r)6 10,100 (gp)6 6,460 (d)14 300 (c)6 |
7,050 (h)3 14,000 (r)5 |
1,330 (h)7 1,590 (r)15 4,990 (m)8 2,190 (gp)15 2,370 (rb)8 7,240 (d)8 224 (d)15 |
5,890 (h)16 11,000 (h)17 ≫10,000 (r)16 25,300 (d)14 |
| 28 | DPSPX | 210 (r)5 140 (r)9 |
1,400 (r)5 790 (r)9 |
568 (m)8 200 (rb)8 721 (d)8 |
183 (s)18 >100,000 (r)9 22,500 (rb)19 |
| 29 | 12 (r)20 | 83 (r)20 | nd | nd | |
| 30 | XAC | 6.8 (h)21 29.1 (h)22 1.2 (r)23 0.49 (r)19 5.49 (gp)19 0.45 (rb)19 0.09 (s)19 0.03 (c)19 159 (d)14 |
18 (h)21 1.00 (h)22 63 (r)23 |
7.8 (h)23 16.0 (h)7 42.7 (r)15 4.51 (m)8 17.8 (gp)15 4.47 (rb)8 29.8 (d)8 3.55 (d)15 |
91.9 (h)22 26 (h)21 71 (h)14 29,000 (r)14 106 (rb)14 180 (s)18,24 138 (d)14 |
| 31 | PD113,297 | 5.59 (r)12 | 70.0 (r)12 | nd | nd |
| 32 | PD115,199 | 14 (r)25 4.05 (r)20 |
16 (r)25 3.86 (rb)26 |
160 (m)27 | nd |
| 33 | NPC-205 | 3.5 (r)28 | 48 (h)28 | 50 (gp)29 | nd |
| 34 | I-ABOPX (BW-A522) | 70 (h)45 37 (r)30 601 (d)14 |
95 (h)45 700 (r)30 |
30 (h)45 | 18 (h)24 1,170 (r)31 1,500 (r)14 179 (rb)14 37.5 (d)14 |
| 35 | BWA1433 | 20 (r)30 132 (d)14 |
nd | 15.6 (h)45 | 54 (h)24 15,000 (r)14 384 (rb)14 1,880 (d)14 |
| 36 | XCC | 175 (h)21 58 (r)23 |
2200 (h)21 595 (h)21 |
13.6 (h)23 40 (h)7 2,200 (r)23 |
3,910 (h)21 75,700 (r)21 |
| A2B-selective 8-phenylxanthine derivatives and heteroaromatically substituted derivatives | |||||
| 37 | MRS1754 | 403 (h)21 16.8 (r)21 |
503 (h)21 612 (r)21 |
1.97 (h)21 12.8 (r)21 16.6 (r)15 3.39 (m)8 9.12 (gp)15 1.79 (rb)8 12.8 (d)8 12.3 (d)15 |
570 (h)21 |
| 38 | MRS1706 | 157 (h)21 38 (r)21 |
112 (h)21 548 (r)21 |
1.4 (h)21 | 230 (h)21 |
| 39 | MRS1765 | 152 (h)21 15.7 (r)21 |
293 (h)21 1640 (r)21 |
2.13 (h)21 | 1270 (h)21 |
| 40 | CVT-5440 | >10,000 (h)32 | >10,000 (h)32 | 50 (h)32 | >10,000 (h)32 |
| 41 | MRS1595 | 3,030 (h)21 11.1 (r) 21 |
1,970 (h) 21 126 (r) 21 |
26.6 (h) 21 | 670 (h) 21 |
| 42 | 100 (r)33 | 97.7 (h)33 | 2.88 (h)33 | 1,290 (h)33 | |
| 43 | PSB-1115 | >10,000 (h) 2 2,200 (r)1 |
24,000 (r)1 | 53.4 (h) 2 | >10,000 (h) 2 |
| 44 | PSB-50 | 60 (r)2 | 199 (r)2 | 6.8 (h) 2 | 477 (h) 2 |
| 45 | PSB-53 | 1,181 (h)2 481 (r)2 |
ca. 10,000 (h)2 3,800 (r)2 |
24 (h) 2 | 4,622 (h) 2 |
| 46 | PSB-55 | 122 (h)2 37 (r) 2 |
ca. 10,000 (r) 2 550 (r) 2 |
1.3 (h) 2 | 475 (h) 2 |
| 47 | PSB-298 | 68 (h)2 35 (r) 2 |
2,139 (r) 2 | 1.2 (h) 2 60 (h)34 |
422 (h) 2 |
| 48 | 3.6 (r)35 | 74 (r)35 | 5.4 (h)35 | ≥10,000 (h)35 | |
| 49 | PSB-601 | 2,067 (h)36 260 (r)36 |
484 (h)36 93.7 (r)36 |
3.6 (h)36 | >1,000 (h)36 |
| 50 | PSB-09120 | >10,000 (h)37 >1,000 (r) 37 |
22.7 (h) 37 122 (r) 37 |
0.157 (h) 37 | >10,000 (h) 37 |
| 51 | PSB-0788 | 2,240 (h)37 386 (r) 37 |
333 (h) 37 1,730 (r) 37 |
0.393 (h) 37 | >1,000 (h) 37 |
| 52 | PSB-603 | >10,000 (h)37 >10,000 (r) 37 |
>10,000 (h) 37 >10,000 (r) 37 |
0.553 (h) 37 KD 0.403 (h) 37 KD 0.351 (m) 37 |
>10,000 (h) 37 |
| 53 | CVT-6694 | >6,000 (h)38 | >5,000 (h)38 | 7 (h)38 | >9,000 (h)38 |
| 54 | CVT-7124 | >6,000 (h)38 | >5,000 (h)38 | 6 (h)38 | >9,000 (h)38 |
| 55 | CVT-6883 | 1,940 (h)39 | 3,280 (h)39 | 22 (h) 39 | 1,070 (h) 39 |
| 56 | MRE-2029-F20 | 200 (h)40 | >1,000 (h) 40 | 5.5 (h) 40 | >1,000 (h) 40 |
| 57 | ATL 802 | 369 (h)41 9,583 (m)41 |
654 (h)41 8,393 (m)41 |
2.36 (h)41 8.58 (m)41 |
>1,000 (h)41 >10,000 (m)41 |
| 58 | ATL 852 | nd | nd | 28.5 (h)c | nd |
| 8-Phenylalkyl-substituted xanthines | |||||
| 59 | MDL 102,503 | 6.9 (r)42 | 157 (r)42 | nd | nd |
| 60 | 60.7 (r)42 | 848 (r)42 | nd | nd | |
| 61 | MDL 102,234 | 23.2 (r)42 | 3,510 (r)42 | nd | nd |
| 62 | L-97-1 | 580 (h)43 | >100,000 (h)43 | >100,000 (h)43 | nd |
| 63 | 102 (r)44 | 83.2 (h)44 | 7.41 (h)44 | 10,000 (h)44 | |
h = human; c = cow; d = dog; gp = guinea pig; m= mouse; r = rat; rb = rabbit; a few A2B data are from functional (cAMP) studies; nd = no data available;
personal communication (J. Linden), also see 41
Several approaches were taken to increase the water solubility. A sulfonate group was introduced at the p-position of the 8-phenyl ring, which greatly increased solubility (Daly et al. 1985; Shamim et al. 1989). However, this modification tended to decrease both affinity and selectivity, in comparison to the uncharged 8-phenyl analogues. Thus, SPT (27) and DPSPX (28), the latter of which is somewhat more potent, were both useful in pharmacological experiments where a blockade of all AR subtypes is required. It has to be kept in mind that these compounds do not block rat A3 receptors but are active at other species like human and sheep (table 2). SPT (27) was shown not to penetrate into the brain due to its high polarity (Baumgold et al. 1992).
2. Adenosine A1 receptor antagonists
2.1. 8-Aryl- and 8-arylalkyl-substituted xanthines
An alternative approach to the introduction of charged groups for increasing water solubility resulted in the synthesis of the 8-aryl derivatives in which the charged group was separated from the phenyl ring by a spacer group. Various substitutions of an 8-phenyl ring indicated that an electron-donating group provided a favorable AR affinity (Jacobson et al. 1985b; Shamim et al. 1988). Thus, a methoxy substituent was elaborated into a carboxymethyloxy group resulting in carboxylic congener XCC 36 and amine congener 8-[4-[[[[(2-aminoethyl)amino]carbonyl]methyl]oxy]phenyl]-l,3-dipropylxanthine (XAC, 30) (fig. 2 and table 2) (Jacobson et al. 1985b; Jacobson et al. 1999). By placing the charged group at a distance from the 8-aryl ring, it was possible to maintain and even enhance the high affinity seen with neutral, but poorly soluble analogues. Thus, XAC was found to have a Ki value of 1.2 nM at the rat A1AR and ~50-fold selectivity in comparison to the rat A2AR. The initial measure of A2AR affinity used in Jacobson et al. (1985b) was the inhibition of cyclic AMP accumulation in rat brain slices, which corresponds more closely to the A2BAR, rather than the A2AAR. However, subsequent tests at the rat A2AAR confirmed that there was still a margin of selectivity of XAC in binding to the A1AR in rat (Ukena et al, 1986c). The substitution of the 1,3-dipropyl groups of XAC with 1,3-diethyl increased affinity at the A2AAR while decreasing it at the A1AR (Jacobson et al. 1987a). The aqueous solubility of XAC was found to be 25 μM, which was an improvement over the uncharged 8-aryl derivatives. Therefore, XAC was suitable for use in pharmacological experiments as a general AR antagonist and was the first such antagonist to display moderate A1AR selectivity at least in rat.
Given the promise of a relatively water-soluble and somewhat selective xanthine derivative, this amine-functionalized derivative of 1,3-dipropyl-8-phenylxanthine was specifically radiolabeled on the 1,3-dipropyl groups by catalytic reduction of a 1,3-diallyl precursor. The resulting [3H]XAC was useful as a radiotracer in binding experiments at rat cerebral cortical A1ARs with a KD value of ~ 1 nM, and was thus the first generally useful antagonist radioligand for study of this receptor (Jacobson et al. 1986a). [3H]XCC 36 was also introduced as a high affinity radioligand for the A1AR (Jarvis et al. 1987).
Another rationale for the design of XAC with a primary amino group was the “functionalized congener approach” to drug design (Jacobson et al. 1986b; Jacobson 2009). By this approach, a chemically functionalized chain is incorporated at an insensitive site on the xanthine pharmacophore and can be extended to enable a conjugation strategy. Such high affinity conjugates are useful for AR characterization and can be coupled to radioactive or spectroscopic reporter groups without losing the ability to bind to the receptor (Jacobson et al. 1987b). XAC was also used as an immobilized high-affinity ligand for the purpose of affinity chromatography leading to the isolation of both A1 and A2A receptors and their purification to homogeneity (Olah et al. 1989; Weiss and Grisshammer 2002). While in XAC the polar, basic residue was connected to the 8-phenyl ring via ether and amide linkages, in another series sulphonamide-linked derivatives were investigated (31,32, fig. 2). Compound 31 and its congeners were potent A1 antagonists, but showed only a moderate degree of selectivity (table 2) (Bruns et al. 1986; Bruns et al. 1987a).
Besides 8-phenylxanthine derivatives 8-benzyl- (62) and 8-phenylethyl-substituted xanthines (59–61) have also been investigated and optimized with respect to A1 affinity (Peet et al. 1993). Among 8-(arylalkyl)-xanthine derivatives, 3-[2-(4-aminophenyl)ethyl]-8-benzyl-7-{2-ethyl(2-hydroxyethyl)amino]ethyl}-1-propyl-3,7-dihydropurine-2,6-dione (L-97-1, 62) is weaker in binding than typical 8-arylxanthine probes, but is a water soluble A1 AR antagonist bearing a basic substituent at N7 that has been proposed for the treatment of asthma (Obiefuna et al., 2005). 1,3-Dipropyl-8-phenylethyl-xanthine derivatives with a methyl (59–60) or ethyl (61) substituent at the α-carbon atom adjacent to the xanthine C8-position showed particularly high affinity and selectivity for A1AR with a configurational preference for the (R)- over the (S)-enantiomer (Peet et al. 1993).
2.2. 8-Cycloalkylxanthines
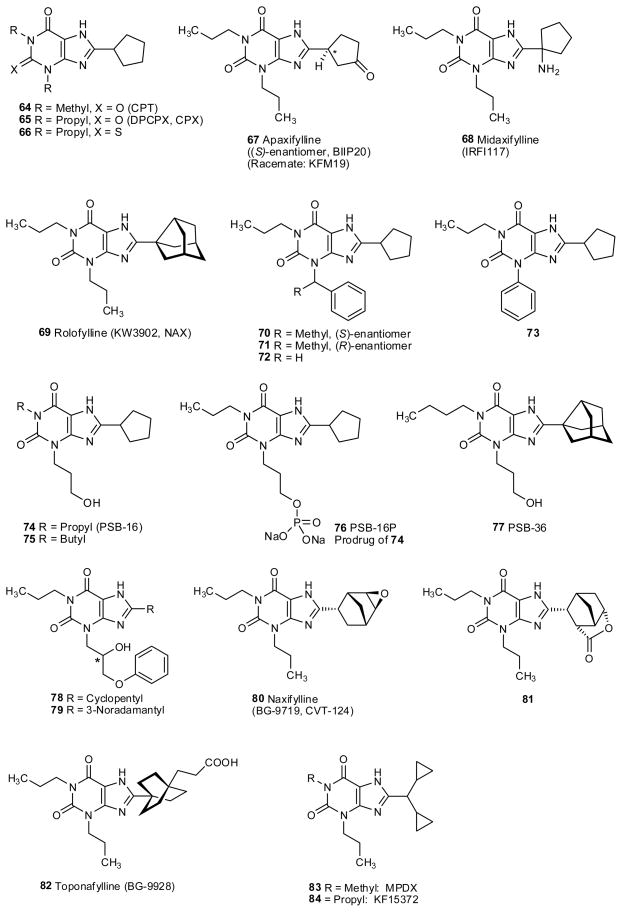
The introduction of 8-cycloalkyl groups instead of 8-aryl groups proved to be beneficial for affinity at the A1AR, and also allowed sufficient aqueous solubility for broad pharmacological application (fig. 3 and table 3). The 8-cycloalkylxanthine derivative that is most widely used as a pharmacological tool is DPCPX (65, also known as CPX), which is ~500-fold selective for rat A1AR in comparison to the A2AAR (Bruns et al. 1987a). Among the human ARs, the A1AR selectivity is less than in the rat (table 3). The corresponding cyclohexyl analogue is similar in its pharmacological profile (Shamim et al. 1989). Curiously, DPCPX was in clinical trials for the treatment of cystic fibrosis through a non-AR related mechanism. It was found to act on the CFTR (cystic fibrosis-related chloride transporter) to enhance chloride in cells systems, an action that is unrelated to its AR antagonism (Cohen et al. 1997; Sorbera et al. 2000).
Fig. 3.
8-Cycloalkylxanthines
Table 3.
Adenosine receptor affinities of 8-cycloalkylxanthines
| Ki (nM)a | |||||
|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | ||
| 64 | 8-Cyclopentyl-theophylline | 24 (r)1 6.3 (r)3 26.1 (gp)3 6.4 (rb)3 2.9 (s)3 1.4 (c)3 |
1,400 (r)1 3,170 (r)27 |
710 (h)2 902 (h)27 |
~100,000 (h)1 >10,000 (r)27 |
| 65 | DPCPX (CPX) | 3.0 (h)4 0.50 (r)4 1.0 (r)5 0.18 (r)3 1.06 (gp)3 3.9 (gp)6 0.21 (rb)3 0.10 (s)3 0.05 (c)3 0.29 (c)6 11.4 (d)7 |
129 (h)8 60 (h)9 157 (r) 10 500 (r)5 |
51 (h)4 63.8 (h)5 186 (r)5 200 (r)11 86.2 (m)12 145 (gp)11 96.0 (rb)12 147 (d)12 132 (d)11 |
795 (h)13 243 (h)4 509 (h)7 3,960 (h)8 >10,000 (r)9 43,000 (r)7 708 (rb)7 115 (d)7 |
| 66 | 2-Thio-CPX | 0.655 (r)14 | 314 (r)14 | 2800 (h)15 | nd |
|
67 KFM19 (rac.) BIIP-20 (S-(−)) |
Apaxifylline (S-(−)-configurated enantiomer) | 10.5 (mk)16,b 3 (r)17 |
1,512 (mk)16,b 2,640 (r)17 |
nd | nd |
|
68 IRFI117 |
Midaxifylline (8-(1-Aminocyclo-pentyl)-1,3-dipropylX | 2618 | 54,60018 | nd | nd |
| 69 KW3902 (NAX) | Rolofylline 1,3-Dipropyl-8-(3-noradamantyl)X | 0.72 (h)19 8.0 (h)20 0.19 (r)21 12.6 (r)19 |
108 (h)19 673 (h)20 380 (r)21 510 (r)19 |
296 (h)20 | 4,390 (h)20 |
| 70 | 1-Propyl-3-(S)-1-methylbenzyl-8-cyclopentylX | 10.1 (r)9 | 3,500 (r)9 | 8,000 (h)9 | 85 (h)9 >10,000 (r)9 |
| 71 | 1-Propyl-3-(R)-1-methylbenzyl-8-cyclopentylX | 23.8 (r)9 | 2,400 (r)9 | 2,960 (h)9 | 370 (h)9 |
| 72 | 1-Propyl-3-benzyl-8-cyclopentylX | 24.3 (h)9 8.70 (r)9 |
511 (r)9 | 8,000 (h)9 | 54.6 (h)9 |
| 73 | 1-Propyl-3-phenyl-8-cyclopentylX | 7.1 (h)9 1.01 (r)9 |
1,200 (h)9 492 (r)9 |
625 (h)9 | 395 (h)9 |
| 74 PSB-16 | 1-Propyl-3-(3-hydroxypropyl)-8-cyclopentylX | 5.74 (h)9 0.57 (r)9 |
664 (r)9 | 194 (h)9 | 3,100 (h)9 |
| 75 | 1-Butyl-3-(3-hydroxypropyl)-8-cyclopentyl X | 0.45 (r)9 | 582 (r)9 | nd | 1,190 (h)9 |
| 77 PSB-36 | 1-Butyl-3-(3-hydroxypropyl)-8- (3-noradamantyl)X | 0.7 (h)9 0.124 (r)9 |
980 (h)9 552 (r)9 |
187 (h)9 | 2,300 (h)9 6,500 (r)9 |
| 78 | 49 (h)22 55 (r)22 |
>10,000 (h)22 >10,000 (r)22 |
nd | 3,550 (h)22 | |
| 79 | 29 (h)22 21 (r)22 |
>10,000 (h)22 >10,000 (r)22 |
nd | >10,000 (h)22 | |
| 80 BG-9719 (CVT-124) | Naxifylline | 0.45 (h)19 12 (h)21 0.67 (r)19 |
1,100 (h)19 1,660 (h)21 1,250 (r)19 |
611 (h)21 1,010 (m)12 470 (rb)12 742 (d)12 |
4,810 (h)21 |
| 81 | 18 (h)21 3.0 (r)21 |
657 (h)21 264 (r)21 |
802 (h)21 | >1,000 (h)21 | |
| 82 BG-9928 | Toponafylline | 7.4 (h)20 3.9 (mk)23 1.3 (r)20 29 (d)23 |
6,410 (h)20 943 (mk)23 2,440 (r)20 4307 (d)23 |
90 (h)20 | >10,000 (h)20 |
| 83 | MPDX (1-Methyl analog of KF 15372) | 4.2 (r)24 | >100 (r)24 | nd | nd |
| 84 | KF 15372 | 0.99 (r) 25 3.0 (r)26 3.0 (gp)25 |
430 (r) 25 | nd | nd |
h = human; c = cow; d = dog; gp = guinea pig; m= mouse; mk = monkey; r = rat; rb = rabbit; a few data are from functional (cAMP) studies; nd = no data available A2B
data for the racemate (KFM-19)
More bulky cycloalkyl substituents in the xanthine 8-position, such as 3-noradamantyl (e.g. rolofylline (KW3902, 69) and 77), (substituted) norbornyl (naxifylline (BG-9719, CVT124, 80), and the lactone 81), dicyclopropylmethyl (MPDX, 83, and KF15372, 84) and bicyclo[2.2.2]octyl (toponafylline (BG-9928, 82)) yielded very potent and selective A1 antagonists (fig. 3 and table 3).
The 2-thio analog of DPCPX (65), 2-thio-DPCPX (66) was similarly potent and selective as 65 showing that in the 2-position a hydrogen bond acceptor (like a keto group) was not required (Jacobson et al. 1989b)). Replacement of the 3-propyl residue in DPCPX by a phenyl (73), benzyl (72), or a chiral methylbenzyl residue (70,71) was well tolerated (Weyler et al. 2006). However, the affinity for the human A3AR was increased by the lipophilic, aromatic residues, and the compounds lost A1-selectivity vs. A3. The introduction of polar hydroxy groups in the 3-position was well tolerated by the A1, but not by the A3AR leading to very potent and highly selective A1 antagonists (74,75,77–79) (Weyler et al. 2006; Massip et al. 2006). In fact, 1-butyl-3-(3-hydroxypropyl)8-(3-noradamantyl)xanthine (PSB-36, 77) is one of the most potent A1 antagonists described to date showing Ki values of 0.124 nM (rat), and 700 nM (human), respectively. The hydroxylated DPCPX derivative 74 (PSB-16) was converted to its phosphoric acid ester disodium salt, yielding a highly water-soluble A1 antagonist prodrug suitable for parenteral application without the need for detergents and organic solvents (Weyler et al. 2006).
Several other polar derivatives and analogs of DPCPX were developed in order to further improve water-solubility and bioavailability. Apaxifylline (67) with a keto group at C3 of the cyclopentyl ring was clinically evaluated as a memory-enhancing drug for the treatment of dementias (Schingnitz et al. 1991). An amino-substituted DPCPX derivative, midaxifylline (68) has also been investigated (Ceccarelli et al. 1995). A promising second-generation compound currently undergoing clinical trials for the treatment of chronic heart failure is toponafylline (82), which contains a carboxylate function (Doggrell, 2005). Rolofylline (69), an A1 antagonist of the first generation, had shown promising results in a pilot phase III study in patients with acute heart failure, but in a recently published larger phase III study (PROTECT) it did not exhibit significant improvement over placebo (Slawsky et al. 2009). Further potential applications for A1AR antagonists include hypertension and renal diseases due to their diuretic and kidney-protective effects. The feasibility of designing kidney-selective prodrugs of an A1AR antagonist has been demonstrated (Barone et al. 1989). Yet other applications are cardiac arrhythmia, asthma and other respiratory disorders, and the prevention of organ damage, e.g. resulting from transplantation (Jacobson et al. 1992a; Müller et al. 1996; Müller 1997; Jacobson et al. 2006; Moro et al. 2006; Givertz 2009).
2.3. Species differences
Among the human ARs, the A1AR affinity and selectivity (vs. A2A and A2BAR) of 8-cycloalkyl and 8-aryl derivatives is typically less than in the rat (see table 2 and 3). Several groups have studied the species dependence of the AR affinity of xanthine derivatives (see Ukena et al. 1986b; Klotz et al. 1991; Müller et al. 1993; Müller 1997; Kull et al. 1999; Fozard et al. 2003; Auchampach 2009). An early conclusion was that the affinity of typical 8-substituted analogues (both aryl and cycloalkyl) was greatest at the bovine A1AR, intermediate at the rat A1AR, and lowest at the porcine A1AR. Later, it was found that the human A1AR most closely resembled the porcine A1AR, in that respect. At the A2A and the A2BAR the opposite is true although differences are moderate: 8-substituted xanthines, such as XAC and DPCPX, are more potent at the human receptor than at the rat ortholog. The largest species differences are observed for the A3 receptor: 8-phenyl- and 8-cyclopentylxanthines are typically much more potent at the human than at the rat A3 receptor (Linden 1994; Ji et al 1994; Jacobson 1998; Müller 2001; Müller 2003).
2.4. Deaza- and azaxanthines
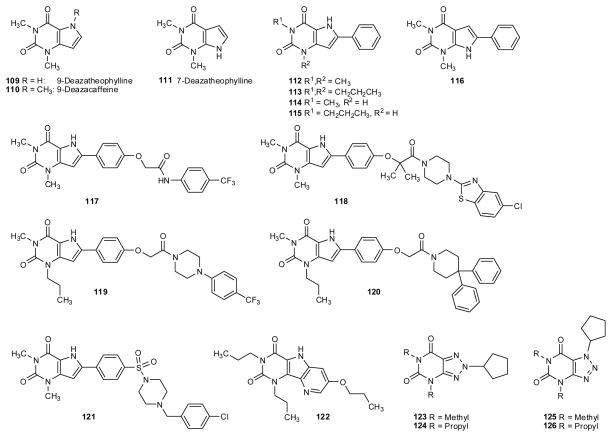
Analogs of xanthine derivatives, such as caffeine, theophylline, and 1,3-dialkyl-8-phenylxanthine have been synthesized which are lacking either the N7 (“7-deazaxanthines”) or the N9 nitrogen atom (“9-deazaxanthines”) in the imidazole partial structure (compounds 109–116, fig. 5 and table 5). It was found that the nitrogen atom in the 9-position was not required for high receptor affinity, the 9-deazaxanthines being even slightly more potent at A1AR in comparison with the corresponding xanthine derivatives (Grahner et al., 1994). To the contrary, 7-deazaxanthines were much less potent proving that the xanthines will bind as 7H-rather than 9H-tautomers to the receptors (Grahner et al., 1994). The addition of another nitrogen atom into the 8-position of xanthines was less successful: 8-azaxanthines (123–126, fig. 5 and table 5) showed only moderate affinity for the receptors (Franchetti et al. 1994) which can be explained by the lacking of the N7-H atom that is required as a hydrogen bond donor for high-affinity binding.
Fig. 5.
Deazaxanthines and azaxanthines
Table 5.
Adenosine receptor affinitities of deazaxanthines and azaxanthines
| Ki (nM)a | |||||
|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | ||
| Deazaxanthines | |||||
| 109 | 9-Deaza-theophylline | 5,400 (r)1 | 12,000 (r)1 | nd | nd |
| 110 | 9-Deazacaffeine | 32,000 (r)1 | 72,000 (r)1 | nd | nd |
| 111 | 7-Deaza-theophylline | 43,000 (r)1 | >250,000 (r)1 | nd | nd |
| 112 | 1,3-Dimethyl-8-phenyl-9-deazaX | 47 (r)1 | 510 (r)1 | nd | nd |
| 113 | 1,3-Dipropyl-8-phenyl-9-daX | 13 (r)1 | 450 (r)1 | nd | nd |
| 114 | 1-Methyl-8-phenyl-9-deazaX | 97 (r)1 | 2000 (r)1 | 520 (h)2 | 2098 (h)2 |
| 115 | 1-Propyl-8-phenyl-9-daX | 45 (h)2 39 (r)1 |
>10,000 (h)2 1,200 (r)1 |
42 (h)2 | 380 (h)2 |
| 116 | 1,3-Dimethyl-8-phenyl-7-deazaX | 3,1001 | 12,0001 | nd | nd |
| 117 | 14.8 (h)3 | 64.6 (h)3 | 3.02 (h)3 | >1,000 (h)3 | |
| 118 | >1,000 (h)4 | 10,000 (h)4 | 11.0 (h)4 | >1,000 (h)4 | |
| 119 | 89.1 (h)4 | 324 (h)4 | 2.04 (h)4 | 2,240 (h)4 | |
| 120 | 676 (h)4 | 3,550 (h)4 | 5.26 (h)4 | >1,000 (h)4 | |
| 121 | 183 (h)5 | nd | 1 (h)5 | 12,260 (h)5 | |
| 122 | Tricyclic 9-DeazaX | 346 (h)6 | 164 (h)6 | nd | 3.82 (h)6 |
| 8-Azaxanthines | |||||
| 123 | 1,3-Dimethyl-8-cyclopentyl-8-azaX | 110,000 (c)7 | 58,000 (c)7 | nd | nd |
| 124 | 1,3-Dipropyl-8-cyclopentyl-8-azaX | 1,300 (c)7 | 13,000 (c)7 | nd | nd |
| 125 | 1,3-Dimethyl-7-cyclopentyl-8-azaX | 11,000 (c)7 | 292,000 (c)7 | nd | nd |
| 126 | 1,3-Dipropyl-7-cyclopentyl-8-azaX | 340 (c)7 | 10,000 (c)7 | nd | nd |
h = human; c = cow; r = rat; a few A2B data may be from functional (cAMP) studies; nd = no data available
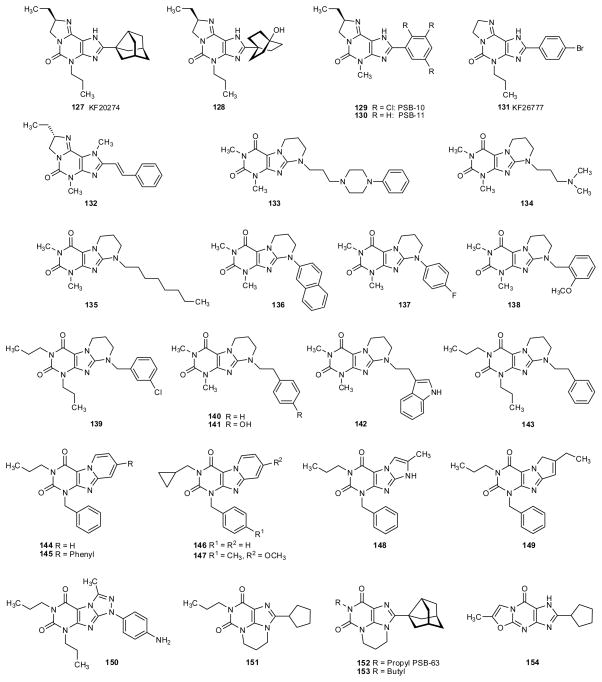
2.5. Tricyclic xanthine derivatives
Several different types of tricyclic xanthine derivatives have been prepared and investigated (fig. 6 and table 6). Cycloalkyl-substituted dihydro-imidazo[2,1-i]purinones (127,128) showed high A1 affinity and selectivity combined with improved water-solubility due to the presence of a basic nitrogen atom that can be protonated (Suzuki et al. 1992; Vu et al. 2006). A new class of heterotricyclic xanthine derivatives in which the 3-alkyl-substituent is tethered to the N9 atom – pyrimido[1,2,3-cd]purinediones (151–153) - was synthesized and investigated (fig. 6) (Weyler et al. 2006). Interestingly, the cyclopentyl-substituted derivative 151, an analog of DPCPX, was only weakly active probably due to the lacking of the N7-H atom. In contrast, the 3-noradamantyl-substituted analogs (152,153) showed relatively high A1 affinity. While propyl derivative 152 (PSB-63) was very selective versus the other AR subtypes, butyl derivative 153 was also quite potent at human A3AR (table 6). Another novel tricyclic analog of DPCPX, the oxazolo[3,2-a]purinone derivative 154, showed only weak affinity for AR (table 6) (Müller 1994). In a series of tricyclic pyrimido[2,1-f]purinediones the N,N-dipropyl-substituted derivative 139 (fig. 6) bearing a m-chlorobenzyl residue attached to the additional ring was a relatively potent A1 antagonist with some selectivity (table 6) (Drabczynska et al. 2007a).
Fig. 6.
Tricyclic xanthine derivatives
Table 6.
Adenosine receptor affinities of tricyclic xanthine derivatives
| Ki (nM)a | |||||
|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | ||
| Imidazo[2,1-i]purin-5-ones | |||||
| 127 | KF20274 | 2.7 (r)1 | 290 (r)1 | nd | nd |
| 128 | 22 (h)2 6 (r)2 |
4,400 (h)2 2,700 (r)2 |
580 (h)2 | >10,000 (h)2 | |
| 129 | PSB-10 | 1,700 (h)3 805 (r)4 |
2,700 (h)3 6,040 (r) 4 |
nd | 0.441 (h) 4 |
| 130 | PSB-11 | 1,640 (h)3 440 (r)3 |
1,280 (h)3 2,100 (r)3 |
2,100 (m)4 | 2.34 (h)3 KD 4.9 (h)5 |
| 131 | KF26777 | 1,800 (h)6 | 470 (h)6 | 620 (h)6 | 0.20 (h)6 |
| 132 | 14,900 (r)4 | 424 (r)4 | 3,700 (m)4 | 30,600 (h)4 | |
| Pyrimido[2,1-f]purinediones | |||||
| 133 | 15,000 (r)7 | 16,000 (r)7 | nd | nd | |
| 134 | 20,000 (r)7 | >250,000 (r)7 | nd | nd | |
| 135 | >25,000 (r)8 | 998 (r) 8 | 5,200 (h)8 | 12,300 (h)8 | |
| 136 | 26,800 (h)9 ≥25,000 (r)9 |
2,870 (h)9 219 (r)9 |
ca. 10,000 (h)9 | >10,000 (h)9 | |
| 137 | 16,700 (h)9 >25,000 (r)9 |
1,880 (h)9 147 (r)9 |
ca. 10,000 (h)9 | >10,000 (h)9 | |
| 138 | >25,000 (r)10 | 11,300 (h)10 699 (r)10 |
nd | nd | |
| 139 | 89 (r)10 | 478 (r)10 | nd | 1,290 (h)10 | |
| 140 | >10,000 (h)11 >25,000 (r)11 |
2,890 (h)11 320 (r)11 |
ca. 10,000 (h)11 | >10,000 (h)11 | |
| 141 | >25,000 (h)11 ca. 25,000 (r)11 |
630 (h)11 230 (r)11 |
7,200 (h)11 | >10,000 (h)11 | |
| 142 | >25,000 (h)11 ca. 25,000 (r)11 |
4,560 (h)11 330 (r)11 |
ca. 10,000 (h)11 | >10,000 (h)11 | |
| 143 | 620(r)11 | 860(r)11 | 590 (h)11 | 3,660 (h)11 | |
| Pyrido[2,1-f]purinediones | |||||
| 144 | 50 (h)12 | 119 (h)12 | nd | 4.0 (h)12 | |
| 145 | >10,000 (h)12 | >10,000 (h)12 | nd | 35 (h)12 | |
| 146 | >1,000 (h)13 | 242 (h)13 | nd | 4.2 (h)13 | |
| 147 | >1,000 (h)13 | >1,000 (h)13 | >1,000 (h)13 | 2.24 (h)13 | |
| Imidazo-, Pyrrolo- and Triazolopurinediones | |||||
| 148 | >1,000 (h)14 | >1,000 (h)14 | >1,000 (h)14 | 0.8 (h)14 | |
| 149 | >1,000 (h)14 | >1,000 (h)14 | >1,000 (h)14 | 3.5 (h)14 | |
| 150 | >10,000 (h)15 | 2,050 (h)15 | >100,000 (h)15 | 1,330 (h)15 | |
| 4,5-Dihydro-6H,8H-pyrimido[1,2,3-cd]purine-8,10-diones | |||||
| 151 | 1,440 (r)16 | 12,400 (r)16 | 42,600 (h)16 | nd | |
| 152 | PSB-63 | 16.9 (r)16 90.6 (h)16 |
22,000 (r)16 34,500 (h)16 |
3,190 (h)16 | >10,000 (h)16 |
| 153 | 40.6 (r)16 13.8 (h)16 |
23,400 (r)16 ca. 25,000 (h)16 |
22,300 (h)16 | 188 (h)16 | |
| Oxazolo[3,2-a]purinone | |||||
| 154 | 770 (r)17 | 20,600 (r)17 | nd | nd | |
h = human; m = mouse; r = rat; a few A2B data may be from functional (cAMP) studies; nd = no data available
3. Adenosine A2A receptor antagonists
A2AAR selective antagonists of both xanthine and nonxanthine classes have been developed and some have entered clinical trials for Parkinson’s disease, based on the opposing action of adenosine and dopamine in the striatal pathways in the brain (Richardson et al. 1997; Schapira et al 2006; Schwarzschild et al. 2006; Müller et al. 2007; Baraldi et al. 2008). The cellular mechanisms of the motor and neuroprotective effects of A2AAR antagonists have been explored (Yu et al. 2008). Recently, ameliorating effects of A2A antagonists including xanthine derivatives on animal models of Alzheimer’s disease and cognitive dysfunction have been reported (Dall’Igna et al. 2007; Cunha et al. 2008; Takahashi et al. 2008). Since the early 1990s, there has been a major medicinal chemical effort to increase the A2AAR selectivity of simple xanthines by structural modification.
Prior to the synthesis of truly A2AAR selective antagonists, certain high affinity xanthines were used in a nonselective fashion as probes of the A2AAR. For example, [3H]XAC (30) was useful as a radiotracer in binding experiments at the A2AAR in human platelets and was therefore the first antagonist radioligand with high affinity at the A2AAR (Ukena et al. 1986a). PD115,199 32 was prepared in tritiated form and shown to bind with high affinity to the rat A2AAR (Bruns et al. 1987b).
The first “selective” A2A receptor antagonist described in the literature was the caffeine analog 3,7-dimethyl-1-propargylxanthine (DMPX, 20, fig. 1 and table 1) (Ukena et al. 1986b). Like caffeine, the compound possesses low A2A receptor affinity and moderate selectivity versus A1 receptors. Nevertheless, this compound has been widely used in in vivo studies because of its good water solubility and bioavailability (Seale et al. 1988; Thorsell et al. 2007). Later on it was found that DMPX is equally potent at A2B as at A2A receptors. The species dependence of affinity at the A2A receptor of 1,3,7- and 1,3,8-trisubstituted xanthines has been reported (Stone et al. 1988).
An early example of a caffeine analogue that displayed selectivity for the A2A receptor was 8-trifluoromethylcaffeine, but the affinity was still low with at Ki value in binding at the rat A2AAR of 29 μM (Jacobson et al. 1993b). This effect of the 8-trifluoromethyl group was not observed in the corresponding (inactive) theophylline derivative. A 8-(trans-2-carboxyvinyl) derivative of caffeine also proved to be similarly selective for the A2A receptor.
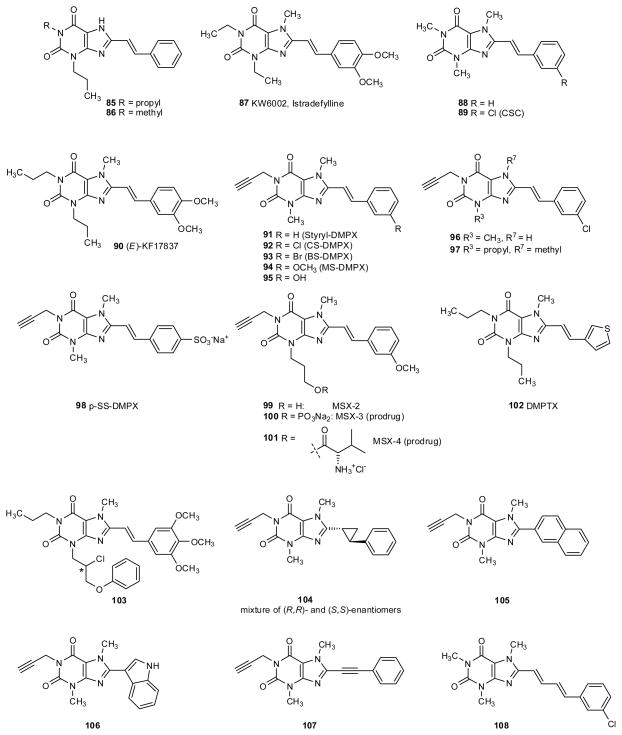
3.1. 8-Styrylxanthines
The observation that N7-methylation in 8-substituted xanthine derivatives was better tolerated by the A2A than the A1 receptor (Shamim et al. 1989), and that the 8-substituent had to be coplanar for achieving high A2A receptor affinity (Erickson et al. 1991) led to the first highly potent and selective A2A receptor antagonists: the 1,3,7-alkyl-substituted 8-styrylxanthine derivatives 87–95 and 99 (table 4).
Table 4.
Adenosine receptor affinities of 8-styrylxanthines and configurationally stable analogs
| Ki (nM)a | |||||
|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | ||
| Styrylxanthinesb | |||||
| 85 | 1,3-Dipropyl-8-stryrylX | 22.2 (r)1 | 85.1 (r)1 | nd | nd |
| 86 | 1-Methyl-3-propyl-8-styrylX | 31.1 (r)1 | 46.5 (r)1 | nd | nd |
| 87 | Istradefylline (KW-6002) (Ki MAO-B = 28,000 nM)2 | 841 (h)c 230 (r)c |
12 (h)3 91.2 (h)c 2.2 (r)4 4.46 (r)5 |
>10,000 (h)c | 4,470 (h)c |
| 88 | 8-Styrylcaffeine (Ki MAO-B = 2,864 nM)6 | 3890 (r)7 | 94 (r)7 | nd | nd |
| 89 | CSC (Ki MAO-B = 80.6 nM MAO-B)5 | 28,000 (r)7 | 54 (r)7 | 8,2008 | >10,000 (r)9 |
| 90 | KF17837 | 390 (r)10 | 7.9 (r)10 (E/Z) 1.0 (r)10 (E) |
1,500 (h)10 | nd |
| 91 | Styryl-DMPX | 1,100 (r)11 | 27 (r)11 | nd | nd |
| 92 | CS-DMPX | 1,300 (r)11 | 13 (r)11 | nd | nd |
| 93 | BS-DMPX | 1,200 (r)11 | 8.2 (r)11 10 (r)12 |
>10,000 (h)13 | >10,000 (h)13 |
| 94 | m-Methoxystyryl-DMPX | 1,280 (r)13 | 12 (r)13 | nd | nd |
| 95 | m-Hydroxystyryl-DMPX | 940 (r)13 | 21 (r)13 | nd | nd |
| 96 | 7-unsubst. analog of CS-DMPX | 250 (r)11 | 410 (r)11 | nd | nd |
| 97 | 3-Propyl analog of CS-DMPX | 102 (r)11 | 5.1 (r)11 | nd | nd |
| 98 | p-SS-DMPX | 4,900 (r)12 | 240 (r)12 | nd | nd |
| 99 | MSX-2 | 900 (r)14 2,500 (h)14 |
8.04 (r)13,14 5.38 (h)14,d 14.5 (h) 14,e |
>10,000 (h)14 2,900 (h)15 |
>10,000 (h)14 |
| 102 | DMPTX (8-(3-thienylethenyl)-1,3-dipropylX) | 561 (r)16 | 19 (r)16 | nd | nd |
| 103 | 44 (r)17 | >10,000 (r)17 | nd | nd | |
| Analogs of Styrylxanthines | |||||
| 104 | Phenylcyclopropyl-DMPX (trans, rac.) | 4,600 (r)18 | 1,700 (r)18 | nd | nd |
| 105 | β-Naphthyl-DMPX | 980 (r)18 | 380 (r)18 | nd | nd |
| 106 | 3-Indolyl-DMPX | 1,000 (r)18 | 300 (r)18 | nd | nd |
| 107 | Phenylethynyl-DMPX | >3,000 (r)18 | 314 (h)c 300 (r)18 |
nd | 5,000 (h)c |
| 108 | PhenylbutadienylX (Ki MAO-B = 42.1 nM)5 | nd | 104 (r)18 | nd | nd |
h = human; c = cow; d = dog; gp = guinea pig; m= mouse; mk = monkey; r = rat; rb = rabbit; a few A2B data are from functional (cAMP) studies; nd = no data available
most data probably represent data from mixture of E/Z isomers since in dilute solutions light-induced isomerization occurs very fast and is difficult to avoid under standard testing conditions
Müller et al., unpublished data
recombinant receptors expressed in CHO cells
native receptors (post-mortem human brain cortex)
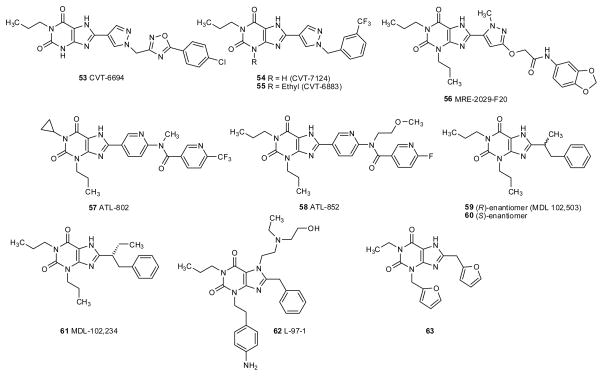
A small alkyl group at N1 (methyl, ethyl, propyl, propargyl) proved to be optimal for high A1 affinity and selectivity, while methylation is required in the 7-position (Jacobson et al. 1993a; Nonaka et al. 1994a; Shimada et al. 1997; Müller et al. 1998a; Müller et al. 2000; Kase 2003). The 8-styryl residue has to be (E)-configured, and meta-chloro or –methoxy substitution improved affinity and selectivity. The m-position of the 8-styryl ring can be substituted with elongated chains with retention of A2A receptor selectivity and enhancement of water solubility (Jacobson et al. 1993a). The phenyl ring in the 8-styryl residue can be substituted by heterocyclic rings, such as a 3-thienyl ring (102) (Del Giudice et al. 1996).
The most common substitutents at N3 in A2A receptor-selective xanthine derivatives have been small alkyl residues, such as methyl, propyl, and 3-hydroxypropyl (reviewed Müller 2000; Cacciari et al. 2003; Vu 2005; Yuzlenko et al. 2006; Müller et al. 2007; Cristalli et al. 2007; Cristalli et al. 2009). Recently, the develoment of a new synthetic approach allowed the preparation of a series of xanthine derivatives with more variations in the 3-position (Massip et al. 2006). It was found that the A2A receptor tolerated bulky, functionalized substituents in the 3-position. For instance, N3-phenoxypropyl-substituted 8-(methoxystyryl)xanthine derivatives (e.g. 103) are potent and selective A2A antagonists (Massip et al. 2006).
Some of the best A2A antagonists were istradefylline (KW6002, 87), m-chlorostyrylcaffeine (CSC, 89), m-bromostyryl-DMPX (93), and MSX-2 (99). (E)-8-(3-chlorostyryl)caffeine (89) is not only a potent A2A antagonist (Ki rA2A 54 nM), but in addition, it has been reported to be a potent inhibitor of monoamineoxidase B (baboon MAO-B, Ki 80.6 nM), an enzyme which metabolizes dopamine (van den Berg et al. 2007; Petzer et al. 2009). This activity may contribute to the potency of CSC in in vivo studies, e.g. in animal models of Parkinson’s disease. All other styrylxanthine derivatives investigated so far, including 8-styrylcaffeine (88) and istradefylline (87), are considerably less potent as MAO-B inhibitors than CSC. Recently, a chain-extended homolog of CSC, 8-(m-chlorophenylbutadienyl)caffeine (108) has been described to show similar dual activity as A2A antagonist and MAO-B inhibitor (Pretorius et al. 2008).
Istradefylline (KW-6002, 87) has been intensively studied in vitro and in a number of animal models. Until recently (Fernandez et al. 2010), it was in Phase IIIb clinical trials for Parkinson’s disease. In phase II clinical trials istradefylline reduced motoric dysfunction without producing dyskinesias (reviewed by Knutsen et al. 2001). An 11C-labelled version of istradefylline has been prepared and used for positron emission tomography (PET) studies in healthy human brain (Hirani et al. 2001).
A major drawback of styrylxanthine derivatives, however, is their high lipophilicity and low water-solubility. Introduction of a polar sulfonate group into 8-styryl-DMPX resulting in compound 98 led to an almost 10-fold reduction in A2A affinity, but increased water-solubility (Müller et al. 1998b). A more successful approach has been the preparation of water-soluble prodrugs, particulary of the 3-(3-hydroxypropyl)-substituted 1-propargyl-8-styrylxanthine derivative MSX-2 (99) (Müller 2009). MSX-3 (100) is a water-soluble phosphate prodrug of MSX-2, which is cleaved in vivo by ubiquitous phosphatases to release the A2A receptor antagonist MSX-2 (Sauer et al. 2000). MSX-3 has proven useful for animal studies and is widely used for studying the in vivo effects of A2A antagonists (Hauber et al. 1998; Strömberg et al. 2000; Hauber et al. 2001; Ferré et al. 2001; Nagel et al. 2003; Blum et al. 2003; Schindler et al. 2004; Schindler et al. 2005; Antoniou et al. 2005; Karcz-Kubich et al. 2003a; Karcz-Kubicha et al. 2003b; Filip et al. 2006; Fuxe et al. 2007; Ishiwari et al. 2007; Farrar et al. 2007; Carriba et al. 2007; Salamone et al. 2008; Marcellino et al. 2008; Ferré et al. 2008; Mott et al. 2009; Worden et al. 2009). Due to its very high water-solubility at physiologic pH of 7.4 (9 mg/mL) it can be directly injected into specific brain areas, but is also an effective A2A antagonist after systemic application. Recently, an amino acid ester prodrug of MSX-2, MSX-4 (101) has been synthesized, which was found to be well soluble in water, highly stable in artificial gastric fluid, but readily cleaved by esterases and may be a suitable prodrug for peroral administration (Vollmann et al. 2008).
Care has to be taken when using the (E)-configurated styrylxanthines since they easily undergo light-induced isomerization in dilute solutions yielding mixtures of (E)- and (Z)-isomers, the (Z)-isomers being only weakly active or inactive (Nonaka et al. 1993; Müller et al. 1998b). This isomerisation does not occur in concentrated solution, e.g. during synthesis of the compounds, or when the compounds are applied as solid dosage forms. However, styrylxanthines can also undergo light-induced dimerization ([2+2]-cycloaddition reaction) in the solid state, and therefore have to be rigorously stored under exclusion of light (Hockemeyer et al. 2004).
3.2. Configurationally stable analogs of 8-styrylxanthines
To overcome the problem of the photoisomerization, the styryl moiety has been replaced with different more stable bioisosteric groups (e.g. replacement of the double bond for a cyclopropyl ring in 104, a 2-naphthyl residue in 105, a triple bond in 107) (Müller et al. 1997c), or a tricyclic constrained structure (133–143) (Kiec-Kononowicz et al. 2001; Drabczynska et al. 2003; Fhid et al. 2003; Drabczynska et al. 2004; Drabczynska et al. 2006; Drabczynska et al. 2007). In most cases a significant loss of affinity was observed by such modifications. The most promising compounds were the pyrimido[2,1-f]purinedione derivative 141 (Ki hA2A 630 nM, rA2A 230 nM) and 8-phenylethynyl-DMPX (107, Ki A2A 314 nM, rA2A 300 nM), both endowed with high selectivity. The latter class of compounds has been optimized towards increased A2A affinity and the obtained highly potent and selective A2A antagonists have been described in a recent patent (Müller et al. 2008). Furthermore, a substitution of the ethenyl group with a diazo structure has been performed. The obtained compounds retained selectivity but showed only moderate affinity (Müller et al. 1997b).
3.3. A2A-selective radiolabelled xanthine derivatives
The tritiated derivative of the 8-styrylxanthine KF17837S (the equilibrium mixture of (E)- and (Z)- KF17837 isomers) was shown to bind to rat striatal membranes in a saturable and reversible way, with KD values at low nanomolar concentration (Nonaka et al. 1994b). Another A2A antagonist radioligand was prepared, [3H]3-(3-hydroxypropyl)-7-methyl-8-(m-methoxystyryl)-1-propargylxanthine ([3H]MSX-2). This molecule showed high affinity (KD =8.0 nM) for rat and human A2A AR, with saturable and reversible binding, and also a selectivity of at least two orders of magnitude versus all other AR subtypes (Müller et al. 2000).
3.4. Heterocyclic compounds related to xanthines
A tricyclic styryl-substituted imidazo[2,1-i]purin-5-one derivative (132, fig. 6 and table 6) showed enhanced water-solubility but reduced A2AR affinity and moderate selectivity (Müller et al. 2002a).
4. Adenosine A2B receptor antagonists
4.1. Aryl-substituted 1,3-dialkylxanthines
From the initial studies of Daly and coworkers using cAMP studies in the brain slice, it was recognized that 1,3,7- and 1,3,8-trisubstituted xanthines have considerable affinity at the A2BAR. Also, the simple xanthine enprofylline 10 was discovered to have slight selectivity for the A2BAR, which was proposed to be responsible for its antiasthmatic action in the clinic (Stefanovich 1989; Daly 2000; Daly 2007). Screening efforts by Bruns (1981) followed by more detailed studies by Müller, Daly and Jacobson showed that 1-monosubstituted xanthine derivatives, such as 1-propylxanthine (6) and 1-butylxanthine (7) were about 10-fold more potent than enprofylline at A2BAR and equally selective (Müller et al. 1993b; Kim et al. 2002).
In fact, the unintended interaction at the A2BAR of widely used xanthine antagonists of the ARs has proven to be a complication in pharmacological studies.
Many known xanthines were screened at the A2BAR to identify leads for the design of novel A2BAR antagonists. The first successful efforts to enhance the activity of 1,3,8-trisubstituted xanthines at the A2BAR by Jacobson and colleagues resulted in one compound of intermediate selectivity at the human, but not rat A2BAR, MRS1595 (41), which is a hydrazide derivative of XCC (fig. 2 and table 2) (Kim et al. 2000). Then, further probing of SAR culminated in the introduction of MRS1754 (37), which was the first selective A2BAR antagonist with nanomolar affinity at the human receptor (Kim et al. 2000). The degree of selectivity for the human A2BAR was >120 fold, but selectivity for the rat A2BAR was considerably less (fig. 2 and table 2). Thus, it remained a challenge to design a rat A2BAR-selective xanthine antagonist. Another drawback in the series of anilide derivatives of XCC is the low aqueous solubility, which is partly remedied in related antagonists such as MRS1706 (38). Nevertheless, [3H]MRS1754 has found application as a useful radioligand of the A2BAR (Ji et al. 2001). Structurally related 8-phenylxanthine derivatives include CVT-5440 (40), in which additional aromatic rings were attached by an ether linkage, and 42 with a modified 3-substituent (3-methoxypropyl), have been developed as potent and selective A2B antagonists (Kim et al. 2000; Nieto et al. 2009). Newer derivatives in this series, which have two pyridine rings linked by an amide group, include the highly selective A2BAR antagonists ATL-802 (57) and ATL-852 (58). [3H]ATL-852 has been reported as a high affinity radioligand at this receptor (Cagnina et al. 2009).
8-Pyrazolyl-substituted xanthines that have been developed as selective human A2BAR antagonists include MRE-2029-F20 (56), which was also reported as a high affinity radioligand (Baraldi et al. 2004). A different series of isomeric 8-pyrazolyl-xanthines yielded the highly potent A2B antagonists 53–55 (CVT-6694, CVT-7124 and CVT-6883) (Kalla et al. 2008; Elzein et al. 2008; Kalla et al. 2009). High selectivity at human receptors has been reported for all of these pyrazolylxanthines, but no data for rodent receptors has been reported. CVT-6883 (55) is a promising candidate for the treatment of diabetes or asthma and has entered phase I clinical trials. Pain treatment is another potential area under consideration for A2BAR antagonists (Abo-Salem et al. 2004; Akkari et al. 2006; Bilkei-Gorzo et al. 2008; Michael et al. 2010).
4.2. 1,8-Disubstituted xanthines
The observation that 1-monosubstituted and 1,8-disubstituted xanthine derivatives showed high affinity and increased selectivity for A2BAR led to the development of a series of 1-alkyl-8-phenylxanthine derivatives (43–52) (Hayallah et al. 2002; Yan et al. 2004; Yan et al. 2006; Borrmann et al. 2009). These compounds also appeared to show reduced affinity at the rat A1 receptor and therefore increased A2B selectivity in rat. 1-Propyl-8-p-sulfophenylxanthine (PSB-1115, 43) was developed as a water-soluble A2B antagonist, useful as a pharmacological tool for in vivo studies (Müller et al. 1993b; Kirfel et al. 1997; Abo-Salem et al. 2004; Bilkei-Gorzo et al. 2008). 1-Butyl-8-(p-carboxyphenyl)xanthine (PSB-53, 45) showed similar affinity and selectivity. The 1-propargyl-8-p-bromophenylxanthine (PSB-50, 44) was more potent, but somewhat less selective and much less water-soluble. The 8-phenylxanthine derivatives PSB-55 (46), a benzylpiperazine derivative, and PSB-298 (47), a hydroxyethylamide, were synthesized to obtain more polar compounds with high A2B affinity. PSB-298 was obtained in tritiated form and was found to have a low degree of non-specific binding (Bertarelli et al. 2006). However, its affinity and selectivity was not satisfactory.
Starting from the sulfonate PSB-1115 (43), sulfonic acid esters (e.g. 48) and sulphonamides (e.g. 49–52) were obtained (Yan et al. 2004; Yan et al. 2006). Compound 48 can be envisaged as a lipophilic prodrug of the highly polar sulfonate 43, which may show peroral bioavailability and release of 43 after absorption (Yan et al. 2004). However, 48 has high A2B affinity itself and is therefore a co-drug rather than a prodrug, although without selectivity versus A1AR (fig. 2 and table 2). The most potent and selective A2B antagonists described to date are the sulphonamide derivatives 50–52, whose development was based on PSB-601 (49) an already very potent and selective A2B antagonist (Ki 3.6 nM). Compounds 50–52 show subnanomolar affinity for A2B receptors and very high selectivity in humans and in rodents. [3H]PSB-603 was prepared as a selective, high-affinity A2B antagonist radioligand with Kd values of 0.403 nM at human and 0.351 nM at mouse A2BAR (Borrmann et al. 2009).
4.3. 9-Deazaxanthines
Several series of 9-deazaxanthine derivatives (115, 117–121) were developed as A2B antagonists, which were structurally related to the 8-phenylxanthine derivatives described above (fig. 5 and table 5). A number of compounds with low nanomolar affinity were obtained, and some were selective for the human A2B receptor (Carotti et al. 2006; Esteve et al. 2006; Stefanachi et al. 2008).
4.4. 8-Furylmethyl-substituted xanthines
8-(2-Furyl)methyl-substituted xanthined derivatives, e.g. 63, have been developed as A2B antagonists (fig. 2 and table 2). Some of them showed high A2B affinity but only moderate selectivity (Balo et al. 2009).
5. Adenosine A3 receptor antagonists
In the search for A3AR antagonists, alkylxanthines were initially rejected as a suitable lead in favor of nonxanthine chemically diverse heterocycles, because of the exceptionally low affinity of alkylxanthines at the rat A3AR. For example, the classical adenosine antagonists caffeine and theophylline have Ki values of >100 μM at the rat A3AR (table 1). Initial SAR studies at the rat A3AR were conducted using multiply substituted xanthines, many of which retained selectivity for the A3AR (van Galen et al. 1994). Only slight A3AR selectivity was observed for analogues containing 8-alkyl and 2-thio substitutions (Kim et al. 1994b). However, when other species orthologues of the A3AR were cloned and studied pharmacologically, such as sheep, and human A3AR, many xanthines were found to display good affinity for those A3 receptors (Linden 1994). Thus, attention returned to the xanthines as a source for A3AR antagonist leads.
One of the earliest approaches to enhancing the affinity of xanthines at the A3AR was to attach a ribose group at the 7-position (21,22). The 5′-uronamide derivative N-methyl-1,3-dibutylxanthine-7-β-D-ribofuronamide (DBXRM, 22) is 140-fold selective for the A3AR, but the presence of the uronamide function increases the efficacy such that it is an agonist at this receptor (van Galen et al. 1994; Kim et al. 1994b; Bridson et al. 1998).
5.1. 8-Aryl-substituted xanthine derivatives
8-Phenylxanthine derivatives bearing a carboxylate group attached to the phenyl ring via an ethylene (35) or an oxymethylene spacer (34) were initially found to be potent antagonists at the human A3AR, but both compounds are also very potent A1 and A2B antagonists (fig. 2 and table 2). [125I]I-ABOPX (BW-A522, 34) has been used as a radioligand for labelling the A1AR as well as human A3 and human A2BARs (Patel et al. 1988; Salvatore et al. 1993; Linden et al. 1999). The corresponding p-azido derivative was previously used for photoaffinity labeling of the A1AR. The xanthine derivative 34 is characterized by a p-amino-m-iodobenzyl residue at N3 of the xanthine core (fig. 2) and is therefore quite lipophilic. As observed for other xanthine antagonists, 34 is much less potent (65-fold) at rat as compared to human A3 receptors.
5.2. Tricyclic xanthine and deazaxanthine derivatives
Cyclized derivatives of xanthines, such as (8R)-8-ethyl-4-methyl-2-phenyl-4,5,7,8-tetrahydro-1H-imidazo[2.1-i]purin-5-one (PSB-11, 130), its trichloro-phenyl-substituted derivative PSB-10 (129), and the 8-unsubstituted 8-bromophenyl derivative 131 are very potent A3-selective antagonists (fig. 6 and table 6) (Müller et al. 2002a; Saki et al. 2002; Ozola et al. 2003). PSB-11 (KD 4.9 nM) was prepared as a radioligand by catalytic hydrogenation from the polychlorinated precursor PSB-10 (Müller et al. 2002b; Burbiel et al. 2003). Due to its increased polarity and solubility in comparison to xanthines [3H]PSB-11 shows only low non-specific binding. Further tricyclic xanthine derivatives, in which an additional ring was attached to the imidazole rather than the pyrimidine ring of the xanthine core structure (pyrido-, imidazo-, pyrrolo- and triazolo-purinediones, 144–150), were developed as A3-selective antagonists (Priego et al. 2002; Baraldi et al. 2005; Pastorin et al. 2005; Priego et al. 2008). An aromatic residue (mostly benzyl) attached to the N3 (xanthine numbering) increases A3 affinity in xanthine derivatives including the tricyclic ones.
Besides tricyclic xanthine derivatives, tricyclic deazaxanthines have also been obtained (Ishiyama et al. 2009). Compound 122 (fig. 5 and table 5) was one of the most potent and selective compounds in this series.
6. Xanthine derivatives used as molecular probes
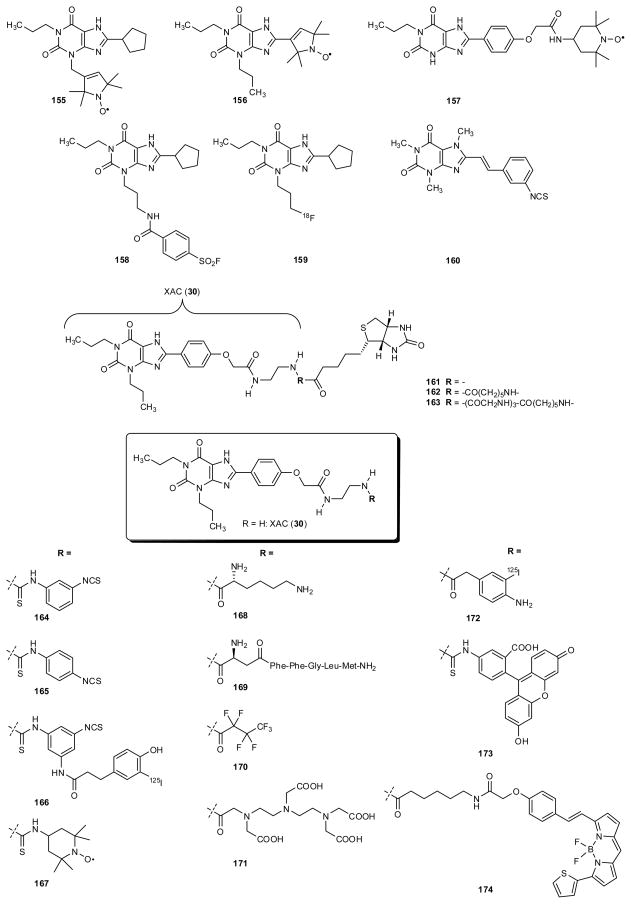
6.1. Irreversible ligand probes
The amine congener XAC (30) was coupled to a variety of bifunctional crosslinking reagents to form products containing a single chemically reactive group (fig. 7 and table 7). For example, 1,3- and 1,4-phenylene diisothiocyanates were conjugated to XAC to form the A1 AR-selective antagonists m-DITC-XAC 164 and p-DITC-XAC 165, which were demonstrated to bind irreversibly at submicromolar concentrations to the A1AR to act as covalent affinity labels. Similarly, prosthetic reagents designed for radiolabeling and photoaffinity labeling could be coupled to XAC and similar amine congeners (Stiles et al. 1988; Jacobson et al. 1989a). The conjugate with the p-aminophenylacetyl moiety was readily iodinated to form the radioligand 172, which could then be converted to the photoactivatable p-azide (Stiles and Jacobson 1987). This azide then served to crosslink a radiolabeled AR antagonist suitable to the A1AR protein, which could be visualized by gel electrophoresis. The sulfonyl fluoride group present in 158 was also means of crosslinking a xanthine derivative to the A1AR (Scammels et al. 1994; van Muijlswijk-Koezen et al. 2001).
Fig. 7.
Functionalized xanthines as molecular probes
Table 7.
Adenosine receptor affinities of functionalized xanthines as molecular probes
| Ki (nM)a | |||||
|---|---|---|---|---|---|
| A1 | A2A | A2B | A3 | ||
| Spin-labeled probes | |||||
| 155 | 5.47 (r)1 | 8,780 (r)1 | >1,000 (h)1 | 1,700 (h)1 | |
| 156 | 8.23 (r)1 | 3,800 (r)1 | 3,100 (h)1 | ca. 10,000 (h)1 | |
| 157 | 15.7 (r)1 | 1,270 (r)1 | 48 (h)1 | 350 (h)1 | |
| 167 | TEMPO-XAC | 4.9 (r)2 0.30 (c)2 |
nd | nd | nd |
| Irreversible ligands | |||||
| 158 | FSCPX3,4 | 10 (r)3 | nd | nd | nd |
| 160 | ISC | 42,600 (r)5 51,400 (gp)5 89,500 (rb)5 63,400 (c)5 |
146 (r)5 160 (gp)5 413 (rb)5 516 (c)5 |
nd | nd |
| 164 | m-DITC-XAC | 2.39 (r)6 52 (r)7 |
nd | nd | nd |
| 165 | p-DITC-XAC | 6.60 (r)6 27 (r)7 |
nd | nd | nd |
| Radioligands | |||||
| 159 | [18F]CPFPX | 1.26 (h)8 0.63 (r)8 1.37(p)8 0.18 (c)8 |
940 (h)8 812 (r)8 |
nd | nd |
| 166 | 40 [IC50] (c) 9 | nd | nd | nd | |
| 172 | [125I]PAPA-XAC | 0.1 (c)10 | nd | nd | nd |
| Biotin conjugates | |||||
| 161 | 54 (r)11,12 | nd | nd | nd | |
| 162 | 50 (r)11,12 | nd | nd | nd | |
| 163 | 60 (r)12 | nd | nd | nd | |
| Various conjugates | |||||
| 168 | D-Lys-XAC | 1.74 [IC50] (r) 13 | 159 [IC50] (r)13 | nd | nd |
| 169 | 35 (r)12 | nd | nd | nd | |
| 170 | 8.1 (r)2 0.8 (c)2 |
nd | nd | nd | |
| 171 | DTPA-XAC | 59.5 (r)2 3.25 (c)2 |
nd | nd | nd |
| Fluorescent ligands | |||||
| 173 | FITC-XAC | 125 (r)2 9.3 (c)2 |
nd | nd | nd |
| 174 | XAC-BY630 | 151 (h)14 | nd | nd | nd |
| Bivalent ligand conjugates | |||||
| 17511,15 | 31 (r) | nd | nd | nd | |
| 176 | A2A antagonist/D2 agonist for A2A/D2 receptor heteromers Ki D2 (s) = 1.0 nM)16 |
nd | 55 (s)16 | nd | nd |
h = human; c = cow; d = dog; gp = guinea pig; p = pig; r = rat; rb = rabbit; s = sheep
Jacobson et al., unpublished
6.2. Spectroscopic probes: spin labeled and fluorescent probes
Other types of reporter groups could be similarly incorporated into xanthine functionalized congeners with retention of moderate AR affinity, for example, chelating groups capable of complexing radioactive metal ions; spin labels for electron spin resonance (ESR) spectroscopy, e.g., 167; a perfluorinated acyl prosthetic group, as in 170, intended for use in fluorine-NMR spectroscopy, and fluorescent dyes, e.g., 173 and 174 (Jacobson et al. 1987b). The fluorescent conjugate 174 of XAC and BODIPY (6-(((4,4-difluoro-5-(2-thienyl)-4-bora-3a,4a-diaza-s-indacene-3-yl)styryloxy)acetic acid), with an intermediate ε-aminocaproyl spacer, has proven useful in fluorescence correlation spectroscopy to characterize ligand complexes of the A1AR (Briddon et al. 2004).
Spin-labeled probes that retained high A1 affinity were obtained by inserting the spin-label into the molecule as part of the pharmacophore. The most potent and A1-selective compounds were the DPCPX analogs 155 (replacement of the 3-substituent by a spin label) and 156 (substitution of the cyclopentyl ring by a structurally related spin label). Both compounds showed affinity for A1AR in the low nanomolar range combined with high selectivity versus the other receptor subtypes (Ilas et al. 2005). The 1-propyl-8-phenyl derivative 157, in which the spin label was integrated into the 8-substituent, showed good affinity for A1 as well as A2B receptors (fig. 7 and table 7).
6.3. Specialized radioligand probes based on conjugation
Trifunctional probes derived from XAC were synthesized for the purpose of crosslinking to both a reporter group and the receptor (Boring et al. 1991). By this means, the xanthine would deliver a radioactive or spectroscopic prosthetic group to the receptor, to which it would react irreversibly by virtue of an electrophilic group such as an isothiocyante. This approach was illustrated with a series of analogues of m-DITC-XAC containing a third substituent in the phenyl isothiocyanate ring. For example, in 166 the third substituent contained a 3-(4-hydroxy-phenyl)propionate moiety for radioiodination (Jacobson et al 1992b). This antagonist derivative effectively radiolabeled the bovine A1 AR in a covalent manner. Similar trifunctional xanthine probes for covalent labeling of ARs that furthermore contained a cleavable disulfide linkage within the chain linked to the xanthine moiety were reported (Jacobson et al. 1995). The intended strategy was to be able to remove the label after isolation of the modified receptor in order to regenerate the binding ability of the receptor.
6.4. Xanthine radioligand probes for positron emission tomography
There is a need for the development of imaging agents based on high affinity ligands for ARs. For example, ligands for in vivo positron emission tomographic (PET) imaging of A1, A2A, and A3 ARs have been developed. The high affinity A1AR antagonist DPCPX gave rise to the high affinity analogue in which a terminal hydrogen of the 3-propyl group has been substitued with radiofluorine: [18F]CPFPX (8-cyclopentyl-1-propyl-3-(3-fluoropropyl)-xanthine, 159), similar in structure to DPCPX). This tracer is being developed for PET imaging of the A1AR in the brain (Holschbach et al. 2002; Bauer et al. 2009).
PET ligands for the A2A AR in the 8-styrylxanthine series that are structurally related to KW6002, have been developed: for example, [7-methyl-11C]-(E)-8-(3,4,5-trimethoxystyryl)-1,3,7-trimethylxanthine ([11C]TMSX) (Ishiwata et al. 2000a). This compound was alternately named [11C]KF18446 ([7-methyl-11C]-(E)-8-(3,4,5-trimethoxystyryl)-1,3,7-trimethylxanthine, (Ishiwata et al. 2000b; Ishiwata et al. 2002; Ishiwata et al. 2003a; Ishiwata et al. 2003b). Ex vivo autoradiography for this molecule showed a high striatal uptake and a high uptake ratio of the striatum in comparison to other brain regions; [11C]KF18446 was therefore proposed as a suitable radioligand for mapping A2AAR of the brain by PET (Mishina et al. 2007). In 2001 the synthesis and the testing of the 8-styrylxanthine derivative [11C]KW-6002 as a PET ligand was reported. This molecule showed high retention in the striatum but it bound also to extra-striatal regions, so its potential as a PET ligand appeared to require further investigation (Hirani et al. 2001; Brooks et al. 2008).
In an earlier study, 11C-labeled (E)-KF17837 was synthesised and tested, and it was proposed as a potential positron emission tomography (PET) radioligand for mapping the A2AAR in the heart and the brain (Ishiwata et al. 1996; Ishiwata et al. 1997). Further studies on radiolabeled xanthine derivatives as A2AAR radioligands were carried out by preparing and testing an 11C-labeled selective antagonist, (E)-8-(3-chlorostyryl)-1,3-dimethyl-7-[11C]methylxanthine [11C]CSC). This molecule was shown to accumulate in the striatum, and PET studies on rabbits showed a fast brain uptake of [11C]CSC, reaching a maximum in less than 2 min (Marian et al. 1999). Few years later, iodinated and brominated styrylxanthine derivatives labeled with 11C were tested as in vivo probes (Ishiwata et al. 2000c). [7-Methyl-11C]-(E)-3,7-dimethyl-8-(3-iodostyryl)-1-propargylxanthine ([11C]IS-DMPX) and [7-methyl-11C]-(E)-8-(3-bromostyryl)-3,7-dimethyl-1-propargylxanthine ([11C]BS-DMPX) showed Ki affinities of 8.9 and 7.7 nM respectively, and high A2A/A1 selectivity values. Unfortunately, biological studies proved that the two ligands were only slightly concentrated in the striatum, and that they were not suitable as in vivo ligands because of low selectivity for the striatal A2A receptors and a high nonspecific binding (Ishiwata et al. 2000c).
6.5. Conjugated ligand probes and bivalent ligands
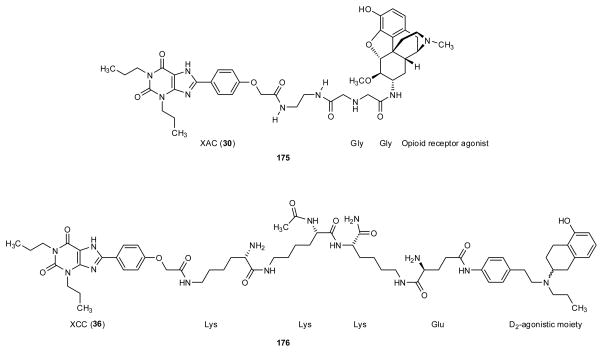
Three biotin conjugates 161–163 of 1,3-dipropyl-8-phenylxanthine (fig 7) were reported as being able to bind competitively to the rat A1 AR, but in the case of 161 and 162 only in the absence of avidin. This was in contrast to similar conjugates of functionalized nucleoside agonists, which more readily bound simultaneously to both avidin and the A1 AR. Results were interpreted in terms of the possible reorientation of the ligands at the receptor binding site (Jacobson et al. 1985a; Jacobson 1990).
Two different pharmacophores, one being a xanthine AR antagonist, have been tethered with the intention to create a dual selectivity in a single functional unit. For example, XAC was coupled covalently through an L-Lys linker to a segment derived from the neurotransmitter peptide substance P (SP) to form a binary drug 169 (Jacobson et al. 1987c). The Lys linker served to increase aqueous solubility and to preserve A1 AR by virtue of a free amino group in the spacer chain. Thus, conjugate 169 bound to the rat A1 receptor with a Ki value of 35 nM and to the NK1 (neurokinin type 1) receptor with a Ki value of 300 nM. Similarly, XAC was coupled to functionalized agonist ligands for opioid receptors, e.g., 175, and for D2 dopamine receptors, e.g., 176 (fig. 7 and table 7) (Jacobson 2009; Soriano et al. 2009). Each of these conjugates bound effectively to both relevant receptors.
7. Conclusions
The pharmacological activity of the natural xanthines currently used in therapy, namely theophylline (as an antiasthmatic) and caffeine (as CNS stimulant, for the treatment of apnoea in newborn babies, and as analgesic in combination therapy e.g. for the treatment of headaches) is mainly mediated by a (non-selective) inhibition of AR subtypes. AR subtype-selective xanthine derivatives with high potency have been developed and are evaluated in animal models and clinical trials.
Fig. 4.
8-Styrylxanthines and configurationally stable analogs
Abbreviations
- AR(s)
Adenosine receptor(s)
- c
calf or cow
- d
dog
- CHO
Chinese hamster ovary
- gp
guinea pig
- h
human
- m
mouse
- MAO
monoaminoxidase
- MAO-B
monoaminoxidase type B
- mk
monkey
- nd
not determined
- p
pig
- r
rat
- rb
rabbit
- s
sheep
- X
xanthine(s)
Contributor Information
Christa Müller, Email: christa.mueller@uni-bonn.de.
Kenneth A. Jacobson, Email: kajacobs@helix.nih.gov.
References
- Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Müller CE. Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther. 2004;308:358–366. doi: 10.1124/jpet.103.056036. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Cooper J, Shine J, Hill SJ. Characterization of the human brain putative A2B adenosine receptor expressed in Chinese hamster ovary (CHO.A2B4) cells. Br J Pharmacol. 1996;119:1286–1290. doi: 10.1111/j.1476-5381.1996.tb16035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Daifoti-Papadopoulou Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, Panlilio L, Müller CE, Goldberg SR, Ferré S. A detailed behavioural analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology. 2005;183:154–162. doi: 10.1007/s00213-005-0173-6. [DOI] [PubMed] [Google Scholar]
- Akkari R, Burbiel JC, Hockemeyer J, Müller CE. Recent progress in the development of adenosine receptor ligands as antiinflammatory drugs. Curr Top Med Chem. 2006;6:1375–1399. doi: 10.2174/15680266106061375. [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol. 1997;52:846–860. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Kreckler LM, Wan TC, Maas JE, van der Hoeven D, Gizewski E, Narayanan J, Maas GE. Characterization of the A2B adenosine receptor from mouse, rabbit, and dog. J Pharm Exp Ther. 2009;329:2–13. doi: 10.1124/jpet.108.148270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balo MC, Brea J, Caamano O, Fernandez F, Garcia-Mera X, Lopez C, Loza MI, Nieto MI, Rodriguez-Borges JE. Synthesis and pharmacological evaluation of novel 1- and 8-substituted 3-furfurylxanthines as adenosine receptor antagonists. Bioorg Med Chem. 2009;17:6755–6760. doi: 10.1016/j.bmc.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Tabrizi MA, Preti D, Bovero A, Romagnoli R, Fruttarolo F, Zaid NA, Moorman AR, Varani K, Gessi S, Merighi S, Borea PA. Design, synthesis, and biological evaluation of new 8-heterocyclic xanthine derivatives as highly potent and selective human A2B adenosine receptor antagonists. J Med Chem. 2004;47:1434–1447. doi: 10.1021/jm0309654. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Preti D, Tabrizi MA, Fruttarolo F, Romagnoli R, Zaid NA, Moorman AR, Merighi S, Varani K, Borea PA. New pyrrolo[2,1-f]purine-2,4-dione and imidazo[2,1-f]purine-2,4-dione derivatives as potent and selective human A3 adenosine receptor antagonists. J Med Chem. 2005;48:4697–4701. doi: 10.1021/jm058008c. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Tabrizi MA, Gessi S, Borea PA. Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility. Chem Rev. 2008;108:238–263. doi: 10.1021/cr0682195. [DOI] [PubMed] [Google Scholar]
- Barone S, Churchill PC, Jacobson KA. Adenosine receptor prodrugs: towards kidney-selective dialkylxanthines. J Pharm Exp Therap. 1989;250:79–85. [PMC free article] [PubMed] [Google Scholar]
- Bauer A, Ishiwata K. Adenosine receptor ligands and PET imaging of the CNS. Handb Exp Pharmacol. 2009;193:617–642. doi: 10.1007/978-3-540-89615-9_19. [DOI] [PubMed] [Google Scholar]
- Baumgold J, Nikodijevic O, Jacobson KA. Penetration of adenosine antagonists into mouse brain as determined by ex vivo binding. Biochem Pharmacol. 1992;43:889–894. doi: 10.1016/0006-2952(92)90257-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertarelli DCG, Diekmann M, Hayallah AM, Rüsing D, Iqbal J, Preiss B, Verspohl EJ, Müller CE. Characterization of human and rodent native and recombinant adenosine A2B receptors by radioligand binding studies. Purinergic Signal. 2006;2:559–571. doi: 10.1007/s11302-006-9012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Abo-Salem OM, Hayallah AM, Michel K, Müller CE, Zimmer A. Adenosine receptor subtype-selective antagonists in inflammation and hyperalgesia. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:65–76. doi: 10.1007/s00210-007-0252-9. [DOI] [PubMed] [Google Scholar]
- Blum D, Galas M-C, Pintor A, Brouillet E, Ledent C, Müller CE, Bantubungi K, Galluzzo M, Gall D, Cuvelier L, Rolland A-S, Popoli P, Schiffmann SN. A dual role of adenosine A2A receptors in the modulation of 3-nitropropionic acid-induced striatal lesions: implications for the neuroprotective potential of A2A antagonists. J Neurosci. 2003;23:5361–5369. doi: 10.1523/JNEUROSCI.23-12-05361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring DL, Ji XD, Zimmet J, Taylor KE, Stiles GL, Jacobson KA. Trifunctional agents as a design strategy for tailoring ligand properties: Irreversible inhibitors of A1 adenosine receptors. Bioconjugate Chem. 1991;2:77–88. doi: 10.1021/bc00008a002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrmann T, Hinz S, Bertarelli DCG, Li W, Florin NC, Scheiff AB, Müller CE. 1-Alkyl-8-(piperazine-1-sulfonyl)phenylxanthines: development and characterization of adenosine A2B receptor antagonists and a new radioligand with subnanomolar affinity and subtype specificity. J Med Chem. 2009;52:3994–4006. doi: 10.1021/jm900413e. [DOI] [PubMed] [Google Scholar]
- Brackett LE, Daly JW. Functional characterization of the A2b adenosine receptor in NIH 3T3 fibroblasts. Biochem Pharmacol. 1994;47:801–814. doi: 10.1016/0006-2952(94)90480-4. [DOI] [PubMed] [Google Scholar]
- Briddon SJ, Middleton RJ, Cordeaux Y, Flavin FM, Weinstein JA, George MW, Kellam B, Hill SJ. Quantitative analysis of the formation and diffucion of A1-adenosine receptor-antagonist complexes in single living cells. Proc Natl Acad Sci. 2004;101:4673–4678. doi: 10.1073/pnas.0400420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridson PK, Lin X, Mleman N, Ji XD, Jacobson KA. Synthesis and adenosine receptor affinity of 7-β-D-ribofuranosylxanthine. Nucleosides Nucleotides. 1998;17:759–768. doi: 10.1080/07328319808004673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ, Doder M, Osman S, Luthra SK, Hirani E, Hume S, Kase H, Kilborn J, Martindill S, Mori A. Positron emission tomography analysis of [11C]KW-6002 binding to human and rat adenosine A2A receptors in the brain. Synapse. 2008;62:671–681. doi: 10.1002/syn.20539. [DOI] [PubMed] [Google Scholar]
- Bruns RF. Adenosine antagonism by purines, pteridines and benzopteridines in human fibroblasts. Biochem Pharmacol. 1981;30:325–333. doi: 10.1016/0006-2952(81)90062-9. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Daly JW, Snyder SH. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci USA. 1980;77:5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RF, Lu GH, Pugsley TA. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986;29:331–346. [PubMed] [Google Scholar]
- Bruns RF, Lu GH, Pugsley TA. In: Topics and perspectives in adenosine research. Gerlach E, Becker BF, editors. 1987a. p. 59. [Google Scholar]
- Bruns RF, Fergus JH, Badger EW, Bristol JA, Santay LA, Hays SJ. PD 115,199: an antagonist ligand for adenosine A2 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987b;335:64–69. doi: 10.1007/BF00165038. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Fergus JH. Solubilities of adenosine antagonists determined by radioreceptor assay. J Pharm Pharmacol. 1989;41:590–594. doi: 10.1111/j.2042-7158.1989.tb06537.x. [DOI] [PubMed] [Google Scholar]
- Bulicz J, Bertarelli DCG, Baumert D, Fülle F, Müller CE, Heber D. Synthesis and pharmacology of pyrido[2,3-d]pyrimidinediones bearing polar substituents as adenosine receptor antagonists. Bioorg Med Chem. 2006;14:2837–2849. doi: 10.1016/j.bmc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Burbiel J, Thorand M, Müller CE. Improved efficient synthesis for multigram-scale production of PSB-10, a potent antagonist at human A3 adenosine receptors. Heterocycles. 2003;60:1425–1432. [Google Scholar]
- Cacciari B, Pastorin G, Spalluto G. Medicinal chemistry of A2A adenosine receptor antagonists. Curr Top Med Chem. 2003;3:403–411. doi: 10.2174/1568026033392183. [DOI] [PubMed] [Google Scholar]
- Cagnina RE, Ramos SI, Marshall MA, Wang G, Frazier CR, Linden J. Adenosine A2B receptors are highly expressed on murine type II alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L467–L474. doi: 10.1152/ajplung.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carotti A, Cadavid MI, Centeno NB, Esteve C, Loza MI, Martinez A, Nieto R, Ravina E, Sanz F, Segarra V, Sotelo E, Stefanachi A, Vidal B. Design, synthesis, and structure-activity relationships of 1-,3-,8- and 9-substituted 9-deazaxanthines at the human A2B adenosine receptor. J Med Chem. 2006;49:282–299. doi: 10.1021/jm0506221. [DOI] [PubMed] [Google Scholar]
- Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Müller C, Woods AS, Hope BT, Ciruela F, Casado V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferré S. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32:2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- Ceccarelli S, Altobelli M, D’Alessandro A, Paesano A. A novel hydrophilic 8-cycloalkylxanthine derivative (IRFI 117) is a highly selective antagonist at A1 adenosine receptors. Res Commun Mol Pathol Pharmacol. 1995;87:101–102. [Google Scholar]
- Cirillo R, Barone D, Franzone JS. Doxofylline, an antiasthmatic drug lacking affinity for adenosine receptors. Arch Int Pharmacodyn Ther. 1988;295:221–237. [PubMed] [Google Scholar]
- Cohen BE, Lee G, Jacobson KA, Kim YC, Huang Z, Sorscher E, Pollard HB. CPX (1,3-dipropyl-8-cyclopentylxanthine) and other alkyl-xanthines differentially bind to wild type and DF508 mutant first nucleotide binding fold (NBF-1) domains of the cystic fibrosis transmembrane conductance regulator. Biochemistry. 1997;36:6455–6461. doi: 10.1021/bi970150v. [DOI] [PubMed] [Google Scholar]
- Cristalli G, Cacciari B, Dal Ben D, Lambertucci C, Moro S, Spalluto G, Volpini R. Highlights on the development of A2A adenosine receptor agonists and antagonists. ChemMedChem. 2007;2:260–281. doi: 10.1002/cmdc.200600193. [DOI] [PubMed] [Google Scholar]
- Cristalli G, Müller CE, Volpini G. Recent development in adenosine A2A receptor ligands. In: Wilson CN, Mustafa SJ, editors. Handbook of Experimental Pharmacology 193: Adenosine Receptors in Health and Disease. 2009. [DOI] [PubMed] [Google Scholar]
- Cunha GM, Canas PM, Melo CS, Hockemeyer J, Müller CE, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol. 2008;210:776–781. doi: 10.1016/j.expneurol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Dall’Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A2A receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp Neurol. 2007;203:241–245. doi: 10.1016/j.expneurol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Daly JW. Adenosine receptors: targets for future drugs. J Med Chem. 1982;25:197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Daly JW, Padgett W, Shamim MT, Butts-Lamb P, Waters J. 1,3-Dialkyl-8-(p-sulfophenyl)xanthines: potent water-soluble antagonists for A1- and A2-adenosine receptors. J Med Chem. 1985;28:487–492. doi: 10.1021/jm00382a018. [DOI] [PubMed] [Google Scholar]
- Daly JW, Padgett WL, Shamim MT. Analogues of caffeine and theophylline: effect of structural alterations on affinity at adenosine receptors. J Med Chem. 1986a;29:1305–1308. doi: 10.1021/jm00157a035. [DOI] [PubMed] [Google Scholar]
- Daly JW, Padgett WL, Shamim MT. Analogues of 1,3-dipropyl-8-phenylxanthine: enhancement of selectivity at A1-adenosine receptors by aryl substituents. J Med Chem. 1986b;29:1520–1524. doi: 10.1021/jm00158a034. [DOI] [PubMed] [Google Scholar]
- Daly JW, Hide I, Müller CE, Shamim M. Caffeine analogs: structure-activity relationships at adenosine receptors. Pharmacology. 1991;42:309–321. doi: 10.1159/000138813. [DOI] [PubMed] [Google Scholar]
- Daly JW. Analogs of caffeine and theophylline: activity as antagonists at adenosine receptors. In: Imai S, Nakazawa M, editors. Role of adenosine and adenine nucleotides in the biological system. Elsevier; Amsterdam: 1991. pp. 119–129. [Google Scholar]
- Daly JW, Jacobson KA. Adenosine and andenine nucleotides: from molecular biology to integrative physiology. Kluwer Academic Publishers; Boston: 1995. p. 155. [Google Scholar]
- Daly JW. Alkylxanthines as research tools. J Autonomic Nervous System. 2000;81:44–52. doi: 10.1016/s0165-1838(00)00110-7. [DOI] [PubMed] [Google Scholar]
- Daly JW. Caffeine analogs: biomedical impact. Cell Mol Life Sci. 2007;64:2153–2169. doi: 10.1007/s00018-007-7051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice MR, Borioni A, Mustazza C, Gatta F, Dionisotti S, Zocchi C, Ongini E. (E)-1-(Heterocyclyl or cyclohexyl)-2-[1,3,7-trisubstituted(xanthin-8-yl)]ethenes as adenosine A2A receptors antagonists. Eur J Med Chem. 1996;31:59–63. [Google Scholar]
- Doggrell SA. BG-9928 (Biogen Idec) Curr Opin Investig Drugs. 2005;6:962–968. [PubMed] [Google Scholar]
- Drabczynska A, Schumacher B, Müller CE, Karolak-Wojciechowska J, Michalak B, Pekala E, Kiec-Kononowicz K. Impact of the aryl substituent kind and distance from pyrimido[2,1-f]purindiones on the adenosine receptor selectivity and antagonistic properties. Eur J Med Chem. 2003;38:397–402. doi: 10.1016/s0223-5234(03)00051-5. [DOI] [PubMed] [Google Scholar]
- Drabczynska A, Müller CE, Schumacher B, Hinz S, Karolak-Wojciechowska J, Michalak B, Pekala E, Kiec-Kononowicz K. Tricyclic oxazolo[2,3-f]purinediones: potency as adenosine receptor ligands and anticonvulsants. Bioorg Med Chem. 2004;12:4895–4908. doi: 10.1016/j.bmc.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Drabczynska A, Müller CE, Lacher SK, Schumacher B, Karolak-Wojciechowska J, Nasal A, Kawczak P, Yuzlenko O, Pekala E, Kiec-Kononowicz K. Synthesis and biological activity of tricyclic aryloimidazo-, pyrimido-, and diazepinopurinediones. Bioorg Med Chem. 2006;14:7258–7281. doi: 10.1016/j.bmc.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Drabczynska A, Müller CE, Karolak-Wojciechowska J, Schumacher B, Schiedel A, Yuzlenko O, Kiec-Kononowicz K. N9-Benzyl-substituted 1,3-dimethyl- and 1,3-dipropyl-pyrimido[2,1-f]purinediones: synthesis and structure-activity relationships at adenosine A1 and A2A receptors. Bioorg Med Chem. 2007a;15:5003–5017. doi: 10.1016/j.bmc.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Drabczynska A, Müller CE, Schiedel A, Schumacher B, Karolak-Wojciechowska J, Fruzinski A, Zobnina W, Yuzlenko O, Kiec-Kononowicz K. Phenylethyl-substituted pyrimido[2,1-f]purinediones and related compounds: structure-activity relationships as adenosine A1 and A2A receptor ligands. Bioorg Med Chem. 2007b;15:6956–6974. doi: 10.1016/j.bmc.2007.07.051. [DOI] [PubMed] [Google Scholar]
- Elzein E, Kalla RV, Li X, Perry T, Gimbel A, Zeng D, Lustig D, Leung K, Zablocki J. Discovery of a novel A2B adenosine receptor antagonist as a clinical candidate for chronic inflammatory airway diseases. J Med Chem. 2008;51:2267–2278. doi: 10.1021/jm7014815. [DOI] [PubMed] [Google Scholar]
- Erickson RH, Hiner RN, Feeney SW, Blake PR, Rzeszotarski WJ, Hicks RP, Costello DG, Abreu ME. 1,3,8-trisubstituted xanthines. Effects of substitution pattern upon adenosine receptor A1/A2 affinity. J Med Chem. 1991;34:1431–1435. doi: 10.1021/jm00108a029. [DOI] [PubMed] [Google Scholar]
- Esteve C, Nueda JL, Beleta J, Cardenas A, Lozoya E, Cadavid MI, Loza MI, Ryder H, Vidal B. New pyrrolopyrimidin-6-ylbenzyenesulfonamides: potent A2B adenosine receptor antagonists. Bioorg Med Chem Lett. 2006;16:3642–3645. doi: 10.1016/j.bmcl.2006.04.074. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Pereira M, Velasco F, Hockemeyer J, Müller CE, Salamone J. Adenosine A2A receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology. 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Ferkany JW, Valentine HL, Stone GA, Williams M. Adenosine A1 receptors in mammalian brain: species differences in their interactions with agonists and antagonists. Drug Dev Res. 1986;9:85–93. [Google Scholar]
- Fernandez HH, Greeley DR, Zweig RM, Wojcieszek J, Mori A, Sussman NM. -US-051 Study Group (2010) Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial. Parkinsonism Relat Disord. 6002;16:16–20. doi: 10.1016/j.parkreldis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Giménez-Llort L, Rimondini R, Müller CE, Strömberg I, Ögren SO, Fuxe K. Adenosine/dopamine interaction: implication for the treatment of Parkinson’s disease. Parkinsonism and Related Disorders. 2001;7:235–241. doi: 10.1016/s1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- Ferré S, Diamond I, Goldberg SR, Yao L, Hourani SM, Huang ZL, Urade Y, Kitchen I. Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry implications for drug addiction, sleep and pain. Prog Neurobiol. 2007;83:332–347. doi: 10.1016/j.pneurobio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, Quiroz C, Justinova Z, Lluis C, Franco R, Goldberg SR. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008;13:2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- Fhid O, Pawlowski M, Jurczyk S, Müller CE, Schumacher B. Pyridin-8-on[2,1-f]theophylline-9-alkylcarboxylic acid amides as A1 and A2A adenosine receptor ligands. Il Farmaco. 2003;58:439–444. doi: 10.1016/s0014-827x(03)00066-1. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Przegalinski E, Müller CE, Agnati LF, Franco R, Roberts DCS, Fuxe K. Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res. 2006;1077:67–80. doi: 10.1016/j.brainres.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Fozard JR, Baur F, Wolber C. Antagonist pharmacology of adenosine A2B receptors from rat, guinea pig and dog. Eur J Pharmacol. 2003;475:79–84. doi: 10.1016/s0014-2999(03)02078-8. [DOI] [PubMed] [Google Scholar]
- Franchetti P, Messini L, Cappellacci L, Grifantini M, Lucacchini A, Martini C, Senatore G. 8-Azaxanthine derivatives as antagonists of adenosine receptors. J Med Chem. 1994;37:2970–2975. doi: 10.1021/jm00044a018. [DOI] [PubMed] [Google Scholar]
- Frédérick R, Ooms F, Castagnoli N, Jr, Petzer JP, Feng JF, Schwarzschild MA, Van der Schyf CJ, Wouters J. (E)-8-(3-Chlorostyryl)-1,3,7-trimethylxanthine, a caffeine derivative acting both as antagonist of adenosine A2A receptors and as inhibitor of MAO-B. Acta Crystallogr. 2005;C61:o531–o532. doi: 10.1107/S0108270105023140. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden KT, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors: a report from the IUPHAR subcommittee. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Fredholm BB, Jacobson KA. John W. Daly and the early characterization of adenosine receptors. Heterocycles. 2009;79:73–83. doi: 10.3987/COM-08-S(D)Memoire-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Genedani S, Agnati L. Adenosine A2A receptors, dopamine D2 receptors and their interactions in Parkinson’s disease. Mov Disord. 2007;22:1990–2017. doi: 10.1002/mds.21440. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Kim SK, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. Structural determinants of A3 adenosine receptor activation: Nucleoside ligands at the agonist/antagonist boundary. J Med Chem. 2002;45:4471–4484. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis U, Grahner B, Pawlowski M, Drabczynska A, Gorczyca M, Müller CE. Tricyclic theophylline derivatives with high water-solubility: structure-activity relationships at adenosine receptors, phosphodiesterases and benzodiazepine binding sites. Pharmazie. 1995;50:333–336. [PubMed] [Google Scholar]
- Givertz MM. Adenosine A1 receptor antagonists at a fork in the road. Circ Heart Fail. 2009;2:519–522. doi: 10.1161/CIRCHEARTFAILURE.109.916072. [DOI] [PubMed] [Google Scholar]
- Grahner B, Winiwarter S, Lanzner W, Müller CE. Synthesis and structure-activity relationships of deazaxanthines: analogs of potent A1- and A2-adenosine receptor antagonists. J Med Chem. 1994;37:1526–1534. doi: 10.1021/jm00036a019. [DOI] [PubMed] [Google Scholar]
- Hayallah AM, Sandoval-Ramírez J, Reith U, Schobert U, Preiss B, Schumacher B, Daly JW, Müller CE. 1,8-Disubstituted xanthine derivatives: synthesis of potent A2B-selective adenosine receptor antagonists. J Med Chem. 2002;45:1500–1510. doi: 10.1021/jm011049y. [DOI] [PubMed] [Google Scholar]
- Hauber W, Nagel J, Sauer R, Müller CE. Motor effects induced by a blockade of adenosine A2A receptors in the caudate-putamen. Neuroreport. 1998;9:1803–1806. doi: 10.1097/00001756-199806010-00024. [DOI] [PubMed] [Google Scholar]
- Hauber W, Neuscheler P, Nagel J, Müller CE. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of A2A receptors in the caudate-putamen of rats. Eur J Neurosci. 2001;14:1287–1293. doi: 10.1046/j.0953-816x.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- Hirani E, Gillies J, Karasawa A, Shimada J, Kase H, Opacka-Juffry J, Osman S, Luthra SK, Hume SP, Brooks DJ. Evaluation of [4-O-methyl-11C]KW-6002 as a potential PET ligand for mapping central adenosine A2A receptors in rats. Synapse. 2001;42:164–176. doi: 10.1002/syn.1110. [DOI] [PubMed] [Google Scholar]
- Hockemeyer J, Burbiel JC, Müller CE. Multigram-scale syntheses, stability, and photoreactions of A2A adenosine receptor antagonists with 8-styrylxanthine structure: potential drugs for Parkinson’s disease. J Org Chem. 2004;69:3308–3318. doi: 10.1021/jo0358574. [DOI] [PubMed] [Google Scholar]
- Holschbach MH, Olsson RA, Bier D, Wutz W, Sihver W, Schüller M, Palm B, Coenen HH. Synthesis and evaluation of no-carrier-added 8-cyclopentyl-3-(3-[18F]fluoropropyl)-1-propylxanthine ([18F]CPFPX): a potent and selective A1-adenosine receptor antagonist for in vivo imaging. J Med Chem. 2002;45:5150–5156. doi: 10.1021/jm020905i. [DOI] [PubMed] [Google Scholar]
- Ilas J, Pekar S, Hockemeyer J, Euler H, Kirfel A, Müller CE. Development of spin-labeled probes for adenosine receptors. J Med Chem. 2005;48:2108–2114. doi: 10.1021/jm049513x. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Bastia E, Ongini E, Monopoli A. Adenosine receptors in neurological disorders. Emerg Ther Targets. 2000;4:635–663. [Google Scholar]
- Ishiwari K, Madson LJ, Farrar AM, Mingote SM, Valenta JP, DiGianvittorio MD, Frank LE, Correa M, Hockemeyer J, Müller CE, Salamone JD. Injections of the selective adenosine A2A antagonist MSX-3 into the nucleus accumbens core attenuate the locomotor suppression induced by haloperidol in rats. Behavioural Brain Res. 2007;178:190–199. doi: 10.1016/j.bbr.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata K, Noguchi J, Toyama H, Sakiyama Y, Koike N, Ishii S, Oda K, Endo K, Suzuki F, Senda M. Synthesis and preliminary evaluation of [11C]KF17837, a selective adenosine A2A antagonist. Appl Radiat Isot. 1996;47:507–511. doi: 10.1016/0969-8043(95)00295-2. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Sakiyama Y, Sakiyama T, Shimada J, Toyama H, Oda K, Suzuki F, Senda M. Myocardial adenosine A2A receptor imaging of rabbit by PET with [11C]KF17837. Ann Nucl Med. 1997;11:219–225. doi: 10.1007/BF03164767. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Noguchi J, Wakabayashi S, Shimada J, Ogi N, Nariai T, Tanaka A, Endo K, Suzuki F, Senda M. 11C-labeled KF18446: a potential central nervous system adenosine A2A receptor ligand. J Nucl Med. 2000a;41:345–354. [PubMed] [Google Scholar]
- Ishiwata K, Ogi N, Shimada J, Nonaka H, Tanaka A, Suzuki F, Senda M. Further characterization of a CNS adenosine A2A receptor ligand [11C]KF18446 with in vitro autoradiography and in vivo tissue uptake. Ann Nucl Med. 2000b;14:81–89. doi: 10.1007/BF02988585. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Shimada J, Wang WF, Harakawa H, Ishii S, Kiyosawa M, Suzuki F, Senda M. Evaluation of iodinated and brominated [11C]styrylxanthine derivatives as in vivo radioligands mapping adenosine A2A receptor in the central nervous system. Ann Nucl Med. 2000c;14:247–253. doi: 10.1007/BF02988206. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Ogi N, Hayakawa N, Oda K, Nagaoka T, Toyama H, Suzuki F, Endo K, Tanaka A, Senda M. Adenosine A2A receptor imaging with [11C]KF18446 PET in the rat brain after quinolinic acid lesion: comparison with the dopamine receptor imaging. Ann Nucl Med. 2002;16:467–475. doi: 10.1007/BF02988643. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Kawamura K, Kimura Y, Oda K, Ishii K. Potential of an adenosine A2A receptor antagonist [11C]TMSX for myocardial imaging by positron emission tomography: a first human study. Ann Nucl Med. 2003a;17:457–462. doi: 10.1007/BF03006434. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Wang WF, Kimura Y, Kawamura K, Ishii K. Preclinical studies on [11C]TMSX for mapping adenosine A2A receptors by positron emission tomography. Ann Nucl Med. 2003b;17:205–211. doi: 10.1007/BF02990023. [DOI] [PubMed] [Google Scholar]
- Ishiyama H, Nakajima H, Nakata H, Kobayashi J. Synthesis of hybrid analogues of caffeine and eudistomin D and their affinity for adenosine receptors. Bioorg Med Chem. 2009;17:4280–4284. doi: 10.1016/j.bmc.2009.05.036. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Kirk KL, Padgett W, Daly JW. Probing the adenosine receptor with adenosine and xanthine biotin conjugates. FEBS Lett. 1985a;184:30–35. doi: 10.1016/0014-5793(85)80646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Kirk KL, Padgett WL, Daly JW. Functionalized congeners of 1,3-dialkylxanthines: preparation of analogues with high affinity for adenosine receptors. J Med Chem. 1985b;28:1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Ukena D, Kirk KL, Daly JW. [3H]xanthine amine congener of 1,3-dipropyl-8-phenylxanthine: an antagonist radioligand for adenosine receptors. Proc Natl Acad Sci USA. 1986a;83:4089–4093. doi: 10.1073/pnas.83.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Kirk KL, Padgett WL, Daly JW. A functionalized congener approach to adenosine receptor antagonists: amino acid conjugates of 1,3-dipropylxanthine. Mol Pharmacol. 1986b;29:126–133. [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Ukena D, Padgett W, Daly JW, Kirk KL. Xanthine functionalized congeners as potent ligands at A2-adenosine receptors. J Med Chem. 1987a;30:211–214. doi: 10.1021/jm00384a037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Ukena D, Padgett W, Kirk KL, Daly JW. Molecular probes for extracellular adenosine receptors. Biochem Pharmacol. 1987b;36:1697–1707. doi: 10.1016/0006-2952(87)90056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Lipowski AW, Moody TW, Padgett W, Pijl E, Kirk KL, Daly JW. Binary drugs: conjugates of purines and a peptide that bind to both adenosine and substance P receptors. J Med Chem. 1987c;30:1529–1532. doi: 10.1021/jm00391a046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, de la Cruz R, Schulick R, Kiriasis L, Padgett W, Pfleiderer W, Kirk KL, Neumeyer JL, Daly JW. 8-Substituted xanthines as antagonists as A1 and A2-adenosine receptors. Biochem Pharmacol. 1988;37:3653–3661. doi: 10.1016/0006-2952(88)90398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Barone S, Kammula U, Stiles GL. Electrophilic derivatives of purines as irreversible inhibitors of A1-adenosine receptors. J Med Chem. 1989a;32:1043–1051. doi: 10.1021/jm00125a019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Kiriasis L, Barone S, Bradbury BJ, Kammula U, Campagne JM, Daly JW, Neumeyer JL, Pfleiderer W. Sulfur-containing xanthine derivatives as selective antagonists at A1-adenosine receptors. J Med Chem. 1989b;32:1873–1879. doi: 10.1021/jm00128a031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA. Probing adenosine receptors using biotinylated purine conjugates. In: Wilchek M, Bayer E, editors. Methods in Enzymology. Vol. 184. 1990. pp. 668–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, van Galen PJM, Williams M. Perspective, adenosine receptors: pharmacology, structure activity relationships and therapeutic potential. J Med Chem. 1992a;35:407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Olah ME, Stiles GL. Trifunctional ligands: A radioiodinated high affinity acylating antagonist for the A1 adenosine receptor. Pharmacol Commun. 1992b;1:145–154. [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gallo-Rodriguez C, Melman N, Fischer B, Maillard M, van Bergen A, van Galen PJ, Karton Y. Structure-activity relationships of 8-styrylxanthines as A2-selective adenosine antagonists. J Med Chem. 1993a;36:1333–1342. doi: 10.1021/jm00062a005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Shi D, Gallo-Rodriguez C, Manning M, Jr, Müller C, Daly JW, Neumeyer JL, Kiriasis L, Pfleiderer W. Effect of trifluoromethyl and other substituents on activity of xanthines at adenosine receptors. J Med Chem. 1993b;36:2639–2644. doi: 10.1021/jm00070a007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Fischer B, Ji XD. A “cleavable trifunctional” approach to receptor affinity labeling: regeneration of binding to A1-adenosine receptors. Bioconjugate Chem. 1995;6:255–263. doi: 10.1021/bc00033a004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA. A3 adenosine receptors: Novel ligands and paradoxical effects. Trends Pharmacol Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, IJzerman AP, Linden J. 1,3-Dialkylxanthine derivatives having high potency as antagonists at human A2B adenosine receptors. Drug Devel Res. 1999;47:45–53. doi: 10.1002/(sici)1098-2299(199905)47:1<45::aid-ddr6>3.0.co;2-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA. Functionalized congener approach to the design of ligands for G protein-coupled receptors (GPCRs) Bioconjugate Chem. 2009;20:1816–1835. doi: 10.1021/bc9000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Jacobson KA, Williams M. Autoradiographic localization of adenosine A1 receptors in rat brain using [3H]XCC, a functionalized congener of 1,3-dipropylxanthine. Neurosci Lett. 1987;81:69–74. doi: 10.1016/0304-3940(87)90342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XD, Stiles GL, Jacobson KA. [3H]XAC (xanthine amine congener) is a radioligand for A2-adenosine receptors in rabbit striatum. Neurochem Internat. 1991;18:207–213. doi: 10.1016/0197-0186(91)90187-i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XD, Gallo-Rodriguez C, Jacobson KA. 8-(3-Isothiocyanatostyryl)caffeine is a selective irreversible inhibitor or striatal A2-adenosine receptors. Drug Devel Res. 1993;29:292–298. doi: 10.1002/ddr.430290407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XD, von Lubitz D, Olah ME, Stiles GL, Jacobson KA. Species differences in ligand affinity at central A3-adenosine receptors. Drug Dev Res. 1994;33:51–59. doi: 10.1002/ddr.430330109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XD, Kim YC, Ahern DG, Linden J, Jacobson KA. [3H]MRS 1754, a selective antagonist radioligand for A2B adenosine receptors. Biochem Pharmacol. 2001;61:657–663. doi: 10.1016/s0006-2952(01)00531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla R, Elzein E, Perry T, Li X, Gimbel A, Yang M, Zeng D, Zablocki J. Selective, high affinity A2B adenosine receptor antagonists: N-1 monosubstituted 8-(pyrazol-4-yl)xanthines. Bioorg Med Chem Lett. 2008;18:1397–1401. doi: 10.1016/j.bmcl.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Kalla R, Zablocki J. Progress in the discovery of selective, high affinity A2B adenosine receptor antagonists as clinical candidates. Purinergic Signal. 2009;5:21–29. doi: 10.1007/s11302-008-9119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Quarta D, Hope BT, Antoniou K, Müller CE, Morales M, Schindler CW, Goldberg SR, Ferré S. Enabling role of adenosine A1 receptors in adenosine A2A receptor-mediated striatal expression of c-fos. Eur J Neurosci. 2003a;18:296–302. doi: 10.1046/j.1460-9568.2003.02747.x. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Müller CE, Fuxe K, Goldberg SR, Popoli P, Ferré S. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacol. 2003b;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Kase H. The adenosine A2A receptor selective antagonist KW6002: research toward a novel nondopaminergic therapy for Parkinson’s disease. Neurology. 2003;61(Suppl 6):S97–S100. doi: 10.1212/01.wnl.0000095219.22086.31. [DOI] [PubMed] [Google Scholar]
- Kiec-Kononowicz K, Drabczynska A, Pekala E, Michalak B, Müller CE, Schumacher B, Karolak-Wojciechowska J, Duddeck H, Rockitt S, Wartchow R. New developments in A1 and A2 adenosine receptor antagonists. Pure Appl Chem. 2001;73:1411–1420. [Google Scholar]
- Kiesman WF, Zhao J, Conlon PR, Petter RC, Jin X, Smits G, Lutterodt F, Sullivan GW, Linden J. Norbornyllactone-substituted xanthines as adenosine A1 receptor antagonists. Bioorg Med Chem. 2006a;14:3654–3661. doi: 10.1016/j.bmc.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Kiesman WF, Zhao J, Conlon PR, Dowling JE, Petter RC, Lutterodt F, Jin X, Smits G, Fure M, Jayaraj A, Kim J, Sullivan G, Linden J. Potent and orally bioavailable 8-bicyclo[2.2.2]octylxanthines as adenosine A1 receptor antagonists. J Med Chem. 2006b;49:7119–7131. doi: 10.1021/jm0605381. [DOI] [PubMed] [Google Scholar]
- Kim HO, Ji XD, Melman N, Olah ME, Stiles GL, Jacobson KA. Structure activity relationships of 1,3-dialkylxanthine derivatives at rat A3-adenosine receptors. J Med Chem. 1994a;37:3373–3382. doi: 10.1021/jm00046a022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, Ji XD, Melman N, Olah ME, Stiles GL, Jacobson KA. Selective ligands for rat A3-adenosine receptors: structure-activity relationships of 1,3-dialkylxanthine-7-riboside derivatives. 1994b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Karton Y, Ji XD, Melman N, Linden J, Jacobson KA. Acyl-hydrazide derivatives of a xanthine carboxylic congener (XCC) as selective antagonists at human A2B adenosine receptors. Drug Devel Res. 1999;47:178–188. doi: 10.1002/(sici)1098-2299(199908)47:4<178::aid-ddr4>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-S, Ji X-d, Melman N, Linden J, Jacobson KA. Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A2B adenosine receptors. J Med Chem. 2000;43:1165–1172. doi: 10.1021/jm990421v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-A, Marschall MA, Melman N, Kim HS, Müller CE, Linden J, Jacobson KA. Structure-activity relationships at human and rat A2B adenosine receptors of xanthine derivatives substituted at the 1-, 3-, 7-, and 8-positions. J Med Chem. 2002;45:2131–2138. doi: 10.1021/jm0104318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirfel A, Schwabenländer F, Müller CE. Crystal structure of 1-propyl-8-(4-sulfophenyl)-7H-imidazo[4,5-d]pyrimidin-2,6(1H,3H)-dione dehydrate, C14H14N4O5S x 2 H2O. Z Kristallographie – New Cryst Struct. 1997;3:447–448. [Google Scholar]
- Klotz KN, Vogt H, Tawfik-Schlieper H. Comparison of adenosine receptors in brain from different species by radioligand binding and photoaffinity labelling. Naunyn-Schmiedeberg’s Arch Pharmacol. 1991;343:196–201. doi: 10.1007/BF00168610. [DOI] [PubMed] [Google Scholar]
- Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, Lohse MJ. Comparative pharmacology of human adenosine receptor subtypes - characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedeberg’s Arch Pharmacol. 1998;357:1–9. doi: 10.1007/pl00005131. [DOI] [PubMed] [Google Scholar]
- Knutsen LJ, Weiss SM. KW-6002 (Kyowa Hakko Kogyo) Curr Opin Investig Drugs. 2001;2:668–673. [PubMed] [Google Scholar]
- Krämer SD, Testa B. The biochemistry of drug metabolism – an introduction part 6. Inter-individual factors affecting drug metabolism. Chem Biodivers. 2008;5:2465–2578. doi: 10.1002/cbdv.200890214. [DOI] [PubMed] [Google Scholar]
- Kull B, Arslan G, Nilsson C, Owman C, Lorenzen A, Schwabe U, Fredholm BB. Differences in the order of potency for agonists but not antagonists at human and rat adenosine A2A receptors. Biochem Pharmacol. 1999;57:65–75. doi: 10.1016/s0006-2952(98)00298-6. [DOI] [PubMed] [Google Scholar]
- Linden J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol Sci. 1994;15:298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Linden J, Taylor HE, Robeva AS, Tucker AL, Stehle JH, Rivkees SA, Fink JS, Reppert SM. Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. Mol Pharmacol. 1993;44:524–532. [PubMed] [Google Scholar]
- Linden J, Thai T, Figler H, Jin X, Robeva AS. Characterization of human A2B adenosine receptors: radioligand binding, western blotting, and coupling to Gq in human embryonic kidney 293 cells and HMC-1 mast cells. Mol Pharmacol. 1999;56:705–713. [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, Tanganelli S, Müller CE, Fisone G, Lluis C, Agnati LF, Franco R, Fuxe K. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioural analysis. Neuropharmacology. 2008;54:815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Marian T, Boros I, Lengyel Z, Balkay L, Horvath G, Emri M, Sarkadi E, Szentmiklosi AJ, Fekete I, Tron L. Preparation and primary evaluation of [11C]CSC as a possible tracer for mapping adenosine A2A receptors by PET. Appl Radiat Isot. 1999;50:887–893. doi: 10.1016/s0969-8043(98)00162-6. [DOI] [PubMed] [Google Scholar]
- Martin PL, Wysocki RJ, Jr, Barrett RJ, May JM, Linden J. Characterization of 8-(N-methylisopropyl)amino-N6-(5′-endohydroxy-endonorbornyl)-9-methyladenine (WRC-0571), a highly potent and selective, non-xanthine antagonist of A1 adenosine receptors. J Pharmacol Exp Ther. 1996;276:490–499. [PubMed] [Google Scholar]
- Massip S, Guillon J, Bertarelli D, Bosc JJ, Leger JM, Lacher S, Bontemps C, Dupont T, Müller CE, Jarry C. Synthesis and preliminary evaluation of new 1- and 3-[1-(2-hydroxy-3-phenoxypropyl)]xanthines from 2-amino-2-oxazolines as potential A1 and A2A adenosine receptor antagonists. Bioorg Med Chem. 2006;14:2697–2719. doi: 10.1016/j.bmc.2005.11.050. [DOI] [PubMed] [Google Scholar]
- Michael S, Warstat C, Michel F, Yan L, Müller CE, Nieber K. Adenosine A2A agonist and A2B antagonist mediate an inhibition of inflammation-induced contractile disturbance of a rat gastrointestinal preparation. Purinergic Signal. 2010 doi: 10.1007/s11302-009-9174-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M, Ishiwata K, Kimura Y, Naganawa M, Oda K, Kobayashi S, Katayama Y, Ishii K. Evaluation of distribution of adenosine A2A receptors in normal human brain measured with [11C]TMSX PET. Synapse. 2007;61:778–784. doi: 10.1002/syn.20423. [DOI] [PubMed] [Google Scholar]
- Moro S, Gao ZG, Jacobson KA, Spalluto G. Progress in the pursuit of therapeutic adenosine receptor antagonists. Med Res Rev. 2006;26:131–159. doi: 10.1002/med.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott AM, Nunes EJ, Collins LE, Port RG, Sink KS, Hockemeyer J, Müller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effects of the dopamine antagonist haloperidol on effort-related decision making in a T-maze cost/benefit procedure. Psychopharmacology. 2009;204:103–112. doi: 10.1007/s00213-008-1441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CE. Formation of oxazolo[3,2-a]purinones from propynyluracils. J Org Chem. 1994;59:1928–1929. [Google Scholar]
- Müller CE. A1-Adenosine receptor antagonists. Exp Opin Ther Patents. 1997;7:419–440. [Google Scholar]
- Müller CE. A2A Adenosine receptor antagonists - future drugs for Parkinson’s disease? Drugs Fut. 2000;25:1043–1052. [Google Scholar]
- Müller CE. A3 Adenosine receptor antagonists. Mini-Rev Med Chem. 2001;1:417–427. doi: 10.2174/1389557510101040417. [DOI] [PubMed] [Google Scholar]
- Müller CE. Medicinal chemistry of adenosine A3 receptor ligands. Curr Top Med Chem. 2003;3:445–462. doi: 10.2174/1568026033392174. [DOI] [PubMed] [Google Scholar]
- Müller CE. Prodrug approaches for enhancing the bioavailability of drugs with low solubility. Chemistry & Biodiversity. 2009;6:2071–2083. doi: 10.1002/cbdv.200900114. [DOI] [PubMed] [Google Scholar]
- Müller CE, Scior T. Adenosine receptors and their modulators. Pharm Acta Helv. 1993a;68:77–111. doi: 10.1016/0031-6865(93)90012-u. [DOI] [PubMed] [Google Scholar]
- Müller CE, Shi D, Manning M, Jr, Daly JW. Synthesis of paraxanthine analogs (1,7-disubstituted xanthines) and other xanthines unsubstituted at the 3-position: structure-activity relationships at adenosine receptors. J Med Chem. 1993b;36:3341–3349. doi: 10.1021/jm00074a015. [DOI] [PubMed] [Google Scholar]
- Müller CE, Stein B. Adenosine receptor antagonists: structures and potential therapeutic applications. Curr Pharm Des. 1996;2:501–530. [Google Scholar]
- Müller CE, Geis U, Hipp J, Schobert U, Frobenius W, Pawlowski M, Suzuki F, Sandoval-Ramirez J. Synthesis and structure-activity relationships of DMPX (3,7-dimethyl-1-propargylxanthine) derivatives, A2A-selective adenosine receptor antagonists. J Med Chem. 1997a;40:4396–4405. doi: 10.1021/jm970515+. [DOI] [PubMed] [Google Scholar]
- Müller CE, Sauer R, Geis U, Frobenius W, Talik P, Pawlowski M. Aza-analogs of 8-styrylxanthines as A2A-adenosine receptor antagonists. Arch Pharm Pharm Med Chem. 1997b;330:181–189. doi: 10.1002/ardp.19973300606. [DOI] [PubMed] [Google Scholar]
- Müller CE, Schobert U, Hipp J, Geis U, Frobenius W, Pawlowski M. Configurationally stable analogs of styrylxanthines as A2A adenosine receptor antagonist. Eur J Med Chem. 1997c;32:709–719. [Google Scholar]
- Müller CE, Sandoval-Ramirez J, Schobert U, Geis U, Frobenius W, Klotz KN. 8-(Sulfostyryl)xanthines: water-soluble A2A-selective adenosine receptor antagonists. Bioorg Med Chem. 1998b;6:707–719. doi: 10.1016/s0968-0896(98)00025-x. [DOI] [PubMed] [Google Scholar]
- Müller CE, Maurinsh J, Sauer R. Binding of [3H]MSX-2 (3-(3-hydroxypropyl)-7-methyl-8-(m-methoxystyryl)-1-propargylxanthine) to rat striatal membranes - a new, selective antagonist radioligand for A2A adenosine receptors. Eur J Pharm Sci. 2000;10:259–265. doi: 10.1016/s0928-0987(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Müller CE, Thorand M, Qurishi R, Diekmann M, Jacobson KA, Padgett WL, Daly JW. Imidazo[2,1-i]purin-5-ones and related tricyclic water-soluble purine derivatives: potent A2A- and A3-adenosine receptor antagonists. J Med Chem. 2002a;45:3440–3450. doi: 10.1021/jm011093d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CE, Diekmann M, Thorand M, Ozola V. [3H]8-Ethyl-4-methyl-2-phenyl-(8R)-4,5,7,8-tetrahydro-1H-imidazo[2,1-i]purin-5-one ([3H]PSB-11), a novel high-affinity antagonist radioligand for human A3 adenosine receptors. Bioorg Med Chem Lett. 2002b;12:501–503. doi: 10.1016/s0960-894x(01)00785-5. [DOI] [PubMed] [Google Scholar]
- Müller CE, Ferré S. Blocking striatal adenosine A2A receptors: a new strategy for basal ganglia disorders. Recent Patents CNS Drug Discov. 2007;2:1–21. doi: 10.2174/157488907779561772. [DOI] [PubMed] [Google Scholar]
- Müller CE, Hockemeyer J, Tzvetkov NT, Burbiel JC. Preparation of 8-ethynyl-xanthine derivatives as selective A2A receptor antagonists (SANOL Arznei Schwarz GmbH, Germany) WO 2008077557 A1 PCT Int Appl. 2008
- Nagel J, Schladebach H, Koch M, Schwienbacher I, Müller CE, Hauber W. Effects of an adenosine A2A receptor blockade in the nucleus accumbens on locomotion, feeding, and prepulse inhibition in rats. Synapse. 2003;49:279–286. doi: 10.1002/syn.10240. [DOI] [PubMed] [Google Scholar]
- Nieto MI, Balo MC, Brea J, Caamano O, Cadavid MI, Fernandez F, Mera XG, Lopez C, Rodriguez-Borges JE. Synthesis of novel 1-alkyl-8-substituted 3-(3-methoxypropyl)xanthines as putative A2B receptor antagonists. Bioorg Med Chem. 2009;17:3426–3432. doi: 10.1016/j.bmc.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Noguchi J, Ishiwata K, Furuat R, Simada J-i, Kiyosawa M, Ishii S-i, Endo K, Suzuki F, Senda M. Evaluatioin of carbon-11 labeled KF15372 and its ethyl and methyl derivatives as a potential CNS adenosine A2 receptor ligand. Nucl Med Biol. 1997;24:53–59. doi: 10.1016/s0969-8051(96)00161-8. [DOI] [PubMed] [Google Scholar]
- Nonaka Y, Shimada J, Nonaka H, Koike N, Aoki N, Kobayashi H, Kase H, Yamaguchi K, Suzuki F. Photoisomerization of a potent and selective adenosine A2 antagonist, (E)-1,3-Dipropyl-8-(3,4-dimethoxystyryl)-7-methylxanthine. J Med Chem. 1993;36:3731–3733. doi: 10.1021/jm00075a031. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Ichimura M, Takeda M, Nonaka Y, Shimada J, Suzuki F, Yamaguchi K, Kase H. KF17837 ((E)-8-(3,4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine), a potent and selective adenosine A2 receptor antagonist. Eur J Pharmacol. 1994a;267:335–341. doi: 10.1016/0922-4106(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Mori A, Ichimura M, Shindou T, Yanagawa K, Shimada J, Kase H. Binding of [3H]KF17837S, a selective adenosine A2 receptor antagonist, to rat brain membranes. Mol Pharmacol. 1994b;46:817–822. [PubMed] [Google Scholar]
- Obiefuna PC, Batra VK, Nadeem A, Borron A, Wilson CN, Mustafa SJ. A novel A1 adenosine receptor antagonist, L-97-1 [3-[2-(4-aminophenyl)-ethyl]-8-benzyl-7-{2-ethyl-(2-hydroxy-ethyl)-amino]-ethyl}-1-propyl-3,7-dihydro-purine-2,6-dione], reduces allergic responses to house dust mite in an allergic rabbit model of asthma. J Pharmacol Exp Ther. 2005;315:329–336. doi: 10.1124/jpet.105.088179. [DOI] [PubMed] [Google Scholar]
- Olah ME, Jacobson KA, Stiles GL. Affinity chromatography of the bovine cerebral cortex A1 adenosine receptor. FEBS Lett. 1989;257:292–296. doi: 10.1016/0014-5793(89)81555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozola V, Thorand M, Diekmann M, Qurishi R, Schumacher B, Jacobson KA, Müller CE. 2-Phenylimidazo[2,1-i]purin-5-ones: structure-activity relationships and characterization of potent and selective inverse agonists at human A3 adenosine receptors. Bioorg Med Chem. 2003;11:347–356. doi: 10.1016/s0968-0896(02)00456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Hoffmann C, Kim HO, Padgett WL, Daly JW, Brambilla R, Motta C, Abbracchio MP, Jacobson KA. Activation and desensitization of rat A3-adenosine receptors by selective adenosine derivatives and xanthine-7-ribosides. Drug Devel Res. 1998;44:97–105. doi: 10.1002/(SICI)1098-2299(199806/07)44:2/3<97::AID-DDR7>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorin G, Bolcato C, Cacciari B, Kachler S, Klotz K-N, Montopoli C, Moro S, Spalluto G. Synthesis, biological and modelling studies of 1,3-di-n-propyl-2,4-dioxo-6-methyl-8-(substituted) 1,2,3,4-tetrahydro[1,2,4]triazolo[3,4-f]purines as adenosine receptor antagonists. Farmaco. 2005;60:643–651. doi: 10.1016/j.farmac.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Patel A, Craig RH, Daluge SM, Linden J. 125I-BW-A844U, an antagonist radioligand with high affinity and selectivity for adenosine A1 receptors, and 125I-azido-BW-A844U, a photoaffinity label. Mol Pharmacol. 1988;33:585–591. [PubMed] [Google Scholar]
- Peet NP, Lentz NL, Dudley MW, Ogden AM, McCarty DR, Racke MM. Xanthines with C8 chiral substituents as potent and selective adenosine A1 antagonists. J Med Chem. 1993;36:4015–4020. doi: 10.1021/jm00077a004. [DOI] [PubMed] [Google Scholar]
- Petzer JP, Steyn S, Castagnoli KP, Chen JF, Schwarzschild MA, Van der Schyf CJ, Castagnoli N. Inhibition of monoamine oxidase B by selective adenosine A2A receptor antagonists. Bioorg Med Chem. 2003;11:1299–1310. doi: 10.1016/s0968-0896(02)00648-x. [DOI] [PubMed] [Google Scholar]
- Petzer JP, Castagnoli N, Jr, Schwarzschild MA, Chen J-F, Van der Schyf CJ. Dual-target-directed drugs that block monoamine oxidase B and adenosine A2A receptors for Parkinson’s disease. Neurotherapeutics. 2009;6:141–151. doi: 10.1016/j.nurt.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister JR, Belardinelli L, Lee G, Lum RT, Milner P, Stanley WC, Linden J, Baker SP, Schreiner G. Synthesis and biological evaluation of the enantiomers of the potent and selective A1-adenosine antagonist 1,3-dipropyl-8-[2-(5,6-epoxynorbornyl)]xanthine. J Med Chem. 1997;40:1773–1778. doi: 10.1021/jm970013w. [DOI] [PubMed] [Google Scholar]
- Pretorius J, Malan SF, Castagnoli N, Jr, Bergh JJ, Petzer JP. Dual inhibition of monoamine oxidase B and antagonism of the adenosine A2A receptor by (E,E)-8-(4-phenylbutadien-1-yl)caffeine analogues. Bioorg Med Chem. 2008;16:8676–8684. doi: 10.1016/j.bmc.2008.07.088. [DOI] [PubMed] [Google Scholar]
- Priego E-M, von Frijtag Drabbe Kuenzel J, IJzerman AP, Camarasa M-J, Pérez-Pérez M-J. Pyrido[2,1-f]purine-2,4-dione derivatives as a novel class of highly potent human A3 adenosine receptor antagonists. J Med Chem. 2002;45:3337–3344. doi: 10.1021/jm0208469. [DOI] [PubMed] [Google Scholar]
- Priego E-M, Pérez-Pérez M-J, von Frijtag Drabbe Kuenzel JK, de Vries H, IJzerman AP, Camarasa M-J, Martín-Santamaría S. Selective human adenosine A3 antagonists based on pyrido[2,1-f]purine-2,4-diones: novel features of hA3 antagonist binding. ChemMedChem. 2008;3:111–119. doi: 10.1002/cmdc.200700173. [DOI] [PubMed] [Google Scholar]
- Richardson PJ, Kase H, Jenner PG. Adenosine A2A receptor antagonists as new agents for the treatment of Parkinson’s disease. Trends Pharmacol Sci. 1997;18:338–344. doi: 10.1016/s0165-6147(97)01096-1. [DOI] [PubMed] [Google Scholar]
- Robeva AS, Woodard RL, Jin X, Gao Z, Bhattarcharya S, Taylor HE, Rosin DL, Linden J. Molecular characterization of recombinant human adenosine receptors. Drug Dev Res. 1996;39:243–252. [Google Scholar]
- Saki M, Tsumuki H, Nonaka H, Shimada J, Ichimura M. KF26777 (2-(4-bromophenyl)-7,8-dihydro-4-propyl-1H-imidazo[2,1-i]purin-5(4H)-one dihydrochloride), a new potent and selective adenosine A3 receptor antagonist. Eur J Pharmacol. 2002;444:133–141. doi: 10.1016/s0014-2999(02)01662-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Betz AJ, Ishiwari K, Felsted J, Madson L, Mirante B, Clark K, Font L, Korbey S, Sager TN, Hockemeyer J, Müller CE. Tremorolytic effects of adenosine A2A antagonists: implications for parkinsonism. Front Biosci. 2008;13:3594–3605. doi: 10.2741/2952. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Ishiwari K, Betz AJ, Farrar AM, Mingote SM, Font L, Hockemeyer J, Müller CE, Correa M. Dopamine/adenosine interactions related to locomotion and tremor in animal models: possible relevance to parkinsonism. Parkinsonism Relat Disord. 2008;14(Suppl 2):S130–134. doi: 10.1016/j.parkreldis.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci USA. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R, Maurinsh J, Reith U, Fülle F, Klotz KN, Müller CE. Water-soluble phosphate prodrugs of 1-propargyl-8-styrylxanthine derivatives, A2A-selective adenosine receptor antagonists. J Med Chem. 2000;43:440–448. doi: 10.1021/jm9911480. [DOI] [PubMed] [Google Scholar]
- Scammels PJ, Baker SP, Belardinelli L, Olsson RA. Substituted 1,3-dipropylxanthines as irreversible antagonists of A1 adenosine receptors. J Med Chem. 1994;37:2704–2712. doi: 10.1021/jm00043a010. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Bezard E, Brotchie J, Calon F, Collingridge GL, Ferger B, Hengerer B, Hirsch E, Jenner P, Le Novere N, Obeso JA, Schwarzschild MA, Spampinato U, Davidai G. Novel pharmacological targets for the treatment of Parkinson’s disease. Nat Rev Drug Discov. 2006;5:845–854. doi: 10.1038/nrd2087. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Karcz-Kubicha M, Thorndike EB, Müller CE, Tella SR, Goldberg SR, Ferré S. Lack of adenosine A1 and dopamine D2 receptor-mediated modulation oft he cardiovascular effects oft he adenosine A2A receptor agonist CGS 21680. Eur J Pharmacol. 2004;484:269–275. doi: 10.1016/j.ejphar.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Karcz-Kubicha M, Thorndike EB, Müller CE, Tella SR, Ferré S, Goldberg SR. Role of central and peripheral adenosine recepotrs in the cardiovascular responses to intraperitoneal injections of adenosine A1 and A2A subtype receptor agonists. Br J Pharmacol. 2005;144:642–650. doi: 10.1038/sj.bjp.0706043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schingnitz G, Küfner-Mühl U, Ensinger H, Lehr E, Kuhn FJ. Selective A1 antagonists for treatment of cognitive deficits. Nucleosides Nucleotides. 1991;10:1067–1076. [Google Scholar]
- Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Seale TW, Abla KA, Shamim MT, Carney JM, Daly JW. 3,7-Dimethyl-1-propargylxanthine: a potent and selective in vivo antagonist of adenosine analogs. Life Sci. 1988;43:1671–1684. doi: 10.1016/0024-3205(88)90478-x. [DOI] [PubMed] [Google Scholar]
- Shamim MT, Ukena D, Padgett WL, Hong O, Daly JW. 8-Aryl and 8-cycloalkyl-1,3-dipropylxanthines: further potent and selective antagonists for A1-adenosine receptors. J Med Chem. 1988;31:613–617. doi: 10.1021/jm00398a020. [DOI] [PubMed] [Google Scholar]
- Shamim MT, Ukena D, Padgett WL, Daly JW. Effects of 8-phenyl and 8-cycloalkyl substituents on the activity of mono-, di-, and trisubstituted alkylxanthines with substitution at the 1-, 3-, and 7-positions. J Med Chem. 1989;32:1231–1237. doi: 10.1021/jm00126a014. [DOI] [PubMed] [Google Scholar]
- Shimada J, Suzuki F, Nonaka H, Ishii A. 8-Polycycloalkyl-1,3-dipropylxanthines as potent and selective antagonists for A1-adenosine receptors. J Med Chem. 1992a;35:924–930. doi: 10.1021/jm00083a018. [DOI] [PubMed] [Google Scholar]
- Shimada J, Suzuki F, Nonaka H, Ishii A, Ichikawa S. (E)-1,3-Dialkyl-7-methyl-8-(3,4,5-trimethoxystyryl)xanthines: potent and selective adenosine A2 antagonists. J Med Chem. 1992b;35:2342–2345. doi: 10.1021/jm00090a027. [DOI] [PubMed] [Google Scholar]
- Shimada J, Koike N, Nonaka H, Shiozaki S, Yanagawa K, Kanda T, Kobayashi H, Ichimura M, Nakamura J, Kase H, Suzuki F. Adenosine A2A antagonists with potent anti-cataleptic activity. Bioorg Med Chem Lett. 1997;7:2349–2352. [Google Scholar]
- Shukla D, Chakraborty S, Singh S, Mishra B. Doxofylline: a promising methylxanthine derivative for the treatment of asthma and chronic obstructive pulmonary disease. Expert Opin Pharmacother. 2009;10:2343–2356. doi: 10.1517/14656560903200667. [DOI] [PubMed] [Google Scholar]
- Slawsky MT, Givertz MM. Rolofylline: a selective adenosine 1 receptor antagonist fort he treatment of heart failure. Expert Opin Pharmacother. 2009;10:311–322. doi: 10.1517/14656560802682213. [DOI] [PubMed] [Google Scholar]
- Solinas M, Ferré S, Antoniou K, Quarta D, Zustinova Z, Hockemeyer J, Pappas LA, Segal PN, Wertheim C, Müller CE, Goldberg SR. Involvement of adenosine A1 receptors in the discriminative-stimulus effects of caffeine in rats. Psychopharmacology. 2005;179:576–586. doi: 10.1007/s00213-004-2081-6. [DOI] [PubMed] [Google Scholar]
- Sorbera LA, Martín L, Castaner J. Drugs Fut. 2000;25:1011–1016. [Google Scholar]
- Soriano A, Ventura R, Molero A, Hoen R, Casadó V, Cortés A, Fanelli F, Albericio F, Lluís C, Franco R, Royo M. Adenosine A2A receptor-antagonist/dopamine D2 receptor agonist bivalent ligands as pharmacological tools to detect A2A-D2 receptor heteromers. J Med Chem. 2009;52:5590–5602. doi: 10.1021/jm900298c. [DOI] [PubMed] [Google Scholar]
- Stefanachi A, Brea JM, Cadavid MI, Centeno NB, Esteve C, Loza MI, Martinez A, Nieto R, Ravina E, Sanz F, Segarra V, Sotelo E, Vidal B, Carotti A. 1-, 3- and 8-substituted 9-deazaxanthines as potent and selective antagonists at the human A2B adenosine receptor. Bioorg Med Chem. 2008;16:2852–2869. doi: 10.1016/j.bmc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Stefanovich V. The xanthines. Drug News Perspect. 1989;2:82–88. [Google Scholar]
- Stiles GL, Jacobson KA. A new high affinity, iodinated adenosine receptor antagonist as a radioligand/photoaffinity crosslinking probe. Mol Pharmacol. 1987;32:184–188. [PMC free article] [PubMed] [Google Scholar]
- Stiles GL, Jacobson KA. High affinity acylating antagonists for the A1 adenosine receptor: identification of binding subunit. Mol Pharmacol. 1988;34:724–728. [PMC free article] [PubMed] [Google Scholar]
- Stone GA, Jarvis MF, Sills M, Weeks B, Snowhill EW, Williams M. Species differences in high affinity adenosine A2 receptors in striatal membranes from mammalian brain. Drug Dev Res. 1988;15:31–46. [Google Scholar]
- Strömberg I, Popoli P, Müller CE, Ferré S, Fuxe K. Electrophysiological and behavioural evidence for an antagonistic modulatory role of adenosine A2A receptors in dopamine D2 receptor regulation in the rat dopamine denervated striatum. Eur J Neurosci. 2000;12:4033–4037. doi: 10.1046/j.1460-9568.2000.00288.x. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Shimada J, Mizumoto H, Karasawa A, Kubo K, Nonaka H, Ishii A, Kawakita T. Adenosine A1 antagonists. 2. Structure-activity relationships on diuretic activities and protective effects against acute renal failure. J Med Chem. 1992a;35:3066–3075. doi: 10.1021/jm00094a022. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Shimada J, Nonaka H, Ishii A, Shiozaki S, Ichikawa S, Ono E. 7,8-Dihydro-8-ethyl-2-(3-noradamantyl)-4-propyl-1H-imidazo[2,1-i]purin-5(4H)-one: a potent and water-soluble adenosine A1 antagonist. J Med Chem. 1992b;35:3578–3581. doi: 10.1021/jm00097a016. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Pamplona FA, Prediger RD. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front Biosci. 2008;13:2614–2632. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Johnson J, Heilig M. Effect of the adenosine A2A receptor antagonist 3,7-dimethyl-propargylxanthine on anxiety-like and depression-like behavior and alcohol consumption in Wistar Rats. Alcohol Clin Exp Res. 2007;31:1302–1307. doi: 10.1111/j.1530-0277.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- Ukena D, Jacobson KA, Kirk KL, Daly JW. A [3H]amine congener of 1,3-dipropyl-8-phenylxanthine. A new radioligand for A2 adenosine receptors of human platelets. FEBS Lett. 1986a;199:269–274. doi: 10.1016/0014-5793(86)80493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena D, Jacobson KA, Padgett WL, Ayala C, Shamim MT, Kirk KL, Olsson RA, Daly JW. Species differences in structure-activity relationships of adenosine agonists and xanthine antagonists at brain A1 adenosine receptors. FEBS Lett. 1986b;209:122–128. doi: 10.1016/0014-5793(86)81096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena D, Daly JW, Kirk KL, Jacobson KA. Functionalized congeners of 1,3-dipropyl-8-phenylxanthine: potent antagonists for adenosine receptors that modulate membrane adenylate cyclase in pheochromocytoma cells, platelets and fat cells. Life Sci. 1986c;38:797–807. doi: 10.1016/0024-3205(86)90596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena D, Schudt C, Sybrecht GW. Adenosine receptor-blocking xanthines as inhibitors of phosphodiesterase isozymes. Biochem Pharmacol. 1993;45:847–851. doi: 10.1016/0006-2952(93)90168-v. [DOI] [PubMed] [Google Scholar]
- van den Berg D, Zoellner KR, Ogunrombi MO, Malan SF, Terre’Blanche G, Castagnoli N, Jr, Bergh JJ, Petzer JP. Inhibition of monoamine oxidase B by selected benzimidazole and caffeine analogues. Bioorg Med Chem. 2007;15:3692–3702. doi: 10.1016/j.bmc.2007.03.046. [DOI] [PubMed] [Google Scholar]
- van Galen PJM, van Bergen AH, Gallo-Rodriguez C, Melman N, Olah ME, IJzerman AP, Stiles GL, Jacobson KA. A binding site model and structure-activity relationships for the rat A3 adenosine receptor. Mol Pharmacol. 1994;45:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- van Muijlwijk-Koezen JE, Timmerman H, van der Sluis RP, van de Stolpe AC, Menge WM, Beukers MW, van der Graaf PH, de Groote M, IJzerman AP. Synthesis and use of FSCPX, an irreversible adenosine A1 antagonist, as a ‘receptor knock-down’ tool. Bioorg Med Chem. 2001;11:815–818. doi: 10.1016/s0960-894x(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Vittori S, Volpini R, Lambertucci C, Taffi S, Klotz KN, Cristalli G. 2-substituted 5′-N-methylcarboxamidoadenosine (MECA) derivatives as A3 adenosine receptor ligands. Nucleosides Nucleotides Nucleic Acids. 2005;24:935–938. doi: 10.1081/ncn-200059296. [DOI] [PubMed] [Google Scholar]
- Vlok N, Malan SF, Castagnoli N, Jr, Bergh JJ, Petzer JP. Inhibition of monoamine oxidase B by analogues of the adenosine A2A receptor antagonist (E)-8-(3-chlorostyryl)caffeine (CSC) Bioorg Med Chem. 2006;14:3512–2351. doi: 10.1016/j.bmc.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Vollmann K, Qurishi R, Hockemeyer J, Müller CE. Synthesis and properties of a new water-soluble prodrug of the adenosine A2A receptor antagonist MSX-2. Molecules. 2008;13:348–359. doi: 10.3390/molecules13020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu CB. Recent advances in the design and optimization of adenosine A2A receptor antagonists. Curr Opin Drug Discov Devel. 2005;8:458–468. [PubMed] [Google Scholar]
- Vu CB, Kiesman WF, Conlon PR, Lin K-C, Tam M, Petter RC, Smits G, Lutterodt F, Jin X, Chen L, Zhang J. Tricyclic imidazoline derivatives as potent and selective adenosine A1 receptor antagonists. J Med Chem (2006) 2006;49:7132–7139. doi: 10.1021/jm060539t. [DOI] [PubMed] [Google Scholar]
- Weiss HM, Grisshammer R. Purification and characterization of the human adenosine A2a receptor functionally expressed in Escherichia coli. Eur J Biochem. 2002;269:82–92. doi: 10.1046/j.0014-2956.2002.02618.x. [DOI] [PubMed] [Google Scholar]
- Weyler S, Fülle F, Diekmann M, Schumacher B, Hinz S, Klotz KN, Müller CE. Improving potency, selectivity, and water-solubility of adenosine A1 receptor antagonists: xanthines modified at position 3 and related pyrimido[1,2,3-cd]purinediones. ChemMedChem. 2006;1:891–902. doi: 10.1002/cmdc.200600066. [DOI] [PubMed] [Google Scholar]
- Worden L, Shahriari M, Farrar A, Sink KS, Hockemeyer J, Müller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effort-related effects of dopamine blockade: differential interaction with D1 and D2 family antagonists. Psychopharmacology. 2009;203:489–499. doi: 10.1007/s00213-008-1396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Müller CE. Preparation, properties, reactions, and adenosine receptor affinities of sulfophenylxanthine nitrophenyl esters: toward the development of sulfonic acid prodrugs with peroral bioavailability. J Med Chem. 2004;47:1031–1043. doi: 10.1021/jm0310030. [DOI] [PubMed] [Google Scholar]
- Yan L, Bertarelli CG, Hayallah AM, Meyer H, Klotz KN, Müller CE. A new synthesis of sulfonamides by aminolysis of p-nitrophenylsulfonates yielding potent and selective adenosine A2B receptor antagonists. J Med Chem. 2006;49:4384–4391. doi: 10.1021/jm060277v. [DOI] [PubMed] [Google Scholar]
- Yu L, Shen HY, Coelho JE, Araujo IM, Huang QY, Day YJ, Rebola N, Canas PM, Rapp EK, Ferrara J, Taylor D, Müller CE, Linden J, Cunha RA, Chen JF. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol. 2008;63:338–346. doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]
- Yuzlenko O, Kiec-Kononowicz K. Potent adenosine A1 and A2A receptors antagonists: recent developments. Curr Med Chem. 2006;13:3609–3625. doi: 10.2174/092986706779026093. [DOI] [PubMed] [Google Scholar]
- Zablocki J, Kalla R, Perry T, Palle V, Varkhedkar V, Xiao D, Piscopio A, Maa T, Gimbel A, Hao J, Chu N, Leung K, Zeng D. The discovery of a selective, high affinity A2B adenosine receptor antagonist for the potential treatment of asthma. Bioorg Med Chem. 2005;15:609–612. doi: 10.1016/j.bmcl.2004.11.044. [DOI] [PubMed] [Google Scholar]