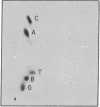
Abstract
In an attempt to isolate cells that could survive with total replacement of thymidine by bromodeoxyuridine in nuclear DNA, cells of a bromodeoxyuridine-dependent Syrian hamster line were cultured in medium containing aminopterin and bromodeoxyuridine but no thymidine. A line of cells, called HAB, was isolated. The HAB cells have been maintained in continuous cultivation for over nine months and have undergone more than 125 population doublings. Direct base analysis showed that the level of substitution of bromodeoxyuridine for thymidine in nuclear DNA was at least 99.8%, and possibly 100%. The existence of such cells raises many questions. The expected high frequency of bromodeoxyuridine-induced base transitions, including errors in both replication and transcription, would seem to be incompatible with the apparently stable transmission and expression of the genetic information in these cells.
Keywords: mammalian cells
Full text
PDF

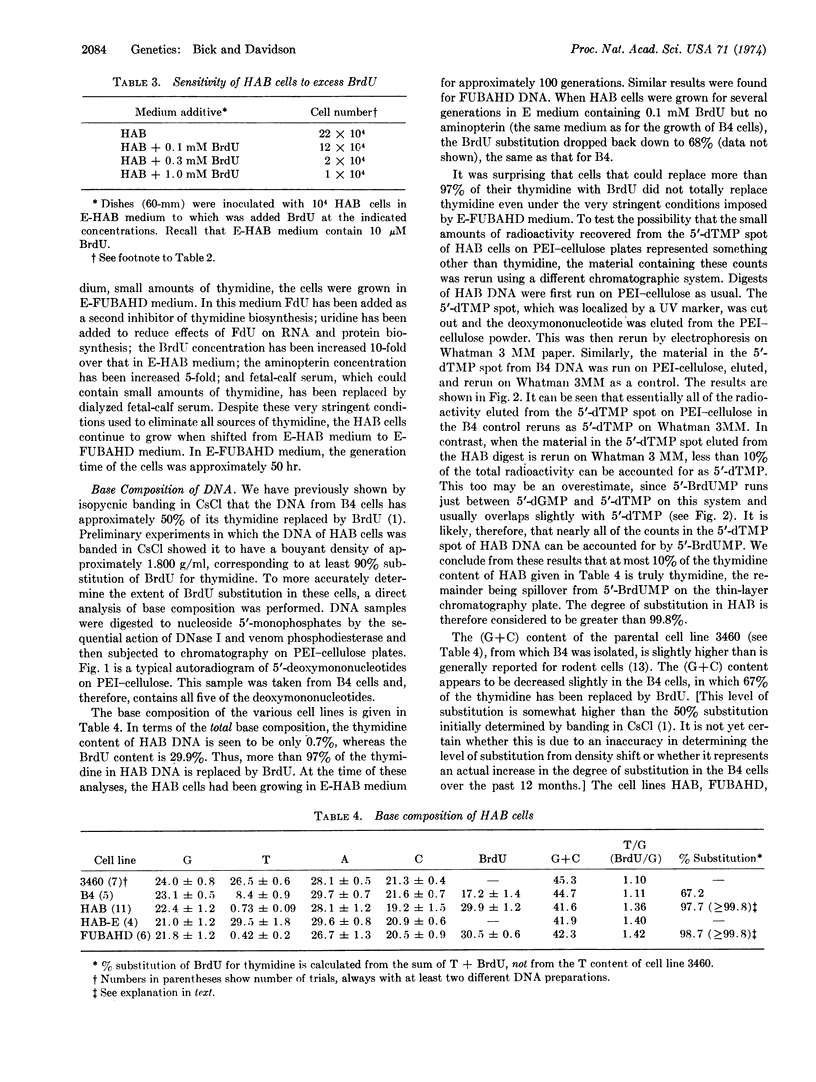
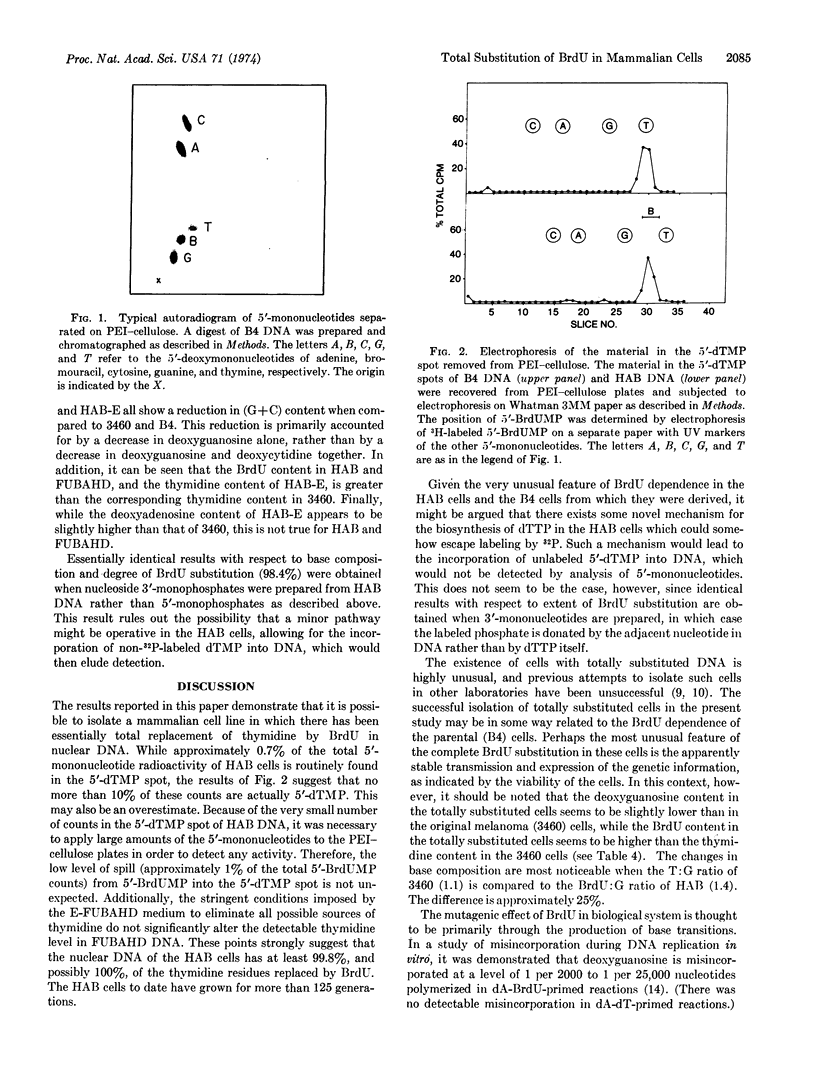

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Couch J. L., Hanawalt P. C. Analysis of 5-bromouracil distribution in partially substituted deoxyribonucleic acids. Anal Biochem. 1971 May;41(1):51–56. doi: 10.1016/0003-2697(71)90190-4. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Bick M. D. Bromodeoxyuridine dependence--a new mutation in mammalian cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):138–142. doi: 10.1073/pnas.70.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKALA M. T. Mode of action of 5-bromodeoxyuridine on mammalian cells in culture. J Biol Chem. 1959 Dec;234:3072–3076. [PubMed] [Google Scholar]
- Hill B. T., Tsuboi A., Baserga R. Effect of 5-bromodeoxyuridine on chromatin transcription in confluent fibroblasts. Proc Natl Acad Sci U S A. 1974 Feb;71(2):455–459. doi: 10.1073/pnas.71.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAJIWARA K., MUELLER G. C. MOLECULAR EVENTS IN THE REPRODUCTION OF ANIMAL CELLS. 3. FRACTIONAL SYNTHESIS OF DEOXYRIBONUCLEIC ACID WITH 5-BROMODEOXYURIDINE AND ITS EFFECT ON CLONING EFFICIENCY. Biochim Biophys Acta. 1964 Nov 15;91:486–493. [PubMed] [Google Scholar]
- Kotzin B. L., Baker R. F. Selective inhibition of genetic transcription in sea urchin embryos. Incorporation of 5-bromodeoxyuridine into low molecular weight nuclear DNA. J Cell Biol. 1972 Oct;55(1):74–81. doi: 10.1083/jcb.55.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITMAN R. M., PARDEE A. B. Production of bacteriophage mutants by a disturbance of deoxyribonucleic acid metabolism. Nature. 1956 Sep 8;178(4532):529–531. doi: 10.1038/178529b0. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac operator analogues: bromodeoxyuridine substitution in the lac operator affects the rate of dissociation of the lac repressor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2574–2576. doi: 10.1073/pnas.69.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. The 5'-terminal nucleotides of T7 bacteriophage deoxyribonucleic acid. J Mol Biol. 1966 Jan;15(1):49–61. doi: 10.1016/s0022-2836(66)80208-5. [DOI] [PubMed] [Google Scholar]
- STOCKDALE F., OKAZAKI K., NAMEROFF M., HOLTZER H. 5-BROMODEOXYURIDINE: EFFECT ON MYOGENESIS IN VITRO. Science. 1964 Oct 23;146(3643):533–535. doi: 10.1126/science.146.3643.533. [DOI] [PubMed] [Google Scholar]
- Silagi S., Bruce S. A. Suppression of malignancy and differentiation in melanotic melanoma cells. Proc Natl Acad Sci U S A. 1970 May;66(1):72–78. doi: 10.1073/pnas.66.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUTNER T. A., SWARTZ M. N., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. X. Influence of bromouracil substitutions on replication. Proc Natl Acad Sci U S A. 1962 Mar 15;48:449–455. doi: 10.1073/pnas.48.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]