Abstract
Nuclear monolayers of WI-38 cells prepared by the method of Tsai and Green were used to determine RNA synthesis in isolated nuclei in situ. In nuclear monolayers, incorporation of [3H]UTP into RNA is dependent on the presence of the other three nucleotide triphosphate and is abolished by actinomycin D. The extent of RNA synthesis under these conditions was measured in density-inhibited WI-38 human diploid fibroblasts at various intervals after cell proliferation was stimulated by a change of medium.
RNA synthesis increases 15 min after the nutritional change and reaches a peak at 18 hr, which is also the peak of DNA synthesis. Thereafter RNA synthesis declines. Essentially similar results are obtained whether the endogenous RNA polymerase or a bacterial polymerase is used. Replacement of the stimulating medium by conditioned medium stops the increase in RNA synthesis that occurs in cultures subject to continuous stimulation. Finally, RNA synthesis in nuclear monolayers, using the endogenous RNA polymerase, occurs by chain elongation only, while re-initiation occurs with the bacterial RNA polymerase.
Keywords: endogenous [WI-38] RNA polymerase, exogenous RNA polymerase, chromatin template activity, gene sites, DNA synthesis
Full text
PDF
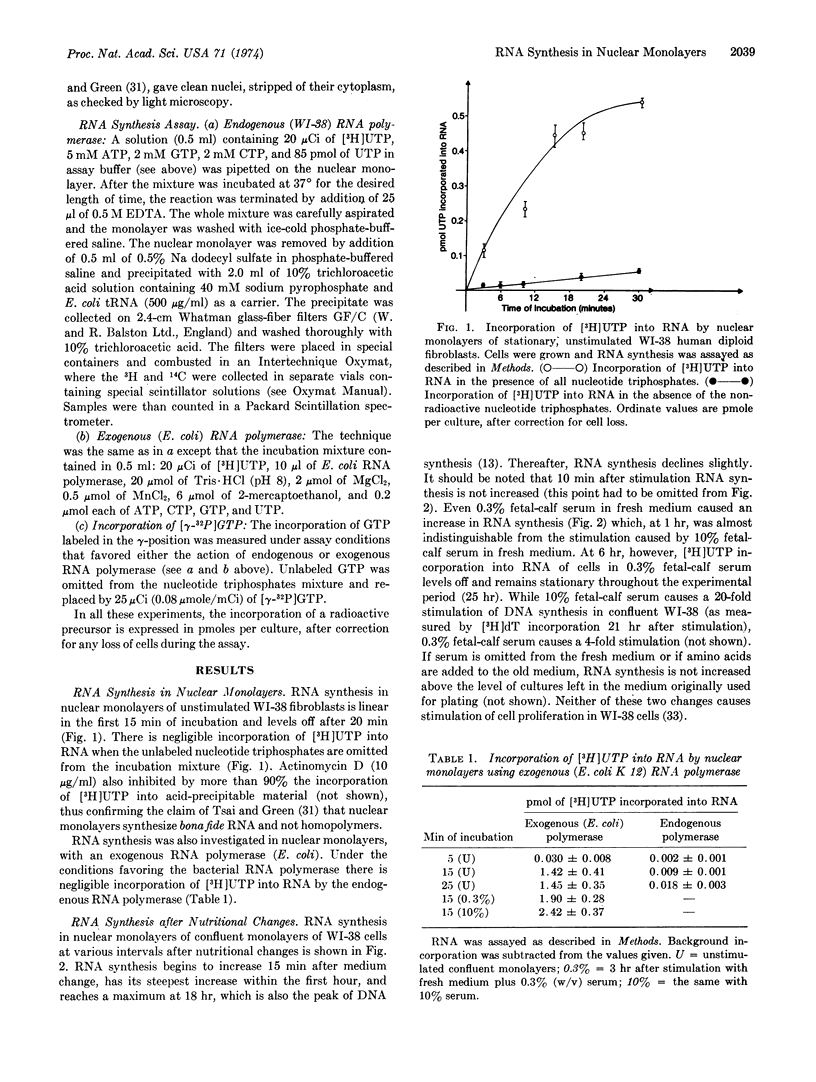
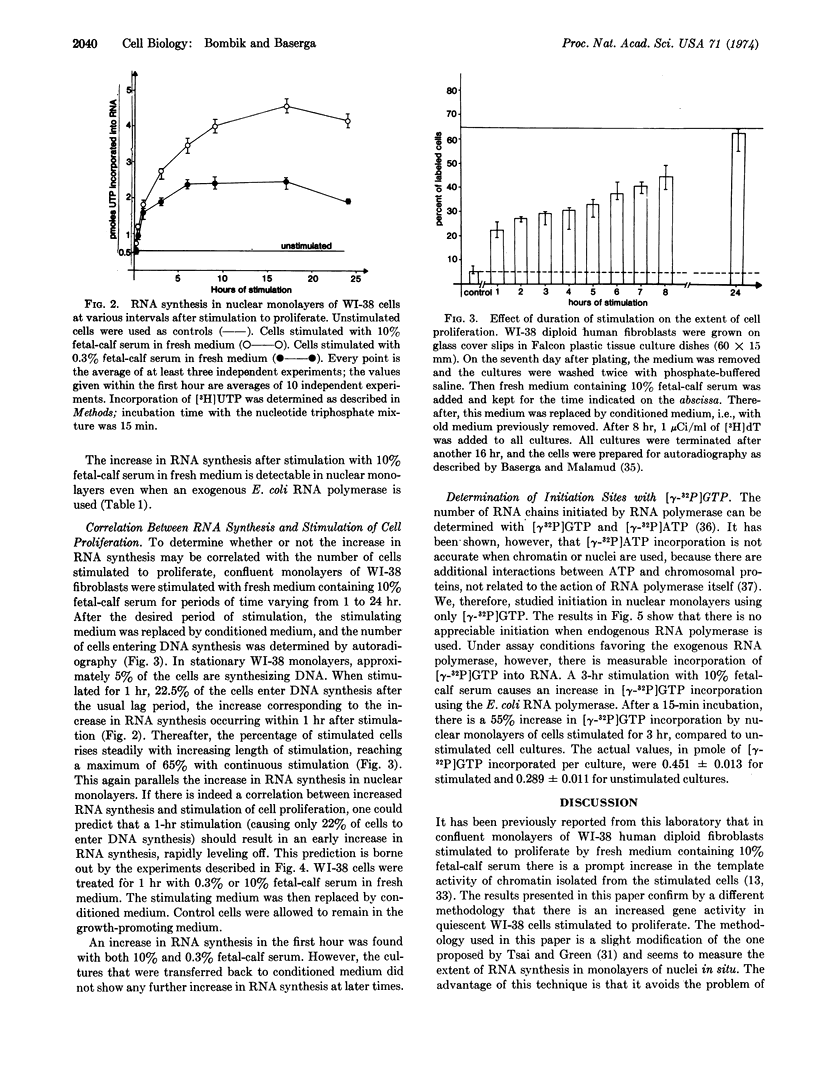
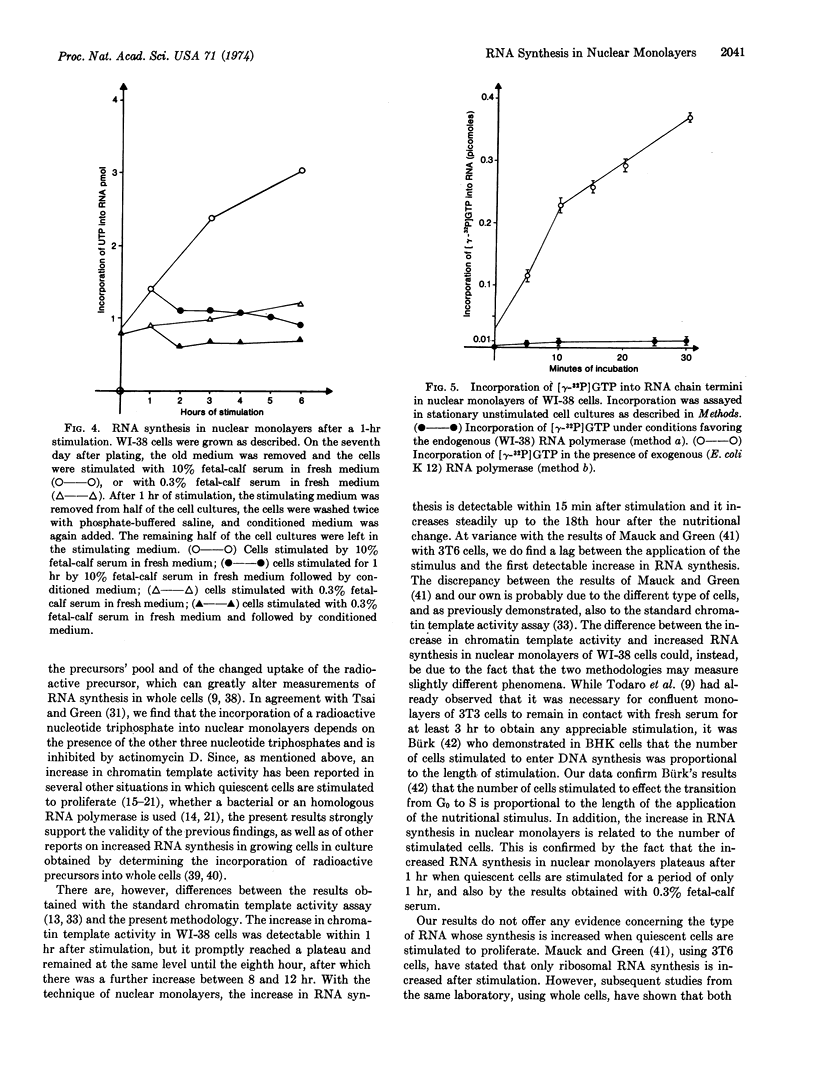

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannai S., Terayama H. Template activity of chromatins isolated from regenerating rat liver. J Biochem. 1969 Sep;66(3):289–295. doi: 10.1093/oxfordjournals.jbchem.a129147. [DOI] [PubMed] [Google Scholar]
- Barker K. L., Warren J. C. Template capacity of uterine chromatin: control by estradiol. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1298–1302. doi: 10.1073/pnas.56.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Butterworth P. H., Cox R. F., Chesterton C. J. Transcription of mammalian chromatin by mammalian DNA-dependent RNA polymerases. Eur J Biochem. 1971 Nov 11;23(2):229–241. doi: 10.1111/j.1432-1033.1971.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Bürk R. R. One-step growth cycle for BHK21-13 hamster fibroblasts. Exp Cell Res. 1970 Dec;63(2):309–316. doi: 10.1016/0014-4827(70)90218-1. [DOI] [PubMed] [Google Scholar]
- Cedar H., Felsenfeld G. Transcription of chromatin in vitro. J Mol Biol. 1973 Jun 25;77(2):237–254. doi: 10.1016/0022-2836(73)90334-3. [DOI] [PubMed] [Google Scholar]
- Comings D. E., Tack L. O. Non-histone proteins. The effect of nuclear washes and comparison of metaphase and interphase chromatin. Exp Cell Res. 1973 Nov;82(1):175–191. doi: 10.1016/0014-4827(73)90260-7. [DOI] [PubMed] [Google Scholar]
- Couch R. M., Anderson K. M. Rat ventral prostate chromatin. Effect of androgens on its chemical composition, physical properties, and template activity. Biochemistry. 1973 Jul 31;12(16):3114–3121. doi: 10.1021/bi00740a027. [DOI] [PubMed] [Google Scholar]
- Cox R. F., Haines M. E., Carey N. H. Modification of the template capacity of chick-oviduct chromatin for form-B RNA polymerase by estradiol. Eur J Biochem. 1973 Feb 1;32(3):513–524. doi: 10.1111/j.1432-1033.1973.tb02636.x. [DOI] [PubMed] [Google Scholar]
- Cunningham D. D., Pardee A. B. Transport changes rapidly initiated by serum addition to "contact inhibited" 3T3 cells. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1049–1056. doi: 10.1073/pnas.64.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson C. P. Regulation of the synthesis and the stability of ribosomal RNA during contact inhibition of growth. Nat New Biol. 1971 Jul 28;232(30):101–106. doi: 10.1038/newbio232101a0. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Rovera G., Baserga R. Effect of homologous RNA polymerase on the increase in chromatin template activity of stimulated fibroblasts. Biochem Biophys Res Commun. 1972 Oct 17;49(2):558–562. doi: 10.1016/0006-291x(72)90447-0. [DOI] [PubMed] [Google Scholar]
- Farber J., Rovera G., Baserga R. Template activity of chromatin during stimulation of cellular proliferation in human diploid fibroblasts. Biochem J. 1971 Apr;122(2):189–195. doi: 10.1042/bj1220189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross K. J., Pogo A. O. Control mechanism of ribonucleic acid synthesis in eukaryotes. The effect of amino acid and glucose starvation and cycloheximide on yeast deoxyribonucleic acid-dependent ribonucleic acid polymerases. J Biol Chem. 1974 Jan 25;249(2):568–576. [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Troll W., Brittinger G., Weissmann G. Template activity of nuclei from stimulated lymphocytes. Nature. 1969 Jun 28;222(5200):1247–1250. doi: 10.1038/2221247a0. [DOI] [PubMed] [Google Scholar]
- Keshgegian A. A., Furth J. J. Comparison of transcription of chromatin by calf thymus and E. coli RNA polymerases. Biochem Biophys Res Commun. 1972 Aug 21;48(4):757–763. doi: 10.1016/0006-291x(72)90671-7. [DOI] [PubMed] [Google Scholar]
- Liao S., Lin A. H. Prostatic nuclear chromatin: an effect of testosterone on the synthesis of ribonucleic Acid rich in cytidylyl(3',5')guanosine. Proc Natl Acad Sci U S A. 1967 Feb;57(2):379–386. doi: 10.1073/pnas.57.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U., Hurwitz H. The role of DNA in RNA synthesis, IX. Nucleoside triphosphate termini in RNA polymerase products. Proc Natl Acad Sci U S A. 1965 Sep;54(3):815–822. doi: 10.1073/pnas.54.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanka D., Gould H. Transcription of rat-liver chromatin with homologous enzyme. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1161–1165. doi: 10.1073/pnas.70.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J. C., Green H. Regulation of RNA synthesis in fibroblasts during transition from resting to growing state. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2819–2822. doi: 10.1073/pnas.70.10.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J. E., Bonner J. A partial sequence of nuclear events in regenerating rat liver (gene regulation-chromosomal RNA). Proc Natl Acad Sci U S A. 1972 Jan;69(1):7–10. doi: 10.1073/pnas.69.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. P., Ringertz N. R. Localization of DNA-dependent RNA polymerase activities in fixed human fibroblasts by autoradiography. Exp Cell Res. 1973 Jan;76(1):223–228. doi: 10.1016/0014-4827(73)90439-4. [DOI] [PubMed] [Google Scholar]
- Novi A. M., Baserga R. Changes in chromatin template activity and their relationship to DNA synthesis in mouse parotid glands stimulated by isoproterenol. J Cell Biol. 1972 Dec;55(3):554–562. doi: 10.1083/jcb.55.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo A. O., Allfrey V. G., Mirsky A. E. Evidence for increased DNA template activity in regenerating liver nuclei. Proc Natl Acad Sci U S A. 1966 Aug;56(2):550–557. doi: 10.1073/pnas.56.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder R. H. Transcription of chromatin by bacterial RNA polymerase. J Mol Biol. 1973 Oct 25;80(2):229–241. doi: 10.1016/0022-2836(73)90169-1. [DOI] [PubMed] [Google Scholar]
- Rovera G., Baserga R. Effect of nutritional changes on chromatin template activity and non-histone chromosomal protein synthesis in WI-38 and 3T6 cells. Exp Cell Res. 1973 Mar 30;78(1):118–126. doi: 10.1016/0014-4827(73)90045-1. [DOI] [PubMed] [Google Scholar]
- STOKER M. Characteristics of normal and transformed clones arising from BHK21 cells exposed to polyoma virus. Virology. 1962 Dec;18:649–651. doi: 10.1016/0042-6822(62)90071-5. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Release from density dependent growth inhibition by proteolytic enzymes. Nature. 1970 Aug 22;227(5260):843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- Summers W. P., Mueller G. C. Properties of RNA synthesis in HeLa nuclei by Micrococcus lysodeikticu or endogenous RNA polymerase. Biochim Biophys Acta. 1968 Dec 17;169(2):316–326. doi: 10.1016/0005-2787(68)90040-3. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971 Oct;78(2):161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Studies on carcinogenesis by avian sarcoma viruses. VI. Differential multiplication of uninfected and of converted cells in response to insulin. J Cell Physiol. 1967 Jun;69(3):377–384. doi: 10.1002/jcp.1040690314. [DOI] [PubMed] [Google Scholar]
- Thaler M. M., Willee C. A. Template activities in normal, regenerating, and developing rat liver chromatin. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2055–2062. doi: 10.1073/pnas.58.5.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrash C. R., Cunningham D. D. Stimulation of division of density inhibited fibroblasts by glucocorticoids. Nature. 1973 Apr 6;242(5397):399–401. doi: 10.1038/242399a0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., Saunders G. F. Transcription of chromatin by human RNA polymerase. Biochem Biophys Res Commun. 1973 Apr 2;51(3):756–765. doi: 10.1016/0006-291x(73)91380-6. [DOI] [PubMed] [Google Scholar]
- Tsai R. L., Green H. Rate of RNA synthesis in ghost monolayers obtained from fibroblasts preparing for division. Nat New Biol. 1973 Jun 6;243(127):168–170. doi: 10.1038/newbio243168a0. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Rucoslahti E., Nordling S. Neuraminidase stimulates division and sugar uptake in density-inhibited cell cultures. Nat New Biol. 1972 Aug 16;238(85):211–212. doi: 10.1038/newbio238211a0. [DOI] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M., Guelstein V. I., Fetisova E. K. Stimulation of DNA synthesis in cultures of mouse embryo fibroblast-like cells. J Cell Physiol. 1970 Jun;75(3):305–313. doi: 10.1002/jcp.1040750307. [DOI] [PubMed] [Google Scholar]
- WIDNELL C. C., TATA J. R. EVIDENCE FOR TWO DNA-DEPENDENT RNA POLYMERASE ACTIVITIES IN ISOLATED RAT-LIVER NUCLEI. Biochim Biophys Acta. 1964 Jul 22;87:531–533. doi: 10.1016/0926-6550(64)90133-1. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Ribosomal RNA turnover in contact inhibited cells. Nat New Biol. 1972 Jan 12;235(54):58–61. doi: 10.1038/newbio235058a0. [DOI] [PubMed] [Google Scholar]
- Wiebel F., Baserga R. Early alterations in amino acid pools and protein synthesis of diploid fibroblasts stimulated to synthesize DNA by addition of serum. J Cell Physiol. 1969 Oct;74(2):191–202. doi: 10.1002/jcp.1040740211. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Hirokawa Y., Yamada M. Synchronized cell division induced by medium change. Exp Cell Res. 1967 Oct;48(1):226–228. doi: 10.1016/0014-4827(67)90309-6. [DOI] [PubMed] [Google Scholar]


