Abstract
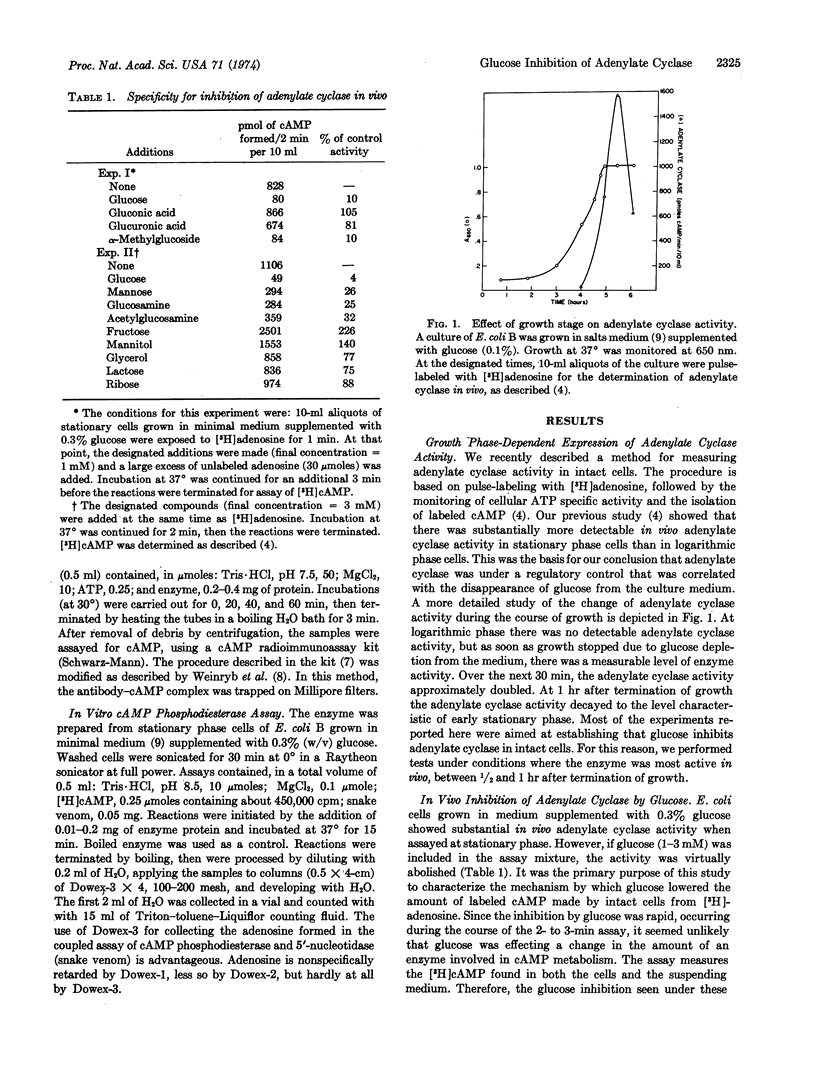
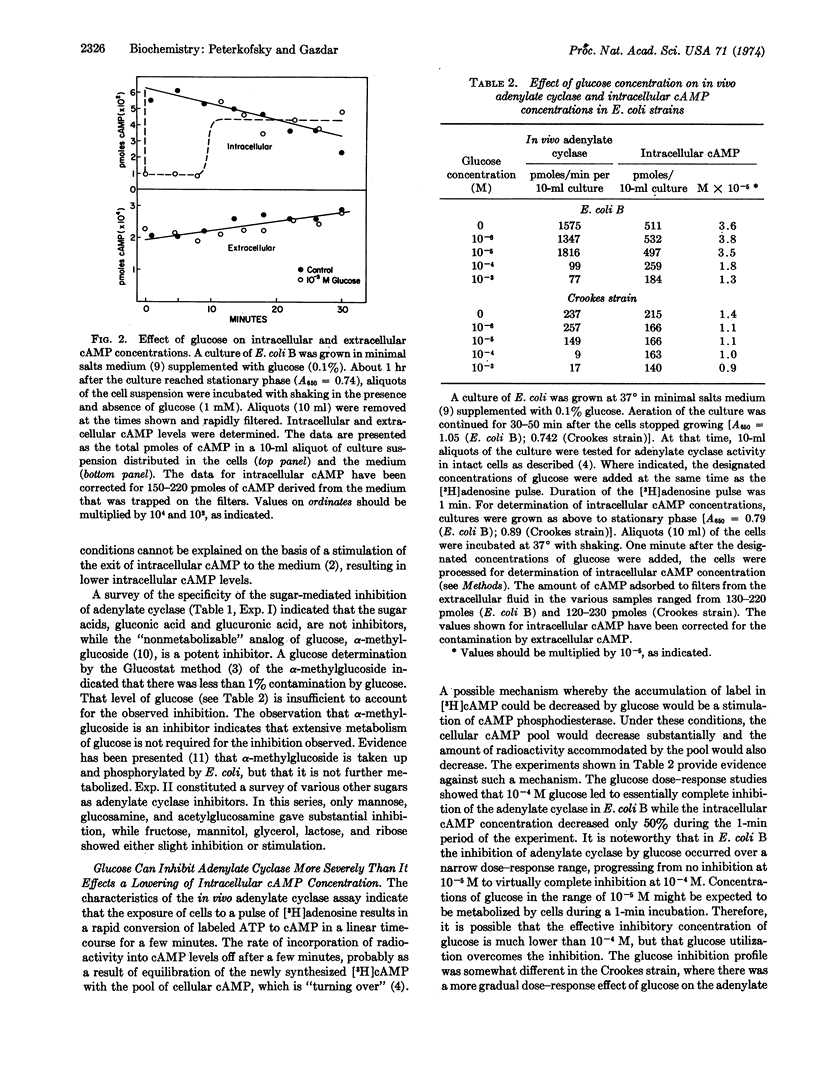
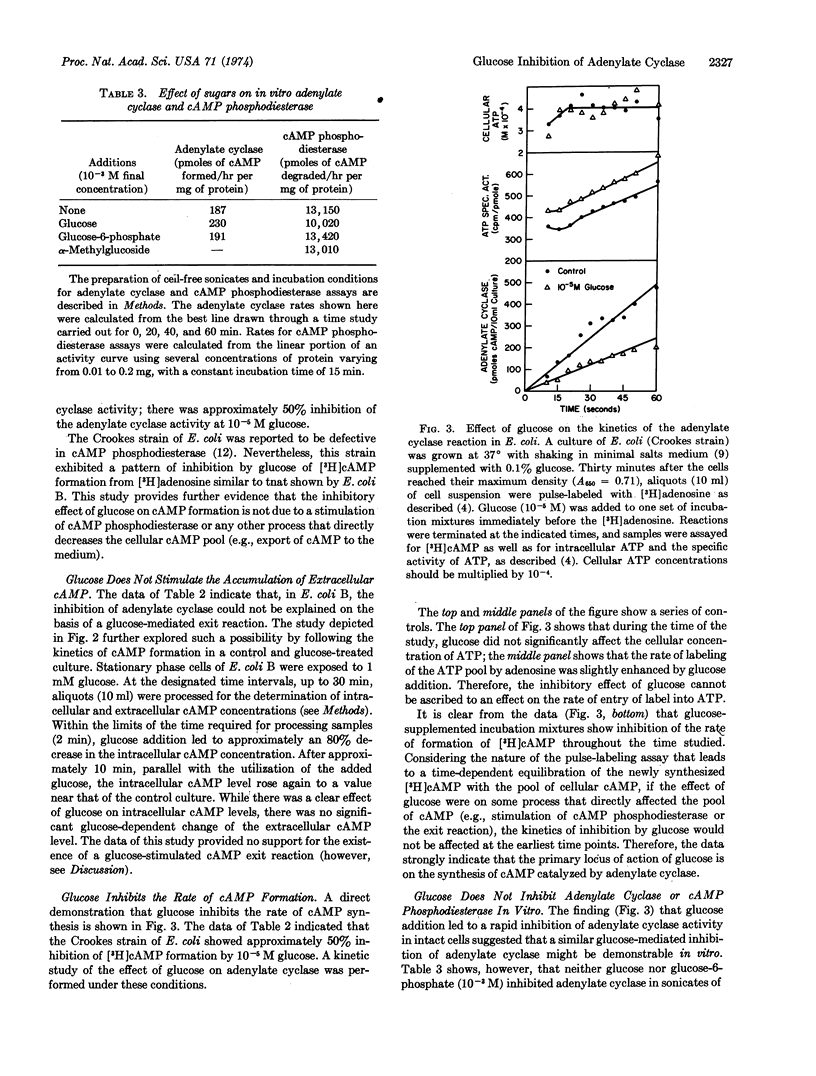
Previous studies in E. coli B have demonstrated an inverse correlation between the presence of glucose in the medium and the accumulation of cyclic AMP in the medium. This observation could not be explained by the action of glucose as a repressor of adenylate cyclase (EC 4.6.1.1) synthesis, as a stabilizer of cyclic AMP phosphodiesterase (EC 3.1.4.17) activity, or as a direct inhibitor of adenylate cyclase activity in cell-free preparations. The recent development of an in vivo assay for adenylate cyclase has provided a basis for further exploring the inhibitory action of glucose in intact cells. With this assay it has been possible to show that, while glucose does not affect adenylate cyclase in vitro, it rapidly inhibits the enzyme activity in intact cells. Extensive metabolism of glucose is not required, since α-methylglucoside also inhibits adenylate cyclase in vivo. When cells are grown on glucose as carbon source, some sugars (mannose, glucosamine) substitute for glucose as adenylate cyclase inhibitors while others (e.g., fructose) do not. Dose-response studies indicate that low concentrations of glucose lead to essentially complete inhibition of adenylate cyclase activity while only moderately decreasing intracellular cyclic AMP concentrations. The evidence presented suggests that the decreased cellular cyclic AMP levels resulting from glucose addition can be accounted for by inhibition of adenylate cyclase without any significant effect on cyclic AMP phosphodiesterase or the transport of cyclic AMP from the cells to the medium.
Keywords: glucose effect, catabolite repression
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buettner M. J., Spitz E., Rickenberg H. V. Cyclic adenosine 3',5'-monophosphate in Escherichia coli. J Bacteriol. 1973 Jun;114(3):1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIHIRA H., WILSON T. H., LIN E. C. STUDIES ON THE GLUCOSE-TRANSPORT SYSTEM IN ESCHERICHIA COLI WITH ALPHA-METHYLGLUCOSIDE AS SUBSTRATE. Biochim Biophys Acta. 1963 Nov 15;78:505–515. doi: 10.1016/0006-3002(63)90912-0. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Nielsen L. D., Monard D., Rickenberg H. V. Cyclic 3',5'-adenosine monophosphate phosphodiesterase of Escherichia coli. J Bacteriol. 1973 Nov;116(2):857–866. doi: 10.1128/jb.116.2.857-866.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose and the metabolism of adenosine 3':5'-cyclic monophosphate in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2794–2798. doi: 10.1073/pnas.68.11.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Measurements of rates of adenosine 3':5'-cyclic monophosphate synthesis in intact Escherichia coli B. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2149–2152. doi: 10.1073/pnas.70.7.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S. Inducible system for the utilization of beta-glucosides in Escherichia coli. I. Active transport and utilization of beta-glucosides. J Bacteriol. 1967 Jan;93(1):254–263. doi: 10.1128/jb.93.1.254-263.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M., Huberman A. Some properties of Escherichia coli adenyl cyclase. Arch Biochem Biophys. 1970 Nov;141(1):236–240. doi: 10.1016/0003-9861(70)90127-x. [DOI] [PubMed] [Google Scholar]
- Tao M., Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci U S A. 1969 May;63(1):86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weinryb I., Michel I. M., Hess S. M. Radioimmunoassay of cyclic AMP without precipitating antibody. Anal Biochem. 1972 Feb;45(2):659–663. doi: 10.1016/0003-2697(72)90229-1. [DOI] [PubMed] [Google Scholar]


