Abstract
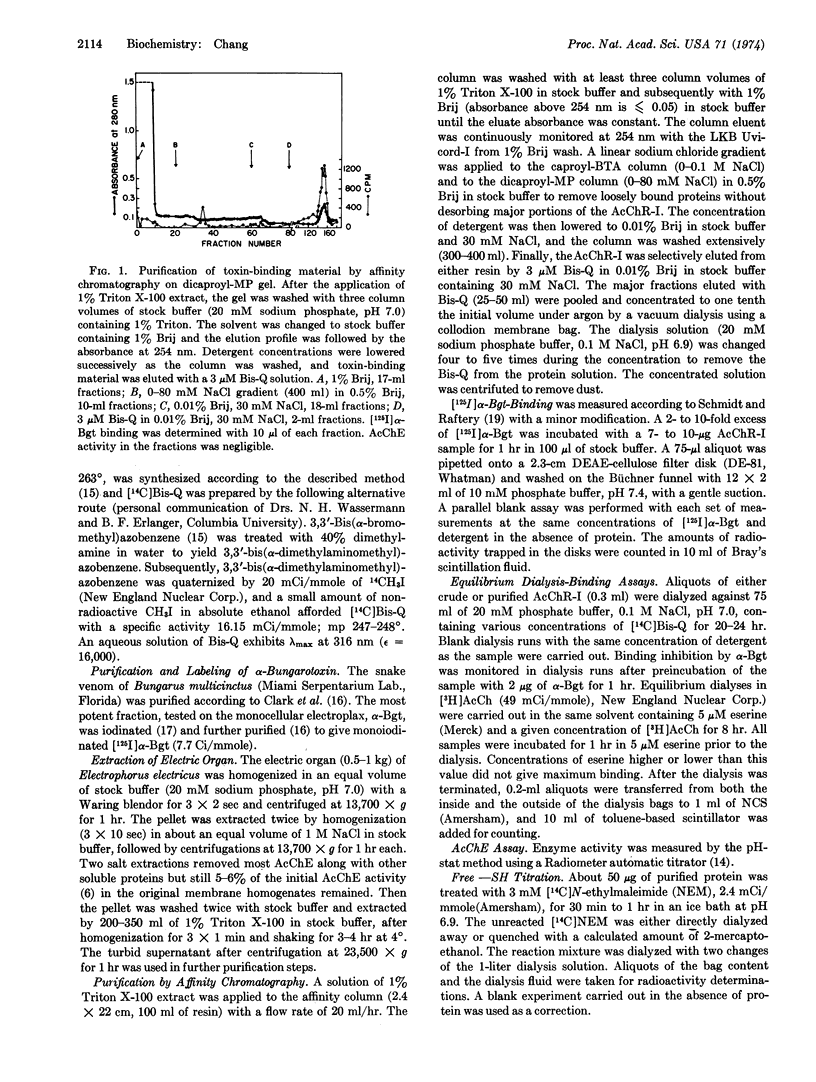
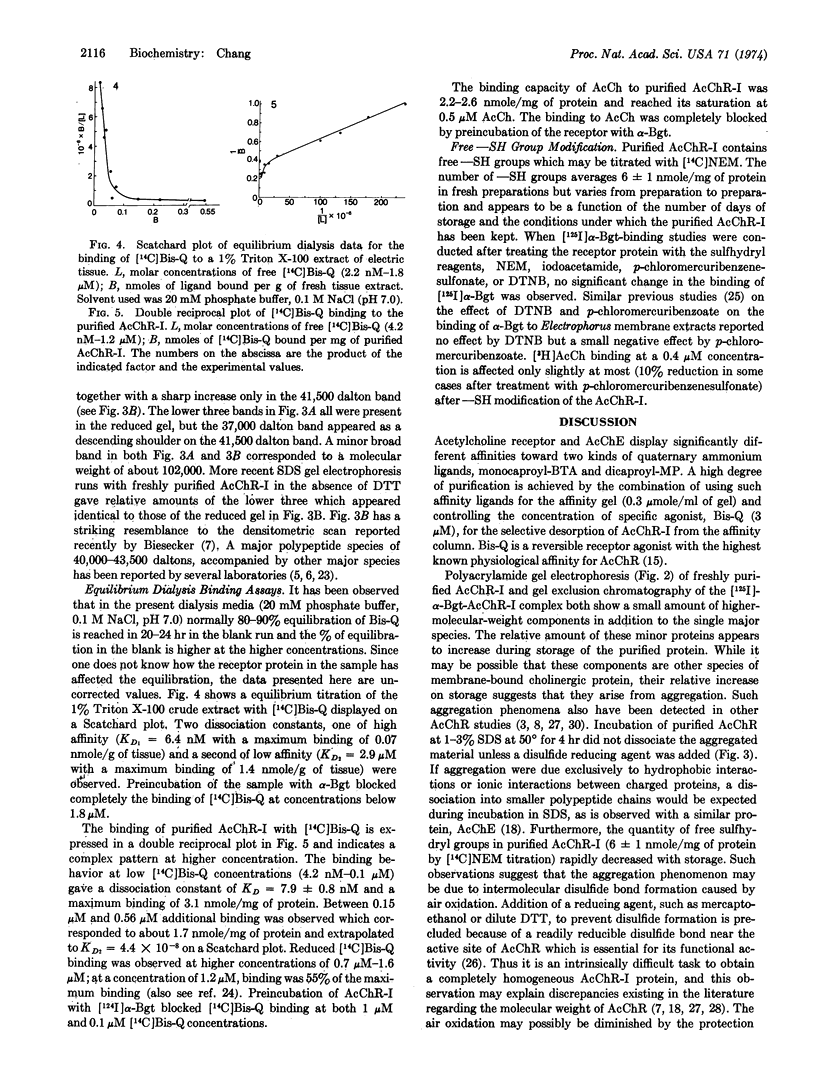
A Triton X-100 extract of electric tissue was subjected to a single step affinity chromatography using either of two affinity gels: [N-(6-aminocaproyl)-p-aminobenzyl]trimethylammonium bromide or methyl[(6-aminocaproyl-6′-aminocaproyl)-3-amino]pyridinium bromide attached to Sepharose 4B. Specific elution of the acetylcholine receptor-I (AcChR-I) with low concentration of a bis-quaternary agonist, 3,3′-bis[α-(trimethylammonium)methyl]-azobenzene bromide (Bis-Q), gave a 35% yield of toxin-binding components in the crude extract. The purified AcChR-I readily underwent aggregation, which appeared to arise from the oxidation of titratable free sulfhydryl on the protein. The protein was characterized by the binding capacities for [125I]α-bungarotoxin (α-Bgt), [3H]acetylcholine, and [14C]Bis-Q; the ratio of these capacities were approximately 2:1:2, respectively, with 5-6:5 nmole of α-Bgt sites per mg of protein. Analysis by sodium dodecyl sulfate gel electrophoresis of the disulfide-reduced and nonreduced polypeptide components indicated that a 41,500 dalton species was the major subunit component of AcChR-I. The binding of [14C]Bis-Q with a Triton X-100 crude extract showed sites with both high and low dissociation constants, whereas purified AcChR-I contained only high-affinity sites. A biphasic double-reciprocal plot and a Hill coefficient of 0.7 suggested negative cooperativity in the binding of Bis-Q with the purified AcChR-I.
Keywords: affinity chromatography, membrane-bound protein, subunit structure, sulfhydryl titration, equilibrium dialysis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels E., Wassermann N. H., Erlanger B. F. Photochromic activators of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1820–1823. doi: 10.1073/pnas.68.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G. Molecular properties of the cholinergic receptor purified from Electrophorus electricus. Biochemistry. 1973 Oct 23;12(22):4403–4409. doi: 10.1021/bi00746a017. [DOI] [PubMed] [Google Scholar]
- Carroll R. C., Eldefrawi M. E., Edelstein S. J. Studies on the structure of the acetylcholine receptor from Torpedo marmorata. Biochem Biophys Res Commun. 1973 Dec 10;55(3):864–872. doi: 10.1016/0006-291x(73)91224-2. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Kasai M., Lee C. Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1241–1247. doi: 10.1073/pnas.67.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. G., Macmurchie D. D., Elliott E., Wolcott R. G., Landel A. M., Raftery M. A. Elapid neurotoxins. Purification, characterization, and immunochemical studies of -bungarotoxin. Biochemistry. 1972 Apr 25;11(9):1663–1668. doi: 10.1021/bi00759a020. [DOI] [PubMed] [Google Scholar]
- Clark D. G., Wolcott R. G., Raftery M. A. Partial characterization of an -bungarotoxin-binding component of electroplax membranes. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1061–1067. doi: 10.1016/0006-291x(72)90816-9. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Robertis E., Lunt G. S., La Torre J. L. Multiple binding sites for acetylcholine in a proteolipid from electric tissue. Mol Pharmacol. 1971 Jan;7(1):97–103. [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T. Purification and molecular properties of the acetylcholine receptor from Torpedo electroplax. Arch Biochem Biophys. 1973 Nov;159(1):362–373. doi: 10.1016/0003-9861(73)90462-1. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., O'Brien R. D. Autoinhibition of acetylcholine binding to Torpedo electroplax; a possible molecular mechanism for desensitization. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2006–2007. doi: 10.1073/pnas.68.9.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Franklin G. I., Potter L. T. Studies of the binding of -bungarotoxin to membrane-bound and detergent-dispersed acetylcholine receptors from Torpedo electric tissue. FEBS Lett. 1972 Nov 15;28(1):101–106. doi: 10.1016/0014-5793(72)80687-2. [DOI] [PubMed] [Google Scholar]
- Fulpius B., Cha S., Klett R., Reich E. Properties of the nicotinic acetylcholine receptor macromolecule of Electrophorus electricus. FEBS Lett. 1972 Aug 15;24(3):323–326. doi: 10.1016/0014-5793(72)80382-x. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho F., Changeux J. P. Molecular weight and quaternary structure of the cholinergic receptor protein extracted by detergents from Electrophorus electricus electric tissue. FEBS Lett. 1973 Dec 15;38(1):11–15. doi: 10.1016/0014-5793(73)80500-9. [DOI] [PubMed] [Google Scholar]
- Karlin A., Cowburn D. The affinity-labeling of partially purified acetylcholine receptor from electric tissue of Electrophorus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3636–3640. doi: 10.1073/pnas.70.12.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Prives J., Deal W., Winnik M. Affinity labeling of the acetylcholine receptor in the electroplax. J Mol Biol. 1971 Oct 14;61(1):175–188. doi: 10.1016/0022-2836(71)90214-2. [DOI] [PubMed] [Google Scholar]
- Karlsson E., Heilbronn E., Widlund L. Isolation of the nicotinic acetylcholine receptor by biospecific chromatography on insolubilized Naja naja neurotoxin. FEBS Lett. 1972 Nov 15;28(1):107–111. doi: 10.1016/0014-5793(72)80688-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. Y. Elapid neurotoxins and their mode of action. Clin Toxicol. 1970 Sep;3(3):457–472. doi: 10.3109/15563657008990119. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J. C., Changeux J. P. Comparison between the affinities for reversible cholinergic ligands of a purified and membrane bound state of the acetylcholine-receptor protein from Electrophorus electricus. FEBS Lett. 1973 May 15;32(1):143–148. doi: 10.1016/0014-5793(73)80758-6. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Meunier J. C., Changeux J. P. Progress in the purification of the cholinergic receptor protein from Electrophorus electricus by affinity chromatography. FEBS Lett. 1972 Nov 15;28(1):96–100. doi: 10.1016/0014-5793(72)80686-0. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Schmidt J., Clark D. G. Specificity of -bungarotoxin binding to Torpedo californica electroplax. Arch Biochem Biophys. 1972 Oct;152(2):882–886. doi: 10.1016/0003-9861(72)90285-8. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Schmidt J., Clark D. G., Wolcott R. G. Demonstration of a specific -bungarotoxin binding component in electrophorus electricus electroplax membranes. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1622–1629. doi: 10.1016/0006-291x(71)90207-5. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Chang H. W., Chen Y. T. Purification of acetylcholinesterase by affinity chromatography and determination of active site stoichiometry. J Biol Chem. 1972 Mar 10;247(5):1555–1565. [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. A simple assay for the study of solubilized acetylcholine receptors. Anal Biochem. 1973 Apr;52(2):349–354. doi: 10.1016/0003-2697(73)90036-5. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. Purification of acetylcholine receptors from Torpedo californica electroplax by affinity chromatography. Biochemistry. 1973 Feb 27;12(5):852–856. doi: 10.1021/bi00729a011. [DOI] [PubMed] [Google Scholar]