Abstract
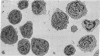
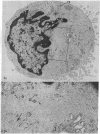
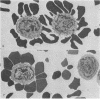
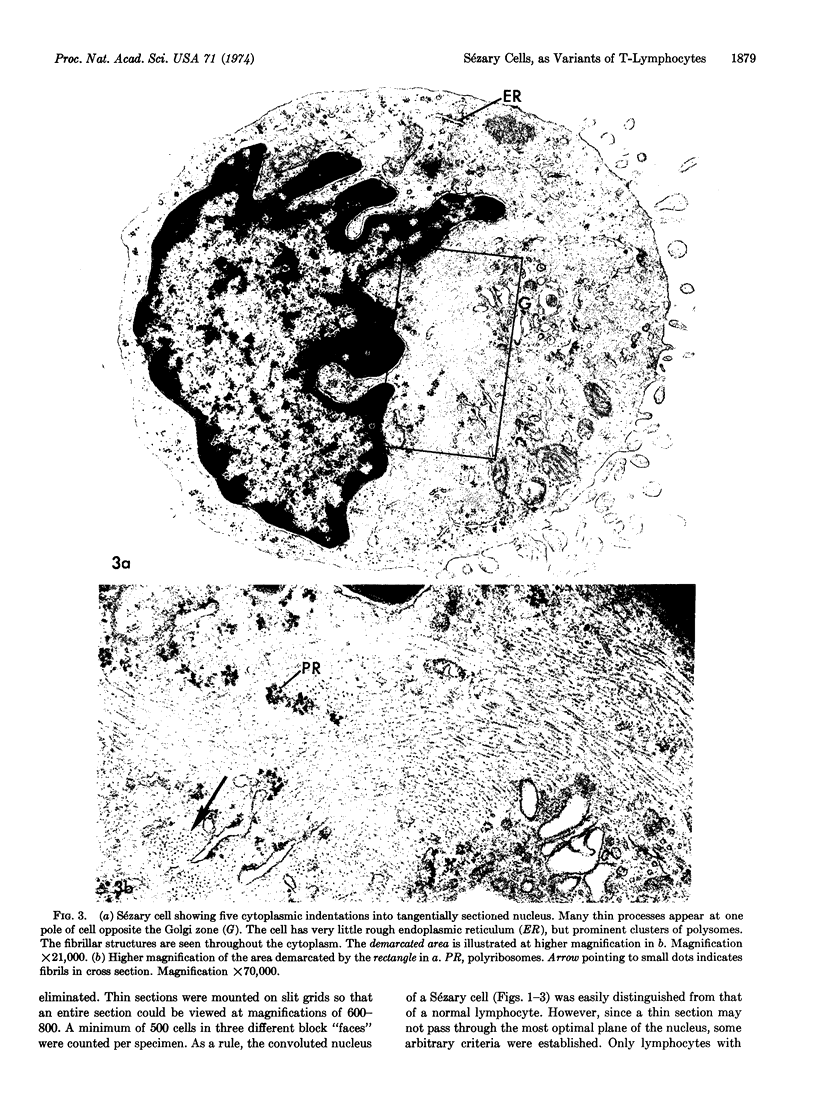
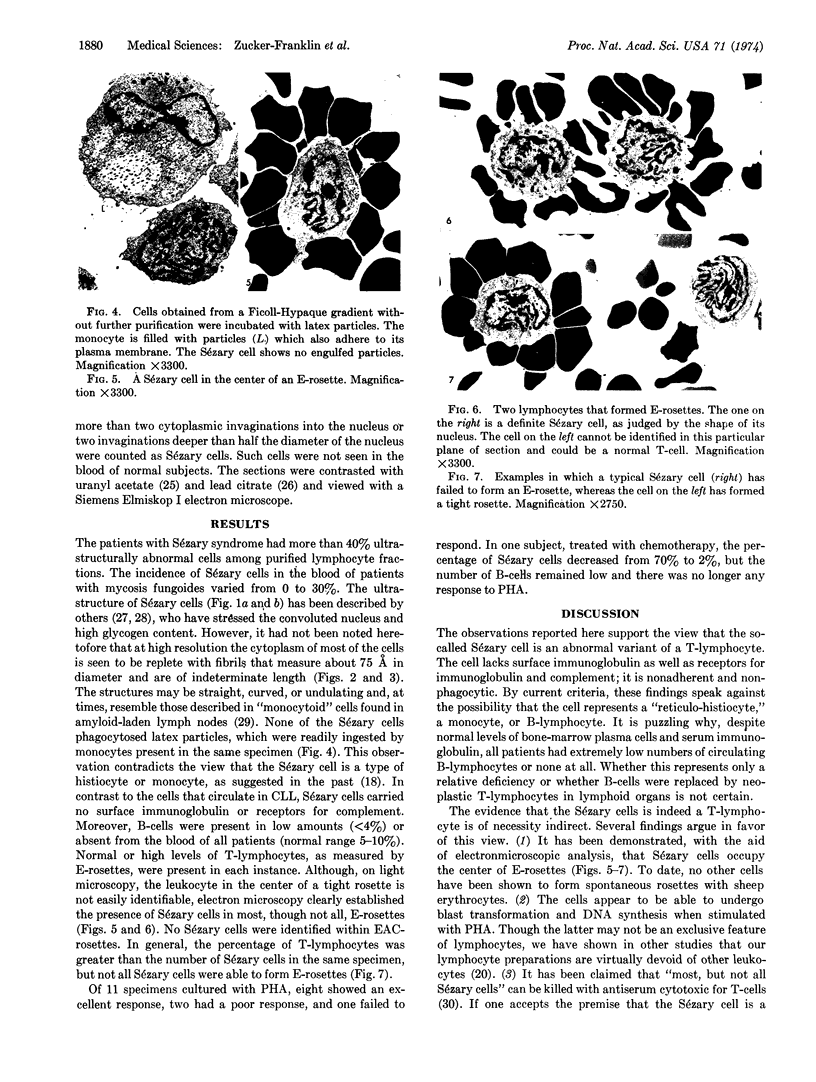
The vast majority of human lymphoid neoplasms examined to date have been associated with a proliferation of bone marrow-dependent (B) lymphocytes. In an effort to delineate human tumors of T-cell (thymusdependent) lineage, use was made of the peripheral blood leukocytes of sixteen subjects with various forms of mycosis fungoides. The abnormal cells in the circulation of these patients are morphologically identical to those that infiltrate their nodes and skin. On electron microscopy, such neoplastic lymphocytes (Sézary cells) had “cerebriform” nuclei and an abundance of cytoplasmic fibrils not described heretofore. Sézary cells were nonadherent and nonphagocytic and usually responded to stimulation with phytohemagglutinin, refuting earlier suggestions that the cells represent monocytes or histiocytes. In contrast to chronic lymphocytic leukemia lymphocytes, the Sézary cells lacked surface immunoglobulin and receptors for complement. Ultrastructural analysis identified Sézary cells in the center of directly formed rosettes (E-rosettes) characterizing the behavior of T lymphocytes in this test. Though some Sézary cells lacked both T and B cell-surface properties, in general, these observations support the view that the Sézary cell is a neoplastic variant of a thymusderived lymphocyte.
Keywords: mycosis fungoides, thymus-derived (T)-cell rosette, lymphoid neoplasms
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Bloch K. J. Immunoglobulins on the surface of neoplastic lymphocytes. N Engl J Med. 1972 Aug 10;287(6):272–276. doi: 10.1056/NEJM197208102870603. [DOI] [PubMed] [Google Scholar]
- BAIRATI A., Jr Submicroscopic structure of Yoshida ascites hepatoma. Cancer Res. 1961 Sep;21:989–992. [PubMed] [Google Scholar]
- Basten A., Sprent J., Miller J. F. Receptor for antibody-antigen complexes used to separate T cells from B cells. Nat New Biol. 1972 Feb 9;235(58):178–180. doi: 10.1038/newbio235178a0. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome J. D., Zucker-Franklin D., Weiner M. S., Bianco C., Nussenzweig V. Leukemic cells with membrane properties of thymus-derived (T) lymphocytes in a case of Sézary's syndrome: morphologic and immunologic studies. Clin Immunol Immunopathol. 1973 Apr;1(3):319–329. doi: 10.1016/0090-1229(73)90049-4. [DOI] [PubMed] [Google Scholar]
- Brouet J. C., Flandrin G., Seligmann M. Démonstration d'une prolifération de cellules lymphocytaires thymodépendantes dans le syndrome de Sezary. C R Acad Sci Hebd Seances Acad Sci D. 1973 Jan 8;276(2):247–249. [PubMed] [Google Scholar]
- Brouet J. C., Flandrin G., Seligmann M. Indications of the thymus-derived nature of the proliferating cells in six patients with Sézary's syndrome. N Engl J Med. 1973 Aug 16;289(7):341–344. doi: 10.1056/NEJM197308162890703. [DOI] [PubMed] [Google Scholar]
- Brownlee T. R., Murad T. M. Ultrastructure of mycosis fungoides. Cancer. 1970 Sep;26(3):686–698. doi: 10.1002/1097-0142(197009)26:3<686::aid-cncr2820260330>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- CLENDENNING W. E., BRECHER G., VANSCOTT E. J. MYCOSIS FUNGOIDES. RELATIONSHIP TO MALIGNANT CUTANEOUS RETICULOSIS AND THE S'EZARY SYNDROME. Arch Dermatol. 1964 Jun;89:785–792. doi: 10.1001/archderm.1964.01590300013005. [DOI] [PubMed] [Google Scholar]
- Crossen P. E., Mellor J. E., Finley A. G., Ravich R. B., Vincent P. C., Gunz F. W. The Sézary syndrome: cytogenetic studies and identification of the Sézary Cell as an abnormal lymphocyte. Am J Med. 1971 Jan;50(1):24–34. doi: 10.1016/0002-9343(71)90201-4. [DOI] [PubMed] [Google Scholar]
- DE PETRIS S., KARLSBAD G., PERNIS B. Filamentous structures in the cytoplasm of normal mononuclear phagocytes. J Ultrastruct Res. 1962 Aug;7:39–55. doi: 10.1016/s0022-5320(62)80025-2. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. H., Adams A. Chromosome studies in chronic lymphocytic leukemia and lymphosarcoma. J Natl Cancer Inst. 1965 Jun;34(6):827–839. [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labaze J. J., Moscovic E. A., Pham T. D., Azar H. A. Histological and ultrastructural findings in a case of the Sézary syndrome. J Clin Pathol. 1972 Apr;25(4):312–319. doi: 10.1136/jcp.25.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- Lutzner M. A., Emerit I., Durepaire R., Flandrin G., Grupper C., Prunieras M. Cytogenetic, cytophotometric, and ultrastructural study of large cerebriform cells of the Sézary syndrome and description of a small-cell variant. J Natl Cancer Inst. 1973 May;50(5):1145–1162. doi: 10.1093/jnci/50.5.1145. [DOI] [PubMed] [Google Scholar]
- Lutzner M. A., Hobbs J. W., Horvath P. Ultrastructure of abnormal cells in Sezary syndrome, mycosis fungoides, and parapsoriasis en plaque. Arch Dermatol. 1971 Apr;103(4):375–386. doi: 10.1001/archderm.103.4.375. [DOI] [PubMed] [Google Scholar]
- Lutzner M. A., Jordan H. W. The ultrastructure of an abnormal cell in Sézary's syndrome. Blood. 1968 Jun;31(6):719–726. [PubMed] [Google Scholar]
- NOWELL P. C., HUNGERFORD D. A. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960 Jul;25:85–109. [PubMed] [Google Scholar]
- Papamichail M., Brown J. C., Holborow E. J. Immunoglobulins on the surface of human lymphocytes. Lancet. 1971 Oct 16;2(7729):850–852. doi: 10.1016/s0140-6736(71)90224-8. [DOI] [PubMed] [Google Scholar]
- Piessens W. F., Schur P. H., Moloney W. C., Churchill W. H. Lymphocyte surface immunoglobulins. Distribution and frequency in lymphoproliferative diseases. N Engl J Med. 1973 Jan 25;288(4):176–180. doi: 10.1056/NEJM197301252880403. [DOI] [PubMed] [Google Scholar]
- Pincus S., Bianco C., Nussenzweig V. Increased proportion of complement-receptor lymphocytes in the peripheral blood of patients with chronic lymphocytic leukemia. Blood. 1972 Sep;40(3):303–310. [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Immunoglobulins on the surface of lymphoid cells in Waldenström's macroglobulinemia. J Clin Invest. 1972 Mar;51(3):701–705. doi: 10.1172/JCI106858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Surface bound immunoglobulins as a cell marker in human lymphoproliferative diseases. Blood. 1972 Dec;40(6):777–794. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. H., Levis W. R. Inherent inability of chronic lymphocytic leukaemia lymphocytes to respond to phytohaemagglutinin. Int Arch Allergy Appl Immunol. 1972;43(6):845–858. doi: 10.1159/000230902. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Herberman R., Frank M. M., Green I. Receptors for complement and immunoglobulin on human leukemic cells and human lymphoblastoid cell lines. J Clin Invest. 1972 Aug;51(8):1933–1938. doi: 10.1172/JCI106999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira N. P., Mendes N. F., Tolnai M. E. Tissue localization of two populations of human lymphocytes distinguished by membrane receptors. J Immunol. 1972 May;108(5):1456–1460. [PubMed] [Google Scholar]
- TASWELL H. F., WINKELMANN R. K. Sezary syndrome--a malignant reticulemic erythroderma. JAMA. 1961 Aug 19;177:465–472. doi: 10.1001/jama.1961.03040330001001. [DOI] [PubMed] [Google Scholar]
- Taranta A., Cuppari G., Quagliata F. Dissociation of hemolytic and lymphocyte-transforming activities of streptolysin S preparations. J Exp Med. 1969 Apr 1;129(4):605–622. doi: 10.1084/jem.129.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet. 1971 Oct 9;2(7728):788–791. doi: 10.1016/s0140-6736(71)92741-3. [DOI] [PubMed] [Google Scholar]
- ZUCKER-FRANKLIN D. THE ULTRASTRUCTURE OF CELLS IN HUMAN THORACIC DUCT LYMPH. J Ultrastruct Res. 1963 Oct;59:325–339. doi: 10.1016/s0022-5320(63)80010-6. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Berney S. Electron microscope study of surface immunoglobulin-bearing human tonsil cells. J Exp Med. 1972 Mar 1;135(3):533–548. doi: 10.1084/jem.135.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Franklin E. C. Intracellular localization of human amyloid by fluorescence and electron microscopy. Am J Pathol. 1970 Apr;59(1):23–41. [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. The percentage of monocytes among "mononuclear" cell fractions obtained from normal human blood. J Immunol. 1974 Jan;112(1):234–240. [PubMed] [Google Scholar]