Abstract
The non-classical Human leukocyte antigen G (HLA-G) differs from classical HLA class I molecules by its low genetic diversity, a tissue-restricted expression, the existence of seven isoforms, and immuno-inhibitory functions. Most of the known functions of HLA-G concern the membrane-bound HLA-G1 and soluble HLA-G5 isoforms, which present the typical structure of classical HLA class I molecule: a heavy chain of three globular domains α1–α2–α3 non-covalently bound to β-2-microglobulin (B2M) and a peptide. Very little is known of the structural features and functions of other HLA-G isoforms or structural conformations other than B2M-associated HLA-G1 and HLA-G5. In the present work, we studied the capability of all isoforms to form homomultimers, and investigated whether they could bind to, and function through, the known HLA-G receptors LILRB1 and LILRB2. We report that all HLA-G isoforms may form homodimers, demonstrating for the first time the existence of HLA-G4 dimers. We also report that the HLA-G α1–α3 structure, which constitutes the extracellular part of HLA-G2 and HLA-G6, binds the LILRB2 receptor but not LILRB1. This is the first report of a receptor for a truncated HLA-G isoform. Following up on this finding, we show that the α1–α3-Fc structure coated on agarose beads is tolerogenic and capable of prolonging the survival of skin allografts in B6-mice and in a LILRB2-transgenic mouse model. This study is the first proof of concept that truncated HLA-G isoforms could be used as therapeutic agents.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-012-1069-3) contains supplementary material, which is available to authorized users.
Keywords: HLA-G, Immune regulation, Inhibitory receptors, Transplantation
Introduction
Human leukocyte antigen G (HLA-G) [1, 2] is a non-classical HLA class I molecule which transcript was first identified in choriocarcinoma cells [3]. Since then, HLA-G has been termed “non-classical” because it differs from classical HLA class I molecules by its genetic diversity, expression, structure, and function. HLA-G is characterized by a low polymorphism [4, 5], and its expression is mainly restricted to trophoblast cells [6], adult thymic medulla [7], and stem cells [8–10]. However, HLA-G neo-expression may be induced in pathological conditions such as cancers [11], transplantation [12], multiple sclerosis [13], inflammatory diseases [14], and viral infections [15, 16].
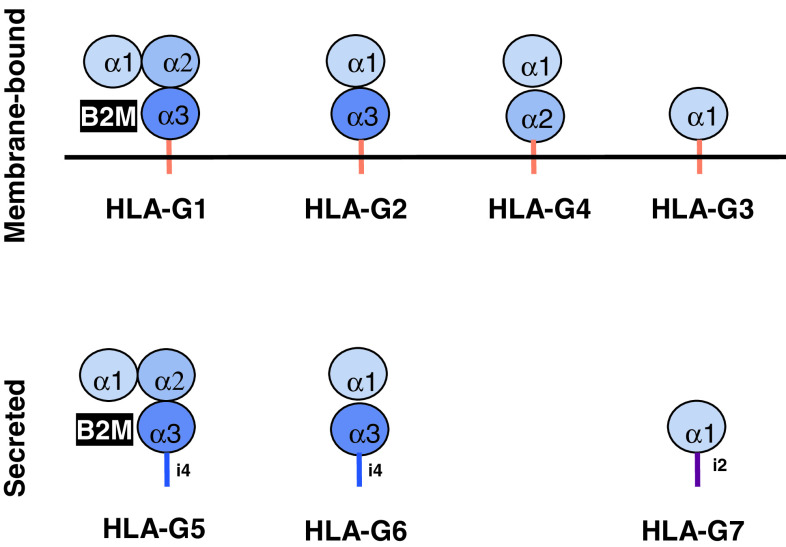
The alternative splicing of the HLA-G primary transcript can generate seven different isoforms, four being membrane-bound (HLA-G1, G2, G3, G4) and three being soluble (HLA-G5, G6, G7). As shown in Fig. 1, HLA-G1 and HLA-G5 isoforms present the typical structure of a classical HLA class I molecule, i.e., a heavy chain of three globular domains non-covalently bound to β-2-microglobulin (B2M) and a peptide, whereas the other isoforms are shorter, lacking one or two domains of the heavy chain, and should not bind B2M. It has to be noted that the α1 domain is present in all HLA-G isoforms.
Fig. 1.
HLA-G isoforms. Alternative splicing of HLA-G primary transcript yields seven isoforms. Excision of one or two exons encoding globular domain generates truncated isoforms, and translation of intron four or intron two yield secreted isoforms that lack the transmembrane domain
No stimulatory function has been reported to date for HLA-G, even responses to allogeneic HLA-G. However, this molecule exerts an immuno-inhibitory function through direct binding to three inhibitory receptors: ILT2/CD85j/LILRB1, ILT4/CD85d/LILRB2, and KIR2DL4/CD158d. LILRB1 is expressed by B cells, some T cells, some NK cells, and all monocytes/dendritic cells [17], whereas LILRB2 is myeloid-specific and its expression is restricted to monocytes/dendritic cells [18]. KIR2DL4 is a specific receptor for HLA-G and only expressed by the CD56bright subset of NK cells [19]. Through interactions with these receptors, HLA-G1 inhibits the cytolytic function of uterine and peripheral blood NK cells [20], the antigen-specific cytolytic function of cytotoxic T lymphocytes [21], the alloproliferative response of CD4+ T cells [22, 23], the proliferation of T cells and peripheral blood NK cells [24–26], and the maturation and function of dendritic cells [27–29]. Furthermore, HLA-G is capable of inducing the generation of suppressive cells [27, 30–32].
HLA-G1 and HLA-G5 are the isoforms that were studied the most extensively, particularly with respect to their structures and their interactions with the inhibitory receptors LILRB1 and LILRB2. HLA-G1 and HLA-G5 (HLA-G1/5) can form dimers [33, 34] through disulphide bonds between unique cysteine residues at positions 42 (Cys42–Cys42), within the α1 domain. It was shown that dimers of B2M-associated HLA-G1 bind LILRB1 and LILRB2 with higher affinity than monomers (Kd of monomers vs. dimers were calculated at 3.5 vs. 0.0067 μM for LILRB1, and 15 vs. 0.75 μM for LILRB2) [35]. This increased affinity of dimers is due to an oblique orientation that exposes the LILRB1- and LILRB2-binding sites of the α3 domain, making it more accessible to the receptors. Both LILRB1 and LILRB2 bind the HLA-G α3 domain at the level of F195 and Y197 residues. However, LILRB1 interacts preferentially with B2M-associated HLA-G, whereas LILRB2 predominantly interacts with B2M-free HLA-G [35]. This difference is very relevant because HLA-G1 and HLA-G5 have been detected as B2M-free heavy chains at the cell surface and in culture supernatants of HLA-G-expressing cells [36]. Furthermore, B2M-free HLA-G heavy chains may be the main structure produced by human villous trophoblast cells [37].
Thus, it seems obvious that HLA-G structure variation plays a role in the biological function of HLA-G and its regulation. Yet the full extent of HLA-G structural diversity remains unknown [2]. In fact, since all HLA-G isoforms have the Cys42 residue, they all should be able to dimerize, as it was shown for HLA-G1 [33] and HLA-G2 and -G6 [38].
Furthermore, even though the structure of B2M-associated HLA-G1 is known [39] and the structural parameters of its association with LILRB1 and LILRB2 have been described [35, 40], nothing is certain concerning the structure of truncated isoforms or the receptors they bind to.
Thus, we investigated the capability of all HLA-G isoforms to form dimers, and the mechanism of action of the HLA-G2 and HLA-G6 isoforms (HLA-G2/6) (α1–α3 domains). We were particularly interested in the α1–α3 structure because its expression has been shown after heart transplantation and is indicative of graft better acceptance [12]. We also investigated whether this isoform was actually capable of promoting allograft tolerance by itself in vivo in an animal model.
Our results show that all isoforms, except for HLA-G3, can dimerize, and we report the existence of the HLA-G4 dimers for the first time. Focusing on the α1–α3 structure (HLA-G2 and -G6 isoforms), we demonstrate its binding to LILRB2 but not LILRB1. Finally, we demonstrate that the α1–α3:LILRB2 interaction is tolerogenic in vivo in an allogeneic skin transplantation murine model.
Materials and methods
Cells lines and transfectants
M8 is an HLA-G-negative melanoma cell line. Transfectants were obtained by electroporation as previously described in [41]. Transfectants used were named M8-HLA-G1, M8-HLA-G2, M8-HLA-G3, M8-HLA-G4, M8-HLAG5, and M8-HLA-G6 according to the HLA-G isoform cDNA transfected [42]. M8 cells transfected with the pcDNA3.1 vector alone were used as a negative control cell line (M8-pcDNA).
The NKL line [43] is an NK cell line that endogenously expresses both KIR2DL4 and LILRB1. This line will thereafter be referred to as NKL-LILRB1+. NKL-LILRB1+LILRB2+ are NKL-LILRB1+ cells transduced with a lentivirus containing the LILRB2 cDNA. The phenotype of NKL-LILRB1+ and NKL-LILRB1+LILRB2+ cells with respect to LILRB1 and LILRB2 expression is shown in Supplemental Fig. 1. As can be seen, both cell lines express the LILRB1 receptor to the same extent, and LILRB2 expression is very high in NKL-LILRB1+LILRB2+ cells whereas NKL-LILRB1+ cells do not express it.
Cells were maintained in RPMI 1640 medium supplemented with 10 % inactivated FCS, 2 mM l-glutamine, 1 mg/ml gentamicin, and fungizone. HLA-G M8 transfectants were selected in medium containing 100 μg/ml hygromycin B (Sigma, St. Louis, MO, USA).
Recombinant protein constructions and coated microspheres
Lentiviruses
HLA-G1 cDNA, HLA-G2 cDNA, and LILRB2 cDNA were amplified by PCR and cloned into the pWPXL lentiviral vector (Addgene) from which GFP had been removed by enzymatic restriction, generating the pWPXL-HLA-G1, pWPXL-HLA-G2, and pWPXL-LILRB2 vectors.
Lentiviral particles were produced by triple-transfecting HEK293T cells by the calcium phosphate method with pWPXL-HLA-G1, pWPXL-HLA-G2, or pWPXL-LILRB2 vectors, the packaging plasmid psPAX2, and the envelope plasmid pMD2.G (Addgene). The supernatants containing lentiviral particles were harvested 48 h after transfection. K562 cells were transduced with HLA-G1 or HLA-G2 lentiviral particles, and NKL-LILRB1+cells were transduced with LILRB2 lentiviral particles. Cell surface expression of the transduced molecules was verified by flow cytometry analysis using anti-HLA-G1 MEM/G-09-PE (Exbio), anti-HLA-G alpha1 domain 4H84 (Exbio), and anti-LILRB2-PE (Beckman Coulter) mAbs, respectively.
HLA-G5-GST and HLA-G6-GST proteins
BL21-Gold Competent bacteria were transformed with pGEX-6P-2-HLA-G5 or pGEX-6P-2-HLA-G6 following the manufacturer’s recommendations (GE Healthcare). Bacteria were selected in 200 μg of ampicillin-LB medium, and cultured in antibiotic free-LB medium at 37 °C at 200 rpm overnight (ON), until a OD = 0.6 was reached. They were then induced with 0.1 mM IPTG and cultured in the same conditions for 3 h. Culture was harvested and centrifuged at 3,000 × g for 40 min. Pellets were collected, and bacteria were lysed with 20 mM Tris–HCl pH = 7.4, 1 mM PMSF buffer, and centrifuged at 15,000 × g for 30 min at 4 °C. Pellets were treated with Urea 8 M in order to dissolve inclusion bodies, and dialyzed against 50 mM Tris–HCl, pH = 7.5, 0.5 M NaCl, 2 mM MgCl2 buffer at 4 °C overnight. Samples were purified by GSTrap columns following the manufacturer’s recommendations (GE Healthcare).
α1–α3-Fc fusion protein
α1–α3 cDNA was cloned into the pFUSE-hFc1 vector (Invitrogen). This pα1–α3-hFc construction was then transfected into HeLa cells (ATCC). The α1–α3-Fc proteins that were used in this report were culture supernatants of α1–α3-Fc-transfected HeLa cells produced in DMEM, 10 % FCS, 2 mM l-glutamine.
For α1–α3-Fc-coated microsphere generation, 1 × 108 sulfate latex beads 4 % w/v 5 μm (Invitrogen) were coated with 20 μg/ml AffiniPure anti-human IgG Fc fragment (Jackson ImmunoResearch) for 2 h at 37 °C followed by a 2-h incubation with BSA (2 mg/ml). After washing, the beads were incubated in culture supernatant containing 0.5 μg/ml of α1–α3-Fc fusion protein at 4 °C for 16 h. Subsequently, the beads were washed twice with PBS. Then, 5 ml of α1–α3-Fc fusion proteins (1 μg/ml) were used for 5 × 106 sulfate latex beads. As a negative control, sulfate latex beads were prepared in an identical manner except that PBS or HeLa mock supernatant was used rather than α1–α3-Fc fusion protein-containing supernatants.
Monoclonal Abs and flow cytometry analysis
The following mAbs were used: from R & D Systems: LILRB1-hFc and LILRB2-hFc; from Exbio (Prague): PE-conjugated anti-HLA-G MEM-G/09 and anti-HLA-G 4H84; from Sigma: goat-anti-mouse HRP and PE-conjugated goat-anti-hFc; from Novus Biologicals: FITC-conjugated anti-GST antibody; and from Beckman Coulter: PE-conjugated anti-LILRB1 and PE-conjugated anti-LILRB2.
Lysis, immunoprecipitation, and Western-blot analysis
Cell lysis
Cells were washed with PBS and then lysed in lysis buffer (50 mM Tris pH = 7.5, 50 mM iodoacetamide, 0.5 % Chaps) at a concentration of 107 cells/ml. Iodoacetamide at this concentration prevents post-lysis multimerization of HLA class I molecules [44].
Lysates were then directly loaded into the gels or subjected to immunoprecipitation.
Immunoprecipitation
Thawed lysates were incubated for 90 min at 4 °C with protein G-sepharose beads coated with isotype control mAb. Precleared lysates were then immunoprecipitated for 90 min at 4 °C with protein G-sepharose beads precoated with LILRB2-Fc. Immunoprecipitates were washed three times with PBS. Proteins bound on beads were divided into two aliquots; one was reduced (reducing condition) by β-mercaptoethanol-containing Laemmli buffer (0.4 % sodium dodecyl sulfate, 5 % glycerol, 1.67 % β-mercaptoethanol, 0.0067 % Bromophenol Blue, 0.4 % DTT, 20.8 mM Tris-HCl, pH = 6.8) while the other was not reduced (Laemmli buffer without β-mercaptoethanol and DTT).
Western blotting
Aliquots of total proteins from M8 transfectants (lysates) or proteins immunoprecipitated from M8 transfectants were separated in 12 % SDS-PAGE. The amounts of lysates loaded were adjusted for each cell line to minimize non-specific background. The gels were blotted onto nitrocellulose membranes (Hybond; Amersham, Buckinghamshire, UK), and the membranes were blocked by a 2-h incubation with PBS containing 0.2 % Tween 20 and 5 % non-fat dry milk. The membranes were then probed overnight at 4 °C with the corresponding antibodies and washed in PBS containing 0.2 % Tween 20. The membranes were subsequently incubated for 1 h at room temperature with peroxidase-conjugated goat-anti-mouse IgG Ab (Sigma), washed thoroughly, stained with enhanced chemiluminescence reagent (Amersham), and exposed to X-ray film.
Cytometry-based HLA-G:LILRB interaction analysis
Luminex bead-based assay
In order to evidence HLA-G:LILRB interactions, recombinant, HLA-G6-GST and control GST proteins were coated on Bio-Plex COOH beads (Bio-Rad) using the Bio-Plex Amine Coupling kit (Bio-Rad) following the provider’s recommendations. Two thousand of these coupled beads were then incubated with sample for 90 min at room temperature, and then again 60 min at room temperature in the presence of 20 μg/ml of human IgG, LILRB1-Fc, or LILRB2-Fc. Beads were then extensively washed, and binding of control IgG, LILRB1-Fc, and LILRB2-Fc to GST-beads or HLA-G6-GST-beads was detected using a PE-conjugated Goat anti-human Fc antibody (Sigma) and flow cytometric analysis of the resulting beads.
Interaction of HLA-G with membrane-bound LILRB receptors
To investigate the binding of B2M-free HLA-G5 and the HLA-G2/G6 isoforms to LILRB molecules, NKL-LILRB1+ and NKL-LILRB1+LILRB2+ cells were incubated in the presence of 1 μg/ml of GST, or B2M-free HLA-G5-GST, or HLA-G6-GST proteins for 30 min on ice. Cells were then washed and the binding of the recombinant proteins to the cells was evaluated using FITC-conjugated anti-GST antibody (Novus Biologicals) and flow cytometry analysis.
In vivo analysis
Specific pathogen-free C57BL/6 (H-2b) mice and LILRB2-transgenic mice (H-2b) [27] were used as skin graft recipients throughout the study. Recipient mice received α1–α3-Fc-coupled microspheres or control microspheres. Donor skin was from MHC class II-disparate B6.CH-2bm12 (bm12, H-2b) mice. Allogeneic skin grafts were performed by standard method as described previously [27]. All skin grafting survival data were analyzed using Kaplan–Meier survival analysis.
Results
Homodimerization of HLA-G isoforms
In order to demonstrate the dimerization of HLA-G isoforms, we used M8 cells transfected with HLA-G1 through HLA-G6 isoforms, and their control: M8-pcDNA cells.
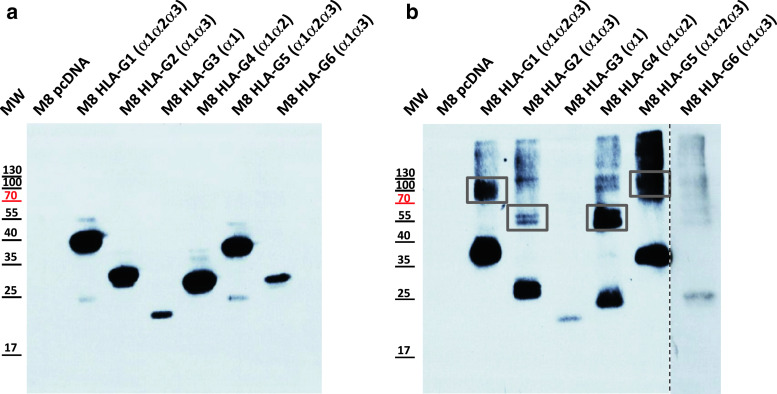
Western-blot analysis of M8 cell lysates under reducing conditions demonstrated that all the isoforms were produced, and also established the size of HLA-G isoform monomers (Fig. 2a). The presence of dimers of HLA-G isoforms was investigated by Western-blot analysis of the same cell lysates after electrophoresis under non-reducing conditions (Fig. 2b). Dimers of HLA-G isoforms were easily observed for HLA-G1 and HLA-G5 (α1–α2–α3 domains, lanes 2 and 6), and HLA-G4 (α1–α2 domains, lane 5). This is the first description of HLA-G4 homodimers. HLA-G2 and HLA-G6 (α1–α3 domains) were more difficult to evidence. In the particular experiment shown in Fig. 2b, HLA-G2 but not HLA-G6 homodimers were observed (lanes 3 and 7, respectively), although their structure is expected to be identical. In other experiments, HLA-G6 dimers were evidenced (not shown). Unexpectedly, homodimers of HLA-G3 (α1 domain, lane 4) were never observed. These results indicate that the dimerization of HLA-G isoforms is uneven, even though they all possess the cysteine in position 42, responsible for HLA-G1 and HLA-G5 dimerization [33].
Fig. 2.
HLA-G isoforms expression and dimerization. a Monomers of HLA-G isoforms were identified from transfected cell lysates by Western blotting in a reducing SDS-PAGE. b Homodimers of HLA-G isoforms were identified from the same lysates by Western blotting in a non-reducing SDS-PAGE. In both cases, 4H84, directed toward the HLA-G α1 domain, was used as a blotting antibody
Binding of HLA-G isoforms to LILRB2
Binding to LILRB1 is dependent on B2M association, and thus concerns only B2M-associated HLA-G1 and HLA-G5 isoforms. However, binding to LILRB2 is not and may concern truncated HLA-G isoforms. Thus, we investigated the HLA-G isoforms capable of binding LILRB2 by immuno-precipitation on cell lysates of isoform-transfected M8 cells (M8pcDNA, M8-HLA-G1, M8-HLA-G2, M8-HLA-G3, M8-HLA-G4, M8-HLA-G5, and M8-HLA-G6) using LILRB2-Fc-coated beads, followed by anti-HLA-G Western blotting. These results are presented in Fig. 3a and show that LILRB2-Fc immunoprecipitated HLA-G1 and HLA-G5 (α1–α2–α3 domains), and HLA-G2 and HLA-G6 (α1–α3 domains) but not HLA-G3 (α1 domain). This hinted that LILRB2 recognized only HLA-G isoforms that contain the α3 domain. However, even though in the experiment shown in Fig. 3a HLA-G4 was not immunoprecipitated, it was in other experiments, yielding to overall inconclusive data concerning the recognition of this isoform by LILRB2.
Fig. 3.
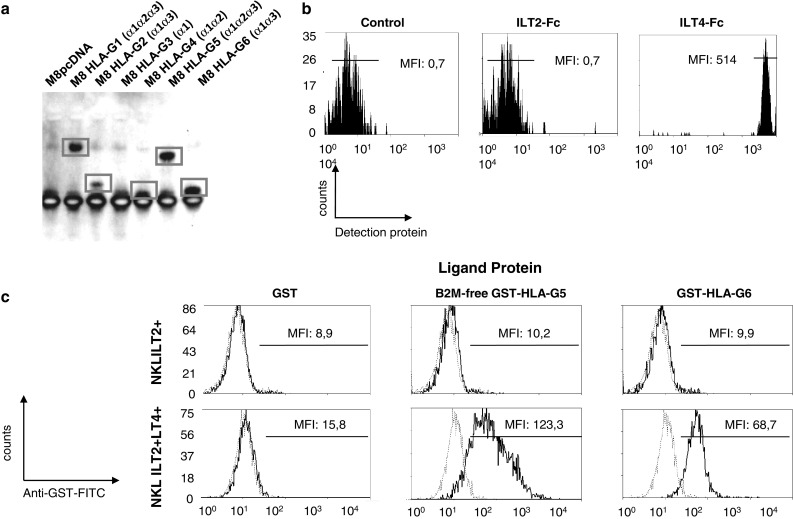
Recognition of the HLA-G α1–α3 structure by LILRB1 and LILRB2. a Immunoprecipitation of HLA-G1 and HLA-G5 (α1–α2–α3 domains), and HLA-G2 and HLA-G6 (α1–α3 domains) with LILRB2-Fc. b Differential direct binding of HLA-G6-GST recombinant protein to LILRB1-Fc and LILRB2-Fc-coated beads. c Differential binding of B2M-free HLA-G5-GST and HLA-G6-GST recombinant proteins to NKL-LILRB1+ and NKL-LILRB1+LILRB2+ cells by flow cytometry analysis, using GST recombinant protein as control and anti-GST antibody for detection
We next focused on the α1–α3 structure (HLA-G2 and HLA-G6) because it was shown to be expressed in heart recipient patients who accepted their transplants [12].
First, we used a cytometry-based HLA-G:LILRB interaction analysis to evaluate the binding of HLA-G2 and -G6 to LILRB1 and LILRB2 using only recombinant proteins. Results are presented in Fig. 3b and show that the HLA-G6-GST recombinant protein was not recognized by LILRB1-Fc, but was recognized by the LILRB2-Fc protein. These results confirm the lack of association between HLA-G2 and LILRB1, in accordance with what is known of the requirement of B2M for LILRB1:HLA-G association. They also confirm the results obtained by immuno-precipitation.
Second, we investigated the binding of HLA-G6-GST recombinant protein and its GST control to NKL-LILRB1+ and NKL-LILRB1+LILRB2+ cells. As shown in Fig. 3c, HLA-G6-GST did not bind NKL-LILRB1+ cells, indicating that neither LILRB1 nor KIR2DL4 recognized the α1–α3 structure. On the other hand, NKL-LILRB1+LILRB2+ cells were strongly positive in this assay, demonstrating that the α1–α3 structure could be recognized by membrane-bound LILRB2, and not only recombinant proteins as in the prior experiments. The same experiment, conducted for B2M-free GST-HLA-G5 recombinant proteins, yielded the same results, confirming that B2M-association is required for HLA-G5 binding to LILRB1, but not LILRB2.
In vivo analysis
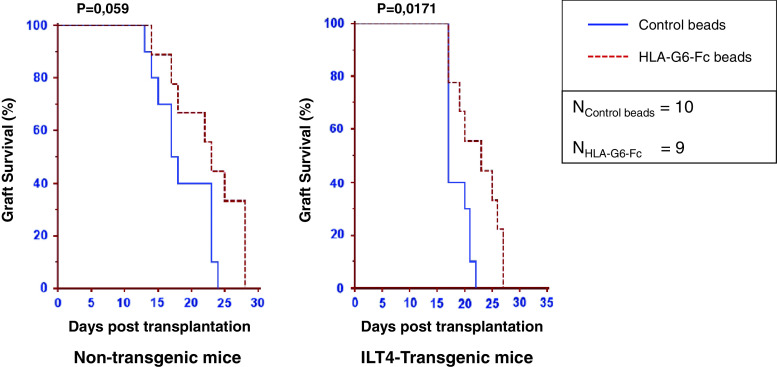
B2M-associated HLA-G1 is capable of delaying skin allograft rejection in a murine animal model [27, 45]. We investigated if the HLA-G α1–α3 structure (HLA-G2 and HLA-G6) also possessed this function. For this, we used α1–α3-Fc fusion proteins, which were produced in a different eukaryotic expression system (HeLa cells). Most of these Fc proteins were dimeric (data not shown). The results obtained for non-transgenic C56BL/6 mice are shown in Fig. 4a. As can be seen, one single IP injection of α1–α3-Fc-coated latex beads 24 h prior to graft and once again at the time of transplantation, improved graft survival. Indeed, the median survival time increased by 5 days, from 18 for control beads (n = 10) to 23 for α1–α3-Fc beads (n = 9, p = 0.059).
Fig. 4.
Tolerogenic function of HLA-G α1–α3 structure (α1–α3-Fc) in vivo. C57BL/6 mice strongly recognize the MHC class II-disparate mutant bm12 mouse that carries the I-Abm12 alloantigen. The capability of α1–α3-Fc-coated beads to delay rejection was evaluated with non-transgenic and LILRB2-transgenic recipient animals. Kaplan–Meier curves representing graft survival are shown for α1–α3-Fc (plain lines) and control treatment (dotted lines). Control treatment: beads coated with mAb but without α1–α3-Fc. Results are expressed as median of graft survival time. Associated values are indicated above the curves
Next, we investigated the tolerogenic function of α1–α3-Fc using LILRB2-transgenic mice as recipients. Results show that α1–α3-Fc-coated beads significantly increased the graft median survival time by 6 days, from 17 days for control mice (n = 10) to 23 days (n = 9, p = 0.0171) (Fig. 4b). These results demonstrate that HLA-G α1–α3 isoforms are capable of increasing graft survival time in both in wild-type and transgenic mice, but that it is more efficient in transgenic mice expressing LILRB2.
Discussion
We based the first part of this work on reports showing that HLA-G1 and HLA-G5 isoforms associated to B2M form homodimers through a disulphide bond between unique cysteine residues at position 42 [33], and that LILRB1 and LILRB2 differ in their recognition of B2M-associated HLA-G1 and HLA-G5, LILRB1 requiring B2M whereas LILRB2 does not [35, 40]. Since these reports concerned only B2M-associated HLA-G1 and HLA-G5 isoforms (α1–α2–α3 domains), we investigated which other isoforms actually formed dimers, and whether HLA-G2 and HLA-G6 (α1–α3 domains) could function through LILRB2.
Our results demonstrate that even though all HLA-G isoforms contain the α1 domain and a cysteine in position 42, they differ in their capability to dimerize. Indeed, as already reported, dimers of α1–α2–α3 isoforms (HLA-G1 and HLA-G5) were easily detected, and so were dimers of HLA-G4 (α1–α2 domains). This indicates that for these structures, the C42 residue is indeed free and accessible for disulfide bond formation [35]. The fact that HLA-G4 behaves as B2M-associated HLA-G1 with respect to dimerization means that the structures adopted by the α1 and α2 domains of HLA-G1 or -G5 and HLA-G4 are similar, which is not surprising since these two domains are known to efficiently assemble into the HLA class I characteristic peptide-binding structure. Hence, C42 is available for intercellular dimerization for the HLA-G1, HLA-G5, and HLA-G4 isoforms.
Dimers of HLA-G2 and -G6 (α1–α3 domains) and HLA-G3 (α1 domain) were difficult or impossible to detect, respectively. This indicates that C42 in these structures may not be available, or not accessible for dimerization. This may also indicate that, lacking the α2 domain it efficiently assembles with, the α1 domain of HLA-G adopts another, unknown configuration. In the case of HLA-G2 and -G6, α1 may assemble with α3, or, as the results obtained for HLA-G3 suggest, may adopt a conformation entirely different, in which no free cysteine is exposed. In the absence of a crystal structure of HLA-G-truncated isoforms, it is not possible to choose between these two hypotheses. However, the fact that LILRB2 recognizes HLA-G2 and -G6 indicates that the structure of the α3 domain may not be different between α1–α2–α3 and α1–α3 structures, and so favors the hypothesis of a different assembly of the α1 domain, and not of a α1–α3 structural association.
The results obtained with HLA-G3 (no dimers) were surprising because we previously published that a synthetic peptide of the HLA-G alpha1 domain efficiently dimerizes, unlike HLA-G3 from M8 cells of this study [46]. This indicates that HLA-G dimerization may depend on the cell type that expresses it, and not only on the presence of the alpha1 domain. If this were proven true, it would mean that the function of HLA-G, which depends on its dimerization, may be regulated by the cells themselves.
We found of particular interest to study α1–α3 (HLA-G2 and -G6) isoforms, since they were detected in the serum of transplanted patients and their physiological relevance was established [12]. Our results show that HLA-G2 and -G6 isoforms are recognized by LILRB2 but not LILRB1. Moreover, an α1–α3-Fc structure coated on agarose beads, through this interaction, is tolerogenic in vivo. These results provide the molecular basis for the biological activity of the HLA-G2 and HLA-G6 isoforms. The fact that these isoforms act through LILRB2 is of particular interest because this receptor is expressed only by cells of the myeloid lineage, i.e., monocytes, dendritic cells, macrophages. Thus, unlike B2M-associated HLA-G1 and -G5 isoforms, HLA-G2 and HLA-G6 would not directly act on lymphocytic effectors but on APC. This is similar to what can be expected of B2M-free HLA-G1 and -G5 isoforms, which can no longer bind LILRB1. This is also compatible with what is known of the in vivo model we used. Indeed, in this transplantation model, HLA-G acts chiefly through the PIRB receptor, which is mainly expressed on APCs.
Taken together, these data clearly show that HLA-G isoforms present important structural variations, which may correspond to different biological functions. As mentioned before, HLA-G1 and HLA-G5 (α1–α2–α3 associated to B2M) are the most extensively studied isoforms, probably because of their alleged higher abundance, their availability for in vitro experiments, and the existence of specific antibodies directed against them. Thus, these isoforms are believed to be the main isoforms responsible for immune regulation in vivo. However, we do not know to which extent this is true. Isoforms and B2M-free HLA-G structures may also play a specific role. Not knowing the precise structure of HLA-G-truncated isoforms implies that we also do not know which receptors they bind to. For instance, in this work, we studied the HLA-G2 or -G6 interaction with LILRB2 because this isoform contains the α3 domain and could potentially bind to LILRB2. However, we do not know what the HLA-G3 receptor could be, or that of HLA-G4, despite our data indicating that this isoform may bind LILRB2. A recent work [47] describes binding of B2M-associated and B2M-free forms of MHC to members of the LILR family, and demonstrates that in addition to LILRB2, B2M-free forms are recognized by several members of the LILR family. In fact, “activating” members of the LILR family show a preference for these forms. It is therefore possible that it is also the case for HLA-G. This would support the notion that HLA-G structural variations may be relevant in biological function modulations. It is also intriguing to consider that, similarly to classical HLA class I molecules, HLA-G has activating receptors. Indeed, it is possible that other HLA-G receptors exist, isoform-specific or not, and studying HLA-G structures other than that of B2M-associated HLA-G1 and -G5 might allow us to identify them. Finally, our data highlight the fact that even though we know the physiological importance of HLA-G, we do not know which of its structures are relevant in vivo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material Phenotype of the NKL-LILRB1+ and NKL-LILRB1+LILRB2+ cells.Cytometry analysis of the cell-surface expression of LILRB1 and LILRB2 receptors on NKLLILRB1+ and NKL-LILRB1+LILRB2+ cells 3 (PPTX 100 kb)
Acknowledgments
We thank Mr. Romain Crolas, Mr. Jeremy Baudhuin, and Dr. Benoit Favier for their technical help in this project. This work was supported by Commissariat a l’Energie Atomique et aux Energies Alternatives, in part by HLA-G Technologies (to A.H.), and by National Institute of Health grant R56 AI055923 (to A. H.).
References
- 1.Carosella ED, Rouas-Freiss N, Paul P, Dausset J. HLA-G: a tolerance molecule from the major histocompatibility complex. Immunol Today. 1999;20(2):60–62. doi: 10.1016/S0167-5699(98)01387-5. [DOI] [PubMed] [Google Scholar]
- 2.Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood. 2008;111(10):4862–4870. doi: 10.1182/blood-2007-12-127662. [DOI] [PubMed] [Google Scholar]
- 3.Ellis SA, Sargent IL, Redman CW, McMichael AJ. Evidence for a novel HLA antigen found on human extravillous trophoblast and a choriocarcinoma cell line. Immunology. 1986;59(4):595–601. [PMC free article] [PubMed] [Google Scholar]
- 4.Kirszenbaum M, Djoulah S, Hors J, Le Gall I, de Oliveira EB, Prost S, Dausset J, Carosella ED. HLA-G gene polymorphism segregation within CEPH reference families. Hum Immunol. 1997;53(2):140–147. doi: 10.1016/S0198-8859(97)00038-4. [DOI] [PubMed] [Google Scholar]
- 5.Kirszenbaum M, Djoulah S, Hors J, Prost S, Dausset J, Carosella ED. Polymorphism of HLA-G gene and protein. J Reprod Immunol. 1999;43(2):105–109. doi: 10.1016/S0165-0378(99)00025-X. [DOI] [PubMed] [Google Scholar]
- 6.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248(4952):220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 7.Mallet V, Blaschitz A, Crisa L, Schmitt C, Fournel S, King A, Loke YW, Dohr G, Le Bouteiller P. HLA-G in the human thymus: a subpopulation of medullary epithelial but not CD83(+) dendritic cells expresses HLA-G as a membrane-bound and soluble protein. Int Immunol. 1999;11(6):889–898. doi: 10.1093/intimm/11.6.889. [DOI] [PubMed] [Google Scholar]
- 8.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 9.Verloes A, Van de Velde H, LeMaoult J, Mateizel I, Cauffman G, Horn PA, Carosella ED, Devroey P, De Waele M, Rebmann V, Vercammen M. HLA-G expression in human embryonic stem cells and preimplantation embryos. J Immunol. 2011;186(4):2663–2671. doi: 10.4049/jimmunol.1001081. [DOI] [PubMed] [Google Scholar]
- 10.Menier C, Rabreau M, Challier JC, Le Discorde M, Carosella ED, Rouas-Freiss N. Erythroblasts secrete the nonclassical HLA-G molecule from primitive to definitive hematopoiesis. Blood. 2004;104(10):3153–3160. doi: 10.1182/blood-2004-03-0809. [DOI] [PubMed] [Google Scholar]
- 11.Paul P, Rouas-Freiss N, Khalil-Daher I, Moreau P, Riteau B, Le Gal FA, Avril MF, Dausset J, Guillet JG, Carosella ED. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci USA. 1998;95(8):4510–4515. doi: 10.1073/pnas.95.8.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lila N, Carpentier A, Amrein C, Khalil-Daher I, Dausset J, Carosella ED. Implication of HLA-G molecule in heart-graft acceptance. Lancet. 2000;355(9221):2138. doi: 10.1016/S0140-6736(00)02386-2. [DOI] [PubMed] [Google Scholar]
- 13.Wiendl H, Feger U, Mittelbronn M, Jack C, Schreiner B, Stadelmann C, Antel J, Brueck W, Meyermann R, Bar-Or A, Kieseier B, Weller M. Expression of the immune-tolerogenic major histocompatibility molecule HLA-G in multiple sclerosis: implications for CNS immunity. Brain. 2005;128(Pt 11):2689–2704. doi: 10.1093/brain/awh609. [DOI] [PubMed] [Google Scholar]
- 14.Aractingi S, Briand N, Le Danff C, Viguier M, Bachelez H, Michel L, Dubertret L, Carosella ED. HLA-G and NK receptor are expressed in psoriatic skin: a possible pathway for regulating infiltrating T cells? Am J Pathol. 2001;159(1):71–77. doi: 10.1016/S0002-9440(10)61675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lozano JM, González R, Kindelán JM, Rouas-Freiss N, Caballos R, Dausset J, Carosella ED, Peña J. Monocytes and T lymphocytes in HIV-1-positive patients express HLA-G molecule. AIDS. 2002;16(3):347–351. doi: 10.1097/00002030-200202150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Lafon M, Prehaud C, Megret F, Lafage M, Mouillot G, Roa M, Moreau P, Rouas-Freiss N, Carosella ED. Modulation of HLA-G expression in human neural cells after neurotropic viral infections. J Virol. 2005;79(24):15226–15237. doi: 10.1128/JVI.79.24.15226-15237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colonna M, Navarro F, Bellón T, Llano M, García P, Samaridis J, Angman L, Cella M, López-Botet M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186(11):1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colonna M, Samaridis J, Cella M, Angman L, Allen RL, O’Callaghan CA, Dunbar R, Ogg GS, Cerundolo V, Rolink A. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J Immunol. 1998;160(7):3096–3100. [PubMed] [Google Scholar]
- 19.Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med. 1999;189(7):1093–1100. doi: 10.1084/jem.189.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouas-Freiss N, Gonçalves RM, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci USA. 1997;94(21):11520–11525. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riteau B, Rouas-Freiss N, Menier C, Paul P, Dausset J, Carosella ED. HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol. 2001;166(8):5018–5026. doi: 10.4049/jimmunol.166.8.5018. [DOI] [PubMed] [Google Scholar]
- 22.Riteau B, Menier C, Khalil-Daher I, Sedlik C, Dausset J, Rouas-Freiss N, Carosella ED. HLA-G inhibits the allogeneic proliferative response. J Reprod Immunol. 1999;43(2):203–211. doi: 10.1016/S0165-0378(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 23.Bainbridge DR, Ellis SA, Sargent IL. HLA-G suppresses proliferation of CD4(+) T-lymphocytes. J Reprod Immunol. 2000;48(1):17–26. doi: 10.1016/S0165-0378(00)00070-X. [DOI] [PubMed] [Google Scholar]
- 24.Bahri R, Hirsch F, Josse A, Rouas-Freiss N, Bidere N, Vasquez A, Carosella ED, Charpentier B, Durrbach A. Soluble HLA-G inhibits cell cycle progression in human alloreactive T lymphocytes. J Immunol. 2006;176(3):1331–1339. doi: 10.4049/jimmunol.176.3.1331. [DOI] [PubMed] [Google Scholar]
- 25.LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, Carosella E. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109(5):2040–2048. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 26.Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26(5):1423–1433. doi: 10.1038/sj.emboj.7601570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35(4):1133–1142. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 28.Gros F, Cabillic F, Toutirais O, Maux AL, Sebti Y, Amiot L. Soluble HLA-G molecules impair natural killer/dendritic cell crosstalk via inhibition of dendritic cells. Eur J Immunol. 2008;38(3):742–749. doi: 10.1002/eji.200736918. [DOI] [PubMed] [Google Scholar]
- 29.Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6–STAT3 signaling pathway. Proc Nat Acad Sci USA. 2008;105(24):8357–8362. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+T cells. Proc Natl Acad Sci USA. 2004;101(18):7064–7069. doi: 10.1073/pnas.0401922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo MG. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116(6):935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 32.Agaugue S, Carosella ED, Rouas-Freiss N. Role of HLA-G in tumor escape through expansion of myeloid-derived suppressor cells and cytokinic balance in favor of Th2 versus Th1/Th17. Blood. 2011;117(26):7021–7031. doi: 10.1182/blood-2010-07-294389. [DOI] [PubMed] [Google Scholar]
- 33.Boyson JE, Erskine R, Whitman MC, Chiu M, Lau JM, Koopman LA, Valter MM, Angelisova P, Horejsi V, Strominger JL. Disulfide bond-mediated dimerization of HLA-G on the cell surface. Proc Natl Acad Sci USA. 2002;99(25):16180–16185. doi: 10.1073/pnas.212643199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonen-Gross T, Achdout H, Gazit R, Hanna J, Mizrahi S, Markel G, Goldman-Wohl D, Yagel S, Horejsí V, Levy O, Baniyash M, Mandelboim O. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J Immunol. 2003;171(3):1343–1351. doi: 10.4049/jimmunol.171.3.1343. [DOI] [PubMed] [Google Scholar]
- 35.Shiroishi M, Kuroki K, Ose T, Rasubala L, Shiratori I, Arase H, Tsumoto K, Kumagai I, Kohda D, Maenaka K. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J Biol Chem. 2006;281(15):10439–10447. doi: 10.1074/jbc.M512305200. [DOI] [PubMed] [Google Scholar]
- 36.Juch H, Blaschitz A, Daxböck C, Rueckert C, Kofler K, Dohr G. A novel sandwich ELISA for alpha1 domain-based detection of soluble HLA-G heavy chains. J Immunol Methods. 2005;307(1–2):96–106. doi: 10.1016/j.jim.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Morales PJ, Pace JL, Platt JS, Langat DK, Hunt JS. Synthesis of beta(2)-microglobulin-free, disulphide-linked HLA-G5 homodimers in human placental villous cytotrophoblast cells. Immunology. 2007;122(2):179–188. doi: 10.1111/j.1365-2567.2007.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales PJ, Pace JL, Platt JS, Phillips TA, Morgan K, Fazleabas AT, Hunt JS. Placental cell expression of HLA-G2 isoforms is limited to the invasive trophoblast phenotype. J Immunol. 2003;171(11):6215–6224. doi: 10.4049/jimmunol.171.11.6215. [DOI] [PubMed] [Google Scholar]
- 39.Clements CS, Kjer-Nielsen L, Kostenko L, Hoare HL, Dunstone MA, Moses E, Freed K, Brooks AG, Rossjohn J, McCluskey J. Crystal structure of HLA-G: a nonclassical MHC class I molecule expressed at the fetal-maternal interface. Proc Natl Acad Sci USA. 2005;102(9):3360–3365. doi: 10.1073/pnas.0409676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiroishi M, Kuroki K, Rasubala L, Tsumoto K, Kumagai I, Kurimoto E, Kato K, Kohda D, Maenaka K. Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d) Proc Natl Acad Sci USA. 2006;103(44):16412–16417. doi: 10.1073/pnas.0605228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul P, Rouas-Freiss N, Moreau P, Cabestre FA, Menier C, Khalil-Daher I, Pangault C, Onno M, Fauchet R, Martinez-Laso J, Morales P, Villena AA, Giacomini P, Natali PG, Frumento G, Ferrara GB, McMaster M, Fisher S, Schust D, Ferrone S, Dausset J, Geraghty D, Carosella ED. HLA-G, -E, -F preworkshop: tools and protocols for analysis of non-classical class I genes transcription and protein expression. Hum Immunol. 2000;61(11):1177–1195. doi: 10.1016/S0198-8859(00)00154-3. [DOI] [PubMed] [Google Scholar]
- 42.Riteau B, Moreau P, Menier C, Khalil-Daher I, Khosrotehrani K, Bras-Goncalves R, Paul P, Dausset J, Rouas-Freiss N, Carosella ED. Characterization of HLA-G1, -G2, -G3, and -G4 isoforms transfected in a human melanoma cell line. Transplant Proc. 2001;33(3):2360–2364. doi: 10.1016/S0041-1345(01)02021-8. [DOI] [PubMed] [Google Scholar]
- 43.Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol. 1996;24(3):406–415. [PubMed] [Google Scholar]
- 44.Bird LA, Peh CA, Kollnberger S, Elliott T, McMichael AJ, Bowness P. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur J Immunol. 2003;33(3):748–759. doi: 10.1002/eji.200323678. [DOI] [PubMed] [Google Scholar]
- 45.Liang S, Baibakov B, Horuzsko A. HLA-G inhibits the functions of murine dendritic cells via the PIR-B immune inhibitory receptor. Eur J Immunol. 2002;32(9):2418–2426. doi: 10.1002/1521-4141(200209)32:9<2418::AID-IMMU2418>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Favier B, HoWangYin KY, Wu J, Caumartin J, Daouya M, Horuzsko A, Carosella ED, LeMaoult J. Tolerogenic function of dimeric forms of HLA-G recombinant proteins: a comparative study in vivo. PLoS One. 2011;6(7):e21011. doi: 10.1371/journal.pone.0021011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones DC, Kosmoliaptsis V, Apps R, Lapaque N, Smith I, Kono A, Chang C, Boyle LH, Taylor CJ, Trowsdale J, Allen RL. HLA class I allelic sequence and conformation regulate leukocyte Ig-like receptor binding. J Immunol. 2011;186(5):2990–2997. doi: 10.4049/jimmunol.1003078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Phenotype of the NKL-LILRB1+ and NKL-LILRB1+LILRB2+ cells.Cytometry analysis of the cell-surface expression of LILRB1 and LILRB2 receptors on NKLLILRB1+ and NKL-LILRB1+LILRB2+ cells 3 (PPTX 100 kb)