Abstract
Recent studies have demonstrated silibinin efficacy against ultraviolet B (UVB)-induced skin carcinogenesis via different mechanisms in cell lines and animal models; however, its role in regulating interleukin-12 (IL-12), an immunomodulatory cytokine that reduces UVB-induced DNA damage and apoptosis, is not known. Here, we report that UVB irradiation causes caspase 3 and PARP cleavage and apoptosis, and addition of recombinant IL-12 or silibinin immediately after UVB significantly protects UVB-induced apoptosis in JB6 cells. IL-12 antibody-mediated blocking of IL-12 activity compromised the protective effects of both IL-12 and silibinin. Both silibinin and IL-12 also accelerated the repair of UVB-caused cyclobutane-pyrimidine dimers (CPDs) in JB6 cells. Additional studies confirmed that indeed silibinin causes a significant increase in IL-12 levels in UVB-irradiated JB6 cells as well as in mouse skin epidermis, and that similar to cell-culture findings, silibinin topical application immediately after UVB exposure causes a strong protection against UVB-induced TUNEL positive cells in epidermis possibly through a significantly accelerated repair of UVB-caused CPDs. Together, these findings for the first time provide an important insight regarding the pharmacological mechanism wherein silibinin induces endogenous IL-12 in its efficacy against UVB-caused skin damages. In view of the fact that an enhanced endogenous IL-12 level could effectively remove UVB-caused DNA damage and associated skin cancer, our findings suggest that the use of silibinin in UVB-damaged human skin would also be a practical and translational strategy to manage solar radiation-caused skin damages as well as skin cancer.
Keywords: Non-melanoma skin cancer, silibinin, DNA damage & repair, cyclobutane-pyrimidine dimers, apoptosis
INTRODUCTION
Non-melanoma skin cancers (NMSCs) account for about 40% of all diagnosed human malignancies in the United States, with an estimated 1.3 million new cases every year [1,2]. Chronic exposure to solar radiation, particularly the ultraviolet B (UVB) component (290–320 nm), is the primary causation for more than 90% of the human NMSCs, and UVB is considered as a complete carcinogen causing initiation, promotion and progression of skin carcinogenesis [3]. Epidermal keratinocytes are the main target of UVB, and the detrimental effects of UVB exposure includes DNA damage, immunosuppression, erythema, premature skin aging, etc.; and eventually NMSCs [4–8]. UVB caused direct DNA damage results from the absorption of high energy photons by cellular DNA leading to the formation of cyclobutane-pyrimidine dimers (CPDs) and 6–4 photoproducts, whereas indirect DNA damage results from the generation of reactive oxygen species that facilitate DNA oxidation [5,9]. CPDs are bulky DNA lesions formed between adjacent pyrimidine nucleotides in the DNA, and if CPDs are not efficiently removed from the genome, they lead to critical mutations, ultimately initiating skin tumors [10]. Higher doses of UVB also lead to apoptotic cell death in epidermal cells, where severity of DNA damage is a crucial molecular trigger [11,12]. Even though apoptosis is a protective mechanism to remove severely damaged cells that are at the risk of becoming malignant, UVB-induced massive apoptosis compromises the natural barrier functions of the skin. Therefore, mechanisms that simultaneously counteract UVB-induced DNA damage as well as the induction of apoptosis in skin cells are crucial in the prevention of cutaneous malignancies.
A considerable body of evidence suggests that the adverse effects of UVB on skin including the photocarcinogenic activity, at least in part, are mediated by immunosuppressive properties of UVB [4,8,13,14]. Interleukin-12 (IL-12) is an immunomodulatory cytokine which has well-evidenced roles in the reversal of UVB-induced DNA damage and immunosuppression as well as in the modulation of UVB-induced apoptosis [14–16]. The antitumor activity of IL-12 is established in various preclinical animal tumor models [17–19] and UVB-induced immunosuppressive as well as inflammatory responses in mice have been shown to be reversed by treatment with recombinant IL-12 (rIL-12) [4,14,20]. Exogenous IL-12 is also known to reverse DNA damage following UVB-irradiation, and it inhibits apoptosis both in vitro and in vivo [21,22].
Cancer is a complex disease and its prevention and/or treatment entirely based upon single or couple of agents might not be plausible; hence, there is a rationale for building an armamentarium of cancer chemopreventive and therapeutics agents. In the past, several naturally occurring phytochemicals have been shown to protect against UVB-induced skin damages and tumorigenesis [23,24], among which silibinin has generated significant attention in recent years because of its promising efficacy against photocarcinogenesis as well as several other epithelial malignancies [25–29]. Extensive studies from our laboratory and elsewhere have shown that silibinin prevents UVB-induced NMSC by both inducing and inhibiting apoptotic cell death depending on the extent of DNA damage [26,30–32]. However, to our knowledge, no detailed mechanistic study has been performed to specifically evaluate the role of IL-12 in the protective effects of silibinin against UVB-induced photodamage in epithelial cells or mouse skin. Results from present study clearly suggest that silibinin protects UVB-damaged cells from apoptosis by accelerating DNA repair in an IL-12-dependent manner both in vitro and in vivo.
MATERIALS AND METHODS
Cell Lines, Animals and UVB Irradiation
The mouse epithelial cell line JB6 was grown in MEM with 5% heat inactivated fetal bovine serum and 25 μg/mL gentamycin (Gibco BRL, Grand Island, NY) under standard conditions. Cells at 70–80% confluence were sham irradiated or irradiated with 50 mJ/cm2 UVB doses as described earlier [30]. The UVB light source was a bank of four FS-40-T-12-UVB sunlamps equipped with UVB Spectra 305 Dosimeter (Daavlin, Bryan, OH), emitting 80% radiation within 280 to 340 nm with 314 nm peak [30]. After irradiation, cells were incubated with MEM containing silibinin, rIL-12, IL-12 antibody or their combinations as indicated.
In other studies, five-week old female hairless SKH-1 mice (Charles River Laboratories, Wilmington, MA) were acclimatized for one week under standard conditions, and then were irradiated as per the protocol described earlier [33]. The UVB and silibinin dose selection was based on previous studies showing silibinin efficacy against the UVB dose-caused skin damages and tumorigenesis [26,34,35]. The treatment groups (n=5) were (a) sham irradiated + 200 μL acetone/mouse topically (Control group), (b) 180 mJ/cm2 UVB + 200 μL acetone/mouse topically after 30 min (UVB group), (c) sham irradiated + 9 mg silibinin in 200 μL acetone/mouse topically (Silibinin group), and (d) 180 mJ/cm2 UVB + 9 mg silibinin in 200 μL acetone/mouse topically after 30 min (UVB+Silibinin group). Mice were sacrificed after 24 and 48 h of treatment, skin samples were collected, a part was fixed in buffered formalin for immunohistochemical (IHC) analyses and the rest was snap frozen in liquid nitrogen. The animal experiment was carried out in accordance with the protocols approved by the Institutional Animal Care and Use Committee.
Reagents and Antibodies
Rabbit polyclonal cleaved caspase-3 and mouse-specific cleaved PARP antibodies were from Cell Signaling Technology (Beverley, MA), HRP-labeled anti-Thymine dimer antibody was from Kamiya Biomedical Company (Seattle, WA), rabbit polyclonal anti-IL-12 antibody was from Abcam (Cambridge, MA), HRP linked anti-mouse IgG antibody was from Amersham Biotech (Piscataway, NJ) and IR800 or IR700 fluorescent dye-labeled anti-mouse and anti-rabbit IgGs were from LI-COR Biosciences (Lincoln, NE). The recombinant mouse IL-12 and neutralizing anti-mouse IL-12 antibody were from R&D Systems (Minneapolis, MN). Mouse IL-12 ELISA Kit was from eBioscience (San Diego, CA) and TUNEL assay kit was from Promega (Madison, WI). Silibinin, mouse monoclonal β-actin antibody, and Hoechst 33342 were from Sigma (St. Louis, MO).
Western Immunoblotting
Cell lysates were prepared in non-denaturing lysis buffer and protein concentration in lysates determined using Bio-Rad DC protein assay kit (Bio-Rad) as per manufacturer’s protocol. For immunoblot analyses, equal protein per sample was denatured in 2X SDS-PAGE sample buffer, resolved on Tris/glycine gels, transferred onto nitrocellulose membranes and probed with specific primary antibodies followed by appropriate IR800 or IR700 dye-labeled secondary antibody, and visualized on Odyssey scanner (LI-COR Biosciences, Lincoln, NE).
Estimation of IL-12 by ELISA
JB6 cells were cultured in 24 well plates up to 70% confluence, UVB irradiated or sham irradiated; incubated for 24 h with or without silibinin and/or IL-12 antibody. The culture supernatants (100 μL each) were subjected to ELISA using the mouse IL-12 ELISA kit following vendor’ protocol. For measuring IL-12 production in mouse skin, ~100 mg of skin samples were homogenized in Tris-HCl (pH 7.5) and centrifuged at 13000 rpm for 30 min, and supernatants (100 μg total protein/sample) were used for the estimation of IL-12 by ELISA kit.
Apoptosis Detection by Annexin V/PI Staining or Hoechst/PI Staining
To analyze the protective role of rIL-12 on UVB-induced apoptosis, JB6 cells were grown to 70–80% confluence, either unexposed or exposed to 50 mJ/cm2 UVB and incubated with rIL-12. After 24 h, cells were collected, stained with Annexin V/PI using Vybrant Apoptosis Assay Kit 2 (Invitrogen, Eugene, OR, USA) following the manufacturer’s protocol and analyzed immediately at the Flow Cytometry Core Facility, University of Colorado Cancer Center. Quantification of apoptotic cell death by Hoechst-PI Staining was carried out as described earlier [36]. Briefly, UVB-irradiated or sham irradiated JB6 cells were treated with silibinin, rIL-12, IL-12 antibody or their combinations as indicated; cells were collected after 24 h and stained with PI (1 mg/mL) and Hoechst 33342 (1 mg/mL). Cells (200/sample) were counted in different fields to score live and apoptotic cells under a fluorescent microscope (Zeiss Axioscope-2 plus-HBO100, Zeiss, Jena, Germany).
Southwestern Slot Blot Assay for CPDs
Genomic DNA was isolated from un-irradiated or UVB irradiated JB6 cells with or without silibinin treatment for different time intervals. DNA was denatured by boiling for 10 min with 10 mM EDTA and 0.4 M NaOH, and transferred to positively charged nitrocellulose membrane by vacuum slot blotting (Bio-Dot Apparatus, Bio-Rad, Hercules, CA). The membranes were dried, blocked with 5% non-fat milk and probed with HRP-labeled anti-CPD antibody. The bands were visualized by enhanced chemiluminiscence method (GE Healthcare, Buckinghamshire, UK).
Immunohistochemical Staining
For the immunohistochemical (IHC) detection of IL-12 and CPDs in mouse skin samples, the sections were processed following procedures described earlier [33,34]. In brief, the sections were incubated with rabbit polyclonal anti-IL-12 antibody at 1:100 dilution overnight at 4 °C or with dilution buffer in a humidified chamber, followed by incubation with anti-rabbit biotinylated secondary antibody for 1 h and HRP conjugated streptavidin for 30 minutes followed by 3,3′-diaminobenzidine (DAB) and counterstained with hematoxylin. The cytoplasmic staining in different treatment groups was scored according to color intensities observed in 5 randomly selected fields per section at x400 magnification.
For CPDs, the sections were rehydrated; nuclear DNA was denatured with 1 N HCl, and permeabilized with 20 μg/mL proteinase-K. The endogenous peroxidase activity and non-specific binding were blocked using 3% hydrogen peroxide in methanol (v/v) and Cas-block reagent, respectively, and the sections were incubated with HRP-labeled anti-CPD antibody at 1:100 dilution for 2 h at room temperature followed by DAB. In situ apoptosis detection was done by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay using colorimetric TUNEL kit as per manufacturer’s instructions. The positive (CPD or TUNEL) cells were counted on five arbitrarily selected fields from each section (x400 magnification) using Zeiss Axioskop- 2 microscope (Carl Zeiss, Inc. Jena, Germany); images were captured using Carl Zeiss AxioCam MRc5 camera and processed by axiovision software 4.6 (Carl Zeiss, Inc.). Percent CPD or TUNEL positive cells are calculated as number of positive cells ×100/total number of cells.
Statistical Analyses
SigmaStat software version 3.5 (Systat Software, Inc., Richmond, CA) was used for all statistical analyses. Quantitative data are presented as mean ± SE. Statistical significance of difference between control and different treatment groups was determined by one way ANOVA followed by Tukey’s test for multiple comparisons and P≤0.05 was considered significant.
RESULTS
Exogenous Interleukin-12 Protects JB6 Cells from UVB-induced Apoptosis
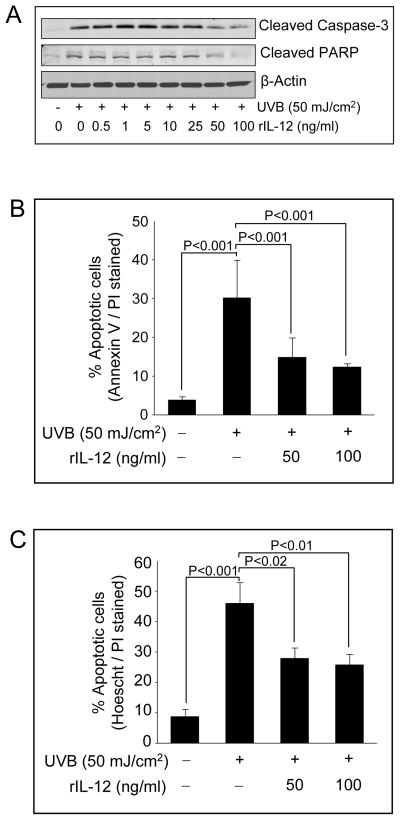
Earlier report has shown that IL-12 inhibits UVB-induced apoptosis by accelerating DNA repair [22], and therefore we established this system under our experimental conditions to facilitate our studies assessing silibinin protective effect on UVB caused apoptosis in JB6 cells and the involvement of IL-12 in that response. As shown in Figure 1A, administration of recombinant IL-12 (0.5–100 ng/ml) to UVB-irradiated JB6 cells resulted in suppression of cleaved caspase-3 and cleaved PARP especially at 50 and 100 ng/ml doses. Quantitative analysis of the UVB-caused apoptotic death and protection by rIL-12 employing AnnexinV/PI staining showed that UVB (50 mJ/cm2) exposure caused 30.1% apoptotic cell population after 24 h, and that rIL-12 (50 and 100 ng/mL) treatment reduced that to 14.8% and 12.4% (P<0.001), respectively (Figure 1B). These observations were confirmed by manual counting of Hoechst/PI stained apoptotic populations of UVB alone and UVB+rIL-12 treated JB6 cells under a fluorescent microscope, which showed a comparable conclusion (Figure 1C).
Figure 1.
Exogenous rIL-12 protects JB6 cells from UVB-induced apoptosis. (A) JB6 cells were irradiated with UVB (50 mJ/cm2) and incubated with various concentrations of rIL-12 for 24 h. Thereafter, cells were harvested, lysates were prepared and Western blotting was performed for cleaved caspase-3 and cleaved PARP. β-actin is shown as loading control. (B) JB6 cells were exposed to 50 mJ/cm2 UVB and treated with 50 or 100 ng/mL concentrations of rIL-12 for 24 h. Cells were harvested and processed for flow cytometric analysis of apoptotic cells by annexin V/PI staining. In each case, quantitative data are mean ± SE of triplicate samples of each treatment. (C) JB6 cells were exposed to 50 mJ/cm2 UVB and treated with 50 or 100 ng/mL concentrations of rIL-12 for 24 h. Cells were collected and stained with Hoechst/PI dye as detailed in ‘Materials and Methods’, counted manually under a fluorescence microscope and percent live and apoptotic cells were calculated. In each case, quantitative data shown are mean ± SE of triplicate samples of each treatment.
Role of IL-12 in the Protective Efficacy of Silibinin Against UVB-induced Apoptosis
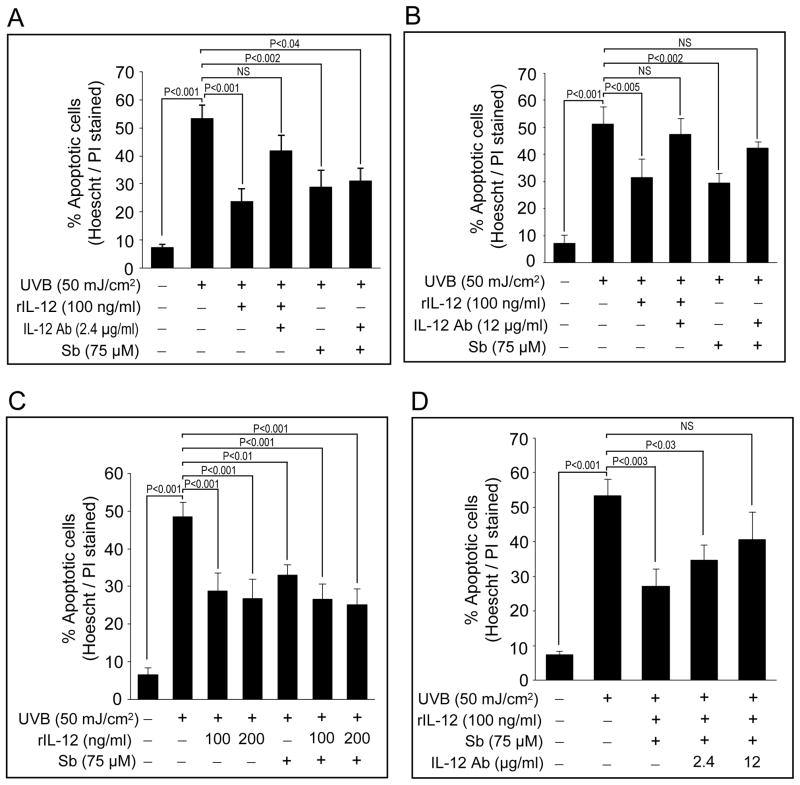
We first optimized that silibinin treatment (75 μM) immediately after UVB exposure (50 mJ/cm2) reverses the apoptosis in terms of inhibiting PARP cleavage and caspase 3 activation (data not shown). Next, we conducted series of experiments to test whether silibinin-mediated reversal of UVB-induced apoptosis is IL-12-dependent or independent. In the first experiment, JB6 cells were UVB-irradiated and incubated with silibinin and/or rIL-12 in the presence or absence of a neutralizing anti-IL-12 antibody. As shown in Figures 2A and 2B, co-administration of 2.4 μg/mL or 12 μg/mL IL-12 antibody reversed the rIL-12-mediated protection against UVB-induced apoptosis. More interestingly, IL-12 antibody co-treatment (at12 μg/mL) along with 75 μM silibinin also reversed silibinin-mediated protection against UVB-caused apoptosis (Figures 2B). These results suggested that IL-12 could be important in silibinin-mediated protective effects against UVB.
Figure 2.
Co-treatment with neutralizing anti-IL-12 antibody reverses both rIL-12 and silibinin-mediated protection against UVB-induced apoptosis in JB6 cells. (A–D) JB6 cells were irradiated with 50 mJ/cm2 UVB and treated with rIL-12 in presence or absence of anti-IL-12 antibody and/or 75 μM silibinin. Cells were harvested after 24 h, and percent apoptotic cells were calculated by Hoechst/PI staining. In each case, quantitative data shown are mean ± SE of triplicate samples of each treatment.
Next, we assessed the possible additive or synergistic protective effect of silibinin and rIL-12 on UVB-induced apoptosis in JB6 cells. As shown in Figure 2C, when UVB-exposed cells were treated with rIL-12, silibinin, or their combination for 24 h, each of them offered significant (P≤0.01 to 0.001) protection when compared to only UVB treated group; however, we did not observe significantly better effect by their combination than each alone (Figure 2C). These results confirmed that silibinin protects JB6 cells from UVB-induced apoptosis through an IL-12-dependent mechanism. Furthermore, co- treatment with IL-12 antibody (2.4 or 12 μg/mL) reversed the protective effect of silibinin and rIL-12 together against UVB-induced apoptosis, substantiating the above mentioned observations (Figure 2D).
Silibinin and rIL-12 Accelerate the Repair of UVB-induced CPDs in JB6 Cells
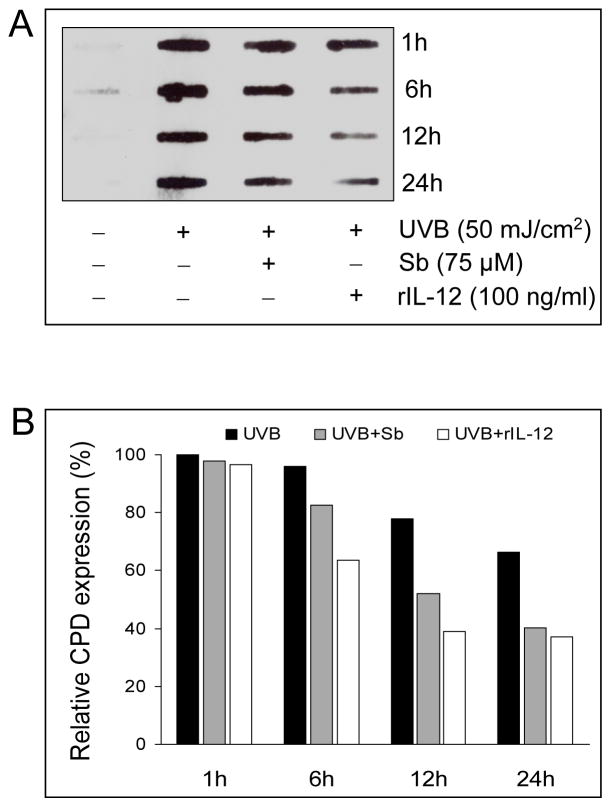
To find out whether the reversal of UVB-induced apoptosis correlates with rapid removal of CPDs by silibinin and rIL-12, a slot blot assay for CPD expression was performed. UVB (50 mJ/cm2) irradiation immediately induced CPD expression in JB6 cells which decreased gradually over the 24 h time-period (Figure 3). Notably, when UVB-irradiated cells were treated with 75 μM silibinin or 100 ng/ml rIL-12, the CPD removal was significantly accelerated (Figure 3A). In densitometric analysis, there was about 34.5% reduction in CPD levels after 24 h of UVB-irradiation as compared to 1 h time point, whereas silibinin or rIL-12 treatment reduced the CPD levels by 59% and 69%, respectively (Figure 3B). These results clearly suggested an association between reversal of UVB-induced apoptosis by both silibinin and rIL-12 and a rapid removal of UVB-caused CDPs possibly through an accelerated DNA repair under these treatments.
Figure 3.
Silibinin and rIL-12 accelerates the removal of UVB-induced CPDs in JB6 cells. (A) JB6 cells were irradiated with 50 mJ/cm2 UVB or sham-irradiated and treated with either DMSO, rIL-12 or 75 μM silibinin for different time intervals as indicated. The genomic DNA was isolated and CPD expression was analyzed in 2 μg DNA per sample. (B) The quantitative data shown are percentage CPD expression calculated from integrated densitometric values using Scion image analysis software (Scion Corporation, Frederick, MD).
Silibinin Enhances the Production and Secretion of IL-12 in UVB-irradiated JB6 Cells and Mouse Epidermis
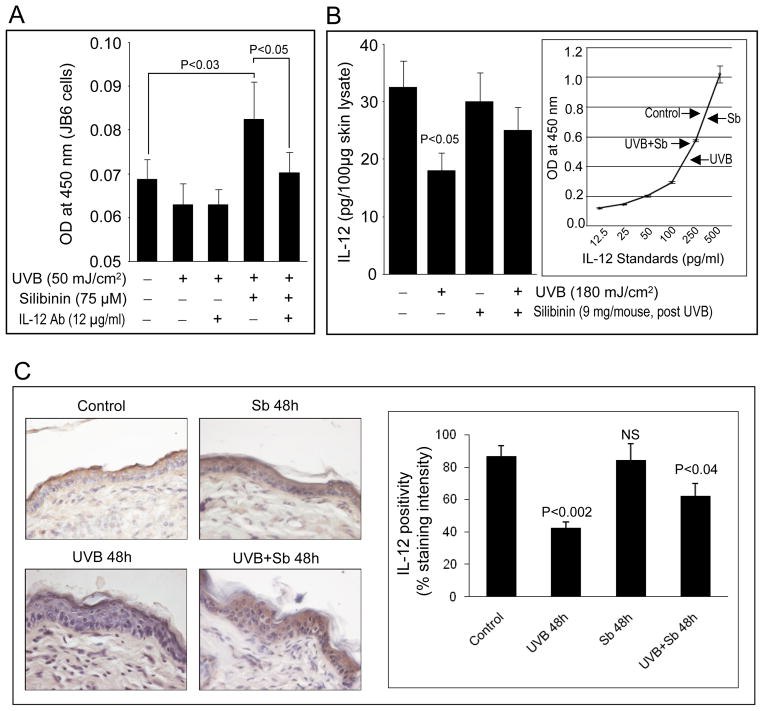
The data shown above suggested that silibinin exerted significant protection against UVB-induced apoptosis via IL-12, and accordingly, next we assessed whether endogenous IL-12 levels are indeed regulated by silibinin treatment in vitro and in vivo. Exposure of JB6 cells to 50 mJ/cm2 UVB alone for 24 h resulted in a slight decrease in the IL-12 levels in cell culture supernatants analyzed by ELISA; however, silibinin treatment following UVB exposure strongly enhanced (P<0.003) the level of IL-12 (Figure 4A). The neutralizing IL-12 antibody co-treatment brought down the silibinin-induced elevation in IL-12 to basal levels in UVB irradiated JB6 cells in 24 h (Figure 4A). This result is in line with our observations that silibinin-mediated protection against UVB-caused apoptosis is compromised when IL-12 activity is blocked by IL-12 antibody (Figure 2).
Figure 4.
Silibinin enhances the production of IL-12 in UVB-irradiated mouse epidermal keratinocytes in vitro and in vivo. (A) JB6 cells were cultured overnight on 96 well plates, sham irradiated or exposed to 50 mJ/cm2 UVB, and incubated with silibinin and/or anti-IL-12 antibody as indicated, in triplicates. After 24 h of incubation, 100 μl of culture supernatants were subjected to ELISA as described in “Materials and Methods”. Data is represented as mean absorbance ± SE of three samples. (B) 100 μg each of the epidermal homogenates from SKH-1 mice; either unexposed (control), exposed to UVB (180 mJ/cm2), topically applied with 9 mg silibinin in 200 μL acetone or UVB exposed and applied with silibinin after 30 min, were subjected to ELISA and the concentration of IL-12 in the epidermal lysates was estimated by comparing with the standard curve. In each case, quantitative data are mean ± SE of triplicate samples of each treatment. (C) Immunohistochemical staining for IL-12 was done in formalin-fixed paraffin-embedded skin sections and representative data are shown for different treatment groups as labeled. In each case quantitative data are mean ± SE of skin samples from three individual mouse in each treatment group from three randomly selected microscopic fields per sample. Representative images are shown at ×400 magnification. Immunostaining was scored based on staining intensities.
In order to assess whether silibinin could also enhance the production of IL-12 following UVB irradiation in vivo, we next carried out ELISA assay to determine IL-12 in the epidermal homogenates prepared from sham or UVB-irradiated and/or silibinin-treated mouse skin. Topical application of silibinin on sham-irradiated mouse did not alter the epidermal expression levels of IL-12 compared to vehicle alone-treated skin (Figure 4B). Importantly, mouse skin exposed to 180 mJ/cm2 UVB dose had significantly lower levels of IL-12 when compared to sham-irradiated control after 48 h (Figure 4B); however, silibinin application after 30 min of UVB exposure restored the epidermal IL-12 levels to near normal levels (Figure 4B). IL-12 expression in mouse skin collected from different treatment groups was also evaluated by IHC. As shown in Figure 4C, silibinin alone had no effect on IL-12 levels in the epidermis whereas UVB exposure significantly suppressed the IL-12 expression in the epidermis. Consistent with the ELISA results, control skin showed 88% IL-12 positivity, whereas 50 mJ/cm2 UVB dose-exposed skin tissue showed reduced levels of IL-12 (52% decrease, P<0.002). Silibinin treatment ameliorated the UVB caused decrease in IL-12 (63% positivity; P<0.04) and IL-12 level was 1.5 fold higher as compared to UVB alone group (Figure 4C).
Silibinin Post-treatment Accelerates the Repair of UVB-induced DNA Damage and Inhibits UVB-induced Apoptosis in Mouse Epidermis
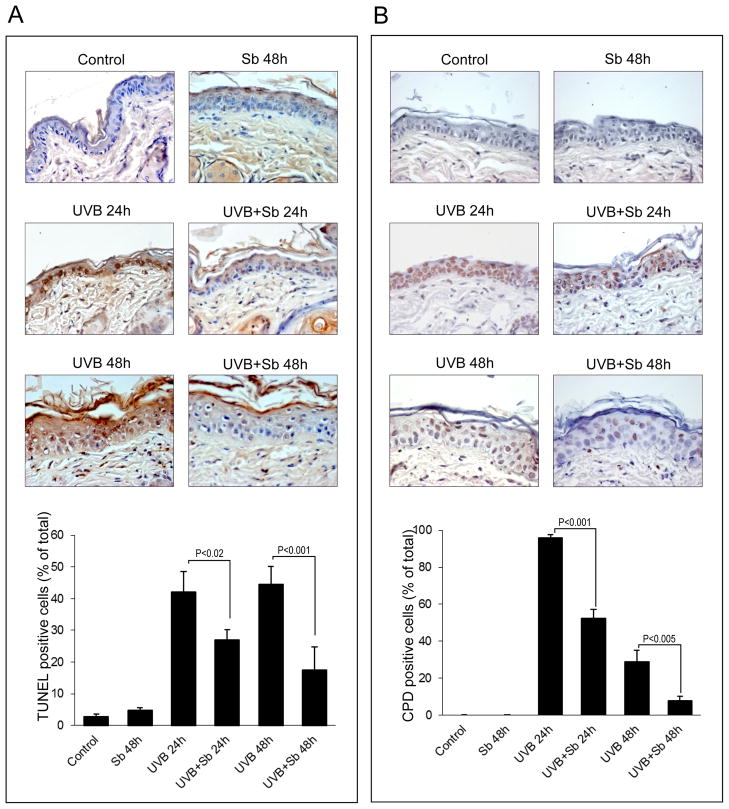
The cell culture results as well as in vivo results showing that silibinin reverses UVB-caused decrease in IL-12 were next associated with an overall efficacy of silibinin in accelerating the repair of UVB-caused CPDs and inhibiting the UVB-caused apoptosis in mouse skin. Sham-irradiated control and silibinin alone-treated mouse skin samples showed low percentage of apoptotic cells (Figure 5A). Mice exposed to 180 mJ/cm2 UVB dose showed 42–44% TUNEL positive (apoptotic) cells after 24–48 h time-points; however, topical silibinin application post-UVB exposure significantly suppressed (37 and 60% after 24 and 48 h, respectively) the UVB-induced apoptosis (Figure 5A). In the studies analyzing CPD expression to establish whether silibinin-mediated inhibition of apoptotic cells in UVB irradiated mouse epidermis is through enhancing DNA repair, sham control or silibinin alone-treated mouse skin samples showed no detectable CPD formation (Figure 5B). However, a 180 mJ/cm2 UVB dose exposure of mice resulted in CPD expression in almost every cell of the epidermis (96%) after 24 h, but silibinin treatment following UVB exposure showed a strong decrease (45%, P<0.001) in CPD formation (Figure 5B). Similarly, after 48 h of UVB exposure, there were about 28% CPD positive cells in the mouse epidermis, whereas silibinin treatment following UVB exposure accelerated the repair and we observed 72% lesser (P<0.005) CPD positive cells (Figure 5B).
Figure 5.
Silibinin suppresses UVB-induced apoptosis in mouse epidermis by accelerating DNA repair. (A & B) The skin samples were collected from SKH-1 hairless mice after 24 h and 48 h of sham/UVB irradiation and/or silibinin treatment, and stained for apoptotic cell death by TUNEL and CPDs as detailed in “Materials and Methods”. Representative skin sections with TUNEL and CPD staining from sham-irradiated, UVB or silibinin (Sb) alone treated and UVB+Sb treated groups are shown at ×400 magnification. Percent TUNEL positive cells (A) and percent CPD positive cells (B) were calculated from different treatment groups by manual counting of positive stained cells. In each case quantitative data are mean ± SE of skin samples from three individual mouse in each treatment group from three randomly selected microscopic fields per sample.
DISCUSSION
UVB-induced DNA damage, particularly CPD, has been recognized as an important molecular trigger for UVB-induced immunosuppression, and experimental and clinical evidences support a strong correlation between immunosuppression and carcinogenesis [37–39]. Immunomodulatory cytokine IL-12 is reported to counteract UVB-induced immunosuppression [14,15] and to rescue epidermal cells from UVB-induced apoptosis by promoting DNA repair [22]. It has been shown that silibinin reverses UVB-induced DNA damage and prevents photocarcinogenesis in mice when applied topically before or after UVB irradiation or given in diet [31,34,35]. Since silibinin reverses UVB-induced DNA damage and protects cells from apoptosis in a similar manner as exogenous IL-12, a possible role of IL-12 in the photoprotective efficacy of silibinin was examined in detail in the present study.
The findings of the present study showed that UVB induces apoptosis in both JB6 cells and SKH-1 mouse epidermis, and that silibinin post-treatment inhibits UVB-induced apoptosis in both in vitro and in vivo systems. When UVB-irradiated JB6 cells were treated with exogenous rIL-12, there was a dose-dependent reversal of UVB-induced apoptosis, and more importantly, a neutralizing antibody against IL-12 compromised both rIL-12 and/or silibinin-mediated protection from UVB-induced apoptosis in JB6 cells. These results, for the first time, established that silibinin exerts its protective role against UVB-induced apoptosis, at least in part, through an IL-12-dependent mechanism. Our additional studies also found that UVB irradiation causes the suppression of endogenous IL-12 levels under both in vitro and in vivo conditions, and consistent with previous reports on the ability of keratinocytes to synthesize and secrete IL-12 [40,41], we found that silibinin enhances the production of IL-12 in UVB-irradiated JB6 cells as well as SKH-1 mouse epidermis. Considering these observations and the well documented role of IL-12 in the repair of DNA damage [22], it is obvious that silibinin prevents UVB-induced apoptotic cell death by inducing IL-12 production and thereby accelerating the DNA repair process. Indeed, our results found a direct evidence for the accelerated repair of photodamage by silibinin in both JB6 cells and mouse epidermis; this rapid removal of UVB-induced CPDs by silibinin suggests the induction of NER pathway; however, more studies are needed in future to support this suggestion.
In the past, several studies have been conducted to understand mechanism/s for IL12-mediated repair of the DNA damage [4,22,42,43]. Schwarz et al. [22] reported that UVB exposure down regulates NER pathway, and IL-12 activates NER through increasing the expression of several NER genes namely DDB2, RPA(p32), RPA(p14), XPC and XPG; however, IL12 did not affect the base-excision repair complex. IL-12 protective efficacy was severely compromised in XPA mice, further confirming the important role of NER pathway in IL-12-mediated repair of the DNA damage caused by UVB [22]. However, signaling molecules or pathways targeted by IL-12 towards activating NER machinery remain unknown. In an another study, Maeda et al. [42] confirmed IL-12 role in DNA repair, and reported that UVB-induced skin tumors develop faster and more frequently in IL-12p40 knockout mice with higher number of sunburn cells, CPDs, and p53 patches in the epidermis. Meeran et al. [43] also reported that UVB- induced DNA damage is repaired faster in wild type mice compared to IL-12 p35 knockout mice. Results from present study showed that silibinin protects against UVB-induced apoptosis, replenishes IL-12 level and accelerates DNA damage repair in cultured mouse keratinocytes. In future, in-depth studies will be conducted in IL-12 knockout mice to analyze whether or not silibinin caused DNA repair as well as protective efficacy against photodamage are dependent upon IL-12 in vivo.
Based upon the proven protective efficacy of IL-12 in animal models, there have been efforts to use IL-12 as a therapeutic agent against cutaneous malignancies in humans [4]. However, there are some concerns related to IL-12 delivery to epidermis as well as cost associated with commercial large scale production of recombinant IL-12 [21]. Results from present study suggest that silibinin could enhance IL-12 level in keratinocytes exposed to UVB; therefore, silibinin could be potentially used instead of recombinant IL-12 to achieve comparable photoprotective effects. Importantly, silibinin use is non-toxic, comparatively inexpensive and has several health benefits including its remarkable hepatoprotective efficacy. Further, silibinin has been tested clinically for its anti-cancer and hepatoprotective efficacies, and lots of data related to silibinin’s human safety is already available [27,44–46]. Therefore, present study revealing silibinin’ protective efficacy and mechanism against UVB-caused photodamage is important and also clinically relevant.
Taken together, our studies substantiated the well-known chemopreventive potential of silibinin against UVB-induced NMSCs by providing new evidences for an IL-12-dependent mechanism in cultured mouse keratinocytes. Importantly, our in vivo experiments clearly showed that when applied topically silibinin specifically enhanced the IL-12 in UVB-irradiated skin and did not affect IL-12 levels in unexposed mouse skin. This finding suggests that silibinin exposure will not affect other IL-12 associated immune responses in the body, and also emphasizes an easy and effective mode of drug delivery. Considering the fact that millions of people get constantly exposed to solar UVB and live with persistent UVB-induced DNA lesions as sunscreens can’t provide adequate protection, the post-damage use of silibinin as an inducer of endogenous IL-12 in UVB-damaged human skin for the repair of DNA damage could be a practical and translational approach in reducing sunlight-caused damages in human skin which eventually lead to skin cancer.
Acknowledgments
Grant support: Supported by NCI R01 grant CA140368.
Abbreviations
- ANOVA
Analysis of variance
- CPD
Cyclobutane-pyrimidine dimer
- DAB
3,3′-diaminobenzidine
- DMSO
Dimethyl Sulfoxide
- ELISA
Enzyme-linked immunosorbent assay
- HRP
Horseradish peroxidase
- IL-12
Interleukin-12
- IR
Infrared
- NMSC
Non-melanoma skin cancer
- PARP
Poly ADP ribose polymerase
- TUNEL
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
- UVB
Ultraviolet B
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.Kraemer KH. Sunlight and skin cancer: another link revealed. Proc Natl Acad Sci U S A. 1997;94(1):11–14. doi: 10.1073/pnas.94.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katiyar SK. Interleukin-12 and photocarcinogenesis. Toxicology and applied pharmacology. 2007;224(3):220–227. doi: 10.1016/j.taap.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. Journal of the American Academy of Dermatology. 2008;58(5 Suppl 2):S139–148. doi: 10.1016/j.jaad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. The British journal of dermatology. 2007;157(5):874–887. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- 7.Phan TA, Halliday GM, Barnetson RS, Damian DL. Spectral and dose dependence of ultraviolet radiation-induced immunosuppression. Frontiers in bioscience: a journal and virtual library. 2006;11:394–411. doi: 10.2741/1807. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz T. Mechanisms of UV-induced immunosuppression. The Keio journal of medicine. 2005;54(4):165–171. doi: 10.2302/kjm.54.165. [DOI] [PubMed] [Google Scholar]
- 9.Moore JO, Wang Y, Stebbins WG, et al. Photoprotective effect of isoflavone genistein on ultraviolet B-induced pyrimidine dimer formation and PCNA expression in human reconstituted skin and its implications in dermatology and prevention of cutaneous carcinogenesis. Carcinogenesis. 2006;27(8):1627–1635. doi: 10.1093/carcin/bgi367. [DOI] [PubMed] [Google Scholar]
- 10.Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1(2):136–142. [PubMed] [Google Scholar]
- 11.Fayolle C, Pourchet J, de Fromentel CC, Puisieux A, Dore JF, Voeltzel T. Gadd45a activation protects melanoma cells from ultraviolet B-induced apoptosis. J Invest Dermatol. 2008;128(1):196–202. doi: 10.1038/sj.jid.5700963. [DOI] [PubMed] [Google Scholar]
- 12.Thyss R, Virolle V, Imbert V, Peyron JF, Aberdam D, Virolle T. NF-kappaB/Egr-1/Gadd45 are sequentially activated upon UVB irradiation to mediate epidermal cell death. EMBO J. 2005;24(1):128–137. doi: 10.1038/sj.emboj.7600501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasahara S, Aizawa K, Okamiya M, et al. UVB irradiation suppresses cytokine production and innate cellular immune functions in mice. Cytokine. 2001;14(2):104–111. doi: 10.1006/cyto.2001.0849. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz A, Grabbe S, Aragane Y, et al. Interleukin-12 prevents ultraviolet B-induced local immunosuppression and overcomes UVB-induced tolerance. The Journal of investigative dermatology. 1996;106(6):1187–1191. doi: 10.1111/1523-1747.ep12347944. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt DA, Owen-Schaub L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J Immunol. 1995;154(10):5114–5120. [PubMed] [Google Scholar]
- 16.Werth VP, Bashir MM, Zhang W. IL-12 completely blocks ultraviolet-induced secretion of tumor necrosis factor alpha from cultured skin fibroblasts and keratinocytes. J Invest Dermatol. 2003;120(1):116–122. doi: 10.1046/j.1523-1747.2003.12012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunda MJ, Luistro L, Warrier RR, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo MP, Vagliani M, Spreafico F, et al. Amount of interleukin 12 available at the tumor site is critical for tumor regression. Cancer Res. 1996;56(11):2531–2534. [PubMed] [Google Scholar]
- 19.Robertson MJ, Ritz J. Interleukin 12: Basic Biology and Potential Applications in Cancer Treatment. Oncologist. 1996;1(1 & 2):88–97. [PubMed] [Google Scholar]
- 20.Schwarz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwarz T. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. The Journal of experimental medicine. 2005;201(2):173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz A, Maeda A, Gan D, Mammone T, Matsui MS, Schwarz T. Green tea phenol extracts reduce UVB-induced DNA damage in human cells via interleukin-12. Photochemistry and photobiology. 2008;84(2):350–355. doi: 10.1111/j.1751-1097.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz A, Stander S, Berneburg M, et al. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol. 2002;4(1):26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- 23.Afaq F, Adhami VM, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat Res. 2005;571(1–2):153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Adhami VM, Syed DN, Khan N, Afaq F. Phytochemicals for prevention of solar ultraviolet radiation-induced damages. Photochemistry and photobiology. 2008;84(2):489–500. doi: 10.1111/j.1751-1097.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 25.Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41(13):1969–1979. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Roy S, Deep G, Agarwal C, Agarwal R. Silibinin prevents ultraviolet B radiation-induced epidermal damages in JB6 cells and mouse skin in a p53-GADD45alpha-dependent manner. Carcinogenesis. 2012;33(3):629–636. doi: 10.1093/carcin/bgr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010;29(3):447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramasamy K, Dwyer-Nield LD, Serkova NJ, et al. Silibinin prevents lung tumorigenesis in wild-type but not in iNOS−/− mice: potential of real-time micro-CT in lung cancer chemoprevention studies. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(4):753–761. doi: 10.1158/1078-0432.CCR-10-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajamanickam S, Velmurugan B, Kaur M, Singh RP, Agarwal R. Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin. Cancer research. 2010;70(6):2368–2378. doi: 10.1158/0008-5472.CAN-09-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhanalakshmi S, Agarwal C, Singh RP, Agarwal R. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. The Journal of biological chemistry. 2005;280(21):20375–20383. doi: 10.1074/jbc.M414640200. [DOI] [PubMed] [Google Scholar]
- 31.Gu M, Dhanalakshmi S, Singh RP, Agarwal R. Dietary feeding of silibinin prevents early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1344–1349. doi: 10.1158/1055-9965.EPI-04-0664. [DOI] [PubMed] [Google Scholar]
- 32.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Dual efficacy of silibinin in protecting or enhancing ultraviolet B radiation-caused apoptosis in HaCaT human immortalized keratinocytes. Carcinogenesis. 2004;25(1):99–106. doi: 10.1093/carcin/bgg188. [DOI] [PubMed] [Google Scholar]
- 33.Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67(7):3483–3491. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 34.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin prevents ultraviolet radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/Cip1 in epidermis. Carcinogenesis. 2004;25(8):1459–1465. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- 35.Mallikarjuna G, Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer research. 2004;64(17):6349–6356. doi: 10.1158/0008-5472.CAN-04-1632. [DOI] [PubMed] [Google Scholar]
- 36.Sharma G, Singh RP, Chan DC, Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23(3B):2649–2655. [PubMed] [Google Scholar]
- 37.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci U S A. 1992;89(16):7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woods GM, Malley RC, Muller HK. The skin immune system and the challenge of tumour immunosurveillance. Eur J Dermatol. 2005;15(2):63–69. [PubMed] [Google Scholar]
- 39.Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of the molecular target for the suppression of contact hypersensitivity by ultraviolet radiation. J Exp Med. 1989;170(4):1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aragane Y, Riemann H, Bhardwaj RS, et al. IL-12 is expressed and released by human keratinocytes and epidermoid carcinoma cell lines. J Immunol. 1994;153(12):5366–5372. [PubMed] [Google Scholar]
- 41.Muller G, Saloga J, Germann T, et al. Identification and induction of human keratinocyte-derived IL-12. J Clin Invest. 1994;94(5):1799–1805. doi: 10.1172/JCI117528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda A, Schneider SW, Kojima M, Beissert S, Schwarz T, Schwarz A. Enhanced photocarcinogenesis in interleukin-12-deficient mice. Cancer Res. 2006;66(6):2962–2969. doi: 10.1158/0008-5472.CAN-05-3614. [DOI] [PubMed] [Google Scholar]
- 43.Meeran SM, Mantena SK, Meleth S, Elmets CA, Katiyar SK. Interleukin-12-deficient mice are at greater risk of UV radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Molecular cancer therapeutics. 2006;5(4):825–832. doi: 10.1158/1535-7163.MCT-06-0003. [DOI] [PubMed] [Google Scholar]
- 44.Flaig TW, Glode M, Gustafson D, et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. The Prostate. 2010;70(8):848–855. doi: 10.1002/pros.21118. [DOI] [PubMed] [Google Scholar]
- 45.Flaig TW, Gustafson DL, Su LJ, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Investigational New Drugs. 2007;25(2):139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 46.Hoh C, Boocock D, Marczylo T, et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(9):2944–2950. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]