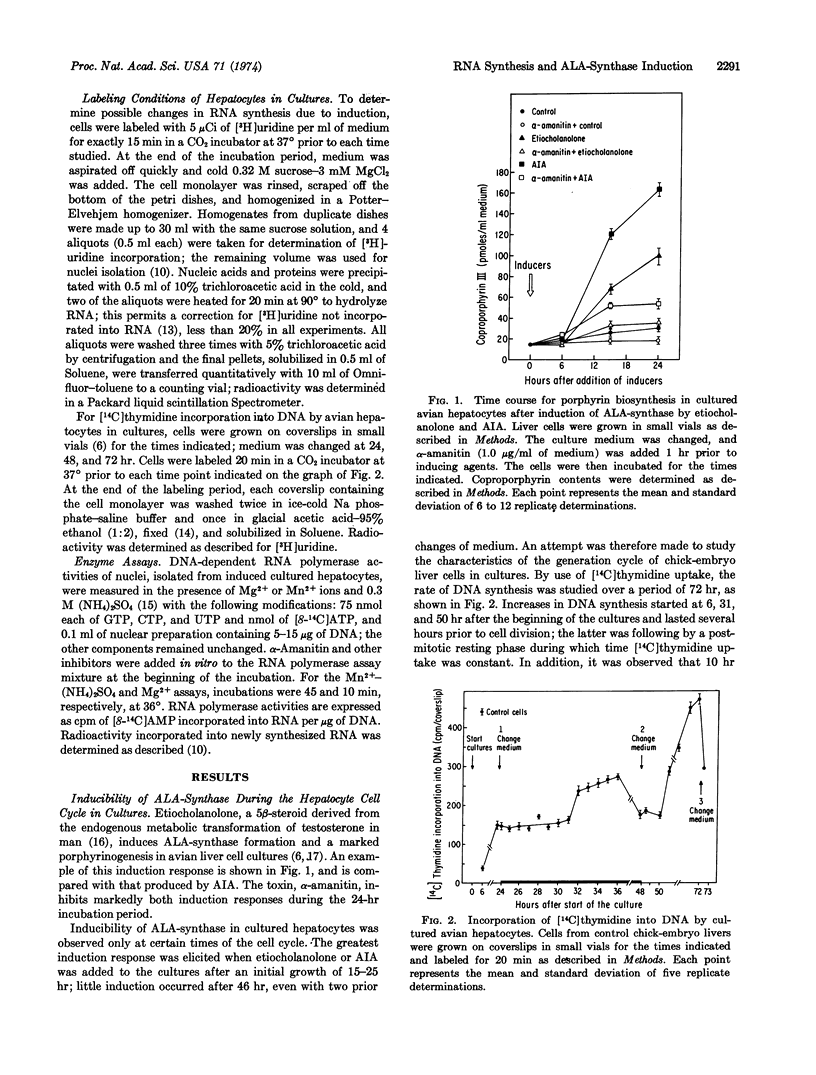
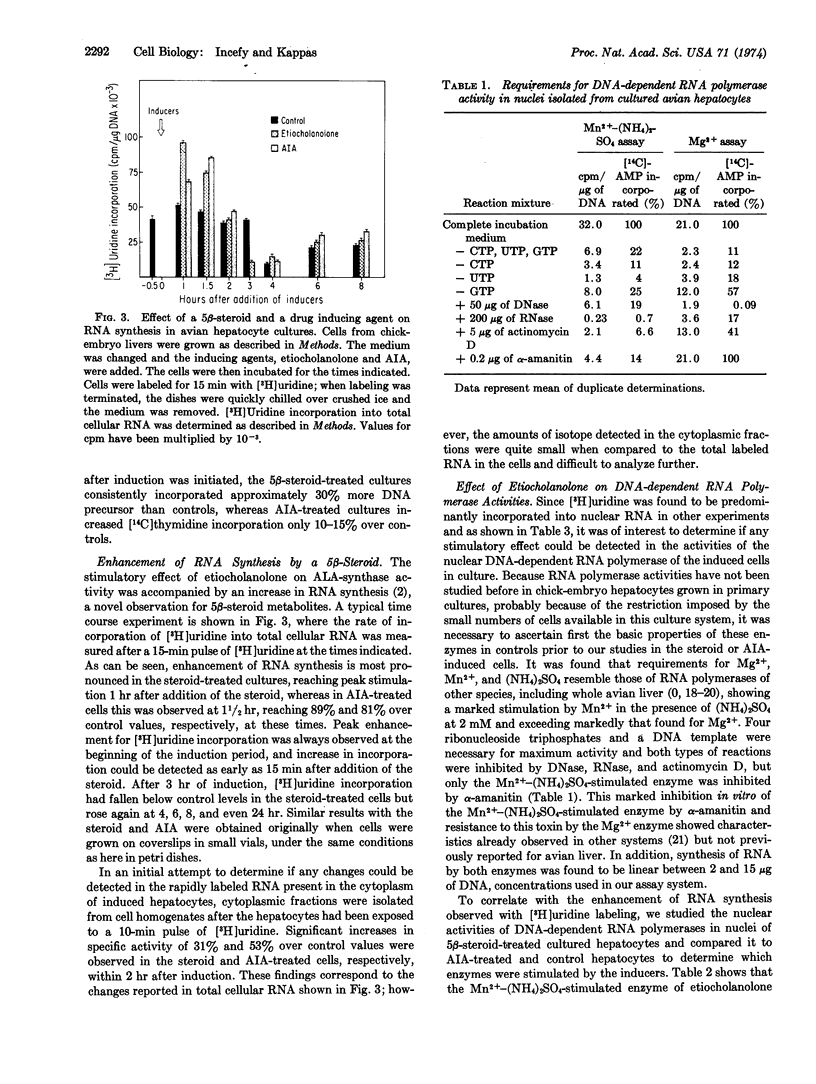
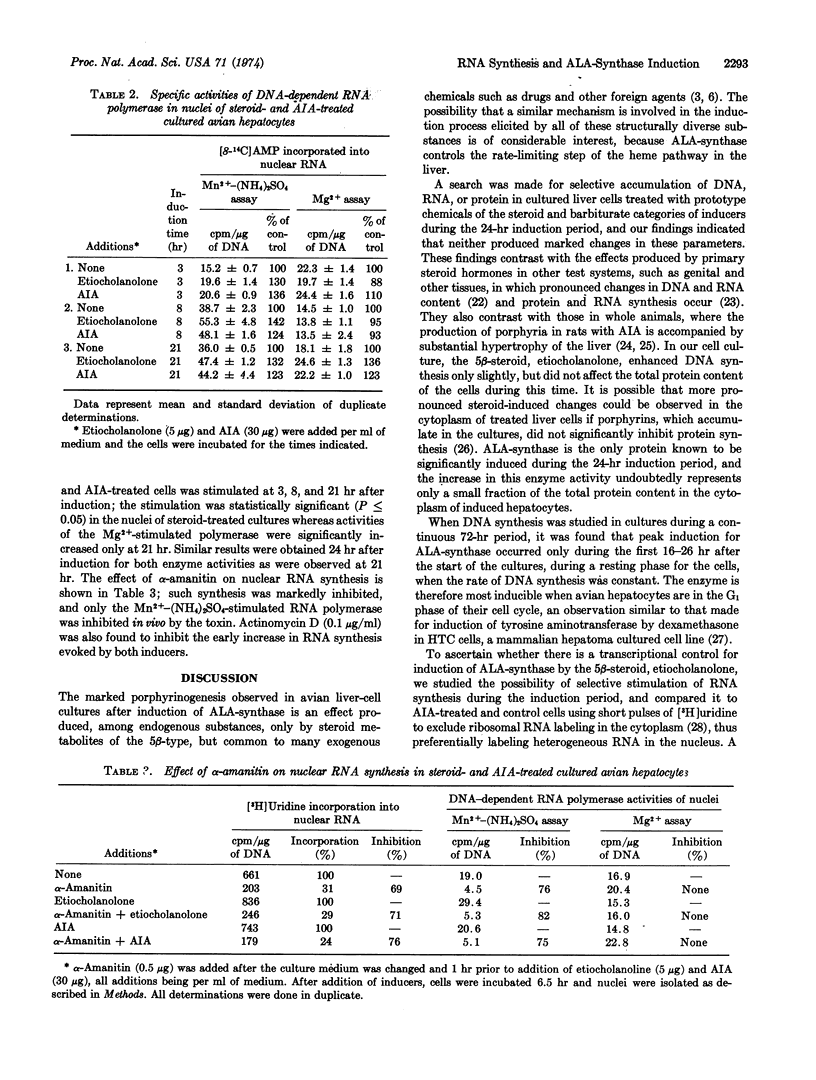
Abstract
The porphyrin-heme pathway is controlled in the liver at the level of the mitochondrial enzyme δ-aminolevulinate synthase (EC 2.3.1.37), a protein inducible in cultured avian hepatocytes by a variety of chemicals including certain 5β-metabolites of steroid hormones. The great sensitivity of the induction process to inhibition by agents known to block transcriptional activity of genetic material suggests that some control mechanism may be operating at this level to regulate the formation of the enzyme. We report here enhancement of nuclear RNA synthesis and of Mn2+-(NH4)2SO4-stimulated DNA-dependent RNA polymerase (EC 2.7.7.6) activities by the 5β-steroid metabolite, 3α-hydroxy-5β-androstan-17-one (etiocholanolone), in cultured avian hepatocytes during induction of the enzyme. These changes were demonstrated in the G1 phase of the hepatocyte cell cycle at a time when DNA synthesis is constant. Our findings support the view that one of the early steps in the process of induction of δ-aminolevulinate synthase by steroid metabolites requires new RNA synthesis, very probably messenger RNA, suggesting a 5β-steroid transcriptional control mechanism for induction of this protein.
Keywords: 3α-hydroxy-5β-androstan-17-one and 2-allyl-2-isopropylacetamide action, porphyrin-heme pathway, RNA polymerases, regulation, protein synthesis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FEIGELSON P., FEIGELSON M. Studies on the mechanism of regulation by cortisone of the metabolism of liver purine and ribonucleic acid. J Biol Chem. 1963 Mar;238:1073–1077. [PubMed] [Google Scholar]
- FRANKLIN R. M., ROSNER J. Localization of ribonucleic acid synthesis in mengovirus-infected L-cells. Biochim Biophys Acta. 1962 Jan 22;55:240–241. doi: 10.1016/0006-3002(62)90960-5. [DOI] [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Granick S., Kappas A. Steroid induction of porphyrin synthesis in liver cell culture. I. Structural basis and possible physiological role in the control of heme formation. J Biol Chem. 1967 Oct 25;242(20):4587–4593. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Hamilton T. H., Widnell C. C., Tata J. R. Synthesis of ribonucleic acid during early estrogen action. J Biol Chem. 1968 Jan 25;243(2):408–417. [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Incefy G. S., Kappas A. Inhibitory effect of a-amanitin on the induction of delta-aminolevulinate synthetase in chick embryo liver. FEBS Lett. 1971 Jun 10;15(2):153–155. doi: 10.1016/0014-5793(71)80045-5. [DOI] [PubMed] [Google Scholar]
- Incefy G. S., Kappas A. Isolation and biochemical characterization of nuclei from chick embryo liver. J Cell Biol. 1971 Aug;50(2):385–398. doi: 10.1083/jcb.50.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incefy G. S., Kappas A. The inhibitory effect of coproporphyrins on amino acid uptake into proteins by porphyric liver cells. FEBS Lett. 1972 Jun 1;23(1):37–40. doi: 10.1016/0014-5793(72)80278-3. [DOI] [PubMed] [Google Scholar]
- Kappas A., Bradlow H. L., Gillette P. N., Gallagher T. F. Abnormal steroid hormone metabolism in the genetic liver disease acute intermittent porphyria. Ann N Y Acad Sci. 1971 Jul 6;179:611–624. doi: 10.1111/j.1749-6632.1971.tb46937.x. [DOI] [PubMed] [Google Scholar]
- Kappas A., Granick S. Steroid induction of porphyrin synthesis in liver cell culture. II. The effects of heme, uridine diphosphate glucuronic acid, and inhibitors of nucleic acid and protein synthesis on the induction process. J Biol Chem. 1968 Jan 25;243(2):346–351. [PubMed] [Google Scholar]
- Kappas A., Song C. S., Levere R. D., Sachson R. A., Granick S. THE INDUCTION OF delta-AMINOLEVULINIC ACID SYNTHETASE in vivo IN CHICK EMBRYO LIVER BY NATURAL STEROIDS. Proc Natl Acad Sci U S A. 1968 Oct;61(2):509–513. doi: 10.1073/pnas.61.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Song C. S., Sassa S., Levere R. D., Granick S. The occurrence of substances in human plasma capable of inducing the enzyme delta-aminolevulinate synthetase in liver cells. Proc Natl Acad Sci U S A. 1969 Oct;64(2):557–564. doi: 10.1073/pnas.64.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedinger C., Gissinger F., Gniazdowski M., Mandel J. L., Chambon P. Animal DNA-dependent RNA polymerases. 1. Large-scale solubilization and separation of A and B calf-thymus RNA-polymerase activities. Eur J Biochem. 1972 Jul 13;28(2):269–276. doi: 10.1111/j.1432-1033.1972.tb01910.x. [DOI] [PubMed] [Google Scholar]
- LOTTSFELDT F. I., LABBE R. F. SOME CYTOLOGIC CHANGES OF RAT LIVER IN EXPERIMENTAL PORPHYRIA. Proc Soc Exp Biol Med. 1965 May;119:226–229. doi: 10.3181/00379727-119-30143. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maul G. G., Hamilton T. H. The intranuclear localization of two DNA-dependent RNA polymerase activities. Proc Natl Acad Sci U S A. 1967 May;57(5):1371–1378. doi: 10.1073/pnas.57.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses H. L., Stein J. A., Tschudy D. P. Hepatocellular changes associated with allylisopropylacetamide-induced hepatic porphyria in rats. Lab Invest. 1970 May;22(5):432–442. [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Pogo A. O., Littau V. C., Allfrey V. G., Mirsky A. E. Modification of ribonucleic Acid synthesis in nuclei isolated from normal and regenerating liver: some effects of salt and specific divalent cations. Proc Natl Acad Sci U S A. 1967 Mar;57(3):743–750. doi: 10.1073/pnas.57.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Granick S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc Natl Acad Sci U S A. 1970 Oct;67(2):517–522. doi: 10.1073/pnas.67.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. Studies on the stimulation by ammonium sulphate of the DNA-dependent RNA polymerase of isolated rat-liver nuclei. Biochim Biophys Acta. 1966 Sep;123(3):478–492. doi: 10.1016/0005-2787(66)90216-4. [DOI] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. Paper disc estimation of radioactive RNA: studies on the presence and elimination of metabolically generated artifacts from labeled purine and pyrimidine precursors. Anal Biochem. 1971 Feb;39(2):319–321. doi: 10.1016/0003-2697(71)90420-9. [DOI] [PubMed] [Google Scholar]