Abstract
Objections to the use of topical nasal anesthesia (TNA) during fiberoptic endoscopic evaluation of swallowing (FEES) with sensory testing (FEESST) have been raised, primarily because of the possibility of desensitizing the pharyngeal and laryngeal mucosa and affecting both the sensory and motor aspects of the swallow. Furthermore, it has been suggested that TNA is not necessary during FEES as it does not improve patient comfort or make the procedure easier for the endoscopist. The purpose of this double-blind, randomized, controlled, crossover clinical trial was to determine how gel TNA during flexible endoscopic evaluation of swallowing with sensory testing affects sensation, swallowing, and comfort rating scores in healthy nondysphagic participants. Laryngopharyngeal sensory thresholds and swallowing durations were compared between two conditions: TNA and sham. Transition duration decreased statistically significantly during the TNA condition compared to the sham for 10 ml only (p < 0.05). All other swallowing measures did not change between the conditions. Laryngopharyngeal sensory thresholds and perceptions did not change between conditions. No change was observed for subject comfort scores, ease of exam, or quality of view. Future studies should evaluate TNA administration variables, including concentration, dosage amount, and method of application, to determine the optimal strategy for providing comfort while avoiding altered swallowing.
Keywords: Topical nasal anesthesia, FEESST, FEES
Introduction
FEES and FEESST
First introduced in the late 1980s, fiberoptic endoscopic evaluation of swallowing (FEES) is now a standard assessment procedure in hospitals across the United States and in many other countries. Due to technological advances, FEES units can now fit into a suitcase, and private practices are sprouting up to provide FEES in nursing homes and rehabilitation centers where patients would otherwise have to be transferred somewhere else for assessment or not receive one. Although videofluoroscopy has historically been considered the gold standard of dysphagia instrumental assessment procedures [1, 2], FEES has secured its status along side videofluoroscopy as a reliable and sensitive assessment tool for many aspects of swallowing function, and videofluoroscopy units do not fit into suitcases!
FEES was first described by Langmoreet al. [3] in 1988 and subsequently by others as fiberoptic visualization of the laryngopharyngeal area during the ingestion of colored food items to determine the presence of dysphagia and define treatment options [4–6]. Upon its introduction, research quickly and exponentially began to proliferate. After all, the tools were already available in most ENT clinics, and the possibility of assessing swallowing without irradiating patients was appealing. Research has shown that FEES is safe to use serially [7], making it most useful for ongoing management of dysphagic patients as they progress with therapy, and a viable option for biofeedback [8]. Researchers can feel secure using FEES to collect swallowing data as the presence of the endoscope in the pharynx does not alter swallowing physiology [9] and FEES is reliable to use with the penetration-aspiration scale (PAS) [10, 11].
Aviv et al. [12] were the first to describe using calibrated pulses of air delivered from the tip of the endoscope to psychophysically test laryngopharyngeal sensation. In this manner they were able to assign a numerical air pressure value to each patient’s laryngopharyngeal sensation threshold. This group later described the use of this same technique for delivering air pulses to the aryepiglottic folds to elicit a brief, involuntary closure of the vocal cords, which can be visualized from the FEES monitor [13]. This closure, a protective mechanism against aspiration, is the laryngeal adductor reflex (LAR) [14, 15]. LAR threshold testing yields sensory thresholds similar to those of psychophysical testing, but it does not require a verbal or physical response from the testee and is therefore a more objective manner with which to measure laryngeal sensation [16]. When a FEES examination is combined with air pulse sensory testing it is called fiberoptic endoscopic evaluation of swallowing with sensory testing (FEESST).
Topical Nasal Anesthesia During FEES and FEESST
Many clinicians utilize topical anesthetics to help reduce the discomfort and anxiety associated with a nasendoscopic exam. ASHA guidelines state that clinicians may choose to use topical nasal anesthetic (TNA) with or without vasoconstriction during nasendoscopy [17]. Some concerns about the use of TNA, especially during swallowing examinations, have been raised, primarily the possibility of the anesthetic dripping into the oropharynx or laryngopharynx and desensitizing the mucosa and potentially affecting both the sensory and the motor aspect of the swallow [17, 18]. Moreover, it has been suggested that TNA is not necessary during an endoscopic examas it does not improve patient comfort andmay make the experience worse because of its unpleasant taste [19, 20].
The argument against potentially desensitizing the laryngopharynx during swallowing is formidable. Research has shown that sensory input and feedback provided by central mechanisms during swallowing are vital to the initiation, implementation, and modulation of the reflexive motor programming planned by the brainstem and executed by the cranial nerves. The internal branch of the superior laryngeal nerve (ISLN) provides afferent innervation to the supraglottic structures, and stimulation of the ISLN can cause laryngeal closure [21], induce swallowing movements [22], and cause central apnea [23]. Studies involving anesthetizing the oropharynx have shown that sensory interruption can result in dysfunction of the normal swallow [24, 25].
Using TNA During FEES/FEESST
Considering the role of sensation in the swallowing process, it is imperative that clinicians choosing to employ TNA during nasendoscopy do so in a manner that does not disrupt the sensory or motor components or induce secondary reactions to the anesthetic. At the location of application, topical anesthetics temporarily interrupt peripheral nerve impulses, effectively reducing or eliminating sensation [26]. It has been recommended that TNA be applied only to the nasal passages and not the pharynx during FEES, and to avoid the use of anesthetic sprays as they may lead to postnasal drainage of the anesthesia into the pharynx [17, 18]. While Langmore [18] recommends using 2 % viscous lidocaine (lignocaine), many other types of TNA are reportedly being used during FEES, including tetracaine, cocaine, benzocaine, xylocaine, and prilocaine [26]. Topical anesthesia can cause adverse reactions if US FDA recommendations are not followed as to proper usage and dosage. Allergic reactions to topical anesthesia are rare and account for only 1 % of all reactions [18].
Several studies have examined the utility of topical anesthetics, vasoconstrictors, and combinations of anesthetics and vasoconstrictors for improving patient comfort level during nasendoscopy. Ten studies that assessed nasendoscopic pain treatment included a control treatment (saline or no treatment) and were therefore able to address efficacy [19, 20, 27–34]. Of these ten studies that focused on the efficacy of prenasendoscopic treatment for patient comfort, one recommended using a vasoconstrictor alone instead of TNA, three recommended using TNA, and five recommended using no treatment. All three studies that advocated TNA were either crossover or split-body designs, meaning each participant was compared to himself and therefore the results are more reliable. Two of the five studies that recommended no treatment used a lubricant on the endoscope; thus, it is reasonable to assume that the suggested alternative treatment of those studies is the use of lubricant jelly to ease passage of the scope through the nasal passages. Two studies specifically on the use of endoscope lubrication during nasendoscopy have been completed [35, 36].
A study by Butler et al. [28] specifically addressed the effects of the anesthetic on swallowing function. They reported anesthetized swallows were less safe and had higher PAS scores but were also more comfortable and led to greater tolerance of the procedure. However, they did use 1 cc of 4 % liquid lidocaine in spray form prior to FEES on swallowing safety and patient tolerance compared to no treatment in the same participants [28]. The authors are currently evaluating whether lower doses of lidocaine can still provide comfort without compromising the swallow.
Although some of the above-mentioned studies reported that no anesthesia is necessary during nasendoscopy and may, in fact, affect swallowing, many clinicians continue to use TNA based on personal experience or patient request. Moreover, the few extant data reporting an effect of TNA on swallowing function were reported after a sprayed application of TNA. The primary method of administering TNA clinically for FEES is to apply gel TNA directly to the nares using a cotton-tip applicator; this is the method described in the originally published FEES protocol [18]. There is currently no evidence that the use of gel TNA during clinical FEES or FEESST would affect either swallowing physiology or the sensation of the laryngopharynx.
The aim of this study was to determine how 0.4 ml of viscous (gel) TNA applied at the onset of FEESST affects the sensory and motor aspects of swallowing in healthy nondysphagic participants. This specific amount (0.4 ml) was chosen as this is approximately the maximum amount that can be inserted into the nares using a cotton-tip applicator two times. This study was a prospective, double blind, controlled, randomized, crossover study of subjects who underwent FEESST twice: once in a topically anesthetized condition (2 % viscous lidocaine) and once in a placebo condition (Surgilube, Savage Laboratories, Melville, NY, USA).
Methods
Participants
The University of Central Arkansas Internal Review Board approved this research for human subjects. Participants were recruited from the University of Central Arkansas by word of mouth and IRB-approved flyers. A power analysis was completed a priori using G Power© (v3.1.3); it was that determined 30 participants were necessary to test the hypotheses. Forty-seven participants consented but 11 dropped out for the following reasons: nasal passages too narrow for scope passage (3), unwilling to attend second visit (6), vasovagal syncope (1), and voluntary discontinuation secondary to discomfort (1). A total of 36 participants (33 females) completed the study (age range = 18–38 years; mean = 23.16). All participants signed a written informed consent and were given the opportunity to ask questions about the study before it began. Participants were screened prior to signing the informed consent and were excluded only if they had a past or current history of neurological disease, thyroid problems, head and neck cancer, swallowing problems, uncontrolled gastroesophageal reflux disease, or milk allergy.
Design
Participants underwent nasendoscopic testing on two separate days under two conditions. In the experimental condition, 0.4 ml of 2 % viscous lidocaine hydrochloride was applied to one side of the nasal cavity twice, one minute each time, via a cotton-tip applicator. This is well below the maximum dosage recommendation of 2 mg per pound of body weight, and 2 % lidocaine is concentrated at 20 mg/ml, which means that a 100-pound person can safely have 10 ml of 2 % viscous lidocaine [26]. In the sham condition, 0.4 ml of Surgilube was applied to one side of the nasal cavity twice via a cotton-tip applicator. The same nasal cavity was used in both conditions and was selected based on patency. The left nostril was chosen for 22/36 (61 %) participants and the right for 14/36 (39 %) participants. The conditions were given in a double-blind fashion so that neither the subject nor the tester knew which agent was being used.
Participants were seated upright in a standard size cushioned chair with a back. While no one but the participant and the coinvestigator were in the room, the coinvestigator applied a gel (viscous lidocaine or Surgilube depending on randomization) to a cotton-tip applicator using a syringe to keep the amount consistent. Half of the gel was applied to the applicator. The applicator was then inserted into the designated nares until it met resistance and was left in place for 1 min. The applicator was then withdrawn, and a second applicator was then prepared and inserted the same way. The coinvestigator removed the second applicator after 1 min and left the room. The participants were unaware that one condition was a sham lubricant to reduce bias. The order of swallowing examination and sensory examination was randomized for each participant.
The principal investigator (PI) placed the sensory sheath (REF 444401, Medtronic Xomed, Jacksonville, FL, USA) over an ENTity endoscope (L0356A, length = 30 cm, width = 3.6 mm; Optim LLC, Sturbridge, MA, USA)) so that the air port in the sheath was in the 6 o’clock position on the endoscope, 180° away from the scope lever. A Stingray camera (09/17-285835485; Allied Vision Technologies, Stadtroda, Germany) recorded the images onto NDOvision software (version 1.0) installed on a MacBook Pro (Apple, Cupertino, CA, USA).
Sensory Testing
Laryngeal Adductor Reflex (LAR)
The LAR is a reflexive adduction of the vocal folds that occurs as a protective mechanism against aspiration [37]. It can be stimulated with air puffs applied to the aryepiglottic folds at rest. In our study, the AP-4000 (Vision Sciences, Inc., Orangeburg, NY, USA) was used to deliver the air puffs. It is a calibrated device that sends duration-controlled (50 ms) and pressure-controlled (0–10 mmHg) pulses of air down the length of an endoscope sheath through an internal port located within the sheath made especially for this purpose. A graduate research assistant sat next to the AP 4000 to adjust pulse delivery during the protocol. With the nasendoscope in place through the same nares to which gel was applied, air puffs were delivered to the aryepiglottic fold. The tip of the scope was held within 2 mm of the tissue surface as described by Aviv and Murry [38].
LAR testing was always conducted prior to perceptual testing as it does not require the subject to give a response and allows the subject time to acclimate to the air puffs. It was conducted according to the protocol outlined by Aviv and Murray [38]. Air puff pressure began at 2.0 mmHg and increased in 0.5-mmHg increments until a LAR was elicited. Then pressure was decreased/increased in 0.1-mmHg increments until a threshold was established. The sensory threshold was determined after a positive LAR had been visualized three times.
Previous research had established that the LAR threshold is comparable to the laryngopharyngeal sensory threshold [39].
Participant Perception of Stimulus Intensity
To determine the effect of TNA on sensory perception, a magnitude estimation sensory rating task was utilized [40]. Participants were instructed to rate the intensity of the air puff stimulation on a scale from 10 to 99 [41, 42]. If they felt the intensity was weak, they were to rate it with a lower number; if they felt the intensity was stronger, they were to rate it with a higher number. The intensities delivered included 2, 4, 6, 8, and 10 mmHg, and the order was randomized. Each intensity was delivered twice (for a total of 10 air puffs) to the aryepiglottic fold on the ipsilateral nares of the lidocaine/lubricant application.
Swallowing Assessment
Each participant drank 10 ± 1 and 20 ± 1 ml of cold 2 % milk (40–44 °F) from a medicine cup, three times for each amount, and a leveled teaspoon of room temperature applesauce three times, for a total of nine swallows per participant per condition. The order of swallows was always 10 ml milk, 20 ml milk, and the applesauce. The participant were instructed to hold the bolus in the mouth and swallow when instructed. A previous study reported that using a command to swallow does not alter the location of the bolus at the time of swallow initiation [43]. All swallows were recorded for later analysis and were reviewed in real time, slow motion, and frame by frame. The PI administered the examinations and analyzed all of the recordings blinded to condition. All recordings were given a random ID number to blind the examiner to the identity of the subject and condition.
Swallowing Measures Analyzed
All swallows were analyzed for bolus dwell time and pharyngeal closure duration [44], as well as residue remaining after the swallow and penetration-aspiration scale (PAS) scores [11] (see Tables 1, 2, 3).
Table 1.
Swallowing duration measures
| Measure | Start | End |
|---|---|---|
| Bolus dwell time at the vallecula (BDT-V) | First frame of bolus head approximation to the vallecula | First frame of complete whiteout |
| Bolus dwell time at the pyriform sinuses (BDT-P) | First frame of bolus head approximation to the pyriform sinus(es) | First frame of complete whiteout |
| Pharyngeal closure duration (PCD) | First frame of complete whiteout | Last frame of complete whiteout |
Table 2.
Residue scale
| Scale | Definition |
|---|---|
| 0 | Absence of residue |
| 1 | Residue coating or a trace amount of residue |
| 2 | More than a coating, but less than 50 % of the bolus remains (moderate residue) |
| 3 | More than 50 % of the bolus remains (severe residue) |
Vallecular residue and pyriform sinus residue were rated independently of each other
Table 3.
Penetration-aspiration scale
| Scale | Definition |
|---|---|
| 1 | No penetration or aspiration of material |
| 2–5 | Laryngeal penetration of material |
| 6–8 | Tracheal aspiration of material |
From Rosenbek et al. [11]
Participant Comfort Scale
Upon completion of the FEESST protocol, patients were asked to rate their comfort/discomfort by marking the intensity of sensation on a general-labeled magnitude scale [45]. The length of the vertical scale was 100 mm with the top labeled ‘‘Strongest sensation of any kind’’ and the bottom labeled ‘‘No sensation.’’ The mark was converted to a 0–100 score based on distance from the bottom (0).
Also upon completion of the FEESST protocol, the endoscopic examiner (PI) rated the ease of passing the scope by marking a general-labeled magnitude scale [45]. The length of the vertical scale was 100 mm with the top labeled ‘‘Most difficulty of any kind’’ and the bottom labeled ‘‘No difficulty.’’ The examiner also rated the quality of endoscopic image obtained during the FEESST protocol by marking on a similar 100-mm general-labeled magnitude scale [45]. The top was labeled ‘‘Worst imaginable quality’’ and the bottom was labeled ‘‘No loss of image.’’ The marks were later converted to a 0–100 score based on distance from the bottom (0).
Analysis
None of the swallowing measures, LAR thresholds, endoscopic ease scores, or quality-of-view scores were normally distributed. Subject comfort scale scores and sensory perception ratings were normally distributed but represent ordinal data. Therefore, all data were analyzed first with the Wilcoxon signed-rank test and then with paired t-tests for comparison. Spearman’s rank order correlation was used for correlation analysis.
Sensory perception data were analyzed using power functions, which were calculated by regressing log ratings on log intensity values using linear regression. Power functions are a log–log plot of the relationship between the physical intensity of sensory stimulation, in this case air puffs to the pharynx, and perception of stimulation intensity, specifically, the participant’s subjective rating. This relationship is summarized by the exponent and constant of the power function, with the exponent equal to the slope of the plotted function and the constant is the y-intercept. Changes in either the slope or the constant across conditions signal a change in perception of stimulation intensity.
Results
Twenty percent of swallowing measures were rescored by the original examiner and compared to the original analysis using interclass correlation coefficient (ICC) to determine intrarater reliability; alphas ranged from 0.65 to 1.0. The lowest intrarater reliability score (α = 0.65) was from PAS scores for 10 ml and differed by only 1/42 swallowing PAS scores. Interrater reliability was also determined using ICC; alphas ranged from 0.52 to 0.96, indicating moderate to strong agreement.
Days between visit 1 and visit 2 ranged from 1 to 23 days (m = 6.8). The number of days between visits did not correlate with any of the experimental measures.
Bolus Dwell Time at the Vallecula (BDT-V) and Pyriforms (BDT-P)
Bolus dwell time at the valleculae (BDT-V) and pyriform sinuses (BDT-P) are FEES measures similar to stage transition duration on videofluoroscopy [44] and are defined as ‘‘the time duration in seconds from the first frame of bolus head approximation to the vallecula or the pyriform sinus(es) until the first frame of whiteout.’’ BDT values are provided in Tables 4, 5 and Fig. 1.
Table 4.
Bolus dwell times at the vallecula means and statistical tests
| Lidocaine | SD | Sham | SD | Wilcoxon z | p value | t test | p value | |
|---|---|---|---|---|---|---|---|---|
| 10 ml | 0.016* | 0.03 | 0.035* | 0.05 | −2.39 | <0.05 | −2.36 | <0.05 |
| 20 ml | 0.049 | 0.08 | 0.038 | 0.06 | −0.99 | 0.32 | 0.7 | 0.49 |
| Puree | 0.037 | 0.09 | 0.029 | 0.09 | −0.98 | 0.33 | 0.93 | 0.306 |
Indicates significant finding
Table 5.
Bolus dwell times at the pyriforms means and statistical tests
| Lidocaine | SD | Sham | SD | Wilcoxon z | p | t-test | p | |
|---|---|---|---|---|---|---|---|---|
| 10 ml | 0.006* | 0.02 | 0.019* | 0.03 | −2.15 | <0.05 | −2.16 | <0.05 |
| 20 ml | 0.018 | 0.05 | 0.022 | 0.05 | −0.4 | 0.69 | −0.43 | 0.67 |
| Puree | 0.00 | 0.00 | 0.00 | 0.00 | – | – | – | – |
Significant finding
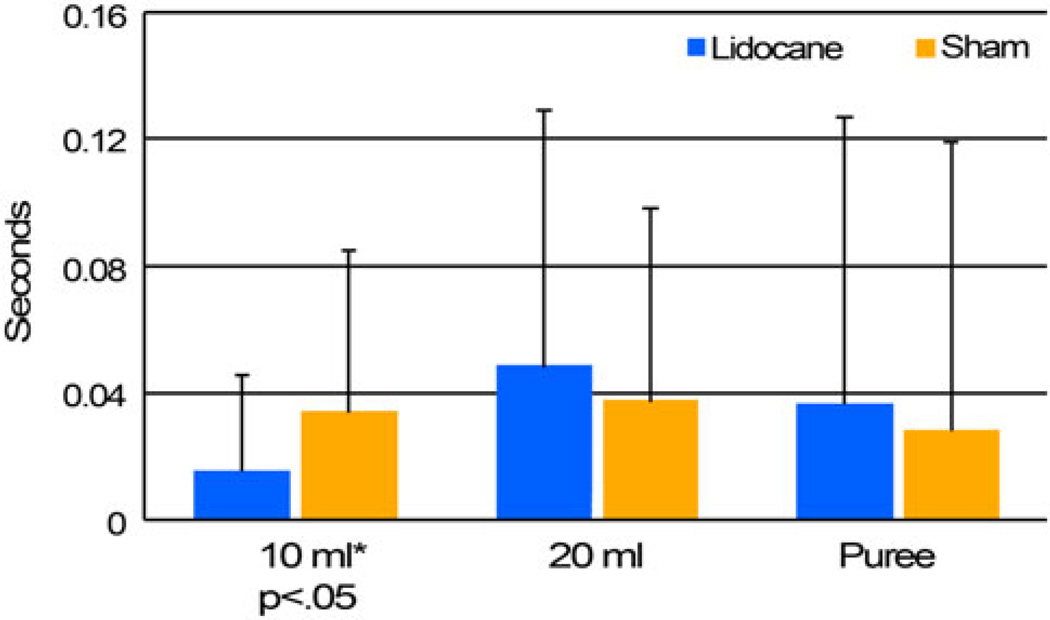
Fig. 1.
Bolus dwell time at the vallecula means by condition. * Significant at p < 0.05
A significant difference was found between the two conditions for bolus dwell time at both the vallecula and pyriforms for 10-ml liquids only (p < 0.05 for both locations on Wilcoxon and t-tests). Examination of the means reveals that the anesthetized condition resulted in a faster initiated swallow than the sham condition for the 10-ml milk swallows. Significant differences in BDT between the lidocaine and sham conditions are not seen for 20-ml milk swallows or puree swallows on either statistical test.
When examining the differences between bolus volumes within the experimental conditions, there was a significant difference between 10- and 20-ml milk swallows for BDT-V in the lidocaine condition only (p < 0.05 for both statistical tests), indicating that when the participants had received lidocaine, the 20-ml boluses remained in the vallecula longer than the 10-ml boluses before the pharyngeal swallow initiated (m = 0.05 vs. 0.02 s), but this difference was not seen in the sham condition. BDT did not significantly change as a function of visit (visit 1 or 2) for any bolus type.
Pharyngeal Closure Duration (PCD)
Pharyngeal closure duration (PCD) was defined as ‘‘the duration of time in seconds from the first to the last frames of complete whiteout.’’ There were no significant differences between the conditions for PCD for either liquid volume or the puree swallows for either statistical test. When comparing bolus types, puree swallows showed a significantly shorter PCD than both 10- and 20-ml liquid swallows (m = 0.52 s vs. 0.59 and 0.62 s, respectively, p < 0.001 and p < 0.001, respectively). This is true both within each condition and with the conditions collapsed. There was a significant difference between PCD for 10- and 20-ml liquid swallows only with conditions collapsed (p = 0.01). When examining visit order with conditions collapsed, participants swallowed faster on 10-ml swallows during their first visit than they did during the second visit (p < 0.01 for both statistical tests). This is not observed for any other bolus type and is perhaps an anxiety effect as well as an order effect because 10 ml was always the first bolus swallowed.
Residue Scales
There were no significant differences in residue severity in the vallecula or pyriforms between lidocaine and sham conditions. Residue scores were compared between bolus types, collapsing conditions and locations. Significant differences were found between 10- and 20-ml bolus residue scores (p < 0.001), 10-ml liquid and puree bolus residue scores (p < 0.01), and 20-ml liquid and puree bolus residue scores (p < 0.001). Puree swallows resulted in the least residue (m = 0.76), followed by 10-ml swallows (m = 0.87) and 20-ml swallows (m = 1.05).
When examined by visit order, pyriform residue was greater for swallows of 10 ml and puree during visit 1 compared to visit 2 (p < 0.05 for both bolus types). Vallecular residue was not significant as a function of visit order.
Penetration-Aspiration Scale (PAS) Scores
No significant differences were observed in PAS scores between lidocaine and sham conditions for any bolus type. Liquid swallow (10 and 20 ml) PAS scores ranged from 1 to 8, indicating that penetration and aspiration occurred during certain liquid swallows. All puree swallows obtained a PAS score of 1, indicating that no penetration or aspiration occurred during any puree swallows. Collapsing conditions for 10-ml swallows, 203/216 (93.9 %) swallows resulted in a PAS score of 1, 12/216 (5.5 %) resulted in a PAS score associated with penetration (PAS scores 2–5), and 1/216 (0.5 %) resulted in a PAS score associated with aspiration (PAS scores 6–8). Collapsing conditions for 20-ml swallows, 197/215 (91.6 %) swallows received a PAS score of 1, 15/215 (6.9 %) received a PAS score associated with penetration, and 3/215 (1.4 %) received a PAS score associated with aspiration. Significant differences were observed between puree PAS scores and 10-ml liquid PAS scores (m = 1.0 vs. 1.14, p < 0.005 for both statistical tests) and 20-ml liquid PAS scores (m = 1.0 vs. 1.25, p < 0.001 for both statistical tests), with the conditions collapsed, but there was no significant difference between 10-ml PAS scores and 20-ml PAS scores (p = 0.16). PAS scores did not differ between visits.
Laryngeal Adductor Reflex (LAR)
All means under both conditions fell well within the range of ‘‘normal’’ laryngeal sensation [12]. No significant difference was found for LAR thresholds between the lidocaine and sham conditions (m = 2.11 vs. 2.05 mmHg, respectively; p = 0.18).
Lidocaine did not improve the participants’ ability to tolerate laryngopharyngeal sensory testing. Two of the 36 participants were not able to tolerate sensory testing during either condition, while 1 during the lidocaine condition and 2 in the sham condition were also not able to tolerate sensory testing. LAR thresholds did not differ between visits.
Sensory Perception Task
Results were analyzed using power functions, which allow for the examination of the relationship between objectively measurable stimuli and the corresponding subjective assessment of those stimuli made by the participants. To determine the effects of anesthesia on laryngopharyngeal sensory perception, all sensory perception data were log10 transformed and the coefficient of determinations (r2), constant means, and exponent means were calculated as a measure of accuracy of the relationship between the actual values (air puff pressure) and the participants’ estimation (sensory perception) of those values. No significant difference was observed for the sensory perception task r2, constant, or exponent values between the lidocaine and the sham condition (p = 0.81, 0.72, and 0.91, respectively).
More participants were unable to tolerate the laryngopharyngeal sensory perception task than the LAR threshold task. During the course of the study, the sensory perception task was discontinued 13 of 72 times. Four of the 36 participants were not able to tolerate the sensory perception task in either the lidocaine or the sham condition, 2 were unable to tolerate the task during the lidocaine condition, and 3 were unable to tolerate the task during the sham condition.
Sensory perception r2 did not differ between visits, an indication that there was no learning effect. More participants asked that the sensory perception task be stopped during visit 2 (8/36) than during visit 1 (5/36).
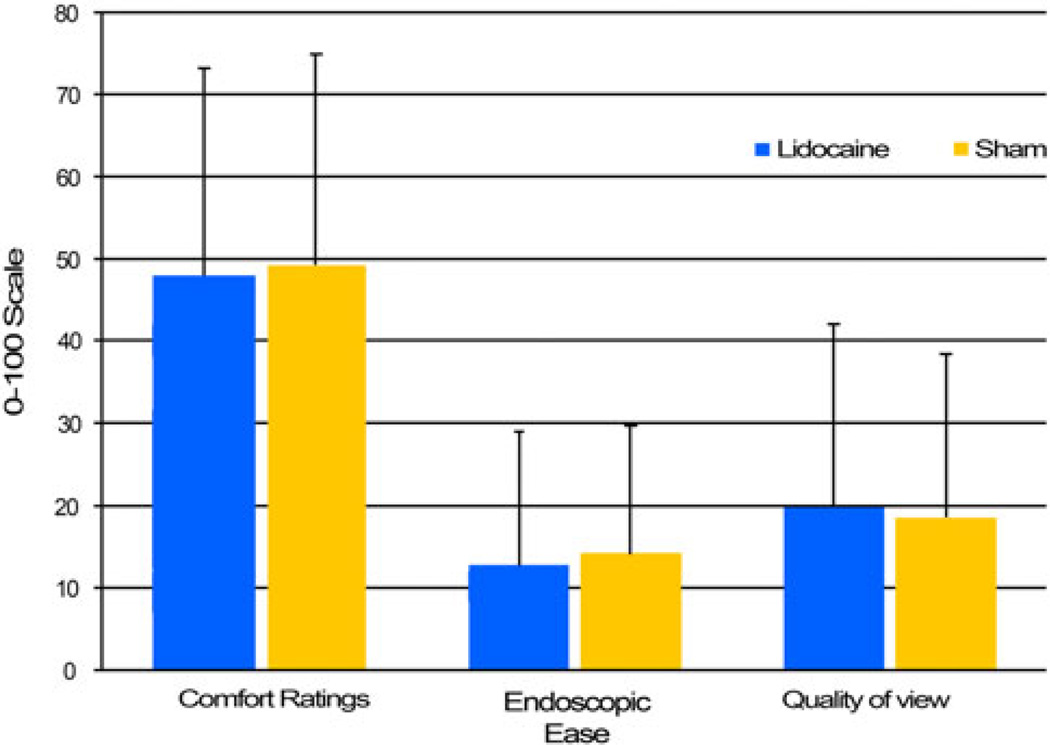
Participant Comfort Ratings
No significant difference was found for comfort scale means between the lidocaine and the sham condition (m = 48 vs. 49.47, respectively; p = 0.74) (Fig. 2). There was no correlation between comfort rating and the length of the exam for either the TNA condition (p = 0.12) or the sham condition (p = 0.08). Comfort ratings did not differ between visits (visit 1: m = 49.66; visit 2: m = 47.8; p = 0.9), indicating that prior experience with nasendoscopy did not change the participants’ subjective measure of discomfort.
Fig. 2.
Scale rating means by condition
Clinician Examination Scales
Endoscopic ease and quality of view were rated on a 0–100 general-label magnitude scale. There was no significant difference between the two conditions for endoscopic ease utilizing the Wilcoxon (p = 0.19) or the t test (p = 0.51) (Fig. 2). Endoscopic ease was significantly better during visit 2 (m = 11.75) compared to visit 1 (m = 15.36), with conditions collapsed using the Wilcoxon (p < 0.05) but not the t-test (p = 0.12).
Likewise, there was no significant difference between the two conditions for quality of view when using either the Wilcoxon (p = 0.68) or the t-test (p = 0.64). Quality of view did not differ between visits 1 and 2 (m = 20.19 vs. 18.83 respectively; p = 0.5).
Exam Length
Endoscopic examination time, which includes all endoscopic procedures, ranged from 7.37 to 19.2 min (m = 13.09 min). The higher examination times are within normal limits for a FEESST exam [46]. The mean duration of the exam for the lidocaine and sham conditions was 13.33 and 12.99 min, respectively. There was no significant difference between length of exam for the two conditions using the Wilcoxon (p = 0.53) or the t-test (p = 0.70). There was a significant difference in exam length between visit 1 and visit 2 (p < 0.01). Endoscopic exams were significantly longer for visit 1 than for visit 2 (m = 14.25 vs. 12.06 min, respectively). There was a slight correlation between quality-ofview scores and exam length for the lidocaine condition (r = 0.50), indicating that the poorer the view, the longer the exam took.
Discussion
Effects of TNA on Swallowing
Results of this study indicate that 0.4 ml of 2 % gel lidocaine applied to the nares unilaterally with a cotton-tip applicator did not affect swallowing physiology or laryngopharyngeal sensation. However, the results of this study do not support using gel TNA to improve patient comfort or ease and the speed of the examination. The amount of lidocaine used in this study is based on the Langmore protocol for FEES, suggesting two placements of the cotton-tip applicator in the nares, each for 1 min. The amount may seem small at 0.4 ml, but this is the maximum amount of gel that can be applied by the prescribed method. Another study that used 1 ml of 4 % lidocaine spray reported significant findings for both increased PAS scores and patient comfort ratings [28], and it is likely that the increased volume and concentration of the anesthesia and the method of administration are responsible for the difference in findings compared to the present study. It can be presumed that the gel anesthesia used in this study did not drip into the pharynx; or, if it did, it was too small an amount to cause alterations to the swallow physiology.
It is possible that the anesthesia could have affected more proximal structures such as the tongue base and soft palate, and as FEESST directly tests only for the laryngeal adductor reflex, these transient changes in sensation would not be quantified. It can be conjectured, however, that if the tongue base was exposed to sufficient anesthesia to alter the swallow, the functional consequence would be increased residual, particularly in the vallecula [2]. However, there was no increase in pharyngeal residue secondary to TNA observed in this study. The majority of swallows resulted in a residue score of 1 for all consistencies and volumes, denoting a trace amount of residue or a residue coating. This amount of residue is considered normal, as it requires two consecutive swallows to clear a bolus, which was also observed in this study but not quantified [18, 47]. Therefore, it can be hypothesized that either the anesthesia did not drip on the tongue base or if it did, it was not a sufficient amount to alter the motor function and bolus clearance.
It is reasonable to suggest that application of anesthesia in otolaryngology offices is done primarily by using atomizers to spray the anesthesia into the nasal cavity. When using FEES in an inpatient medical setting, however, gel TNA is likely easier since it does not require an atomizer with disposable covers. Moreover, since the introduction of FEES [3], the official protocol, still used by clinicians around the world, is to apply gel TNA using the cotton tip applicator, partly because of the likelihood of a nasal spray anesthetizing the laryngopharynx. Our findings, compared with the findings of Butler et al. [28], support this assumption.
The only significant difference observed in this study as the result of anesthesia was a faster bolus dwell time (or faster stage transition) for 10-ml liquid swallows in the lidocaine condition. It is unclear why these swallows would be initiated faster in the anesthetized condition, but this trend is not observed for any other bolus size or type. It might be reasonable to point out that while the cause is uncertain, the result is not a detrimental one. Essentially, the use of lidocaine gel did not delay the initiation of the pharyngeal swallow, and in this respect the swallows with TNA are just as safe as those without. Other significant findings unrelated to the experimental conditions were observed for 10-ml liquid swallows, including shorter pharyngeal closure duration and increased residue during the first visit compared to the second visit, independent of the experimental condition. It is possible that these three effects for 10 ml occurred as a result of the order of bolus type, as this was not randomized. Ten-milliliter liquid swallows were always the first taken during the swallowing portion of the FEESST exam. Interestingly, the shorter pharyngeal closure times and increased residue scores were not significantly correlated. Thus, shorter pharyngeal contraction times did not result in increased severity of pharyngeal residue.
Bolus dwell times differ from those previously reported in the literature. Butler et al. [44] used FEES with 10-ml boluses and reported maximum dwell times of 2.75 and 2.67 s in the vallecula and pyriforms, respectively, compared to 0.50 and 0.37 s reported in this study. Similarly, Dua et al. [48] reported bolus dwell times of 3.2 and 1.4 s, though it was unclear whether they were reporting means or maximum times. Differences in methods and the age range of participants are possible explanations for the discrepancies. Butler et al. [44] included both young and older normal participants in the data reported, and age has been found to significantly increase bolus dwell time and pharyngeal delay time [44, 49]. In Butler’s study [44], syringes were used to administer the boluses, while in Dua’s study [48] self-feeding in a natural mealtime environment was used. Other studies using VFSS have reported pharyngeal delay times of 0.12 s [50] and −0.18 s [49] in younger normal participants, although the definition of pharyngeal delay time can vary. Dua et al. [48] reported that liquid was seen in or past the vallecula before swallow initiation in 60 % of participants during a natural mealtime, which is even more than the 22 % reported in this study.
The use of TNA also did not alter pharyngeal closure durations in this study. Since pharyngeal closure duration is measured from the first frame of whiteout to the last frame of whiteout, it is essentially measuring the duration of pharyngeal contraction against the tongue base for bolus propulsion. Lack of sensory input has an effect on pharyngeal duration as demonstrated by Mansson and Sandberg [51], who reported that extensive anesthetizing of the oropharynx resulted in significantly prolonged pharyngeal contraction. This again validates that the nasal anesthesia did not anesthetize the pharynx in this experiment. The PCD means reported in this study are similar to those reported previously for healthy participants [18, 44]. Of note, PCD increased as volumes increased, with 5-ml puree having the shortest times and 20-ml liquid having the longest times, with 10-ml liquid falling in between. This volume effect has been previously reported in the literature [18].
The PAS scores reported in this study are also similar to those reported in other studies using FEES. Butler et al. [44] reported penetration and aspiration rates of 0.5 % each for healthy participant swallows, whereas Jafari et al. [25] described a 2 % incidence of penetration and 0 % aspiration. This investigation yielded penetration and aspiration rates of 5.5 and 0.5 %, respectively. Complete anesthetization of the swallowing mechanism results in a much higher incidence of unsafe swallows, with 43 % of swallows penetrated and 24 % aspirated [25].
Effects of TNA on Sensation
In addition to .TNA having no detrimental effects on swallowing physiology, there were also no effects on laryngopharyngeal sensory thresholds or sensory perception. What was interesting about the FEESST portion of the study was the number of participants who could not tolerate the sensory testing, which requires the participant to inhibit coughing or swallowing while the endoscope is advanced past the epiglottis and held approximately 2 mm from the arytenoid cartilage or aryepiglottic fold in order to deliver the calibrated burst of air. Fourteen percent of the participants were not able to tolerate the LAR threshold testing and 25 % of the participants were not able to tolerate the sensory perception testing at some point during the study. Aviv et al. [16, 52] reported that 100 % of healthy participants and 96.5 % of patients were able to tolerate FEESST, though the reason for discontinuation of the 3.5 % of patients was not given. The participants in our study for whom sensory testing was discontinued experienced increased coughing and gagging when the scope was introduced into the supraglottic region, even after strategies were introduced to minimize discomfort, such as neck extension or humming while the scope was lowered. This study’s participants were young (mean age = 23.6 years), and it is possible that they were more sensitive to supraglottic trespass than the healthy participants in the Aviv et al. study who were slightly older (mean age = 34 years) [16, 52]. It may also be considered that if a patient cannot tolerate FEESST secondary to coughing and gagging because of the supraglottic presence of the scope, their supraglottic sensation for the purposes of swallowing should be adequate.
Effects of TNA on Rating Scales
Nasal anesthesia did not improve the ease of scope advancement through the nasal passageways either. The endoscopic ease ratings were, on average, quite low on the 0–100 scale, indicating relative ease of scope passage, and they are comparable to ratings reported by Cain et al. [29] who used cophenylcaine. Notably, there was a difference in endoscopic ease ratings comparing means across visits, indicating that the examiner found endoscope insertion to be generally easier during the second visit. This is perhaps due to the familiarity with each participant’s nasal anatomy, having already navigated it once. Similarly, exam length was found to be shorter during visit 2, although exam length and endoscopic ease were not correlated, indicating that the visit 2 exams were not shorter because the endoscopic insertion was easier. It is possible that the visit 2 exam length was shorter because each participant knew what to expect and required less direction to complete the tasks.
Quality of view during the exam did not differ between the two experimental conditions. Essentially, the lidocaine did not obscure the lens any more than the lubricant; and, again, the means reported in this study are comparable to those reported by Cain [29], which are low on the 0–100 scale. Similarly, TNA did not improve participant comfort ratings, which indicates that the amount and concentration used in this study was not entirely effective in numbing the nares against the feeling of the endoscope. Pain/comfort ratings for previous investigations on nasal anesthesia range from 5 to 40 for the TNA condition and from 8 to 57 for the placebo/no treatment condition [20, 27, 29, 32, 33]. With means of 48 and 49.47, respectively, the comfort rating means reported in this study are comparable to previous comfort rating means, if not slightly on the higher end.
Summary
The most clinically significant finding from this study is that TNA, applied in gel form directly to the nares, did not delay onset of the pharyngeal swallow or otherwise increase the participants’ risk of penetration or aspiration of the test materials during the FEES exam. However, it is important to keep in mind that the number of participants included in this study was small (36) and gives this study low statistical power, thus increasing the risk of a type II error. Butler et al. [28] reported increased PAS scores after administration of spray TNA. It can be surmised that the amount and concentration of spray used in the Butler study [28] was suprathreshold to altering the sensory and/or motor aspects of the swallow resulting in a less safe swallow, but was also suprathreshold to improving patient comfort and tolerance during the procedure. Although the amount and concentration used in this study did not alter the safety of the swallows, it was also not effective in improving participant comfort, which contradicts the primary purpose of its use. Future studies should evaluate older normal participants and patients with dysphagia and should account for the various factors of TNA, including concentration, dosage amount, and method of application, to determine the optimal strategy for providing comfort while avoiding altered swallowing. Cain et al. [29] reported a correlation between pre-examination anxiety and pain scores, whereas Singh et al. [34] reported a correlation between deviated nasal septum and pain scores. Future studies may find that patients with high anxiety scores as well as those with narrow nasal passageways are the patients that benefit most from receiving TNA.
Acknowledgments
This study was supported in whole by NIH/ NINDS R21 HD055677 and in part by NICHHD HD055269, NCRR RR20146, DC011824, GM103425, and UL1TR000039.
Footnotes
Disclosures The authors have no conflicts of interest to disclose.
Contributor Information
Erin E. Kamarunas, Department of Audiology and Speech Pathology, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 826, Little Rock, AR 72205, USA ekamarunas@gmail.com; Arkansas Consortium for the Ph.D. in Communication Sciences, 4301 West Markham, Slot 826, Little Rock, AR 72205, USA.
Gary H. McCullough, Department of Speech-Language Pathology, University of Central Arkansas, 201 Donaghey Ave, UCA Box 4985, Conway, AR 72034, USA.
Tiffany J. Guidry, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 826, Little Rock, AR 72205, USA.
Mark Mennemeier, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 826, Little Rock, AR 72205, USA.
Keith Schluterman, Department of Neurology, Conway Regional Hospital, 2200 Ada Ave, St 305, Conway, AR 72034, USA.
References
- 1.Dodds WJ, Logemann JA, Stewart ET. Radiologic assessment of abnormal oral and pharyngeal phases of swallowing. AJR Am J Roentgenol. 1990;154(5):965–974. doi: 10.2214/ajr.154.5.2108570. [DOI] [PubMed] [Google Scholar]
- 2.Logemann JA. Manual for the videofluorographic study of swallowing. 2nd ed. Austin: Pro-Ed; 1993. [Google Scholar]
- 3.Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia. 1988;2(4):216–219. doi: 10.1007/BF02414429. [DOI] [PubMed] [Google Scholar]
- 4.Bastian RW. The videoendoscopic swallowing study: an alternative and partner to the videofluoroscopic swallowing study. Dysphagia. 1993;8(4):359–367. doi: 10.1007/BF01321780. [DOI] [PubMed] [Google Scholar]
- 5.Bastian RW. Videoendoscopic evaluation of patients with dysphagia: an adjunct to the modified barium swallow. Otolaryngol Head Neck Surg. 1991;104(3):339–350. doi: 10.1177/019459989110400309. [DOI] [PubMed] [Google Scholar]
- 6.Rosevear WH, Hamlet SL. Flexible fiberoptic laryngoscopy used to assess swallowing function. Ear Nose Throat J. 1991;70(8):498–500. [PubMed] [Google Scholar]
- 7.Leder SB. Serial fiberoptic endoscopic swallowing evaluations in the management of patients with dysphagia. Arch Phys Med Rehabil. 1998;79(10):1264–1269. doi: 10.1016/s0003-9993(98)90273-8. [DOI] [PubMed] [Google Scholar]
- 8.Leder SB, Novella S, Patwa H. Use of fiberoptic endoscopic evaluation of swallowing (FEES) in patients with amyotrophic lateral sclerosis. Dysphagia. 2004;19(3):177–181. doi: 10.1007/s00455-004-0009-2. [DOI] [PubMed] [Google Scholar]
- 9.Suiter DM, Moorhead MK. Effects of flexible fiberoptic endoscopy on pharyngeal swallow physiology. Otolaryngol Head Neck Surg. 2007;137(6):956–958. doi: 10.1016/j.otohns.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Colodny N. Interjudge and intrajudge reliabilities in fiberoptic endoscopic evaluation of swallowing (FEES) using the penetration-aspiration scale: a replication study. Dysphagia. 2002;17(4):308–315. doi: 10.1007/s00455-002-0073-4. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 12.Aviv JE, Martin JH, Keen MS, Debell M, Blitzer A. Air pulse quantification of supraglottic and pharyngeal sensation: a new technique. Ann Otol Rhinol Laryngol. 1993;102(10):777–780. doi: 10.1177/000348949310201007. [DOI] [PubMed] [Google Scholar]
- 13.Aviv JE, Sacco RL, Thomson J, et al. Silent laryngopharyngeal sensory deficits after stroke. Ann Otol Rhinol Laryngol. 1997;106(2):87–93. doi: 10.1177/000348949710600201. [DOI] [PubMed] [Google Scholar]
- 14.Bhabu P, Poletto C, Mann E, Bielamowicz S, Ludlow CL. Thyroarytenoid muscle responses to air pressure stimulation of the laryngeal mucosa in humans. Ann Otol Rhinol Laryngol. 2003;112(10):834–840. doi: 10.1177/000348940311201002. [DOI] [PubMed] [Google Scholar]
- 15.Murakami Y, Kirchner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59–71. doi: 10.1177/000348947208100106. [DOI] [PubMed] [Google Scholar]
- 16.Aviv JE, Kim T, Thomson JE, Sunshine S, Kaplan S, Close LG. Fiberoptic endoscopic evaluation of swallowing with sensory testing (FEESST) in healthy controls. Dysphagia. 1998;13(2):87–93. doi: 10.1007/PL00009561. [DOI] [PubMed] [Google Scholar]
- 17.ASHA Special Interest Division 13. Swallowing and swallowing disorders (dysphagia) committee on endoscopic evaluation of swallowing guidelines. [Accessed 13 Jan 2012];Role of the speech-language pathologist in the performance and interpretation of endoscopic evaluation of swallowing: guidelines. 2004 http://www.asha.org/policy/GL2004-00059.htm.
- 18.Langmore SE. Endoscopic evaluation and treatment of swallowing disorders. 1st ed. New York: Thieme Medical Publishers; 2001. [Google Scholar]
- 19.Frosh AC, Jayaraj S, Porter G, Almeyda J. Is local anaesthesia actually beneficial in flexible fibreoptic nasendoscopy? Clin Otolaryngol Allied Sci. 1998;23(3):259–262. doi: 10.1046/j.1365-2273.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 20.Leder SB, Ross DA, Briskin KB, Sasaki CT. A prospective, double-blind, randomized study on the use of a topical anesthetic, vasoconstrictor, and placebo during transnasal flexible fiberoptic endoscopy. J Speech Lang Hear Res. 1997;40(6):1352–1357. doi: 10.1044/jslhr.4006.1352. [DOI] [PubMed] [Google Scholar]
- 21.Ogura JH, Lam RL. Anatomical and physiological correlations on stimulating the human superior laryngeal nerve. Laryngoscope. 1953;63(10):947–959. doi: 10.1288/00005537-195310000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19(1):44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 23.Iscoe S, Feldman JL, Cohen MI. Properties of inspiratory termination by superior laryngeal and vagal stimulation. Respir Physiol. 1979;36(3):353–366. doi: 10.1016/0034-5687(79)90047-1. [abstract] [DOI] [PubMed] [Google Scholar]
- 24.Ertekin C, Kiylioglu N, Tarlaci S, Keskin A, Aydogdu I. Effect of mucosal anaesthesia on oropharyngeal swallowing. Neurogastroenterol Motil. 2000;12(6):567–572. doi: 10.1046/j.1365-2982.2000.00232.x. [DOI] [PubMed] [Google Scholar]
- 25.Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550(1):287–304. doi: 10.1113/jphysiol.2003.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCoy GK, Snow EK, Kester L, et al., editors. American hospital formulary service drug information 2010. Bethesda: American Society of Health-System Pharmacists; 2010. Local anesthetics; American Society of Health-System Pharmacists: Local anesthetics; pp. 3297–3305. [Google Scholar]
- 27.Bonaparte JP, Javidnia H, Kilty S. A double-blind randomised controlled trial assessing the efficacy of topical lidocaine in extended flexible endoscopic nasal examinations. Clin Otolaryngol. 2011;36(6):550–557. doi: 10.1111/j.1749-4486.2011.02403.x. [DOI] [PubMed] [Google Scholar]
- 28.Butler S, Lester S, Langmore S, Lintzenich C, Wright S. Effects of topical nasal anesthetic on flexible endoscopic evaluation of swallowing; 20th annual meeting of the dysphagia research society meeting; Toronto, ON, Canada. 2012. Mar, pp. 8–10. [Google Scholar]
- 29.Cain AJ, Murray DP, McClymont LG. The use of topical nasal anaesthesia before flexible nasendoscopy: a double-blind, randomized controlled trial comparing cophenylcaine with placebo. Clin Otolaryngol Allied Sci. 2002;27(6):485–488. doi: 10.1046/j.1365-2273.2002.00608.x. [DOI] [PubMed] [Google Scholar]
- 30.Georgalas C, Sandhu G, Frosh A, Xenellis J. Cophenylcaine spray versus placebo in flexible nasendoscopy: a prospective double-blind randomised controlled trial. Int J Clin Pract. 2005;59(2):130–133. doi: 10.1111/j.1742-1241.2005.00476.x. [DOI] [PubMed] [Google Scholar]
- 31.Johnson PE, Belafsky PC, Postma GN. Topical nasal anesthesia and laryngopharyngeal sensory testing: a prospective, doubleblind crossover study. Ann Otol Rhinol Laryngol. 2003;112(1):14–16. doi: 10.1177/000348940311200104. [DOI] [PubMed] [Google Scholar]
- 32.Johnson PE, Belafsky PC, Postma GN. Topical nasal anesthesia for transnasal fiberoptic laryngoscopy: a prospective, doubleblind, cross-over study. Otolaryngol Head Neck Surg. 2003;128(4):452–454. doi: 10.1016/S0194-59980223294-5. [DOI] [PubMed] [Google Scholar]
- 33.Sadek SA, De R, Scott A, White AP, Wilson PS, Carlin WV. The efficacy of topical anaesthesia in flexible nasendoscopy: a doubleblind randomised controlled trial. Clin Otolaryngol Allied Sci. 2001;26(1):25–28. doi: 10.1046/j.1365-2273.2001.00400.x. [DOI] [PubMed] [Google Scholar]
- 34.Singh V, Brockbank MJ, Todd GB. Flexible transnasal endoscopy: is local anaesthetic necessary? J Laryngol Otol. 1997;111(7):616–618. doi: 10.1017/s0022215100138125. [DOI] [PubMed] [Google Scholar]
- 35.Pothier DD, Awad Z, Whitehouse M, Porter GC. The use of lubrication in flexible fibreoptic nasendoscopy: a randomized controlled trial. Clin Otolaryngol. 2005;30(4):353–356. doi: 10.1111/j.1365-2273.2005.01040.x. [DOI] [PubMed] [Google Scholar]
- 36.Pothier DD, Raghava N, Monteiro P, Awad Z. A randomized controlled trial: is water better than a standard lubricant in nasendoscopy? Clin Otolaryngol. 2006;31(2):134–137. doi: 10.1111/j.1749-4486.2006.01173.x. [DOI] [PubMed] [Google Scholar]
- 37.Amin MR, Postma GN. Office evaluation of swallowing. Ear Nose Throat J. 2004;83(7 Suppl 2):13–16. [PubMed] [Google Scholar]
- 38.Aviv JE, Murry T. FEESST: flexible endoscopic evaluation of swallowing with sensory testing. San Diego: Plural Publishing, Inc; 2005. FEESST safety; pp. 87–96. [Google Scholar]
- 39.Aviv JE, Martin JH, Kim T, et al. Laryngopharyngeal sensory discrimination testing and the laryngeal adductor reflex. Ann Otol Rhinol Laryngol. 1999;108(8):725–730. doi: 10.1177/000348949910800802. [DOI] [PubMed] [Google Scholar]
- 40.Stephen SS. Psychophysics: introduction to its perceptual, neural, and social prospects. 2nd ed. Piscataway: Transaction Publishers; 1986. [Google Scholar]
- 41.Prather RW. Connecting neural coding to number cognition: a computational account. Dev Sci. 2012;15(4):589–600. doi: 10.1111/j.1467-7687.2012.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 43.Almeida RCA, Haguette RCB, Andrade ISN. Swallowing with and without verbal commands: videofluoroscopic findings. Rev Soc Bras Fonoaudiol. 2011;16(3):291–297. [Google Scholar]
- 44.Butler SG, Stuart A, Kemp S. Flexible endoscopic evaluation of swallowing in healthy young and older adults. Ann Otol Rhinol Laryngol. 2009;118(2):99–106. doi: 10.1177/000348940911800204. [DOI] [PubMed] [Google Scholar]
- 45.Bartoshuk LM, Fast K, Snyder DJ. Differences in our sensory worlds. Curr Direct Psychol Sci. 2005;14(3):122–125. [Google Scholar]
- 46.Rees CJ. Flexible endoscopic evaluation of swallowing with sensory testing. Curr Opin Otolaryngol Head Neck Surg. 2006;14(6):425–430. doi: 10.1097/MOO.0b013e328010ba88. [DOI] [PubMed] [Google Scholar]
- 47.Dziadziola J, Hamlet S, Michou G, Jones L. Multiple swallows and piecemeal deglutition: observations from normal adults and patients with head and neck cancer. Dysphagia. 1992;7(1):8–11. doi: 10.1007/BF02493415. [DOI] [PubMed] [Google Scholar]
- 48.Dua KS, Ren J, Bardan E, Xie P, Shaker R. Coordination of deglutitive glottal function and pharyngeal bolus transit during normal eating. Gastroenterology. 1997;112(1):73–83. doi: 10.1016/s0016-5085(97)70221-x. [DOI] [PubMed] [Google Scholar]
- 49.Kim Y, McCullough GH, Asp CW. Temporal measurements of pharyngeal swallowing in normal populations. Dysphagia. 2005;20(4):290–296. doi: 10.1007/s00455-005-0029-6. [DOI] [PubMed] [Google Scholar]
- 50.Tracy JF, Logemann JA, Kahrilas PJ, Jacob P, Kobara M, Krugler C. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia. 1989;4(2):90–94. doi: 10.1007/BF02407151. [DOI] [PubMed] [Google Scholar]
- 51.Mansson I, Sandberg N. Effects of surface anesthesia on deglutition in man. Laryngoscope. 1974;84(3):427–437. doi: 10.1288/00005537-197403000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Aviv JE, Murry T, Zschommler A, Cohen M, Gartner C. Flexible endoscopic evaluation of swallowing with sensory testing: patient characteristics and analysis of safety in 1,340 consecutive examinations. Ann Otol Rhinol Laryngol. 2005;114(3):173–176. doi: 10.1177/000348940511400301. [DOI] [PubMed] [Google Scholar]