Abstract
An ethyl diazomalonyl derivative of cymarin was synthesized in order to photoaffinity label the cardiac glycoside-binding site on (Na+ + K+) adenosinetriphosphate (EC 3.6.1.3). When a noncovalent complex of the enzyme and this cardiac glycoside derivative was photolyzed, a covalent bond was formed between the ligand and the larger of the two polypeptide subunits of the enzyme. Several control experiments demonstrate that this photochemical reaction occured while the ligand was bound to the site at which it inhibits the enzyme activity. Another specific inhibitor, tentatively identified as the ethyl chloromalonyl derivative of cymarin, produced similar photoaffinity labeling of the larger subunit, demonstrating that the photolytic dissociation of the diazo group may not be responsible for the photochemical reaction. Since the cardiac glycoside-binding site, which is accessible from the outside surface of the plasma membrane, and the phosphorylation site, which is accessible from the inside surface, are both on the larger polypeptide subunit of (Na+ + K+) adenosinetriphosphatase, this polypeptide has sequences exposed to both sides of the membrane.
Keywords: cardiac glycoside, membrane enzyme, active transport
Full text
PDF
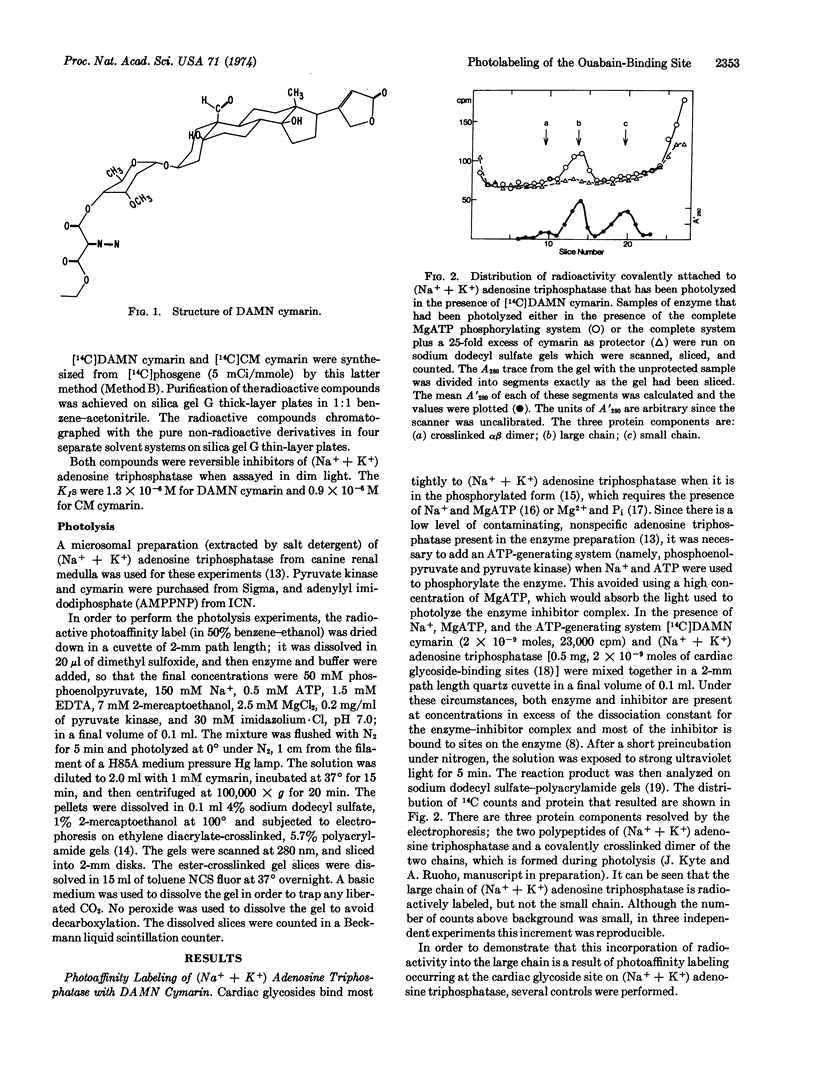


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERS R. W., FAHN S., KOVAL G. J. THE ROLE OF SODIUM IONS IN THE ACTIVATION OF ELECTROPHORUS ELECTRIC ORGAN ADENOSINE TRIPHOSPHATASE. Proc Natl Acad Sci U S A. 1963 Sep;50:474–481. doi: 10.1073/pnas.50.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choules G. L., Zimm B. H. An acrylamide gel soluble in scintillation fluids: its application to electrophoresis at neutral and low pH. Anal Biochem. 1965 Nov;13(2):336–344. doi: 10.1016/0003-2697(65)90202-2. [DOI] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet G. W., Knowles J. R., Porter R. R. The antibody binding site. Labelling of a specific antibody against the photo-precursor of an aryl nitrene. Biochem J. 1972 Jul;128(3):499–508. doi: 10.1042/bj1280499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrow C. E., Rasmussen H., Brunswick D. J., Cooperman B. S. Specific photoaffinity labeling of the adenosine 3':5'-cyclic monophosphate receptor in intact ghosts from human erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3344–3346. doi: 10.1073/pnas.70.12.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hexter C. S., Westheimer F. H. Intermolecular reaction during photolysis of diazoacetyl -chymotrypsin. J Biol Chem. 1971 Jun 25;246(12):3928–3933. [PubMed] [Google Scholar]
- Hokin L. E., Mokotoff M., Kupchan S. M. Alkylation of a brain transport adenosinetriphosphatase at the cardiotonic steroid site by strophanthidin-3-haloacetates. Proc Natl Acad Sci U S A. 1966 Apr;55(4):797–804. doi: 10.1073/pnas.55.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J. Phosphorylation of a purified (Na + +K + ) adenosine triphosphatase. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1259–1265. doi: 10.1016/s0006-291x(71)80008-6. [DOI] [PubMed] [Google Scholar]
- Kyte J. Properties of the two polypeptides of sodium- and potassium-dependent adenosine triphosphatase. J Biol Chem. 1972 Dec 10;247(23):7642–7649. [PubMed] [Google Scholar]
- Kyte J. Purification of the sodium- and potassium-dependent adenosine triphosphatase from canine renal medulla. J Biol Chem. 1971 Jul 10;246(13):4157–4165. [PubMed] [Google Scholar]
- Kyte J. The titration of the cardiac glycoside binding site of the (Na+ + K+)-adenosine triphosphatase. J Biol Chem. 1972 Dec 10;247(23):7634–7641. [PubMed] [Google Scholar]
- Martin N., Warner N. L., Roeder P. E., Singer S. J. Affinity labeling of a mouse IgG2a myeloma protein with binding affinity for nitrophenyl ligands. Biochemistry. 1972 Dec 19;11(26):4999–5005. doi: 10.1021/bi00776a020. [DOI] [PubMed] [Google Scholar]
- Matsui H., Schwartz A. Mechanism of cardiac glycoside inhibition of the (Na+-K+)-dependent ATPase from cardiac tissue. Biochim Biophys Acta. 1968 Mar 25;151(3):655–663. doi: 10.1016/0005-2744(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Ruoho A. E., Hokin L. E., Hemingway R. J., Kupchan S. M. Hellebrigenin 3-haloacetates: potent site-directed alkylators of transport adenosinetriphosphatase. Science. 1968 Mar 22;159(3821):1354–1355. doi: 10.1126/science.159.3821.1354. [DOI] [PubMed] [Google Scholar]
- Ruoho A. E., Kiefer H., Roeder P. E., Singer S. J. The mechanism of photoaffinity labeling. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2567–2571. doi: 10.1073/pnas.70.9.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Siegel G. J., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase. IV. Characterization of the phosphoprotein formed from orthophosphate in the presence of ouabain. J Biol Chem. 1969 Jun 25;244(12):3264–3269. [PubMed] [Google Scholar]
- Singer S. J. Covalent labeling of active sites. Adv Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Martin N., Thorpe N. O. Affinity labeling of the active sites of antibodies and myeloma proteins. Ann N Y Acad Sci. 1971 Dec 31;190:342–351. doi: 10.1111/j.1749-6632.1971.tb13546.x. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Stefanovsky Y., Westheimer F. H. Diazoacetyl subtilisin. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1132–1136. doi: 10.1073/pnas.70.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan R. J., Westheimer F. H. A titration method for bovine trypsin. Anal Biochem. 1969 May;29(2):305–310. doi: 10.1016/0003-2697(69)90314-5. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J. 1962 Jul;84:110–118. doi: 10.1042/bj0840110. [DOI] [PMC free article] [PubMed] [Google Scholar]


