Abstract
Elevated serum uric acid levels are a frequent finding in persons with obesity, hypertension, cardiovascular and kidney disease as well as in those with the cardiorenal metabolic syndrome (CRS). The increased consumption of a fructose-rich Western diet has contributed to the increasing incidence of the CRS, obesity and diabetes especially in industrialized populations. There is also increasing evidence that supports a causal role of high dietary fructose driving elevations in uric acid in association with the CRS. Animal and epidemiological studies support the notion that elevated serum uric acid levels play an important role in promoting insulin resistance and hypertension and suggest potential pathophysiological mechanisms that contribute to the development of the CRS and associated cardiovascular disease and chronic kidney disease. To this point, elevated serum levels of uric acid appear to contribute to impaired nitric oxide production/endothelial dysfunction, increased vascular stiffness, inappropriate activation of the renin-angiotensin-aldosterone system, enhanced oxidative stress, and maladaptive immune and inflammatory responses. These abnormalities, in turn, promote vascular, cardiac and renal fibrosis as well as associated functional abnormalities. Small clinical trials have suggested that uric acid-lowering therapies may be beneficial in such patients; however, a consensus on the treatment of asymptomatic hyperuricemia is lacking. Larger randomized controlled trials need to be performed in order to critically evaluate the beneficial effect of lowering serum uric acid in patients with the CRS and those with diabetes and/or hypertension.
Key Words : Uric acid, Fructose, Cardiorenal metabolic syndrome, Chronic kidney disease
Introduction
The prevalence of obesity continues to increase throughout the world, and childhood-adolescent obesity is emerging as a major public health issue [1,2]. Currently, more than half of the adults in the Unites States are either overweight or obese, and more than 13 million children are obese [3,4,5]. Obesity is associated with an increased prevalence of the cardiorenal metabolic syndrome (CRS), a constellation of interactive cardiovascular disease (CVD) and chronic kidney disease (CKD) risk factors comprising obesity, insulin resistance, metabolic dyslipidemia, hypertension, cardiac diastolic dysfunction and renal abnormalities including proteinuria [2,3,4,5,6]. Increased fructose consumption has been implicated in the development of the obesity epidemic in the United States. Indeed, the consumption of high-fructose corn syrup (HFCS) has increased for the last three decades, and the increase in the intake of HFCS far exceeds the changes in the intake of any other food or food group [2,7,8]. The percentage of HFCS sweeteners increased from 16% in 1978 to 42% in 1998, and since then it has stabilized [9].
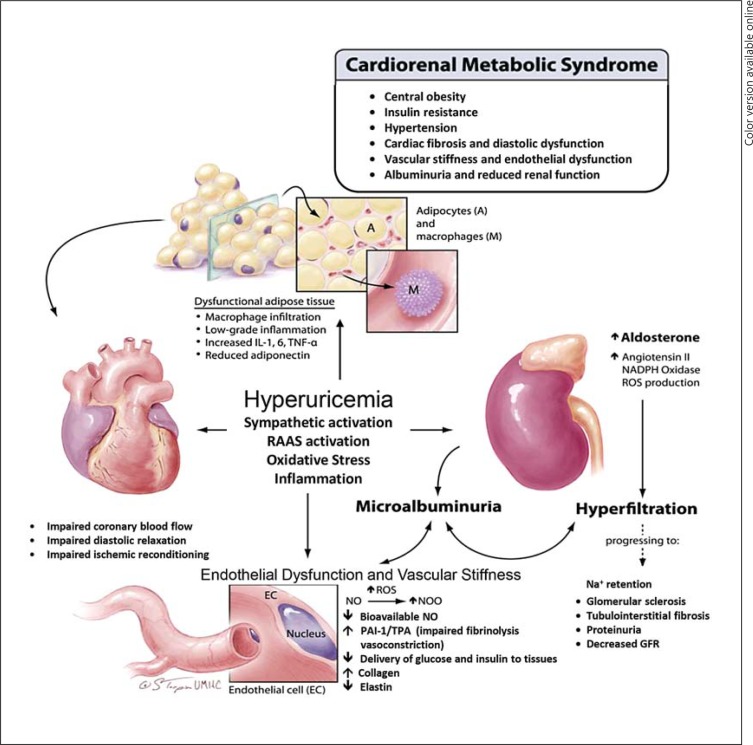
Accumulating evidence suggests that hyperuricemia is one of the important factors that may significantly contribute to the development and progression of the CRS. The Swedish pharmacist Carl Wilhelm Scheele discovered uric acid in 1776 in a bladder calculus [10]. Subsequent reports indicated a relationship between elevated serum uric acid levels, gout, hypertension and CKD [11,12,13,14]. Elevated levels of uric acid have been associated with inflammation, oxidative stress, insulin resistance, dysglycemia, endothelial dysfunction, vascular, renal and cardiac stiffness, cardiac diastolic dysfunction, renal hyperfiltration and proteinuria, all components of the CRS [2,5,6] (fig. 1). The significance of a Westernized diet, high in fructose, and hyperuricemia in the development of the CRS is underscored by the relationship between increased consumption of sugar-sweetened beverages, hyperuricemia and all components of this syndrome [2,15,16] (fig. 1).
Fig. 1.
Mechanisms by which elevated uric acid promotes components of the CRS.
Uric Acid Metabolism and Development of Hyperuricemia with a High-Fructose Diet
Uric acid production and metabolism are complex processes involving various factors that regulate hepatic production, and renal and gut excretion of this compound. Uric acid is the end product of an exogenous pool of purines and endogenous purine metabolism. The exogenous pool varies significantly with diet, and animal proteins contribute significantly to this purine pool. The endogenous production of uric acid is mainly from the liver, intestines and other tissues like muscles, kidneys and the vascular endothelium. Uric acid formation from purine catabolism occurs by a series of enzymatic reactions that ultimately involve the xanthine oxidase enzyme. An intermediate product of this metabolism is inosine. This intermediate is converted by the purine nucleoside phosphorylase to hypoxanthine. Xanthine oxidase converts hypoxanthine to xanthine and subsequently to uric acid [2,6,7,15,16].
The production and catabolism of purines are relatively constant between 300 and 400 mg per day. The kidneys eliminate approximately two-thirds, while the gastrointestinal tract eliminates one-third of the uric acid load [6,15]. Almost all uric acid is filtered from glomeruli, while post-glomerular reabsorption and secretion regulate the amount of uric acid excretion [17,18]. The proximal tubule is the site of uric acid reabsorption and secretion, and approximately 90% is reabsorbed into blood. This is primarily accomplished at the proximal tubular level by transporters that exchange intracellular anions for uric acid [17,18]. Almost all reabsorption of uric acid occurs at the S1 segment of the proximal tubule. In the S2 segment of the proximal tubule, uric acid is secreted to a greater extent than that which undergoes reabsorption. Post-secretory reabsorption occurs at a more distal site of the proximal tubule, and approximately 10% of the filtered uric acid appears in the urine [17,18,19].
Increased serum uric acid levels as seen in obese patients and in persons with renal impairment are driven by multiple mechanisms. In obesity, especially with increased HFCS consumption, there is an increased hepatic production of uric acid [6,7,8,9]. As the glomerular filtration rate (GFR) falls, serum uric acid levels increase progressively, and approximately 50% of renal patients become hyperuricemic by the time they start dialysis [11,20,21,22]. Although hyperuricemia and hyperinsulinemia are closely linked, the mechanisms behind this association remain unclear. One mechanism likely relates to the fact that hyperuricemia promotes insulin resistance and associated hyperinsulinemia [2,3]. Renal tubular function is influenced by insulin metabolic signaling, and urinary uric acid clearance decreases with decreasing insulin-mediated glucose disposal [23]. Recent studies have shown that adipose tissue may contribute as an endogenous source of uric acid, and that uric acid increases the inflammatory macrophage infiltration and inflammation in adipose tissue [24,25].
Uric acid is a by-product of uncontrolled fructose metabolism, which is mediated through increased fructokinase activation. Fructokinase has no negative feedback system, and adenosine triphosphate is used as a source of phosphorylation [26,27]. This results in intracellular phosphate depletion and the rapid generation of uric acid due to activation of adenosine monophosphate deaminase [2,26,28]. Serum uric acid increases rapidly after ingestion of fructose, resulting in increments as high as 2 mg/dl within 1 h [28]. The induction of the fructokinase enzyme by fructose in the liver provides a feed-forward mechanism for continued production of uric acid in the setting of high-fructose Western diet consumption [2,26,28].
Uric Acid, Cardiorenal Metabolic Syndrome and Diabetes
Epidemiological studies have confirmed the association of hyperuricemia with the CRS [2,7,8,23,24,27]. A cross-sectional analysis of 1,370 adolescents aged 12-17 years using data from the National Health and Nutrition Examination Survey (NHANES) [29] showed that the prevalence of the syndrome was <1% in the lowest quartile of uric acid serum levels, 3.7% in the second quartile, 10.3% in the third quartile and 21.1% in the highest quartile. Fructose-rich diets can raise uric acid production and induce the components of the syndrome through mechanisms independent of energy intake or weight gain [2,30,31], effects typically not observed with solely glucose-rich diets [2,30,31]. In the Finnish Diabetes Prevention Study [32], which involved lifestyle intervention in high-risk, middle-aged subjects with impaired glucose tolerance, elevated baseline uric acid and its increase over time predicted a two-fold increase in the likelihood of developing type 2 diabetes mellitus (T2DM). A meta-analysis of 11 cohort studies with 42,834 participants that reported >3,000 incident cases of T2DM with a follow-up period ranging from 2.0 to 13.5 years suggested that serum uric acid levels had a positive correlation with the development of T2DM regardless of various study characteristics [33].
Uric Acid and Cardiovascular Disease
Hyperuricemia has been associated with hypertension in multiple studies, and it has been hypothesized that elevated uric acid might play a role in the pathogenesis of primary hypertension [34,35,36]. In an early study, hyperuricemia was reported in 25-40% of untreated hypertensive subjects and in 75% of malignant hypertensive subjects [34]. A study of more than 3,000 Framingham Study participants showed that elevated serum uric acid levels were an independent predictor of hypertension incidence and longitudinal progression at a 4-year follow-up [35]. In a study of children referred for evaluation of hypertension, uric acid levels were directly correlated with both systolic and diastolic blood pressure in the 63 subjects with hypertension [20]. In the 1999-2006 NHANES study of 6,036 adolescents between 12-17 years of age [21], 17% were obese [body mass index (BMI) ≥95th percentile) and 3.3% had elevated blood pressure. Further, 34% had a uric acid level ≥5.5 mg/dl and, when compared to participants with uric acid levels <5.5 mg/dl, had a 2.03-fold higher odds of having elevated blood pressure. Elevated serum uric acid has also been independently associated with a nondipper circadian pattern. In a study of 112 persons with essential hypertension, of which 60 were nondippers, the nondippers had higher serum uric acid levels [36]. This nondipping pattern is also seen in persons with obesity, salt sensitivity, the CRS and renal disease [2,3].
There is mounting evidence that lowering of serum uric acid levels is a strategy that may help lower elevated blood pressures. For example, in a randomized, double-blind, placebo-controlled trial involving 30 adolescents with newly diagnosed, never-treated stage 1 essential hypertension and serum uric acid levels ≥6 mg/dl, allopurinol treatment was superior to placebo in reducing mean systolic and diastolic as well as 24-hour ambulatory blood pressures [37]. In a study of pregnant women, the mean serum uric acid values for women with preeclampsia were high, and serum uric acid levels of ≥5.5 mg/dl indicated an increased likelihood of preeclampsia in hypertensive pregnant patients [38]. Moreover, in a randomized controlled trial, men who were administered 200 g fructose daily for 2 weeks displayed an increase in 24-hour ambulatory blood pressures, which was prevented with concomitant administration of allopurinol [39]. These results support the notion that consumption of high-fructose diet and consequent hyperuricemia raise blood pressure, and that lowering uric acid with allopurinol lowers blood pressure as well as the associated CVD risk. A meta-analysis of 16 studies including 238,449 adults found that hyperuricemia was associated with a higher risk of both stroke incidence and mortality [40]. This association of elevated uric acid with increased risk for stroke could be observed in both diabetic and nondiabetic populations.
Elevated serum uric acid levels have been shown to be associated with impaired endothelium-mediated relaxation, vascular stiffness and a restrictive left ventricular filling pattern/diastolic dysfunction [2,3,5]. Increasing serum levels of uric acid have also been significantly linked to the progression of congestive heart failure [41,42]. In a randomized, placebo-controlled, double-blind crossover study of patients with New York Heart Association class II-III chronic heart failure comparing 300 mg allopurinol daily (1 month) versus placebo, allopurinol significantly increased the forearm blood flow in response to acetylcholine as compared to placebo [41]. Another study assessing the development of CVD in patients with hypertensive nephropathy and impaired kidney function [estimated GFR (eGFR) <45 ml/min/1.73 m2] found that the use of allopurinol decreased CVD morbidity and all-cause mortality [42]. These observations underscore the notion that elevated uric acid promotes endothelial dysfunction, vascular and cardiac stiffness as well as CVD [2,3].
Elevated Uric Acid and Chronic Kidney Disease
It is unclear whether uric acid is a marker or an independent risk factor for the initiation and progress of CKD. However, uric acid can accelerate renal disease in experimental animals and is epidemiologically associated with progressive renal disease in humans. In animal models of CKD, hyperuricemia leads to worsening proteinuria and renal failure along with associated glomerular sclerosis and tubulointerstitial fibrosis [2,43,44]. In rodent models, high dietary fructose-associated hyperuricemia produces CRS with glomerular hyperfiltration, renal hypertrophy and subsequent increases in proteinuria and reductions in creatinine clearance [44]. Epidemiologic studies have shown that hyperuricemia is an independent risk factor for renal dysfunction in the normal population and in patients with hypertension, diabetes and CKD. The CRS is strongly associated with CKD (defined as GFR <60 ml/min/1.73 m2) and microalbuminuria, with the risk of CKD increasing progressively with the number of criteria that constitute the syndrome [16,27,29]. It has been proposed that increased HFCS consumption causes renal disease in concert with abnormalities characterizing the CRS via increases in uric acid production [2]. Recently, a relationship between sugar-sweetened soda consumption and hyperuricemia and kidney disease has been found in an analysis of data from the ARIC study [45]. As compared to the participants who drank less than one soda per day, the odds ratio for CKD significantly increased to 2.59 among participants who drank more than one soda per day and had a serum uric acid level of >9.0 mg/dl. A study of 21,475 healthy volunteers followed prospectively for a median of 7 years found that a slightly elevated uric acid level (7.0-8.9 mg/dl) was associated with almost twice the risk for incident kidney disease (OR 1.74), and further elevations (≥9.0 mg/dl) were associated with more than three times the risk (OR 3.12) [46]. A recently conducted Swiss population-based, cross-sectional study of >5,000 participants aged 35-75 years found that uric acid levels are an independent risk factor for CKD in both men and women [47].
There is emerging evidence that lowering of uric acid is a key strategy for reducing progression of renal disease. In a prospective randomized trial of 113 patients with an eGFR <60 ml/min/1.73 m2, subjects received treatment with allopurinol 100 mg per day versus standard therapy. In the standard therapy group, the eGFR decreased by 3.3 ± 1.2 ml/min/ 1.73 m2, and in the allopurinol group, the eGFR increased by 1.3 ± 1.3 ml/min/1.73 m2 after 24 months [48]. In a randomized controlled trial of hyperuricemic CKD patients treated with either allopurinol or usual therapy for 12 months, 16% patients in the allopurinol group reached the combined end points of significant deterioration in renal function and dialysis dependence compared with 46.1% in the control group [49]. In an analysis of 838 patients with CKD stage III/IV, a 1 mg/dl greater baseline uric acid level was associated with a 17% increased risk of all-cause mortality and a 16% increased risk of CVD mortality [50]. In a study of >300 patients with IgA nephropathy, hyperuricemia independently predicted renal survival at 1, 3 and 5 years after adjustment for GFR. In the same study, a greater number of hypertensive and hyperuricemic subjects had a reduction in antihypertensive drug dosage when treated with allopurinol [51]. The results of a meta-analysis of 11 randomized controlled trials with a total of 753 participants showed that uric acid-lowering therapy was associated with a decrease in serum creatinine and an increase in eGFR, suggesting that lowering of uric acid slows the decline of kidney function [52]. A post hoc analysis of the Febuxostat Open-Label Clinical Trial of Urate-Lowering Efficacy and Safety study [53] in 116 hyperuricemic patients with gout treated with febuxostat for 5 years demonstrated an inverse correlation between serum uric acid reduction and rate of eGFR decline. Individuals with the greatest reduction in serum uric acid following febuxostat treatment experienced the slowest rate of renal function decline, and for every 1 mg/dl decrease in uric acid, the model projected an expected improvement in eGFR of 1 ml/min/1.73 m2 from the untreated value [53]. Further, a post hoc analysis of data from participants (patients with type II diabetes and nephropathy) in the Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL) trial showed that the risk of a kidney event end point declined by 6% per 0.5 mg/dl decrement in uric acid levels [54]. After adjustments, approximately 20% of the renoprotective effect of losartan was attributed to this drug's uric acid-lowering properties.
Uric Acid, Endothelial Dysfunction and Vascular Stiffness
Endothelial dysfunction and arterial stiffness play a central role in the development and progression of hypertension cardiac diastolic dysfunction as well as CVD and CKD [2,3,5]. Accumulating evidence suggests that increased serum uric acid levels may be associated with endothelial dysfunction and vascular stiffness, especially in the presence of the CRS [55]. A cross-sectional evaluation of the ARIC study population in the United States showed that serum uric acid levels were associated with intimal medial thickness in both sexes [56]. A cross-sectional study of 3,772 Chinese adults found that serum uric acid was associated with increased carotid femoral pulse wave velocity independent of conventional CVD risk factors [57]. A study of 366 hypertensive individuals between the ages of 34 and 75 years showed that uric acid levels positively correlated with the mean maximum pulse wave velocity and carotid intimal medial thickness [58]. Further, in a cross-sectional data analysis of 982 Japanese individuals who underwent health screening, multivariate analysis after adjustment for classical risk factors showed an association between uric acid and high brachial-ankle pulse wave velocity in subjects with the metabolic syndrome as well as in those without [59]. Another study of 940 Chinese participants, of which 22% were hypertensive patients, serum uric acid was positively associated with carotid-femoral pulse wave velocities and central systolic blood pressure in all subjects. Patients with hyperuricemia had significantly higher central systolic blood pressures than those with normal serum uric acid [60].
Cellular and Molecular Mechanisms of Cardiovascular/Renal Injury
The mechanisms by which uric acid promotes cardiovascular and renal injury are multiple, comprising cardiac, vascular, renal hepatic and adipocyte maladaptive immune and inflammatory response, inappropriate activation of the renin-angiotensin-aldosterone system (RAAS), enhanced oxidative stress and impaired nitric oxide availability.
Adipocyte Dysfunction, Maladaptive Immune and Inflammatory Response and Insulin Resistance
Insulin resistance plays an important role in the development of the CRS and T2DM. Hyperuricemia was found to be an independent risk factor for progression to hyperinsulinemia in the 11-year follow-up of nondiabetic participants in the Atherosclerosis Risk in Communities Study [61]. White adipose tissue immune and inflammatory responses in obesity contribute significantly to adipose tissue and systemic insulin resistance [24,62]. Dysregulated adipocyte function also results in increased adipose tissue lipolysis and increased secretion of cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6) and resistin, and decreased secretion of adiponectin [2,24]. Recently, hyperuricemia has been shown to mediate proinflammatory response in the adipose tissue in a murine model of the CRS associated with hyperuricemia. Uric acid induced upregulation of monocyte chemoattractive protein expression and increased macrophage infiltration and proinflammatory responses in adipose tissue [24]. These effects of uric acid were contributed to the intracellular effects of uric acid [24] (fig. 1).
Perivascular Adipose Tissue, Endothelial Dysfunction and Vascular Stiffness
In addition to visceral adipose tissue dysfunction, perivascular adipose tissue also contributes significantly to inflammation, insulin resistance, endothelial dysfunction and vascular stiffness [2,63]. Although perivascular fat exerts protective vasoregulatory actions in lean mice, this protective effect of perivascular fat is lost in the setting of obesity [63]. Significant infiltration of macrophages and T cells in perivascular adipose tissue in obesity has been shown to be associated with endothelial dysfunction [63,64]. Decreased secretion of adiponectin and increased production of cytokines from dysfunctional adipose tissue may significantly contribute to vascular inflammation, insulin resistance, vascular stiffness and impaired relaxation [63]. Elevated uric acid predisposes to all of these adipose tissue abnormalities in conjunction with the CRS [2].
Uric Acid, Inflammasome and Activation of Toll-Like Receptor Signaling
In addition to immune cell polarization and dysregulation of immunity, activation of Toll-like receptor (TLR)-4 and perhaps other TLRs by uric acid may contribute to immune activation and proinflammatory response. Accumulating evidence suggests that inflammasome activation, through IL-1β activation, may contribute to insulin resistance and T2DM [24,63,64]. Insulin sensitivity improves when mice deficient in central inflammasome molecules are fed high-fat diets, and this improvement is accompanied by a suppression of immune and inflammatory responses [24,63]. This inflammasome activation response is seen after exposure to pathogens or activation of danger-associated signals. Although activation of inflammasomes by palmitate and ceramide has been reported in obesity, uric acid-induced activation of inflammasomes suggests that uric acid is another endogenous factor that contributes to the inflammasome response in high fructose diet-induced obesity. Rodent models, in which the uricase gene has been knocked out, show extensive tubular damage due to crystal deposition, renal failure and death in a few weeks [12]. However, crystal-independent injury has also been demonstrated, and hyperuricemia has been shown to cause hypertension and an ischemic type of renal injury with collagen deposition, macrophage infiltration and tubulointerstitial fibrosis.
Uric Acid, RAAS Activation, Endothelial Dysfunction, Vascular Stiffness and Progression of the Cardiorenal Metabolic Syndrome
The role of inappropriate activation of the systemic RAAS and the RAAS in adipose tissue, vasculature and kidney has been increasingly recognized as a significant factor causing cardiovascular and renal injury in obesity and diabetes [2,3]. In addition to direct effects of angiotensin II (Ang II) and aldosterone, indirect effects of elevated uric acid occur through maladaptive macrophage and T cell polarization [2,3,64]. Moreover, increased production of vascular Ang II/aldosterone by perivascular fat causes vascular inflammation and impairment of vascular function. In this regard, elevated uric acid may promote RAAS activation in vascular tissues and kidney [63]. The significance of uric acid-promoting activation of renin-angiotensin is underscored by the modulation of aldosterone secretion through Ang II in the adipose tissue, production of angiotensinogen in the perivascular adipose tissue, increased plasma and adipose tissue aldosterone levels in obesity as well as aldosterone-induced endothelial dysfunction and vascular stiffness [2,3,63].
Role of Oxidative Stress and Nitric Oxide
Enhanced production of reactive oxygen specifics and impaired production and bioavailability of nitric oxide play a central role in endothelial dysfunction, arterial stiffness, impaired diastolic function, hypertension and CKD [2,3]. Production of vasoactive factors and cytokines by perivascular fat has been shown to modulate vascular function by modulating oxidative stress, vascular relaxation and vascular stiffness [2,3,64]. Ang II and aldosterone may also cause insulin resistance indirectly through innate and acquired immune and inflammatory mediated oxidative stress [3,63,64]. Thus, these are additional mechanisms by which elevated uric acid may promote the abnormalities characteristic of the CRS [2].
Gender, Uric Acid, RAAS and the Cardiorenal Metabolic Syndrome
Obesity in the setting of TDM2 has been shown to have a more deleterious impact on diastolic dysfunction in women than in men [65]. Moreover, nondiabetic obese women also display an increased risk for cardiac dysfunction [65] despite the fact that nondiabetic premenopausal women exhibit less incidence of CVD compared to age-matched men. Population-based studies have documented higher ventricular and peripheral arterial stiffness in women, independent of body weight, as a potential factor contributing to the increased incidence of diastolic dysfunction in obese females even before the appearance of other cardiovascular risk factors. Left ventricular mass correlates positively with glucose intolerance and insulin resistance, especially in women [63,65]. Indeed, modulation of the RAAS by uric acid as well as high-salt and high-fructose diets in the setting of obesity may be contributing factors for the abrogation of antagonism of RAAS effects by estrogen and loss of cardiorenal protection in obese premenopausal women [3,65].
Conclusion
There is increasing evidence that uric acid plays a role in the pathogenesis of the CRS, T2DM, CVD and CKD. Potential mechanisms include the role for crystal-induced inflammatory response and intracellular soluble uric acid-mediated oxidative stress, activation of RAAS, impaired nitric oxide availability, maladaptive immune and inflammatory response as well as insulin resistance. Hyperuricemia may be one of the important factors contributing to abrogation of sex differences in cardiorenal protection in premenopausal women in the setting of insulin resistance and obesity. Though several small trials have shown beneficial effects of lowering serum uric acid (table 1), strong clinical data are lacking that reveal whether treating hyperuricemia in such conditions will lead to improved outcomes, and currently there is no consensus in the medical community regarding the use of serum uric acid-lowering therapy in such patients. Larger randomized controlled trials are needed to establish whether uric acid-lowering therapy is beneficial in such patients in order to be incorporated in patient management.
Table 1.
Randomized controlled trials of lowering uric acid
| Study | Patients, n | Intervention | End points | Outcome | Comments |
|---|---|---|---|---|---|
| Doehner et al. [66], 2002 | 14 | Allopurinol vs. placebo | Peripheral blood flow, uric acid, allantoin | ↓ uric acid and allantoin levels; allopurinol: ↑ post-ischemic blood flow | CHF patients; placebo-controlled crossover RCT |
| Siu et al. [49], 2006 | 54 | Allopurinol vs. usual treatment | Serum creatinine, initiation of dialysis therapy, death | ↓ uric acid; there was a trend toward a lower serum creatinine level in the treatment group; 16% in the allopurinol group reached the combined end points of significant deterioration in renal function and dialysis dependence compared with 46.1% in the control group (p = 0.015) | CKD patients |
| Malaguarnera et al. [67], 2009 | 38 | Rasburicase (n = 20) vs. placebo (n = 18) | Serum creatinine, serum uric acid, creatinine clearance | ↓ creatinine and uric acid; ↑ creatinine clearance | Elderly, hyperuricemic patients |
| Goicoechea et al. [48], 2010 | ↓13 | Allopurinol vs. usual treatment | Renal disease progression, cardiovascular events, hospitalizations of any causes | Allopurinol: ↓ serum uric acid and C-reactive protein, risk of hospitalization and cardiovascular events; in the control group, eGFR I3.3 ± 1.2 ml/min/1.73 m2 and in the allopurinol group, eGFR ↑ 1.3 ± 1.3 ml/ min/1.73 m2 after 24 months | CKD patients |
| Kanbay et al. [68], 2011 | 105 | Allopurinol | FMD as a marker of endothelial dysfunction, ambulatory BP monitoring, GFR hsCRP, serum uric acid | ↓ serum uric acid, systolic BP and hsCRP, ↑ eGFRand FMD compared with baseline values | |
BP = Blood pressure; hsCRP = high-sensitivity C-reactive protein; FMD = flow-mediated dilation; RCT = randomized control trial.
Acknowledgements
This research was supported by the National Institutes of Health (R01-HL73101 and R01-HL1079100) and Veterans Affairs Merit System 0018. The authors wish to thank Brenda Hunter for her assistance.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Sowers JR, Whaley-Connell A, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61:943–947. doi: 10.1161/HYPERTENSIONAHA.111.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden MR, Tyagi SC. Uric acid: a new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: the urate redox shuttle. Nutr Metab (Lond) 2004;1:10. doi: 10.1186/1743-7075-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8:609–617. doi: 10.1016/j.hfc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jalal DI, Chonchol M, Chen W, Targher G. Uric acid as a target of therapy in CKD. Am J Kidney Dis. 2013;61:134–146. doi: 10.1053/j.ajkd.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khitan Z, Kim DH. Fructose: a key factor in the development of metabolic syndrome and hypertension. J Nutr Metab. 2013;2013:682673. doi: 10.1155/2013/682673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sánchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 9.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139:1228S–1235S. doi: 10.3945/jn.108.098277. [DOI] [PubMed] [Google Scholar]
- 10.Steele TH. Urate secretion in man: the pyrazinamide suppression test. Ann Intern Med. 1973;79:734–737. doi: 10.7326/0003-4819-79-5-734. [DOI] [PubMed] [Google Scholar]
- 11.Suliman ME, Johnson RJ, García-López E, Qureshi AR, Molinaei H, Carrero JJ, Heimbürger O, Bárány P, Axelsson J, Lindholm B, Stenvinkel P. J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis. 2006;48:761–771. doi: 10.1053/j.ajkd.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 13.Tseng CH. Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int. 2005;68:796–801. doi: 10.1111/j.1523-1755.2005.00459.x. [DOI] [PubMed] [Google Scholar]
- 14.Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M. Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron. 2001;87:333–339. doi: 10.1159/000045939. [DOI] [PubMed] [Google Scholar]
- 15.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6:2364–2373. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 17.Wright AF, Rudan I, Hastie ND, Campbell H. A ‘complexity’ of urate transporters. Kidney Int. 2010;78:446–452. doi: 10.1038/ki.2010.206. [DOI] [PubMed] [Google Scholar]
- 18.Caulfield MJ, Munroe PB, O'Neill D, Witkowska K, Charchar FJ, Doblado M, Evans S, Eyheramendy S, Onipinla A, Howard P, Shaw-Hawkins S, Dobson RJ, Wallace C, Newhouse SJ, Brown M, Connell JM, Dominiczak A, Farrall M, Lathrop GM, Samani NJ, Kumari M, Marmot M, Brunner E, Chambers J, Elliott P, Kooner J, Laan M, Org E, Veldre G, Viigimaa M, Cappuccio FP, Ji C, Iacone R, Strazzullo P, Moley KH, Cheeseman C. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5:e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutman AB, Yu TF. Uric acid nephrolithiasis. Am J Med. 1968;45:756–779. doi: 10.1016/0002-9343(68)90209-x. [DOI] [PubMed] [Google Scholar]
- 20.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loeffler LF, Navas-Acien A, Brady TM, Miller ER, 3rd, Fadrowski JJ. Uric acid level and elevated blood pressure in US adolescents: National Health and Nutrition Examination Survey, 1999-2006. Hypertension. 2012;59:811–817. doi: 10.1161/HYPERTENSIONAHA.111.183244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266:3008–3011. [PubMed] [Google Scholar]
- 24.Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, Johnson RJ, Sautin YY. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258–1269. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, Shirakura T, Kato K, Imaizumi K, Takahashi H, Tamura M, Maeda N, Funahashi T, Shimomura I. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem 2013, E-pub ahead of print. [DOI] [PMC free article] [PubMed]
- 26.Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, Ishimoto T, Le M, Garcia GE, Thomas JB, Rivard CJ, Andres-Hernando A, Hunter B, Schreiner G, Rodriguez-Iturbe B, Sautin YY, Johnson RJ. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One. 2012;7:e47948. doi: 10.1371/journal.pone.0047948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120:442–447. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 28.Fiaschi E, Baggio B, Favaro S, Antonello A, Camerin E, Todesco S, Borsatti A. Fructose-induced hyperuricemia in essential hypertension. Metabolism. 1977;26:1219–1223. doi: 10.1016/0026-0495(77)90114-7. [DOI] [PubMed] [Google Scholar]
- 29.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 30.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 31.Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, Keim NL, Cummings BP, Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94:1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niskanen L, Laaksonen DE, Lindström J, Eriksson JG, Keinänen-Kiukaanniemi S, Ilanne-Parikka P, Aunola S, Hämäläinen H, Tuomilehto J, Uusitupa M. Serum uric acid as a harbinger of metabolic outcome in subjects with impaired glucose tolerance: the Finnish Diabetes Prevention Study. Diabetes Care. 2006;29:709–711. doi: 10.2337/diacare.29.03.06.dc05-1465. [DOI] [PubMed] [Google Scholar]
- 33.Kodama S, Saito K, Yachi Y, Asumi M, Sugawara A, Totsuka K, Saito A, Sone H. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. N Engl J Med. 1966;275:457–464. doi: 10.1056/NEJM196609012750902. [DOI] [PubMed] [Google Scholar]
- 35.Sundström J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 36.Turak O, Ozcan F, Tok D, Işleyen A, Sökmen E, Taşoğlu I, Aydoğdu S, Sen N, McFann K, Johnson RJ, Kanbay M. Serum uric acid, inflammation, and nondipping circadian pattern in essential hypertension. J Clin Hypertens (Greenwich) 2013;15:7–13. doi: 10.1111/jch.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim KH, Friedman SA, Ecker JL, Kao L, Kilpatrick SJ. The clinical utility of serum uric acid measurements in hypertensive diseases of pregnancy. Am J Obstet Gynecol. 1998;178:1067–1071. doi: 10.1016/s0002-9378(98)70549-6. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Pozo SE, Schold J, Nakagawa T, Sánchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–461. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 40.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61:885–892. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 42.Terawaki H, Nakayama M, Miyazawa E, Murata Y, Nakayama K, Matsushima M, Miyazaki M, Sato H, Sato M, Sato T, Taguma Y, Ito S. Effect of allopurinol on cardiovascular incidence among hypertensive nephropathy patients: the Gonryo study. Clin Exp Nephrol. 2013;17:549–553. doi: 10.1007/s10157-012-0742-z. [DOI] [PubMed] [Google Scholar]
- 43.Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013;15:175–181. doi: 10.1007/s11906-013-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sánchez-Lozada LG, Tapia E, Jiménez A, Bautista P, Cristóbal M, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292:F423–F429. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 45.Bomback AS, Derebail VK, Shoham DA, Anderson CA, Steffen LM, Rosamond WD, Kshirsagar AV. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 2010;77:609–616. doi: 10.1038/ki.2009.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19:2407–2413. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponte B, Pruijm M, Marques-Vidal P, Martin PY, Burnier M, Paccaud F, Waeber G, Vollenweider P, Bochud M. Determinants and burden of chronic kidney disease in the population-based CoLaus study: a cross-sectional analysis. Nephrol Dial Transplant. 2013;28:2329–2339. doi: 10.1093/ndt/gft206. [DOI] [PubMed] [Google Scholar]
- 48.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, Arroyo D, Luño J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y, Chen W, Jalal D, Li Z, Chen W, Mao H, Yang Q, Johnson RJ, Yu X. Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidney Blood Press Res. 2012;35:153–160. doi: 10.1159/000331453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Wei Y, Kong X, Xu D. Effects of urate-lowering therapy in hyperuricemia on slowing the progression of renal function: a meta-analysis. J Ren Nutr. 2013;23:389–396. doi: 10.1053/j.jrn.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17:7–13. doi: 10.1097/RHU.0b013e318204aab4. [DOI] [PubMed] [Google Scholar]
- 54.Miao Y, Ottenbros SA, Laverman GD, Brenner BM, Cooper ME, Parving HH, Grobbee DE, Shahinfar S, de Zeeuw D, Lambers Heerspink HJ. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58:2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. [DOI] [PubMed] [Google Scholar]
- 55.Gagliardi AC, Miname MH, Santos RD. Uric acid: a marker of increased cardiovascular risk. Atherosclerosis. 2009;202:11–17. doi: 10.1016/j.atherosclerosis.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Iribarren C, Folsom AR, Eckfeldt JH, McGovern PG, Nieto FJ. Correlates of uric acid and its association with asymptomatic carotid atherosclerosis: the ARIC Study. Atherosclerosis Risk in Communities. Ann Epidemiol. 1996;6:331–340. doi: 10.1016/s1047-2797(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 57.Liang J, Li Y, Zhou N, Teng F, Zhao J, Zou C, Qi L. Synergistic effects of serum uric acid and cardiometabolic risk factors on early stage atherosclerosis: the cardiometabolic risk in Chinese study. PLoS One. 2012;7:e51101. doi: 10.1371/journal.pone.0051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gómez-Marcos MA, Recio-Rodríguez JI, Patino-Alonso MC, Agudo-Conde C, Rodríguez-Sánchez E, Gómez-Sánchez L, Gómez-Sánchez M, García-Ortiz L, Vasorisk group Relationship between uric acid and vascular structure and function in hypertensive patients and sex-related differences. Am J Hypertens. 2013;26:599–607. doi: 10.1093/ajh/hps097. [DOI] [PubMed] [Google Scholar]
- 59.Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Higher serum uric acid is associated with increased arterial stiffness in Japanese individuals. Atherosclerosis. 2007;192:131–137. doi: 10.1016/j.atherosclerosis.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Li Y, Sheng CS, Huang QF, Zheng Y, Wang JG. Association of serum uric acid with aortic stiffness and pressure in a Chinese workplace setting. Am J Hypertens. 2010;23:387–392. doi: 10.1038/ajh.2009.277. [DOI] [PubMed] [Google Scholar]
- 61.Carnethon MR, Fortmann SP, Palaniappan L, Duncan BB, Schmidt MI, Chambless LE. Risk factors for progression to incident hyperinsulinemia: the Atherosclerosis Risk in Communities Study, 1987-1998. Am J Epidemiol. 2003;158:1058–1067. doi: 10.1093/aje/kwg260. [DOI] [PubMed] [Google Scholar]
- 62.Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation – mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32:1771–1776. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism 2013, E-pub ahead of print. [DOI] [PMC free article] [PubMed]
- 64.Schiffrin EL. The immune system: role in hypertension. Can J Cardiol. 2013;29:543–548. doi: 10.1016/j.cjca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Manrique C, Demarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, Hayden MR, Sowers JR. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a western diet. Endocrinology 2013, E-pub ahead of print. [DOI] [PMC free article] [PubMed]
- 66.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 67.Malaguarnera M, Vacante M, Russo C, Dipasquale G, Gargante MP, Motta M. A single dose of rasburicase in elderly patients with hyperuricaemia reduces serum uric acid levels and improves renal function. Expert Opin Pharmacother. 2009;10:737–742. doi: 10.1517/14656560902781972. [DOI] [PubMed] [Google Scholar]
- 68.Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I, Duranay M, Covic A, Johnson RJ. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6:1887–1894. doi: 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]