Abstract
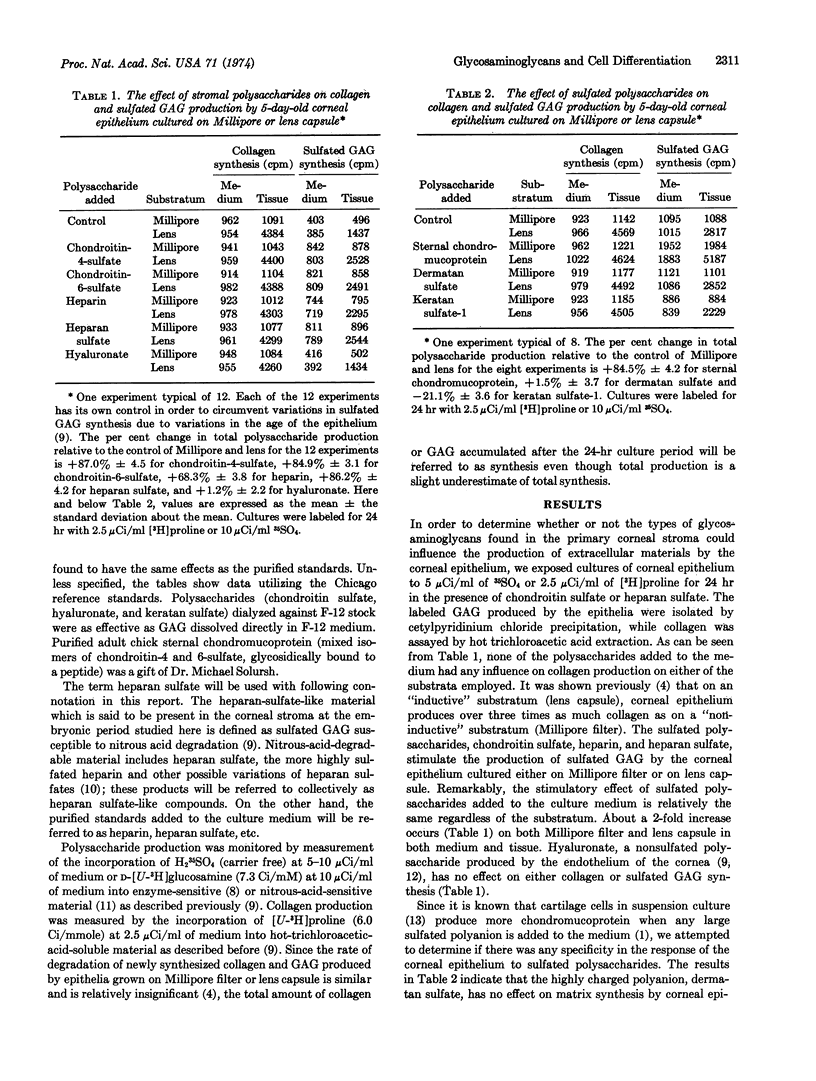
Previously, it was demonstrated that the embryonic corneal epithelium produces the chondroitin sulfate and heparan-sulfate-like compounds and the collagen of the primary corneal stroma. Synthesis of all of these extracellular materials is greatly enhanced in vitro when isolated epithelium is grown on collagenous substrata instead of Millipore filters. We report here that chondroitin sulfate, heparin, and heparan sulfate added to the culture medium at a concentration of 200 μg/ml enhance the synthesis by the epithelium of chondroitin sulfate and heparan-sulfate-like compounds 2-fold, whether or not collagenous substrata are employed. Collagen synthesis is unaffected by adding glycosaminoglycan to the medium. Chondroitin sulfate proteoglycan (chondromucoprotein) has the same stimulatory effect as chondroitin sulfate, but dermatan sulfate and hyaluronate have no measurable effect on glycosaminoglycan production by epithelial cells. Keratan sulfate however, seems to depress glycosaminoglycan synthesis. Thus, in this system, only sulfated polyanions like those produced by the corneal epithelium have a stimulatory effect on glycosaminoglycan synthesis. The results are discussed in terms of how the tissues of the cornea (epithelium, endothelium, keratocytes) may interact by changing the composition of the stromal extracellular matrix.
Keywords: epithelium, tissue interaction
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernfield M. R., Banerjee S. D., Cohn R. H. Dependence of salivary epithelial morphology and branching morphogenesis upon acid mucopolysaccharide-protein (proteoglycan) at the epithelial surface. J Cell Biol. 1972 Mar;52(3):674–689. doi: 10.1083/jcb.52.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad G. W. Collagen and mucopolysaccharide biosynthesis in mass cultures and clones of chick corneal fibroblasts in vitro. Dev Biol. 1970 Apr;21(4):611–635. doi: 10.1016/0012-1606(70)90080-1. [DOI] [PubMed] [Google Scholar]
- Dodson J. W., Hay E. D. Secretion of collagenous stroma by isolated epithelium grown in vitro. Exp Cell Res. 1971 Mar;65(1):215–220. doi: 10.1016/s0014-4827(71)80069-1. [DOI] [PubMed] [Google Scholar]
- Hay E. D., Dodson J. W. Secretion of collagen by corneal epithelium. I. Morphology of the collagenous products produced by isolated epithelia grown on frozen-killed lens. J Cell Biol. 1973 Apr;57(1):190–213. doi: 10.1083/jcb.57.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay E. D., Revel J. P. Fine structure of the developing avian cornea. Monogr Dev Biol. 1969;1:1–144. [PubMed] [Google Scholar]
- Kosher R. A., Lash J. W., Minor R. R. Environmental enhancement of in vitro chondrogenesis. IV. Stimulation of somite chondrogenesis by exogenous chondromucoprotein. Dev Biol. 1973 Dec;35(2):210–220. doi: 10.1016/0012-1606(73)90018-3. [DOI] [PubMed] [Google Scholar]
- Kosher R. A., Searls R. L. Sulfated mucopolysaccharide synthesis during the development of Rana pipiens. Dev Biol. 1973 May;32(1):50–68. doi: 10.1016/0012-1606(73)90219-4. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. I. Membrane-associated and cell-sap species in Chinese hamster cells. Biochemistry. 1971 Apr 13;10(8):1437–1445. doi: 10.1021/bi00784a026. [DOI] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Synthesis of sulfated glycosaminoglycans by embryonic corneal epithelium. Dev Biol. 1973 Dec;35(2):318–331. doi: 10.1016/0012-1606(73)90027-4. [DOI] [PubMed] [Google Scholar]
- Nevo Z., Dorfman A. Stimulation of chondromucoprotein synthesis in chondrocytes by extracellular chondromucoprotein. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2069–2072. doi: 10.1073/pnas.69.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Z., Horwitz A. L., Dorfmann A. Synthesis of chondromucoprotein by chondrocytes in suspension culture. Dev Biol. 1972 May;28(1):219–228. doi: 10.1016/0012-1606(72)90139-x. [DOI] [PubMed] [Google Scholar]
- Solursh M., Meier S. A conditioned medium (CM) factor produced by chondrocytes that promotes their own differentiation. Dev Biol. 1973 Feb;30(2):279–289. doi: 10.1016/0012-1606(73)90089-4. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Jackson G., Gross J. Hyaluronate in morphogenesis: inhibition of chondrogenesis in vitro. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1384–1386. doi: 10.1073/pnas.69.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P., Trelstad R. L. Hyaluronate production and removal during corneal development in the chick. Dev Biol. 1971 Sep;26(1):28–35. doi: 10.1016/0012-1606(71)90104-7. [DOI] [PubMed] [Google Scholar]


