Abstract
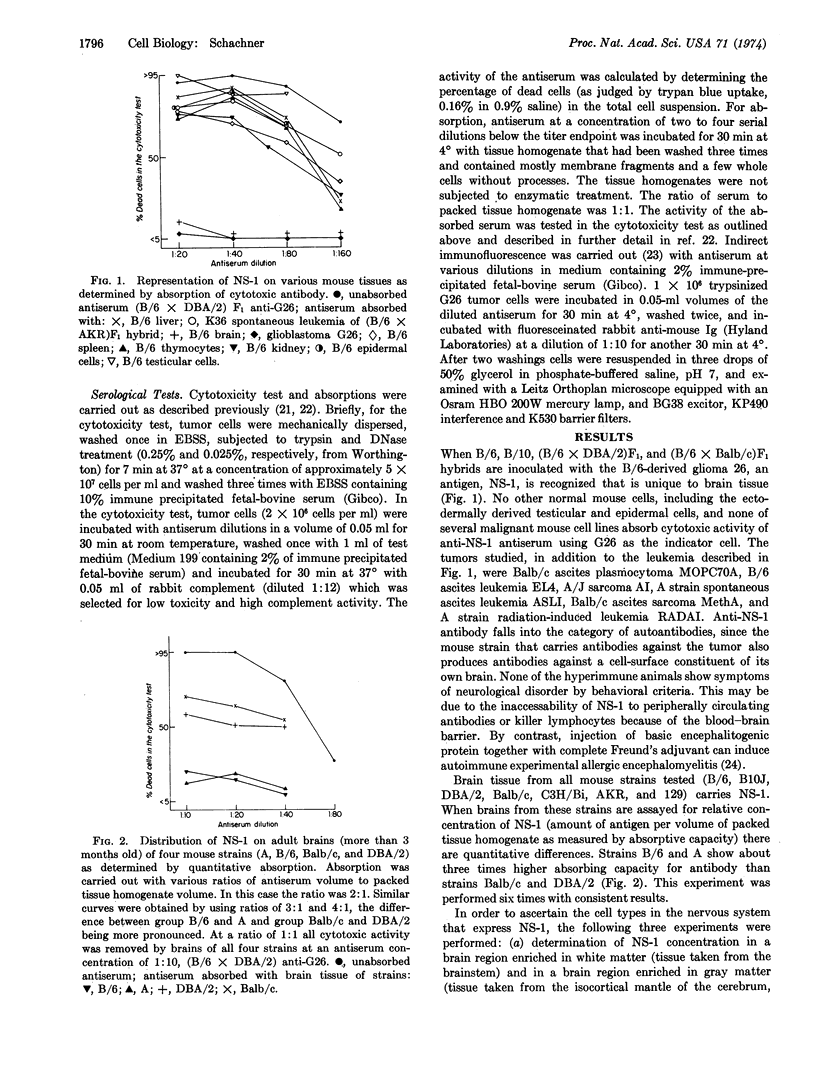
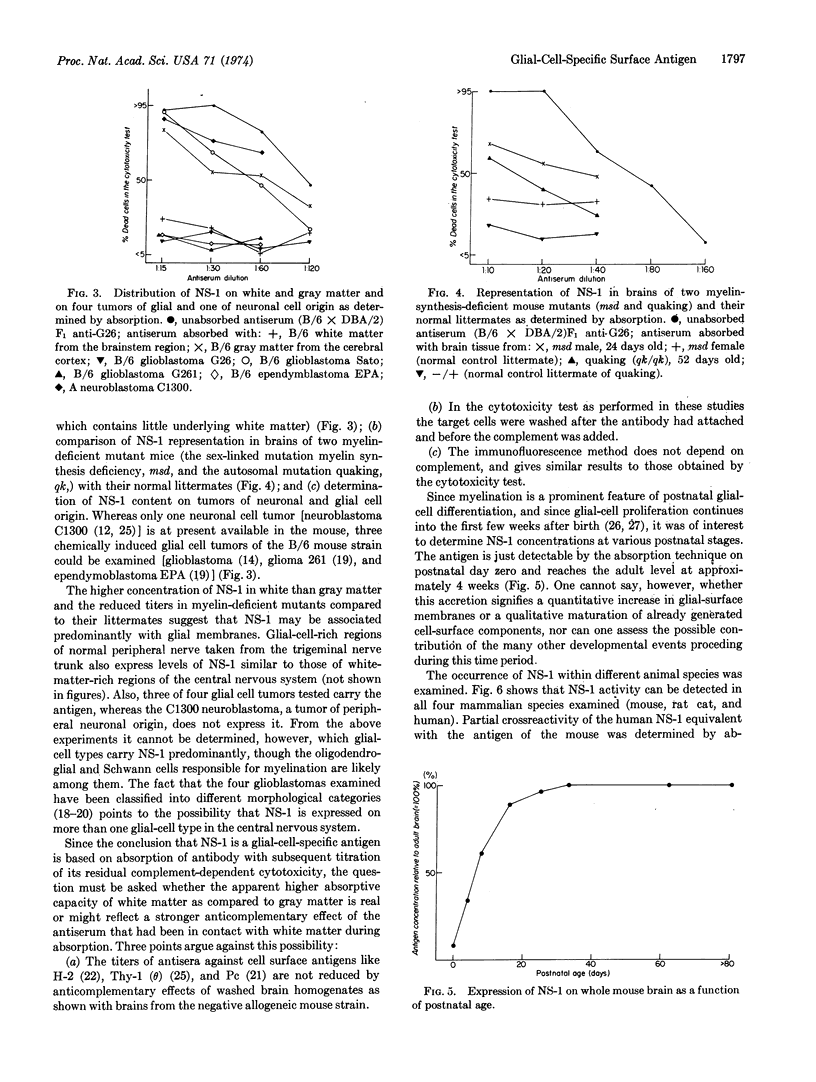
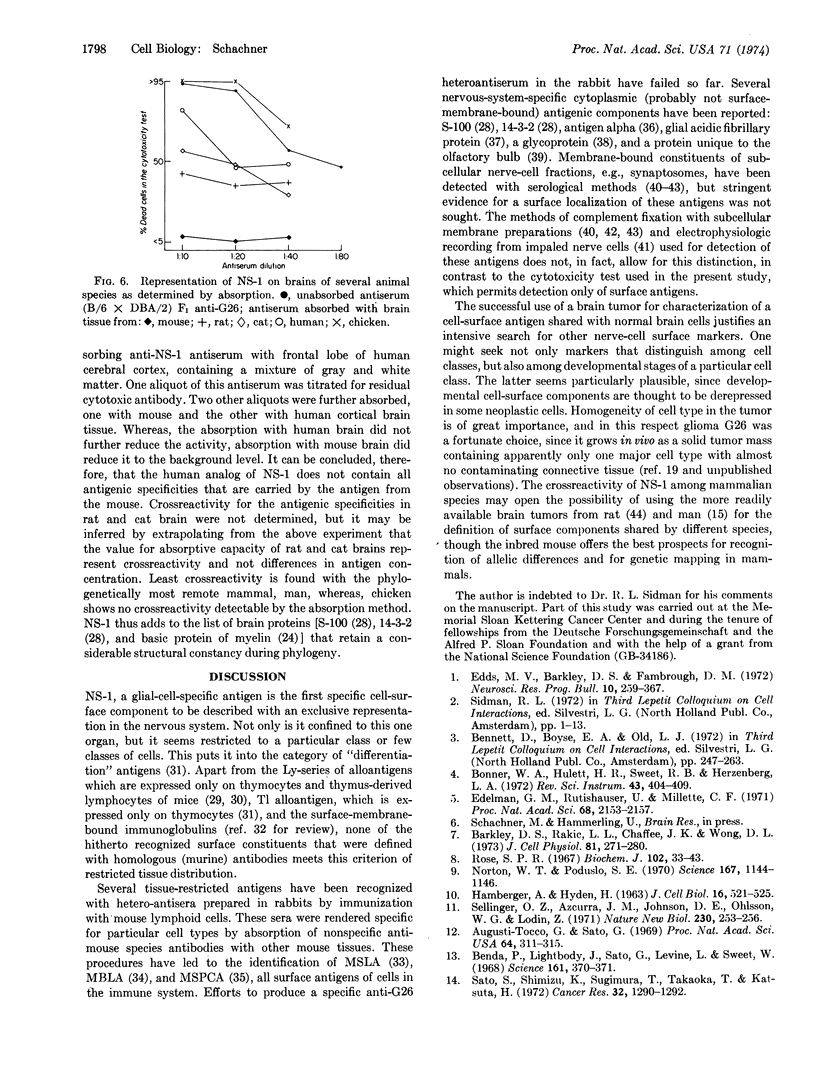
A methylcholanthrene-induced glioblastoma of the C57BL/6 inbred mouse strain was used to raise antibodies in C57BL/6 and C57BL/10 inbred mice and in (C57BL/6 × DBA/2) and (C57BL/6 × Balb/c) F1 hybrids. When examined by the cytotoxicity test, these antibodies define a cell-surface component (or components) found exclusively on brain tissue of all mouse strains studied and of several other mammalian species including man. The antigen, named NS-1 (nervous system antigen-1), is present on cells of three of the four mouse-glial-cell tumors tested, but not on the C1300 neuroblastoma, a tumor of neuronal origin. NS-1 occurs in higher concentration in regions of the nervous system richer in white than in gray matter, and in lower than normal concentrations in brains of myelindeficient neurological mutant mice. The concentration of NS-1 gradually increases postnatally and reaches the adult level between the third and fourth week. The existence of more than one allele or genetic locus controlling NS-1 activity is suggested by the occurrence of higher amounts of NS-1 in brains of the A and C57BL/6 than of the Balb/c and DBA/2 mouse strains. NS-1 is the first cellsurface component to be described that is not only unique to nervous tissue, but specific for glial cells.
Keywords: murine oligodendroglioma G26, glial-cell-surface-specific antigen, myelin and myelin synthesis, crossreactivity among mammalian species
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausman J. I., Shapiro W. R., Rall D. P. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970 Sep;30(9):2394–2400. [PubMed] [Google Scholar]
- BUONASSISI V., SATO G., COHEN A. I. Hormone-producing cultures of adrenal and pituitary tumor origin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1184–1190. doi: 10.1073/pnas.48.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley D. S., Rakic L. L., Chaffee J. K., Wong D. L. Cell separation by velocity sedimentation of postnatal mouse cerebellum. J Cell Physiol. 1973 Apr;81(2):271–279. doi: 10.1002/jcp.1040810215. [DOI] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Bennett G. S., Edelman G. M. Isolation of an acidic protein from rat brain. J Biol Chem. 1968 Dec 10;243(23):6234–6241. [PubMed] [Google Scholar]
- Bonner W. A., Hulett H. R., Sweet R. G., Herzenberg L. A. Fluorescence activated cell sorting. Rev Sci Instrum. 1972 Mar;43(3):404–409. doi: 10.1063/1.1685647. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Itakura K., Stockert E., Iritani C. A., Miura M. Ly-C: a third locus specifying alloantigens expressed only on thymocytes and lymphocytes. Transplantation. 1971 Mar;11(3):351–353. [PubMed] [Google Scholar]
- Boyse E. A., Miyazawa M., Aoki T., Old L. J. Ly-A and Ly-B: two systems of lymphocyte isoantigens in the mouse. Proc R Soc Lond B Biol Sci. 1968 Jun 11;170(1019):175–193. doi: 10.1098/rspb.1968.0032. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Rutishauser U., Millette C. F. Cell fractionation and arrangement on fibers, beads, and surfaces. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2153–2157. doi: 10.1073/pnas.68.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- HAMBERGER A., HYDEN H. Inverse enzymatic changes in neurons and glia during increased function and hypoxia. J Cell Biol. 1963 Mar;16:521–525. doi: 10.1083/jcb.16.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Cotman C., Matthews D. A. Serologic specificities of brain subcellular organelles. I. Antisera to synaptosomal fractions. J Immunol. 1972 May;108(5):1362–1369. [PubMed] [Google Scholar]
- Kornguth S. E., Anderson J. W., Scott G. Isolation of synaptic complexes in a caesium chloride density gradient: electron microscopic and immunohistochemical studies. J Neurochem. 1969 Jun;16(3):1017–1024. doi: 10.1111/j.1471-4159.1969.tb08992.x. [DOI] [PubMed] [Google Scholar]
- Margolis F. L. A brain protein unique to the olfactory bulb. Proc Natl Acad Sci U S A. 1972 May;69(5):1221–1224. doi: 10.1073/pnas.69.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Neuronal soma and whole neuroglia of rat brain: a new isolation technique. Science. 1970 Feb 20;167(3921):1144–1145. doi: 10.1126/science.167.3921.1144. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S. E., Kornblith P. L., Cares H. L., Seals J., Levine L. S-100 protein in human acoustic neurinomas. Brain Res. 1972 Jun 8;41(1):187–193. doi: 10.1016/0006-8993(72)90627-0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S. E., Wechsler W. Biochemically differentiated neoplastic clone of Schwann cells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2885–2889. doi: 10.1073/pnas.69.10.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Nase S., Mitchison N. A. Mouse specific bone marrow-derived lymphocyte antigen as a marker for thymus-independent lymphocytes. Nature. 1971 Mar 5;230(5288):50–51. doi: 10.1038/230050a0. [DOI] [PubMed] [Google Scholar]
- Rose S. P. Preparation of enriched fractions from cerebral cortex containing isolated, metabolically active neuronal and glial cells. Biochem J. 1967 Jan;102(1):33–43. doi: 10.1042/bj1020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Shimizu K., Sugimura T., Takaoka T., Katsuta H. Aldolase C in cultured mouse glioblastoma cells. Cancer Res. 1972 Jun;32(6):1290–1292. [PubMed] [Google Scholar]
- Schachner M. Serologically demonstrable cell surface specificities on mouse neuroblastoma C1300. Nat New Biol. 1973 May 23;243(125):117–119. [PubMed] [Google Scholar]
- Schachner M., Sidman R. L. Distribution of H-2 alloantigen in adult and developing mouse brain. Brain Res. 1973 Sep 28;60(1):191–198. doi: 10.1016/0006-8993(73)90857-3. [DOI] [PubMed] [Google Scholar]
- Sellinger O. Z., Azcurra J. M., Johnson D. E., Ohlsson W. G., Lodin Z. Independence of protein synthesis and drug uptake in nerve cell bodies and glial cells isolated by a new technique. Nat New Biol. 1971 Apr 21;230(16):253–256. doi: 10.1038/newbio230253a0. [DOI] [PubMed] [Google Scholar]
- Shek R. P., MacPherson C. F. Immune response of rats to subcellular fractions of isologous brain and liver. Immunology. 1971 Aug;21(2):333–341. [PMC free article] [PubMed] [Google Scholar]
- Shigeno N., Hämmerling U., Arpels C., Boyse E. A., Old L. J. Preparation of lymphocyte-specific antibody from anti-lymphocyte serum. Lancet. 1968 Aug 10;2(7563):320–323. doi: 10.1016/s0140-6736(68)90530-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Hsu C. J., Boyse E. A. A new differentiation antigen of plasma cells. Eur J Immunol. 1971 Dec;1(6):478–482. doi: 10.1002/eji.1830010614. [DOI] [PubMed] [Google Scholar]
- Warecka K. Isolation of brain-specific glycoprotein. J Neurochem. 1970 Jun;17(6):829–830. doi: 10.1111/j.1471-4159.1970.tb03358.x. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN H. M. The nature of gliomas as revealed by animal experimentation. Am J Pathol. 1955 Jan-Feb;31(1):1–29. [PMC free article] [PubMed] [Google Scholar]


