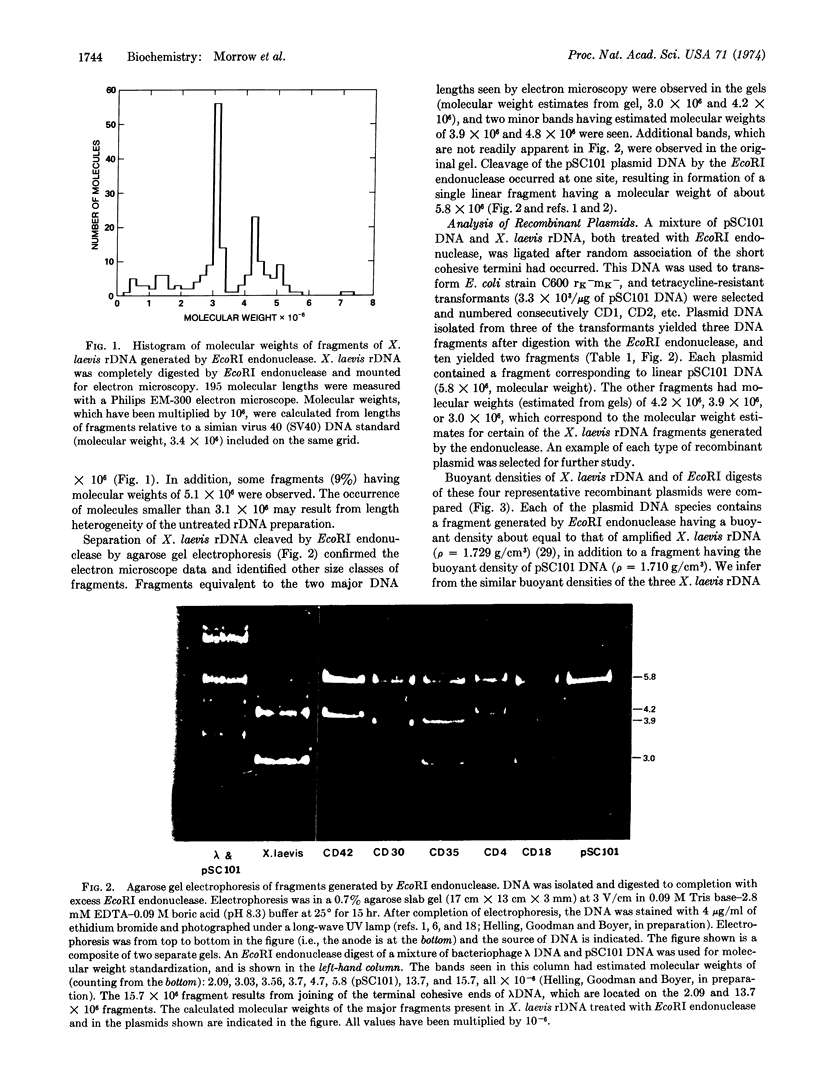
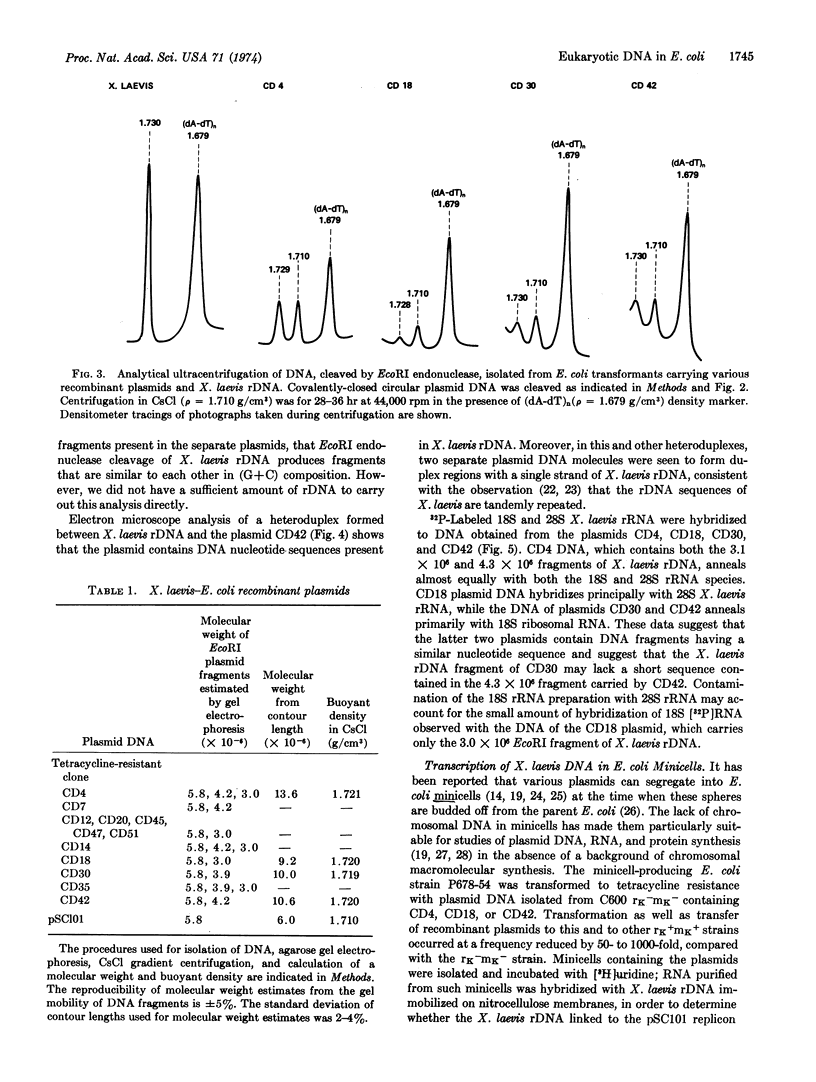
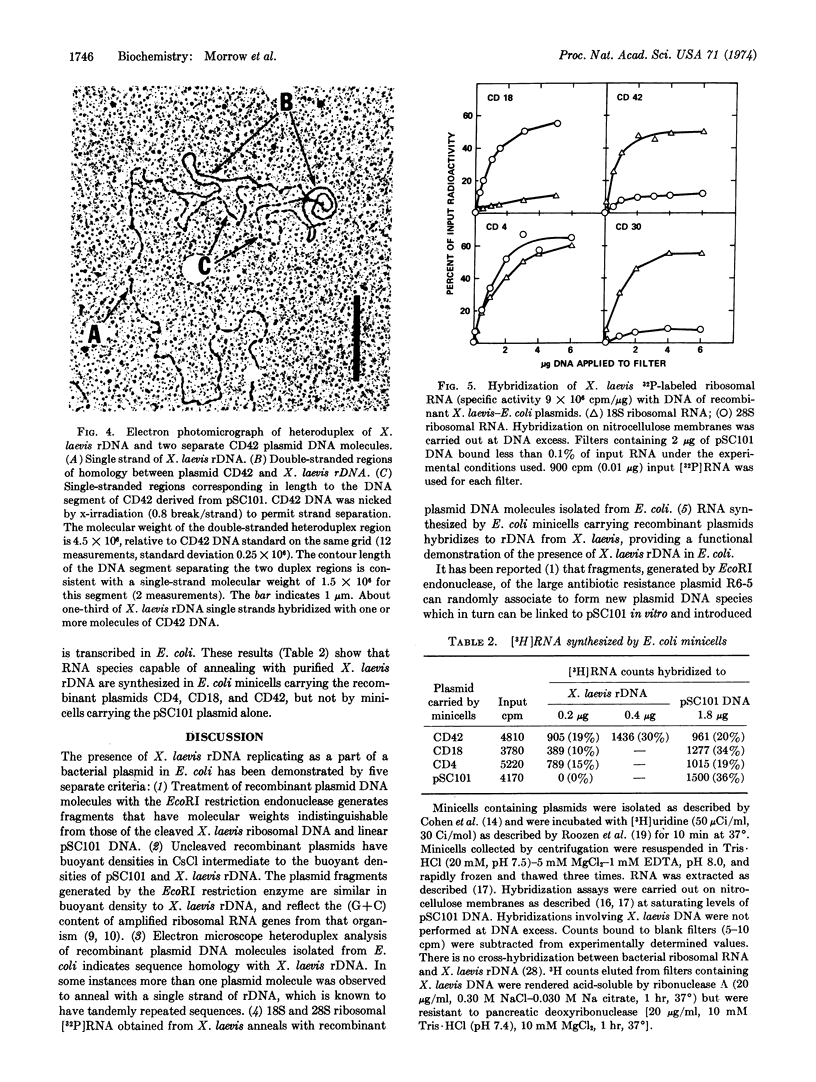
Abstract
Fragments of amplified Xenopus laevis DNA, coding for 18S and 28S ribosomal RNA and generated by EcoRI restriction endonuclease, have been linked in vitro to the bacterial plasmid pSC101; and the recombinant molecular species have been introduced into E. coli by transformation. These recombinant plasmids, containing both eukaryotic and prokaryotic DNA, replicate stably in E. coli. RNA isolated from E. coli minicells harboring the plasmids hybridizes to amplified X. laevis rDNA.
Keywords: restriction, plasmid, transformation, recombination, ribosomal DNA
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Chipchase M., Speirs J. The ribosomal RNA cistrons. Prog Nucleic Acid Res Mol Biol. 1971;11:351–389. doi: 10.1016/s0079-6603(08)60332-3. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Weber C. S. Gene linkage by RNA-DNA hybridization. I. Unique DNA sequences homologous to 4 s RNA, 5 s RNA and ribosomal RNA. J Mol Biol. 1968 Jun 28;34(3):661–680. doi: 10.1016/0022-2836(68)90188-5. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Genome construction between bacterial species in vitro: replication and expression of Staphylococcus plasmid genes in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1030–1034. doi: 10.1073/pnas.71.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Hurwitz J. Genetic transcription in bacteriophage lambda: studies of lambda m RNA synthesis in vivo. J Mol Biol. 1968 Nov 14;37(3):387–406. doi: 10.1016/0022-2836(68)90110-1. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Silver R. P., McCoubrey A. E. Isolation of catenated forms of R factor DNA from minicells. Nat New Biol. 1971 Jun 23;231(25):249–252. doi: 10.1038/newbio231249a0. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Brown D. D., Reeder R. H. Composition and structure of chromosomal and amplified ribosomal DNA's of Xenopus laevis. J Mol Biol. 1970 Jul 28;51(2):341–360. doi: 10.1016/0022-2836(70)90147-6. [DOI] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Derepression of anthranilate synthase in purified minicells of Escherichia coli containing the Col-trp plasmid. J Bacteriol. 1973 Aug;115(2):615–622. doi: 10.1128/jb.115.2.615-622.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Dressler D., Wolfson J. The amplification of ribosomal RNA genes involves a rolling circle intermediate. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2926–2930. doi: 10.1073/pnas.70.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. Segregation into and replication of plasmid deoxyribonucleic acid in chromosomeless segregants of Escherichia coli. J Bacteriol. 1970 Jun;102(3):642–647. doi: 10.1128/jb.102.3.642-647.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., Norman P. Segregation of transferable R factors into Escherichia coli minicells. Nature. 1970 Aug 8;227(5258):606–607. doi: 10.1038/227606a0. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Anraku Y., Lehman I. R. Deoxyribonucleic acid ligase. Isolation and physical characterization of the homogeneous enzyme from Escherichia coli. J Biol Chem. 1973 Nov 10;248(21):7495–7501. [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgaramella V. Enzymatic oligomerization of bacteriophage P22 DNA and of linear Simian virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3389–3393. doi: 10.1073/pnas.69.11.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Sinclair J. H., Brown D. D. Retention of common nucleotide sequences in the ribosomal deoxyribonucleic acid of eukaryotes and some of their physical characteristics. Biochemistry. 1971 Jul 6;10(14):2761–2769. doi: 10.1021/bi00790a017. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Brown D. D. Denaturation map of the ribosomal DNA of Xenopus laevis. J Mol Biol. 1971 Sep 14;60(2):235–247. doi: 10.1016/0022-2836(71)90290-7. [DOI] [PubMed] [Google Scholar]
- van Embden J., Cohen S. N. Molecular and genetic studies of an R factor system consisting of independent transfer and drug resistance plasmids. J Bacteriol. 1973 Nov;116(2):699–709. doi: 10.1128/jb.116.2.699-709.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]




