Abstract
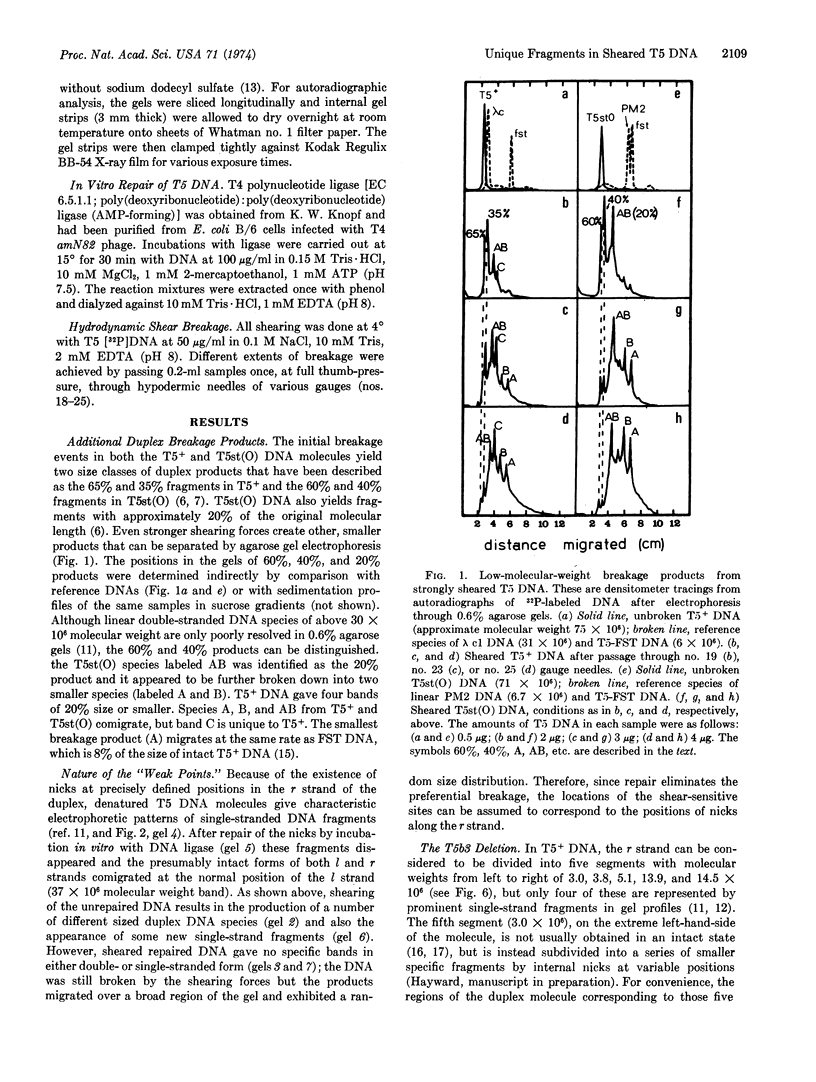
Nicks within one strand of the bacteriophage T5 DNA molecule act as “ weak points” for a novel kind mechanical breakage that can be utilized for “dissecting” the genome. The products from sheared T5+ DNA include five unique double-stranded segments of the molecule and various combinations of adjacent segments. These specific fragments are not obtained after repair of the nicks with DNA ligase (EC 6.5.1.1). The duplex fragments and most of their single-stranded components have been separated, identified, and mapped by means of agarose gel electrophoresis. Even the complementary strands of the unique fragments separate in agarose gels; hence, there are now three useful classes of DNA fragments available from T5: the natural r-strand fragments, their complements from the normally intact l strand, and the corresponding duplex segments. By summing the apparent molecular weights of their single-stranded components, the unique duplex fragments from T5+ DNA can be assigned molecular weights of approximately 6 × 106 (A), 8 × 106 (B), 11 × 106 (C), 27 × 106 (D), and 30 × 106 (E) from left to right along the genome. The most abundant overlapping fragments are segments AB (14 × 106) and ABC (26 × 106). Differences in the number and relative positions of nicks within two distinct groups of heatstable deletion mutants [represented by T5st(O) and T5b3] account for the large differences observed in their patterns of breakage products.
Keywords: agarose gel electrophoresis, DNA mapping, deletion mutants, DNA ligase
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Jeppesen P. G., Katagiri K. J., Delius H. Mapping the DNA fragments produced by cleavage by lambda DNA with endonuclease RI. Nature. 1973 Jan 12;241(5385):120–123. doi: 10.1038/241120a0. [DOI] [PubMed] [Google Scholar]
- Bowman R. D., Davidson N. Hydrodynamic shear breakage of DNA. Biopolymers. 1972;11(12):2601–2624. doi: 10.1002/bip.1972.360111217. [DOI] [PubMed] [Google Scholar]
- Bujard H., Hendrickson H. E. Structure and function of the genome of coliphage T5. 1. The physical structure of the chromosome of T5 + . Eur J Biochem. 1973 Mar 15;33(3):517–528. doi: 10.1111/j.1432-1033.1973.tb02711.x. [DOI] [PubMed] [Google Scholar]
- Bujard H. Location of single-strand interruptions in the DNA of bacteriophage T5. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1167–1174. doi: 10.1073/pnas.62.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgi E., Hershey A. D., Ingraham L. Preferred breakage points in T5 DNA molecules subjected to shear. Virology. 1966 Jan;28(1):11–14. doi: 10.1016/0042-6822(66)90301-1. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Danna K., Nathans D. Specific cleavage of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2913–2917. doi: 10.1073/pnas.68.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D., GOLDBERG E., BURGI E., INGRAHAM L. Local denaturation of DNA by shearing forces and by heat. J Mol Biol. 1963 Mar;6:230–243. doi: 10.1016/s0022-2836(63)80072-8. [DOI] [PubMed] [Google Scholar]
- HERTEL R., MARCHI L., MULLER K. Density mutants of phage T5. Virology. 1962 Dec;18:576–581. doi: 10.1016/0042-6822(62)90060-0. [DOI] [PubMed] [Google Scholar]
- Hayward G. S. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virology. 1972 Jul;49(1):342–344. doi: 10.1016/s0042-6822(72)80042-4. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J Mol Biol. 1972 Feb 14;63(3):383–395. doi: 10.1016/0022-2836(72)90435-4. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. II. Arrangement of the single-stranded DNA fragments in the T5 + and T5st(O) chromosomes. J Mol Biol. 1972 Feb 14;63(3):397–407. doi: 10.1016/0022-2836(72)90436-6. [DOI] [PubMed] [Google Scholar]
- Hayward S. D., Smith M. G. The chromosome of bacteriophage T5. 3. Patterns of transcription from the single-stranded DNA fragments. J Mol Biol. 1973 Oct 25;80(2):345–359. doi: 10.1016/0022-2836(73)90177-0. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. E., Bujard H. Structure and function of the genome of coliphage T5. 2. Regions of transcription of the chromosome. Eur J Biochem. 1973 Mar 15;33(3):529–534. doi: 10.1111/j.1432-1033.1973.tb02712.x. [DOI] [PubMed] [Google Scholar]
- Jacquemin-Sablon A., Richardson C. C. Analysis of the interruptions in bacteriophage T5 DNA. J Mol Biol. 1970 Feb 14;47(3):477–493. doi: 10.1016/0022-2836(70)90316-5. [DOI] [PubMed] [Google Scholar]
- Labedan B., Crochet M., Legault-Demare J., Stevens B. J. Location of the first step transfer fragment and single-strand interruptions in T5stO bacteriophage DNA. J Mol Biol. 1973 Apr 5;75(2):213–234. doi: 10.1016/0022-2836(73)90017-x. [DOI] [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyeritz R. E., Schlegel R. A., Thomas C. A., Jr Hydrodynamic shear breakage of DNA may produce single-chained terminals. Biochim Biophys Acta. 1972 Jul 31;272(4):504–509. doi: 10.1016/0005-2787(72)90505-9. [DOI] [PubMed] [Google Scholar]
- Rhoades M., Rhoades E. A. Terminal repetition in the DNA of bacteriophage T5. J Mol Biol. 1972 Aug 21;69(2):187–200. doi: 10.1016/0022-2836(72)90224-0. [DOI] [PubMed] [Google Scholar]
- Rubenstein I. Heat-stable mutants of T5 phage. I. The physical properties of the phage and their DNA molecules. Virology. 1968 Nov;36(3):356–376. doi: 10.1016/0042-6822(68)90161-x. [DOI] [PubMed] [Google Scholar]
- Skalka A., Burgi E., Hershey A. D. Segmental distribution of nucleotides in the DNA of bacteriophage lambda. J Mol Biol. 1968 May 28;34(1):1–16. doi: 10.1016/0022-2836(68)90230-1. [DOI] [PubMed] [Google Scholar]





