Abstract
The production of different types of blood cells including their formation, development, and differentiation is collectively known as haematopoiesis. Blood cells are divided into three lineages erythriod (erythrocytes), lymphoid (B and T cells), and myeloid (granulocytes, megakaryocytes, and macrophages). Haematopoiesis is a complex process regulated by several mechanisms including microRNAs (miRNAs). miRNAs are small RNAs which regulate the expression of a number of genes involved in commitment and differentiation of hematopoietic stem cells. Evidence shows that miRNAs play an important role in haematopoiesis; for example, myeloid and erythroid differentiation is blocked by the overexpression of miR-15a. miR-221, miR-222, and miR-24 inhibit the erythropoiesis, whereas miR-150 plays a role in B and T cell differentiation. miR-146 and miR-10a are downregulated in megakaryopoiesis. Aberrant expression of miRNAs was observed in hematological malignancies including chronic myelogenous leukemia, chronic lymphocytic leukemia, multiple myelomas, and B cell lymphomas. In this review we have focused on discussing the role of miRNA in haematopoiesis.
1. Background
MicroRNAs (miRNAs) are 20–22 nucleotides long small noncoding RNAs that can bind to the 3′UTR or 5′UTR or in ORF of target mRNA resulting in translational repression or mRNA degradation based on degree of homology. It is believed that miRNAs regulate gene expression in multicellular organisms, but miRNAs are also identified in unicellular algae Chlamydomonas reinhardtii [1]. Interestingly it has been shown that miRNAs can activate the translation. miRNA-122 is specifically expressed in liver where; it plays vital role in fatty acid metabolism and enhances the replication of hepatitis C virus (HCV) RNA by binding to its 5′UTR [2–4]. Ørom et al. found that miR-10a binds to the messenger RNAs (mRNAs) encoding ribosomal proteins to enhance the translation of proteins and ribosomal biogenesis [5]. Due to increase in cloning and computational approaches, there has been a tremendous increase in the number of newly found miRNAs. A total of 9169 miRNAs have been found in different species among which human genome codes for 1424 miRNAs [5]. It has been found that 60% of the human mRNA contains miRNA binding sites. Each mRNA is targeted by many miRNAs conversely and each miRNA can target many mRNAs. miRNAs exhibit different characteristics in plants and mammals. In plants, miRNAs require perfect match with their target mRNAs, whereas in mammals miRNA complementarily covers 2–7 bases, also known as the seed region [6, 7]. In mammals, miRNA target sites are mostly in the 3′UTR region and rarely in 5′UTR and coding regions also, whereas, in case of plants target sites are mostly in the coding region. The mechanism by which a miRNA can diminish protein expression is unclear, but several proposals are there from different experimental evidences. miRNAs can interfere with translation process at the stage of initiation (Figure 2) or elongation (Figure 3), or target mRNA may be affected by isolating it from ribosomal machinery [8–10].
Figure 2.
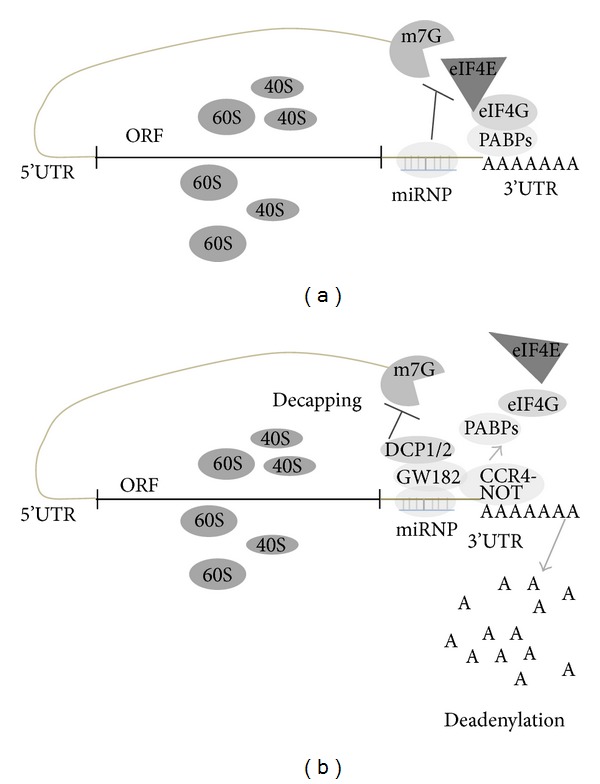
miRNA mediated translation repression. (a) At initiation stage the miRNP (miRNA ribonucleoprotein complex) impairs the recognition of cap by eIF4E there by inhibiting the recruitment of ribosomal subunits onto the mRNA. (b) miRNA mediated degradation of mRNA by deadenylation of 3′−5′ exonuclease after recruiting CCR4-NOT to the polyadenylation site where GW182 is required to bind to miRNPs. Replacement of cap by decapping enzymes DCP1/2 hampers the translation initiation.
Figure 3.
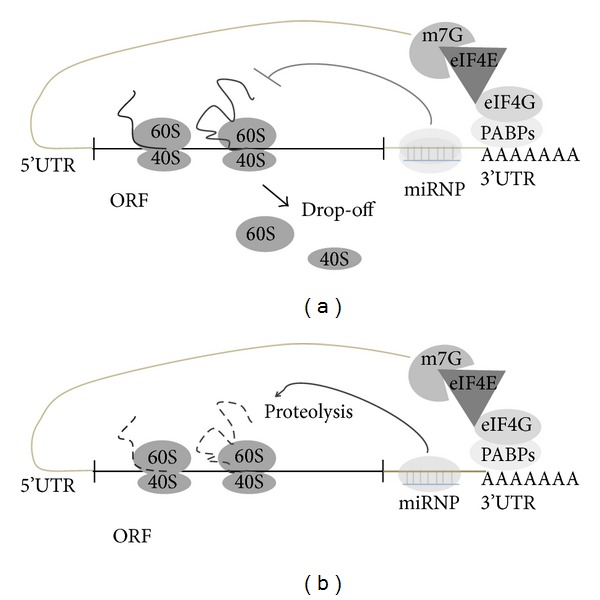
miRNA mediated regulation of translation at postinitiation stage. (a) Ribosome drop-off is the proposed mechanism where translation is initiated and miRNA directed ribosomes to inhibit the translation prematurely. (b) Other possible mechanisms of miRNA mediated translation repression are nascent polypeptides which are degraded by proteosomes.
The experimental evidences indicate that miRNA regulates translation inhibition at initiation (Figure 2) or later stages of translation (Figure 3). Binding of eIF4E to the cap region of mRNA is the initiation of the assembly of the initiation complex; it is identified that miRNA interfere with the eIF4E and impairs its function and poly(A) tail function is also inhibited [11]. There are other evidences suggesting that miRNAs repress translation at later stages of initiation. miRNA lin-4 target the lin-14 and lin-28 mRNAs, but under inhibitory conditions mRNAs of lin-14, lin-28 are not altered indicating that miRNAs inhibit translation after the initiation stage. Interestingly in both cap dependent and cap independent translation mRNAs are inhibited by synthetic miRNA suggesting postinitiation inhibition. Another mechanism by which miRNA inhibit translation is by ribosome drop off, in which ribosomes which are engaged in translation are directed to terminate translation prematurely (Figure 3(a)). There is other proposed mechanisms that miRNAs are degrading the nascent polypeptides by recruiting the proteolytic enzymes (Figure 3(b)) [12, 13].
2. Biogenesis of miRNA
There are various proteins involved in miRNA biogenesis (Table 2). miRNAs are synthesised from coding or noncoding part of genes (promoter, introns, and exons) by RNA polymerase II into a precursor called pri-miRNA. The pri-miRNA is processed by the enzyme Drosha and cleaved into 70–120 nucleotides called precursor miRNA (pre-miRNA). The recombinant Drosha is unable to produce pre-miRNA suggesting that other cofactors may be required for its action. DGCR8 an important cofactor is required for the processing of pri-miRNA and is believed to recognize the cleavage site between ssRNA and stem of pri-miRNA. It is approximately 11 base pairs away from the dsRNA-ssRNA junction [79]. Interestingly some miRNAs are processed independent of Drosha to generate pre-miRNA from introns with the association of debranching enzyme and spliceosome [80]. Pre-miRNA is exported from the nucleus to the cytoplasm [81] by Exportin-5 and it is processed to 19–22 nucleotide duplex by Dicer [82]. Like Drosha, Dicer contains associated proteins TRBP, PACT which increases Dicer stability and activity. There are various isoforms of Dicer and the roles of these isoforms are not known [83, 84]. The mature miRNA then associates with a protein complex known as RNA induced silencing complex (RISC) at 3′UTR or 5′UTR or in ORF of target genes [10] (Figure 1). Complementarity between target mRNA and miRNA particularly in seed region is important in determining the miRNA target sites. The mechanism of miRNA mediated gene silencing is not clearly explored till now and it has been known that miRNA suppresses the expression of a gene by inhibiting the translation of its mRNA. Other major functions of the miRNA are removal of mRNA from translation machinery and associating it with processing bodies (P-bodies) where mRNA is degraded [85]. It has been reported that miRNA association with mRNA degrades the mRNA through decapping and deadenylation of mRNA [86]. By repressing the mRNAs, miRNAs are involved in gene expression changes leading to regulation of different biological aspects including proliferation, differentiation, apoptosis, immune response, ageing, and metabolism.
Table 2.
Proteins involved in miRNA biogenesis.
| Gene | Description | Location | Function | Domains | Reference |
|---|---|---|---|---|---|
| Dicer | Dicer 1, ribonuclease type II | 14q31 | miRNA processing | Type 111 restriction enzyme, RNase3, DEAH, helicase, dsRBD, PAZ | [14] |
|
| |||||
| AGO3 | Argonaute 3 | 1p34-p34 | Short-interfering-RNA-mediated gene silencing | PAZ, PIWI, DUF1785, DUF2344, DUF2678 | [15] |
|
| |||||
| Gemin3 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 20 | 1p13.2 | RNA helicase | DEAD, Type 111 restriction enzyme, helicase C | [16] |
|
| |||||
| Drosha | Drosha, ribonuclease type II | 5p14-p13 | Pri-miRNA processing | RNase3 domain, Double-stranded RNA binding motif | [17] |
|
| |||||
| Exportin-5 | Karyopherin family | 6p21.1 | Transport of small RNAs | Importin-beta N-terminal domain, exportin 1-like protein | [18] |
|
| |||||
| FMRP | Fragile X mental retardation 1 | Xq27.3 | mRNA trafficking | KH domain | [19] |
|
| |||||
| ADAR | Adenosine deaminase, RNA-specific | 1q21.3 | RNA and miRNA editing | z-Alpha, Dsrm, Editase | [20] |
|
| |||||
| TRBP | TAR (HIV-1) RNA binding protein | 12q12, q-13 | Dicer stabilization | DZF, dsRBD | [21] |
|
| |||||
| Importin-8 | GTPase Ran mediate nuclear import | 12p11.21 | Nuclear localization of Argonaute proteins | Cse1 | [22] |
|
| |||||
| ELAV1 | (Embryonic lethal, abnormal vision, Drosophila-like 1) | 19p13.2 | Repression of target sites | RBD | [23] |
|
| |||||
| Dnd1 | Dead end homolog 1 | 5q31.3 | Inhibiting miRNA-mediated repression | PMP-22 | [24] |
Figure 1.
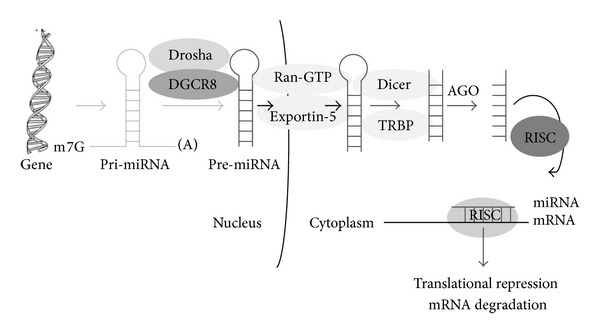
Biogenesis of miRNA. miRNAs are transcribed into pri-miRNA and are capped and polyadenylated. This pri-miRNA is processed by Drosha and DGCR8 into pre-miRNA, which by Ran-GTP and Exportin-5 are transported into the cytoplasm and further processed by Dicer. The miRNA dissociates and with help of RISC gets involved in gene silencing by translation repression or degradation of target mRNA.
3. Detection of miRNA
miRNA detection enable us to study the regulation of miRNA in various biological processes. There are various methods to detect the mature forms of miRNA which include deep sequencing [87], microarrays [88], northern blot [89], and real-time PCR [90]. In microarray analysis the first step is isolation of the total RNA which contains miRNA and here enrichment of small RNA enhances the sensitivity of the detection. This RNA is labeled and hybridized to designed probes specific for a miRNA and later studying the signal intensities we can measure the levels of miRNA in a sample. Microarray analysis can give the data of known miRNAs and it cannot be quantified, but it is useful to assess the miRNA in two different samples relatively. To find new miRNAs deep sequencing can be used, but it is not very established technique; it includes sequencing RNA and analyzing the folding properties and validating the data by northern blot or real-time PCR [87].
4. Prediction of miRNA Targets
Many studies indicate that deregulation of miRNAs results in various disorders including cancer, diabetes, metabolic disorders, and cardiovascular disorders, so understanding of miRNA regulation in various pathologies enables us to solve the diagnostic and therapeutic challenges. To understand the association of miRNA and mRNA many computational based databases have been developed which predict possible targets of miRNAs by using different algorithms (Table 3). All these tools recognize miRNA targets based on seed sequence of miRNA and 3′UTR of target genes. Hence it becomes necessary to validate these targets by experimental approaches such as luciferase assay, RNA interference, microarray, pulsed (p) SILAC, and Argonaute HITS-CLIP [88–91]. The nomenclature of miRNA is a sequential process given in Table 1.
Table 3.
miRNA target prediction tools.
| Database | Description | URL | Reference |
|---|---|---|---|
| miRSystem | Predict the target genes and pathways | http://mirsystem.cgm.ntu.edu.tw/ | [25] |
| miRanda | Predict targets by finding high complementarity regions in 3'UTR | http://www.microrna.org | [26] |
| TargetScan | Detect target genes by perfect complementarity to the seed region | http://www.targetscan.org | [27] |
| PicTar | Seed match, binding energy, conservation | http://pictar.mdc-berlin.de/ | [28] |
| DIANA-microT | Based on affinity interaction between miRNA and mRNA | http://diana.cslab.ece.ntua.gr/ | [29] |
| Mire | Based on miRNA; mRNA duplex stability properties | http://didattica-online.polito.it/eda/miREE/ | [30] |
| RNA22 | Detect targets by pattern recognition and folding energy | http://cbcsrv.watson.ibm.com/rna22.html | [31] |
| Tar Base | Curated database for experimentally tested miRNA targets |
http://diana.cslab.ece.ntua.gr/tarbase/ | [32] |
| miRNA MAP | Collection of experimentally verified miRNA targets | http://mirnamap.mbc.nctu.edu.tw/ | [33] |
| MiRSel | Extraction of miRNA; gene interactions from the literature | http://services.bio.ifi.lmu.de/mirsel/ | [34] |
| miRecords | Targets identified by 11 target prediction programmes | http://mirecords.biolead.org/ | [35] |
| MiRTarBase | Targets collected manually from the literature | http://mirtarbase.mbc.nctu.edu.tw/ | [36] |
| miRWalk | Target identification by complimentarity (perl language) | http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/ | [37] |
| Star Base | Use CLIP-Seq and Degradome-Seq data for miRNA target identification | http://starbase.sysu.edu.cn/ | [38] |
| VHoT | It gives information of viral miRNA relation with host | http://acl.snu.ac.kr/vhot/ | [39] |
| OMIT | Based on ontology design, data integration | http://bioportal.bioontology.org/ontologies/OMIT | [40] |
| MiRPara | It uses support vector machine based software | http://159.226.126.177/mirpara/ | [41] |
Table 1.
miRNA nomenclature.
| Notation | Description |
|---|---|
| “hsa” (Eg. hsa-miR-21) | Species name (Homo sapiens) Eg. Mmu—mus musculus rno—Rattus norvegicuscel—Caenorhabditis elegans ath—Arabidopsis thalianadme- Drosophila melanogaster |
| “miR” (Eg. hsa-mir-17) | Denotes immature form of miRNA (pre-miRNA) or primary transcript or genomic locus |
| “miR” (Eg. has-miR-10) | Refers to the mature form of miRNA |
| a and b notation (Eg. miR-147a, miR-147b) | When two miRNAs are similar except in 2 or 3 nt, then they are denoted by lowercased letters |
| Additional numbers in names (Eg. miR-16-1, miR-16-2) |
In case of two miRNAs are 100% similar, but they are located on different chromosomes and then they are denoted by extra dash followed by number |
| “∗” notation (Eg. miR-56/ miR-56*) | If the same precursor miRNA produces two miRNAs, then the less predominant one is denoted by∗ |
| 3p- and 5p- notation (Eg. miR-56-3p, miR-56-5p) |
If the data is not sufficient to know which one is predominant, then it is written as 3p- or 5p-. 3p- and 5p- indicate that it is derived from 3′, 5′ arms, respectively. |
4.1. Ago HITS-CLIP
Genome-wide functional protein-RNA interaction sites on RNA can be identified by HITS-CLIP, high throughput sequencing of RNAs isolated by cross linking, and immunoprecipitation (Figure 4). In this method RNA and bound protein Argonaute are cross linked by ultraviolet light which gives Arg-miRNA and Arg-RNA binding sites. The remaining RNA is digested and then the RNA and RNA complexes are isolated by immunoprecipitation which are sequenced by next generation sequencers. Comparison of these two datasets helps us to know the miRNA target sites [91].
Figure 4.
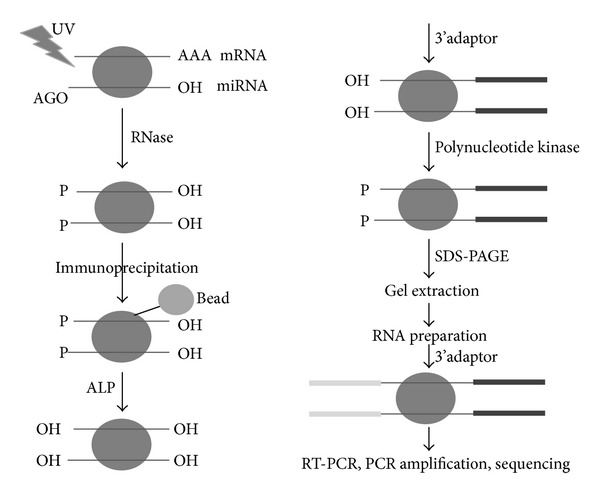
Scheme of Argonaute high-throughput sequencing of RNAs by cross linking and immunoprecipitation. RNA and protein are cross linked by UV and then RNA is digested by RNase, a treatment which is finally immunoprecipated. 5′ ends are dephosphorylated and 3′ ends are adapter ligated followed by phosphate addition at 5′ ends. The complexes of RNA and protein are separated by SDS-PAGE and RNA are amplified after 5′-adaptor ligation. These amplified products will be sequenced by next generation sequencers and finally computational approaches will help identify the miRNA target.
4.2. Microarray and qRT-PCR
miRNAs target the corresponding mRNA and reduce its stability by degrading it. miRNA target can be detected by overexpressing the miRNA in a cell line that does not express the miRNA. Comparison of control and miRNA overexpression in cell lines using qRT-PCR and microarray will enable the detection of miRNA target or its associated pathway [87].
4.3. SILAC
Baek et al. have shown the use of stable isotope labeling using amino acids in cell culture (SILAC) in the identification of miRNA targets [92]. In this method proteins of the culture medium are labeled with radio-labeled amino acids. For the identification of miRNA targets SILAC method is slightly modified, instead of radio-labeled amino acid, and heavy isotope is added to the growth medium for short time to know the newly synthesized proteins. Heavy and medium heavy isotope signal intensities are measured, if the mRNA is the target of a miRNA, the heavy medium isotope signal intensity is decreased; otherwise there is no change in the intensities (i.e., normal and malignant tissue). Protein analysis by mass spectrometry combined with SILAC has strong correlation with miRNA activity [93] (Figure 5).
Figure 5.
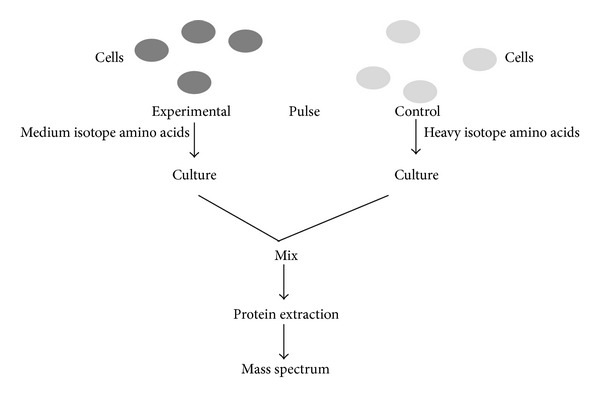
Schematic representation of pulsed SILAC. Proteins of the culture medium of control and experimental sample are labelled with heavy and medium isotopes, respectively, by adding them to growth medium. After short time comparison of heavy and medium isotope signal intensities miRNA target mRNA will be detected.
5. Biological Roles of miRNA
miRNAs regulate several biological processes such as apoptosis, insulin secretion, lipid metabolism, stem cell differentiation, heart development, muscle differentiation, cardiomyocyte hypertrophy, antigen presentation, and ageing. In this section biological roles of miRNA are described briefly.
5.1. Apoptosis
Apoptosis is a regulated process of cell death necessary for normal development and homeostasis. Aberrant expression of miRNA leads to failure of apoptosis finally resulting in evolution of cancer. Even though the exact role of miRNA in the apoptosis is not well understood, there are several instances of its role in apoptosis. Cimmino et al. showed that miR-15 and miR-16 induce apoptosis by targeting Bcl2 [94], whereas miR-330 induces apoptosis in PC-3 cells by reducing the E2F1 mediated Akt phosphorylation [95]. Interestingly, miR-21 acts as an antiapoptotic factor and its knockdown activates caspase activity and increased apoptotic cell death in glioblastoma cells [96].
5.2. Insulin Secretion
Diabetes is the most common metabolic disorder in the world. miRNAs have been found to be involved in the several physiological processes including glucose homeostasis. It was reported that some miRNAs are important for the release of insulin. miR-375 is essential for β-cell survival, proliferation, and division. The expression level of transcription factor Onecut-2 was reduced, whereas the level of granuphilin was increased by miR-9 which is a negative regulator of insulin release [97]. Surprisingly, knockdown of miR-24, miR-26, miR-182, or miR-148 in cultured β-cells also resulted in reduced insulin mRNA level [98].
5.3. Lipid Metabolism
miRNAs have been shown to be important regulators of lipid metabolism also. miR-33 downregulates ABC transporters there by controlling the cholesterol efflux and HDL biogenesis. Decreased serum cholesterol levels were observed due to miR-122 inhibition and it has been shown that it maintains the hepatic cell phenotype [99, 100]. In addition, miR-33a is involved in the cholesterol export and β-oxidation of fatty acids. Its inhibition leads to increased levels of HDL in mice [101, 102].
5.4. Immunity
It has been reported that CD34+ hematopoietic stem cells express 33 miRNAs, among which miR-155 regulates both myelopoiesis and erythropoiesis [103]. In ES (expand) cells, miRNAs regulate Sox2, Nanog, and Oct-4 pluripotency transcription factors [104]. They play major role in immunity where overexpression of miR-181a results in reduction in CD8+ T cells and removal of Dicer in early B cell development leads to prevention of pre-B cell to pro-B cell transition [105, 106]. Liu et al. showed that miR-148 and miR-152 negatively regulate the antigen presentation of DCs and inhibited the production of cytokines [107]. Antigen specific T-cell mediated immunity is suppressed by miR-155 and inhibition of miR-155 reversed this effect [108].
6. Haematopoiesis
The production of different types of blood cells including their formation, development, and differentiation of blood cells is collectively known as haematopoiesis. Blood cells are divided into three lineages erythroid cells, lymphocytes, and myeloid cells. From many studies on miRNA expression including murine and hematopoietic system, it has been shown that miRNAs are not only involved in the normal haematopoiesis but also play a vital role in every stage of haematopoiesis. Dicer is an enzyme essential for miRNA biogenesis, whose deficiency leads to embryonic death and lack of stem cells in hematopoietic system [109]. Pro-B cell to pre-B cell transition is blocked due to removal of Dicer in B-cell progenitors [106]. T-cell development and differentiation is impaired in conditional deletion of Dicer and reduction in the number of CD8+, CD 4+T cells in thymus [110].
6.1. Megakaryopoiesis
From the hematopoietic stem cells through series of commitment steps megakaryocytes (MKs) are generated. MKs undergo a unique maturation process including megakaryoblast formation and polyploidization to produce proplatelets (Figure 6). Platelets are shed from these proplatelets into bone marrow sinusoids. Earlier studies have shown that miRNAs control the MK development and release of platelets (Table 4). Opalinska et al. studied miRNA expression in murine system where they examined 435 miRNAs among them 13 were upregulated and 81 were downregulated [46]. Overexpressing the miR-155 in K562 cells decreased the differentiation of megakaryocyte and erythroid cells by regulating the targets Meis-1 and Ets-1 [44]. The miR-34a contributes to MK differentiation by targeting cMyb, CDKs and it targets MEK1, thereby repressing proliferation [42, 43]. Interestingly, miR-146a, miR-145 are involved in megakaryopoiesis by activating innate immunity targets TIRAP and TRAF6 [111] which mediates the 5q syndrome phenotype [45]. During megakaryocyte development increase in miR-146a levels is repressed by PLZF transcription factor and its target CXCR4 [112]. miR-150 favors the differentiation of megakaryocyte-erythroid progenitors into megakaryocyte lineage rather than erythroid lineage. TPO induces miR-150 expression which in turn targets cMyb in TPO cells [47, 48]. It was detected in myeloproliferative neoplasm patient platelets that miRNA-28 is overexpressed which prevents megakaryocyte differentiation from CD34+cells by targeting TPO receptor [49]. RUNX1 upregulates miR-27a by binding to it and miR-27a targets RUNX1 and decreases its levels and miR181a inhibit Ca2+ induced differentiation and accelerates apoptosis [50, 51]. LIN28 is repressed by miR-181, thereby interrupting the LIN28/let-7 and axis then let-7 is upregulated; finally it promotes megakaryocyte differentiation identifying that miR-130 targets MAFB and miR-10a expression inversely correlate with HOX A1 in differentiated megakaryocytes [53, 113].
Figure 6.
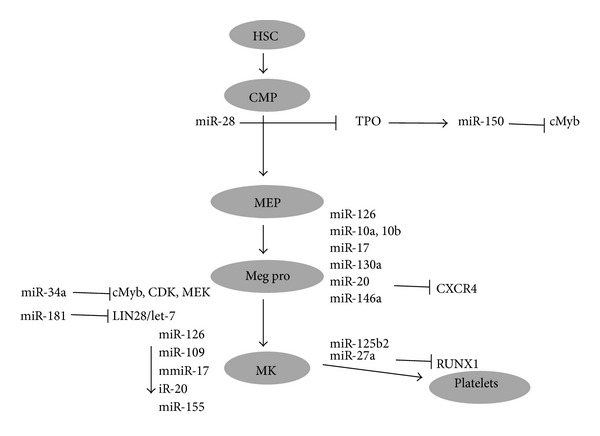
miRNA in megakaryopoiesis. miRNAs playing crucial role in the development of megakaryocyte (MegP-megakaryocyte progenitor, CMP-common myeloid progenitor, MEP-megakaryocyte erythroid progenitor).
Table 4.
miRNA involved in megakaryopoiesis.
| miRNA | Function | Putative targets | Reference |
|---|---|---|---|
| miR-34a | MK differentiation of K562 cells, targets cMyb, CDKs, and MEK1 | HMGN4, CCDC52, KLRK1, RGS17, NFATCG | [42, 43] |
|
| |||
| miR-155 | Downregulated in megakaryopoiesis, targets Meis-1 and Ets-1 | MMP16, SLC11A2, C2orf18 | [44] |
|
| |||
| miR-146a, miR-145 | Involved in megakaryopoiesis by activating innate immunity and mediates 5q syndrome phenotype | SOX11, SP1 | [45] |
|
| |||
| miR-146a | Increased in MK development and targets CXCR4 | CREBL2, NOTCH2, TRAK2, TBX18, RIN2, RAD23B, SLC1A2 | [46, 47] |
|
| |||
| miR-150 | Favours megakaryocyte lineage differentiation; it targets cMyb, induced by TPO | SLC24A4 | [47, 48] |
|
| |||
| miR-28 | It targets TPO receptor and prevents MK differentiation from CD34+ cells | [49] | |
|
| |||
| miR-27a | miR-27a targets and decrease RUNX1 levels | TRIM9, CYB5B, EGR2, BASP1 | [50] |
|
| |||
| miR-181a | miR-181a inhibit Ca2+ induced differentiation of MKs | [51] | |
|
| |||
| miR-125b-2 | Induces proliferation and differentiation of MKs | RAD98, ZNF100, PDS5B, SHE, CDR2 | [52] |
|
| |||
| miR-181 | Mediates MK differentiation by disrupting LIN28/let-7 axis | FOXP1, CCN8, HOXA1 | [53] |
6.2. Erythropoiesis
The term erythropoiesis refers to the process of production of red blood cells. In humans erythropoiesis occurs in red bone marrow. Kidneys respond to low levels of oxygen by releasing a hormone erythropoietin, which triggers erythropoiesis. It has been identified that certain miRNAs play crucial role in erythroid homeostasis (Figure 7). miR-144 and miR-451 are required for erythroid homeostasis (Table 5). Mice deficient of miR-144 and miR-451 have shown to undergo impairment in late erythrocyte maturation which further leads to splenomegaly, mild anaemia, and erythroid hyperplasia. GATA1 controls the erythropoiesis through regulating the two conserved miRNAs, miR-451 and miR-144, which in turn regulate GATA1 expression [54]. Defect in erythroid differentiation and reduction in hematocrit are observed in miR-451−/− mice. miR-451 upregulation rescued the defect in erythroid differentiation by targeting 14-3-3ζ [56]. LMO2 is a transcriptional regulator which is important for HSC development and erythropoiesis. Blood formation is affected in mice lacking this gene and miR-223. LMO2 levels inversely correlated to this miRNA and transduction of miR-223 reduces the commitment of erythroid progenitors [57, 58]. miR-15b, miR-16, and miR-22 have shown strong correlation with the erythroid surface markers CD36, CD235a, and CD71, whereas miR-28 was negatively correlated and CD71 transcriptional activities favour miR-320 in reticulocytes [59, 60]. miR-221, miR-222, and miR-24 inhibit normal erythropoiesis and miR-24 targets ALK4. cMyb and miR-15a are involved in the transition from BFU-E to CFU-E stage [61–63]. Fetal haemoglobin (α2 γ2) is the crucial oxygen carrying protein from 2nd to 3rd trimester development stage which has been observed to be completely replaced by (α2 β2) in adults. It was identified that miR-96 inhibits the γ-globin gene expression, thereby repressing the erythropoiesis [114].
Figure 7.
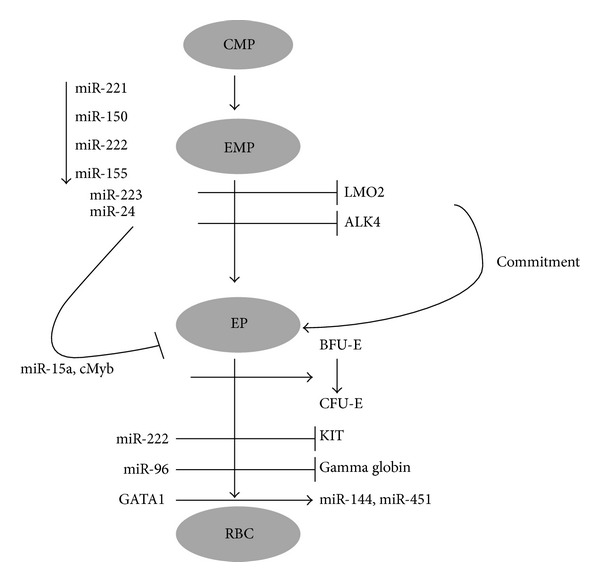
miRNA in erythropoiesis. miRNA in the erythrocyte development. (EP-erythroid progenitor, BFU-E-burst forming unit erythroid, CFU-E-colony forming unit erythroid).
Table 5.
miRNAs involved in erythropoiesis.
| MiRNA | Function | Putative targets | Reference |
|---|---|---|---|
| miR-144, miR-451 | Erythroid homeostasis, deficiency leads to splenomegaly, mild anaemia, and erythroid hyperplasia, controlled by GATA1 | TSPAN12, HMGCR, FBN2, MAP3K8, CXCL16, EREG, ATF2, CDKN2B | [54, 55] |
|
| |||
| miR-451 | Erythroid differentiation defect and reduction in haematocrit in miRNA-451−/− mice | CDKN2B, CXCL16, EREG, ATF2 | [56] |
|
| |||
| miR-223 | Reduces the commitment of erythroid progenitors | LIN54, FOXO1, USP42, ALCAM, BCLAF1, SLC11A2 | [57, 58] |
|
| |||
| miR-15b, mi16, miR-22 | Positive correlation with erythroid markers CD36, CD235a, and CD71 | PRDM4, KIF1B, LAMP3, SWAP70, LIN7C, AKT3, LAMC1 | [59, 60] |
|
| |||
| miR-28 | Negatively correlate with CD71 | [59, 60] | |
|
| |||
| miR-320 | Favours CD71 transcriptional activities | [60] | |
|
| |||
| miR-221, miR-222 | Inhibit normal erythropoiesis | TAF9B, MYLIP, RAB18, CYP7A1, KIF16B, MAT2A, NXN | [61] |
|
| |||
| miR-24 | Targets ALK4 | TRIB3, CBX5, KCNJ2, DGA52 | [62] |
|
| |||
| miR-15a | Transition from BFU-E to CFU-E stage | GFAP, SLC9A8, ZNRF2, FAM81A | [63] |
6.3. Mast Cells
Mast cells are resident cells of tissues throughout body and contain granules rich in histamine and heparin. Mast cells are bone marrow derived and their survival depends on stem cell factor. Biological roles of mast cells include wound healing, innate immunity defense against pathogens, and angiogenesis. miRNAs play important role in mast cells; for example, miR-221 is involved in the adhesion of mast cells, degranulation, and migration towards SCF (expand) and cytokine production [115]. miR-126 favors mast cell proliferation and cytokine production by targeting Spred1. miR-221 and 222 are upregulated after mast cell activation; overexpression of these miRNAs causes defect in cell morphology and cell cycle regulation without affecting viability [116, 117].
6.4. Myelopoiesis
Myelopoiesis is the formation of myeloid cells including granulocytes, monocytes, eosinophils, basophiles, and neutrophils. Many studies reported the role of miRNA in myelopoiesis and in myeloid malignancies. Eva et al. showed that miR-125b affects myelopoiesis in several ways and finally cause of blocking the G-CSF induced differentiation of granulocytes. They found that the Stat3 and Bak1 are the direct targets of miR-125b [118]. AML1 regulates myelopoiesis by recruiting chromatin remodeling enzymes on pre-miR-223 gene and silencing cell differentiation [119]. NF1-A negatively regulates granulocyte and monocyte differentiation. miR-223 and miR-424 repress NF1- A and these miRNA are activated by C/EBPα and PU.1, respectively. AML1 is known to be targeted by miR-17-5p-92 cluster, where this cluster is downregulated in monocytopoiesis and thereby AML1 is upregulated causing induction of M-CSF [120]. Zinc finger protein growth factor independent-1 (Gfi-1) is essential for normal granulocyte differentiation. A mutation in GFI1 causes severe congenital neutropenia (SCN). miR-196B and miR-21 are downregulated in SCN and up- or down- regulation of these miRNAs severely affects myelopoiesis [121]. miR-299-5p is involved in the CD34+ progenitor cell regulation and also in the modulation of monocyte differentiation [122].
Granulocytes. Granulocytes are classified as basophils, eosinophils, and neutrophils based on their morphology and staining properties of their cytoplasm. Basophils are comprised of <1% of white blood cells and eosinophils 1–3% and neutrophils constitute 50–70% of white blood cells. Basophils are nonphagocytic and release certain pharmacologically active substances from their cytoplasmic granules which are involved in certain allergic reactions. These granulocytes have lobed nucleus, granular cytoplasm, and stains with methylene blue which is a basic dye. There are no reports on miRNA regulation of basophils but identified that miR-31, miR-107, and miR-222 are highly expressed in basophils [123].
Eosinophils. Eosinophils are motile phagocytic cells that can migrate from blood to tissue spaces. They have bilobed nucleus, granular cytoplasm and can be stained with compounds like eosin red, an acidic dye. Very few studies have been reported on the miRNA regulation of eosinophils. miR-126 inhibition has been found to cause blockade of the recruitment of eosinophils at the airway walls in allergic asthma, which results in suppressing the development of airway disease [124]. miR-935 is found to be upregulated in eosinophils (5.6 fold) compared to DCs. Inhibition of miR-145 suppresses the eosinophil inflammation and mucous secretion. On treating mice with anti-miR-145 and dexamethasone, a similar effect was shown by both reducing eosinophils infiltration and suppression of mucous secretion in air ways [125, 126]. Wong et al. reported that miR-21* regulate the eosinophil apoptosis by enhancing GM- CSF (expand) which activates and sustains the survival of eosinophils through ERK pathway. Inhibition of miR-21* diminishes its survival activity and activates apoptosis in eosinophils [127].
Neutrophils. Neutrophils are called as polymorph nuclear leucocyte and they have multilobed nucleus. The granulated cytoplasm can stain with both acidic and basic stains. After being produced in bone marrow these are released into the peripheral blood and circulate 7–10 hours after which these migrate into the tissues. miR-29B regulates the neutrophil differentiation and PU1, Myc are the transcriptional regulators of miR-29B [128]. miR-223 is highly expressed in neutrophils and it plays a crucial role in granulocyte progenitor proliferation and function [129]. miRNA clusters regulate apoptosis and survival of several cancers recently. Ward et al. showed that miRNA clusters are expressed in human neutrophils. miR-17-92 cluster (miR-17, miR-19a, miR-19b, miR-20a, miR-92a, miR-18a, and miR-17*) contains seven mature miRNAs five of them (miR-17, miR-19a, miR-19b, miR-20a, miR-92a) are expressed in human neutrophils, whereas miR-18a, miR-17* were not detected in human neutrophils. This suggests that these clusters may play a crucial role in regulating neutrophil functions [130]. Proinflammatory signals upregulate miR-9 in neutrophils through NF-κb [131]. Neutrophil elastase (NE), a serine protease, stimulates the secretion of mucus in pulmonary tracts by inducing MUC5AC. miR-146a negatively regulates the hypersecretion of the mucus by MUC5AC [132]. After spinal cord injury miR-223 was upregulated and highly expressed after 12 hours. Immunohistochemistry revealed that this miR-223 observed in Gr-1 positive neutrophil which indicates that miR-223 regulates neutrophil after spinal cord injury [133]. miR-133a and miR-1 are downregulated in myeloproliferative disorder neutrophils; these have also been reported previously in certain cancers [134]. Radom-Aizik et al. showed the neutrophil miRNA expression pattern after exercise, interestingly 20 of 38 miRNAs downregulated immediately after exercise; remaining 18 miRNAs were upregulated. These miRNAs which were affected are regulating the genes that are involved in the apoptosis and immune processes [135].
Mononuclear Phagocytes. Mononuclear phagocytes are comprised of monocytes and macrophages and monocytes are present in blood and macrophages reside in tissues. In bone marrow granulocyte-monocyte progenitors differentiate into promonocytes and on entering the blood stream these mature into monocytes. After being circulated in blood for 8 hours these enlarge and enter into tissues and differentiate into tissue specific macrophages. MiRNAs have been found to play a crucial role in regulation of monocyte/macrophages. miR-146a increases in monocytes/macrophages upon induction of LPS and it negatively regulates innate immune response as it targets TRAF6 which is involved in TLR signalling, this regulation is dependent on Relb [136, 137]. Pauley et al. showed that in Sjogren's syndrome miR-146a increases the phagocytic activity of monocytes and it suppresses the inflammatory cytokine production [138]. On treating monocytes and U937 cell lines with LPS it was found that there is an upregulation of miR-525-5p and its putative target VPAC1 was shown to be down-regulated suggesting that miR-525-5p regulates the control of immune homeostasis [139]. miR-124 has been found to have role in macrophage activation and its overexpression in bone marrow macrophages leads to inhibition of TNFα [140]. In human monocytes, resveratrol increases miR-663 which target genes such as JunD and JunB decreasing the AP1 activity induced by LPS, hence altering the immune response [141]. Sharbati et al. showed that miR-21, miR-222, miR-23b, miR-24, and miR-27a are upregulated in monocyte differentiation and miR-29 in monocyte infection which suggests that these miRNAs may regulate monocyte defence mechanisms through TGF-β signalling [142].
T-Lymphocytes. T-lymphocytes belonging to white blood cells are produced in bone marrow and mature in thymus and express T cell receptors. T cell receptors recognize an antigen which is bound to major histocompatibility protein, a membrane protein by the process known as antigen presentation. When a T-cell recognizes an antigen which is bound to MHC on a cell it proliferates and differentiates into memory cell and effector cells (Figure 8). There are three subpopulations of T cells they are T helper cell (Th), T-cytotoxic cell (Tc), and T suppressor cell (Ts). T helper cells can be distinguished from T-cytotoxic cells based on the membrane protein CD4 and CD8, respectively. Like other biological processes regulated by miRNA T-cell development in thymus and their activation is controlled by miRNAs. Wu et al. studied the miRNA profiling of naive, effector, and CD8 memory T-cells and they found that miR-16, miR-21, miR-142-3p, miR-142-5p, miR-150, miR-15b, and let-7f are upregulated sevenfold than other miRNAs. They also observed that miRNA expression in effector T-cells was down-regulated compared to naive T cells but their levels are restored back in memory T-cells, suggesting that miRNA may have important in regulation of T-cell development and differentiation [143]. Proliferating T cells contain shorter 3′UTR compared to the normal cells due to which they have less miRNA targeting sites; hence proliferating cells are resistant to regulation by miRNA [144]. Deletion of Dicer in T-cell lineage results in reduction in the number of Treg cells and its suppressor activity. Since Dicer is required for miRNA biogenesis and absence of Dicer is found to hinder in Treg cell development in thymus, it indicates that miRNAs are required for the T-cell development [145, 146]. miRNA-181a is involved in the T and B cell differentiation; the miRNA expression is found to be low in matured and peripheral T-cells, whereas it is highly expressed in the double positive T lymphocytes which are sensitive to low affinity peptide antigens, indicating that miRNA regulates the sensitivity of T-cell receptor [147]. Virts and Thoman showed the age related expression of miRNA in thymopoiesis and they observed that 53% of miRNAs are upregulated in the aged TN1 cells [148]. Ohyashiki et al. showed that miR-92a reduced in CD8+ T-cells progressively with age [149]. miRNA-146a is upregulated, whereas miR-363, miR-498 are downregulated in rheumatoid arthritis CD4+ T-cells and miR-146a is involved in the suppression of apoptosis and play a role in rheumatoid arthritis pathogenesis [150]. Almanza et al. showed that cell fate determination takes place based on the balancing effects of different miRNAs such as miR-150, the let-7, and miR-155 and they found that miR-150 targets KChIP which is upregulated in CD8 T-cell [151].
Figure 8.
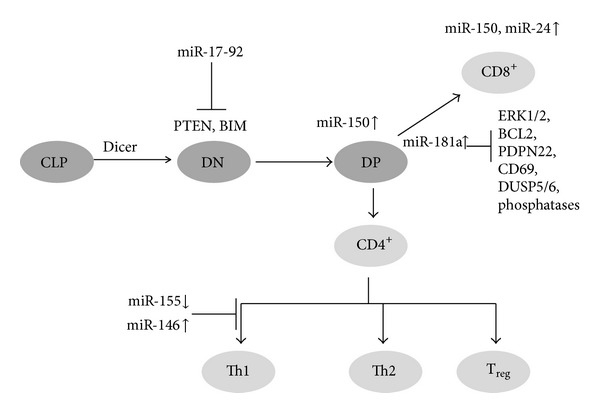
miRNA in T cell development. miRNA regulation at different developmental stages of T-cell development (CLP-common lymphoid progenitor, DN-double negative, DP-bouble positive).
B Lymphocytes. B lymphocytes produced in the bone marrow as immature cells and migrate to the secondary lymphoid organs where they transformed into mature B cell. They express a membrane bound antibody, molecule called B-cell receptor. When a naive B-cell finds an antigen which matches its membrane bound antibody then it starts proliferating and they differentiate into memory B-cell and effector B-cell which produce antibody molecules. Antibodies are glycoproteins and they consist of two light chain polypeptides and two heavy chain polypeptides. Several observations suggest that the role of miRNA in B-cell development and function (Figure 9). Tan et al. studied the miRNA profiling of germinal center, memory, and naive B cells. They observed that several miRNAs are elevated in germinal center of B-cell, which include miR-106a, miR-181b, and miR-17-5p. miR-150 is upregulated in all the three subsets and found that it is the target of survivin, cMyb which is critical for B Cell development and its premature expression inhibits the B- cell early development [152]. These results suggest that miRNAs regulate B-cell maturation and development and very little is known about their regulation in B-cell. MiR-150 reduces the mature B cell levels in circulation but has little effect on myeloid lineage cells. It blocks the transition from pro-B cell to pre-B cell by targeting cMyb, which is crucial for the lymphocyte maturation [153]. miR-155 is required for the B-Cell responses to both thymic dependent and independent antigens and the B-Cell which lacks miR-155 decreases in its response to antigen and production of high affinity IgG1 antibody; interestingly it has been found that miR-155 targets the PU1 transcription factor [154]. miR-15 and miR-16 are deleted (13q14) or downregulated in most of the B-CLLs indicating their involvement in the pathogenesis of B-cell chronic lymphocytic leukemias [155]. Chen et al. identified that miR-181 increase the B lineage cells suggesting that it may play a crucial role in B-cell differentiation [105].
Figure 9.
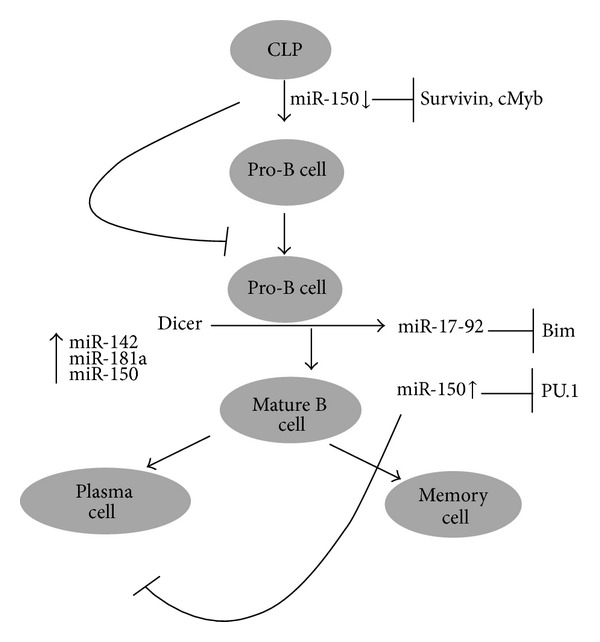
miRNA in B cell development and miRNA regulation of B-cell development from common lymphoid progenitor cell to produce memory and plasma cell.
7. miRNA in Haematological Malignancies
Haematological malignancies are malignant neoplasms that affect blood, lymph node, bone marrow, and other parts of the lymphatic system. These malignancies may arise from myeloid and lymphoid cell lineages lymphoma. Lymphocytic leukaemia and myeloma arise from lymphoid lineage cells, whereas AML, CML, and myelodisplastic syndromes arise from myeloid lineage cells. Haematological malignancies account for 9.5% and lymphomas are more common than any other malignancies in the USA. Leukemias are classified into acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML), acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), acute monocytic leukemia (AMOL), and so forth. Lymphomas are classified into Hodgkin's lymphomas and non-Hodgkin's lymphomas [156]. Expression profiles of miRNAs have shown that they are involved in the haematological malignancies.
7.1. Acute Myeloid Leukemia
Acute myelogenous leukemia is the most common type of leukemia in adults also known as acute myeloblastic leukemia, acute nonlymphocytic leukemia, and acute granulocytic leukemia and is a rapidly growing malignant neoplasm in which many WBCs are found in blood and bone marrow. In acute myeloid leukemia more immature cells are produced abruptly and progression is very fast. Many studies have shown deregulation of miRNAs in AMLs (Table 6). Li et al. showed that miR-126/126* upregulation is associated with chromosomal translocations and inhibited apoptosis and enhances cell viability and proliferation. They also observed that miR-126 targets the tumour suppressor protein PLK2 (polo-like kinase2) which is involved in the cell cycle checkpoint [64]. Transcription factor C/EBPα regulates miR-223 expression. miR-223 inhibits the cell cycle regulator protein E2F1 which leads to suppression of granulopoiesis. They also found that E2F1 binds to the miR-223 promoter in AML cells and blocks its expression indicating that E2F1 is a transcriptional inhibitor of miR-223 in AML. miRNA-221 has been shown to be overexpressed in several solid tumours and it is highly expressed in AML showing its significant role in oncogenesis. miRNAs are epigenetically deregulated in AML. miR-223 is silenced by the hypermethylation of its upstream elements [65]. Cammarata et al.described the oncogenic role of miRNA-221 in AML and it has been found that it inhibits the CDK inhibitor p27. They also observed that the tumour suppressor miRNA let-7b is down-regulated in AML [66]. C/EBPα is epigenetically silenced in AML, thereby blocking the myeloid differentiation and leads to leukemia. It was identified by computational approach that miR-124a target the C/EBPα [67]. Hypoxia-inducible factor 1 (HIF-1) is also playing a crucial role in the miRNA signaling network by modulating the miR-20a and miR-17 which can inhibit the p21 [68]. Garzon et al. suggested miR-29b as a tumour suppressor miRNA in AML. Its expression is low in AML restoration which leads to reduction in the tumorigenicity and have shown the relation between miRNA and DNA hypermethylation [69]. miR-29b overexpression resulted in the reduced expression of DNA methyl transferases DNMT1, DNMT3B, and DNMT3A. ERG is an oncogene which is deregulated in AML and T-ALL. It has been shown that miR-196a and miR-196b regulate the ERG mRNA; its aberrant expression indicates its role in AML [70]. Protooncogene c-Kit overexpress in AML and it has been shown that miR-193b is downregulated in AML and its overexpression resulted in downregulation of c-Kit. It clearly indicated that c-Kit is direct target of miR-193b and it may be therapeutic target in c-Kit positive AML cases [71].
Table 6.
miRNAs involved in AML.
| miRNA | Function | Putative targets | Reference |
|---|---|---|---|
| miR-126* | Upregulation leads to chromosomal translocations and inhibits apoptosis | ADAT2,FOF1, LMO7, | [64] |
|
| |||
| miR-126 | Targets tumour suppressor PLK2 | TOM1, CKMT2, ZNF131, RGS3, | [64] |
|
| |||
| miR-223 | C/EBPα regulates its expression in turn it inhibits E2F1 in AML cells | ANKH, SCN1A, SCN3A, CBFB, CDH11, NEBL, RILPL1, CENPN | [65] |
|
| |||
| miR-221 | Oncogenic miRNA, it inhibits the CDK inhibitor p27 | HIPK1, RAB18, DNM3, ZNF547, | [66] |
|
| |||
| miR-124a | Target the C/EBPα, silenced and block differentiation gives leukemia phenotype | [67] | |
|
| |||
| miR-20a and miR-17 | Inhibits the p21 | POLQ, KLF12, STK38, CENTD1, NUP35, GNB5, CTSK | [68] |
|
| |||
| miR-29b | Tumour suppressor in AML and reduce tumorigenicity | SCML2, C1orf96, COL3A1, COL7A1, COL11A1 | [69] |
|
| |||
| miR-29b | Reduced expression of DNA methyltransferases | CD93, HBP1, SNX21, GNS, HMGCR, HNF4G, DNMT3B | [69] |
|
| |||
| miR-196a and miR-196b | They target ERG expression | FOS, GATA6, HOXB6, HOXC8, ZNF24, CCDC47 | [70] |
|
| |||
| miR-193b | Downregulated in AML and it targets c-Kit | MMP19, ARMC1, ARPC5 |
[71] |
7.2. Chronic Myelogenous Leukemia
Chronic myelogenous leukemia or chronic granulocytic leukemia is a neoplasm of blood cells especially myeloid cells in which uncontrolled growth of granulocytes (eosinophils, basophils, and neutrophils) is observed. This leukemia is characterized by Philadelphia chromosome translocation and it is a translocation between two chromosomes chromosome 9 and 22, it is denoted as t(9; 22)(q34; q11) [157]. Due to this translocation BCR gene on chromosome 22 and ABL gene on chromosome 9 is fused and this fusion product of BCR-ABL protein is a tyrosine kinase. It causes genomic instability by inhibiting DNA repair and finally leads to accumulation of genetic abnormalities [157]. Recently it has been shown that miR-203 inhibits the expression of BCR-ABL and thereby inhibited cell growth and colony formation. In another study it is shown that overexpression of miR-29b inhibited cell growth and colony formation by inhibiting ABL1 and BCR/ABL1 [76]. Xu et al. showed the feedback regulation between BCR, BCR/ABL1, GATA1 and miR-138. In this study they demonstrated that overexpression of miR-138 represses BCR/ABL1 and CCND3 by binding to the coding and 3′UTR regions, respectively [77]. Interestingly, miR-138 expression is increased by GATA1, and repressed by BCR/ABL in addition to imatinib resistance in CML.Turrini et al. suggested that miRNA may play a role in imatinib distribution in CML therapy and they found that miR-212 increases the ABCG2 expression upon treatment with imatinib in CML [78]. To study the pathology of CML it is essential to study the signaling pathways. Specific miRNAs miR-155, miR-564, and miR-31 are downregulated in CML and extrapolation of these results suggested that VEGF, mTOR, ErbB, and MAPK are the main signaling pathways related to the miRNAs [158]. In a different study it was shown that miR-181a, miR-221, miR-20a, miR-17, miR-19a, miR-103, miR-144, miR-155, miR-150, and miR-222 are downregulated in CML and the targets of these miRNAs are associated with EGFR, ERBB, TGFB1, MAPK, and p53 pathways [159]. RalA is a downstream molecule of Bcr-Abl in Ras signalling pathway and is targeted by miR-181a which plays an important role in CML [160]. Bcr-Abl decreases the tumor suppressor miRNAs and increases the oncogenic miRNAs that leads to leukemic transformation. It was confirmed that knockdown of BCR-ABL results in reduction of miR-212, miR-425-5p, miR-130a, miR-130b and miR-148a. Interestingly, overexpression of these miRNAs is correlated with reduction in CCND3 suggesting that BCR-ABL induced oncogenic miRNAs are involved in the downregulation of CCND3 in CML [161]. miRNAs which are involved in CML pathogenesis are described in Table 7.
Table 7.
miRNAs involved in CML.
| miRNA | Function | Putative targets | Reference |
|---|---|---|---|
| miR-17-92 | Down-regulated in imatinib treated CML cells | IRF9, RAB10, TXNIP, TET2 | [72] |
|
| |||
| miR-21 | Antisense inhibition leads to inhibition of migration and cell growth and induces apoptosis | TXPAN2, LUM, SUZ12, MSH2, PDZD2 | [73] |
|
| |||
| miR-203 | Methylated in AML, CML, ALL, CLL. Inhibit the expression of BCR-ABL | RTKN2, AAK1, MYST4 CD109, IL21, PLD2 | [74] |
|
| |||
| miR-451 | Associated with Bcr-Abl | TSC1, ACADSB, GRSF1, MAML1, GDI1, NAMPT | [75] |
|
| |||
| miR-29b | Inhibits ABL1 and BCR/ABL1 there by inhibiting cell growth and colony formation | HAS3, SNX24, CD93, SCML2, COL7A1, ZNF396, HMGCR, ICOS | [76] |
|
| |||
| miR-138 | Represses BCR/ABL1 and CCND3, increases by GATA1 | KLF12, H3F3B, MYO5C, NXN, NEBL, PDPN, STK38 | [77] |
|
| |||
| miR-212 | Increases the ABCG2 expression | APAF1, EP300, EDNRA, CFL2, NOS1, SOX4, SOX11 | [78] |
7.3. Multiple Myeloma
Myeloma is a clonal B-cell malignancy characterized by the aberrant accumulation of plasma cells (PCs) within bone marrow (BM) and extramedullary sites [162, 163]. Myeloma arises from the multifocal proliferation of long-lived PCs and, despite all available therapies, remains invariably fatal [164]. Several studies have identified miRNAs that are deregulated during myelomagenesis, and subsequent studies have explored the role of miRNAs as diagnostics to detect disease or to monitor myeloma progression [165, 166]. These studies have found that the vast majority of miRNAs that are aberrantly expressed in MM cells are upregulated compared with their expression in normal PCs [165]. Zhou et al. compared miRNA expression profiles (miREP) of 52 newly diagnosed MM patients with that obtained from PCs of two healthy donors. Among 464 miRNAs analyzed, 95 had a higher mean expression in PC samples of MM patients compared with those of healthy donors [167]. In related studies, miRNA-15a was downregulated in relapsed and/or refractory MM and found to regulate tumor progression in MM cell lines (MMCLs) [168]. Separately, it was found that miRNAs-15a and miRNAs-16 expression levels were often elevated in the PCs from newly diagnosed MM patients in comparison with healthy PCs [169]. The miRNA-17-92 cluster, which targets the apoptosis facilitator Bcl-2, was also reported to confer tumourigenicity in MM [170]. miRNA-29b has been reported to downregulate Mcl-1 and to induce apoptosis of myeloma cells [171]. The miRNA-193b-365 cluster is overexpressed in MM and three miRNAs-720, 1308, and 1246 were significantly higher in PCs of myeloma patients than healthy controls [166, 172]. HOX9, c-Myc, Bcl-2, and SHP1/SHP2 represent targets of miRNA-146b, 140, 145, 125a, 151, 223, 155, and Let-7f, and changes in expression of these miRNAs may be involved in myelomagenesis and associated with overall prognosis [173]. miRNA-17-92 clusters are other pivotal miRNAs activated by Myc and are highly linked to the progression of MM. Gao et al. reported that in addition to miRNA-17-92 cluster expression; miRNA-15a and 16-1 are also linked to poor prognosis in MM patients [174]. Those with elevated miRNA-17, 20, and 92 levels had shorter progression-free survival than those with reduced miRNA levels. Higher miRNA-20a and 148a levels in myeloma patients are associated with a shorter relapse-free survival, to suggest a possible association between miRNA-20a and poor prognosis [175]. Expression of miRNAs-153, 490, 455, 642, 500, and 296 is associated with a better event-free survival, whereas miRNAs-548d, 373, 554, and 888 expression correlates with a poorer outcome [175]. The precise role of miRNAs in myeloma needs to be further elucidated, although they are predicted to be involved in PC growth, survival, growth factor response, homing, drug resistance, and BM interaction.
8. Conclusions
miRNAs are important regulators of haematopoiesis and control the gene expression of several transcription factors essential for the commitment, proliferation, differentiation, and apoptosis of hematopoietic stem cells. Hematological malignancies arise not only due to the aberrant expression of proteins but also by the regulators of the genes such as miRNAs. Deregulation of miRNA expression results in haematological malignancies. A single miRNA targets many genes and it is not clear whether targeting a single gene or multiple genes leads to hematological malignancies. miRNAs can function through several pathways which are involved in disease manifestations. Gain of function and loss of function experiments will give a better idea about the clinical use of miRNAs. In future more studies need to be focussed on the animal models for the consistent and reliable results which will enable us to understand the pathophysiology of haematological malignancies.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This work was supported by DBT-IYBA, DBT-RGYI, DBT-NIAB, DST, ICMR, UPE-UoH, and UGC grants of Government of India. The authors appreciate the funding in form of CSIR and UGC fellowships from Government of India.
References
- 1.Molnár A, Schwach F, Studholme DJ, Thuenemann EC, Baulcombe DC. miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature. 2007;447(7148):1126–1129. doi: 10.1038/nature05903. [DOI] [PubMed] [Google Scholar]
- 2.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 3.Molnár A, Schwach F, Studholme DJ, Thuenemann EC, Baulcombe DC. miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature. 2007;447(7148):1126–1129. doi: 10.1038/nature05903. [DOI] [PubMed] [Google Scholar]
- 4.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309(5740):1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 5.Ørom UA, Nielsen FC, Lund AH. MicroRNA-10a Binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Molecular Cell. 2008;30(4):460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Friedman RC, Farh KK-H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of MicroRNAs. Genome Research. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siomi H, Siomi MC. Posttranscriptional regulation of MicroRNA biogenesis in animals. Molecular Cell. 2010;38(3):323–332. doi: 10.1016/j.molcel.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Nottrott S, Simard MJ, Richter JD. Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nature Structural and Molecular Biology. 2006;13(12):1108–1114. doi: 10.1038/nsmb1173. [DOI] [PubMed] [Google Scholar]
- 9.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends in Cell Biology. 2007;17(3):118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Moretti F, Thermann R, Hentze MW. Mechanism of translational regulation by miR-2 from sites in the 5′ untranslated region or the open reading frame. RNA. 2010;16(12):2493–2502. doi: 10.1261/rna.2384610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphreys DT, Westman BJ, Martin DIK, Preiss T. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(47):16961–16966. doi: 10.1073/pnas.0506482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Developmental Biology. 1999;216(2):671–680. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- 13.Petersen CP, Bordeleau M-E, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Molecular Cell. 2006;21(4):533–542. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409(6818):363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Carmell MA, Rivas FV, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305(5689):1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 16.Mourelatos Z, Dostie J, Paushkin S, et al. miRNPs: a novel class of ribonucleoproteins containing numerous MicroRNAs. Genes and Development. 2002;16(6):720–728. doi: 10.1101/gad.974702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, Ahn C, Han J, et al. The nuclear RNase III Drosha initiates MicroRNA processing. Nature. 2003;425(6956):415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 18.Bohnsack MT, Czaplinski K, Görlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10(2):185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caudy AA, Myers M, Hannon GJ, Hammond SM. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes and Development. 2002;16(19):2491–2496. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight SW, Bass BL. The role of RNA editing by ADARs in RNAi. Molecular Cell. 2002;10(4):809–817. doi: 10.1016/s1097-2765(02)00649-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y, Hur I, Park S-Y, Kim Y-K, Mi RS, Kim VN. The role of PACT in the RNA silencing pathway. The EMBO Journal. 2006;25(3):522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinmann L, Höck J, Ivacevic T, et al. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell. 2009;136(3):496–507. doi: 10.1016/j.cell.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of MicroRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125(6):1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Kedde M, Strasser MJ, Boldajipour B, et al. RNA-binding protein Dnd1 inhibits MicroRNA access to target mRNA. Cell. 2007;131(7):1273–1286. doi: 10.1016/j.cell.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 25.Lu TP, Lee CY, Tsai MH, et al. miRSystem: an integrated system for characterizing enriched functions and pathways of MicroRNA targets. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0042390.e42390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biology. 2004;2(11, article e363) doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are MicroRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Krek A, Grün D, Poy MN, et al. Combinatorial MicroRNA target predictions. Nature Genetics. 2005;37(5):495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 29.Maragkakis M, Reczko M, Simossis VA, et al. DIANA-MicroT web server: elucidating MicroRNA functions through target prediction. Nucleic Acids Research. 2009;37(supplement 2):W273–W276. doi: 10.1093/nar/gkp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes-Herrera PH, Ficarra E, Acquaviva A, Macii E. miREE: miRNA recognition elements ensemble. BMC Bioinformatics. 2011;12, article 454 doi: 10.1186/1471-2105-12-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miranda KC, Huynh T, Tay Y, et al. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 32.Vergoulis T, Vlachos IS, Alexiou P, et al. TarBase 6.0: capturing the exponential growth of miRNA targets with experimental support. Nucleic Acids Research. 2012;40(D1):D222–D229. doi: 10.1093/nar/gkr1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu S-D, Chu C-H, Tsou A-P, et al. miRNAMap 2.0: genomic maps of MicroRNAs in metazoan genomes. Nucleic Acids Research. 2008;36(supplement 1):D165–D169. doi: 10.1093/nar/gkm1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naeem H, Küffner R, Csaba G, Zimmer R. miRSel: automated extraction of associations between MicroRNAs and genes from the biomedical literature. BMC Bioinformatics. 2010;11, article 135 doi: 10.1186/1471-2105-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao F, Zuo Z, Cai G, Kang S, Gao X, Li T. miRecords: an integrated resource for MicroRNA-target interactions. Nucleic Acids Research. 2009;37(supplement 1):D105–D110. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu S-D, Lin F-M, Wu W-Y, et al. miRTarBase: a database curates experimentally validated MicroRNA-target interactions. Nucleic Acids Research. 2011;39(supplement 1):D163–D169. doi: 10.1093/nar/gkq1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk—database: prediction of possible miRNA binding sites by “ walking” the genes of three genomes. Journal of Biomedical Informatics. 2011;44(5):839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Yang J-H, Li J-H, Shao P, Zhou H, Chen Y-Q, Qu L-H. StarBase: a database for exploring MicroRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Research. 2011;39(supplement 1):D202–D209. doi: 10.1093/nar/gkq1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H, Park S, Min H, Yoon S. vHoT: a database for predicting interspecies interactions between viral MicroRNA and host genomes. Archives of Virology. 2012;157(3):497–501. doi: 10.1007/s00705-011-1181-y. [DOI] [PubMed] [Google Scholar]
- 40.Huang J, Townsend C, Dou D, Liu H, Tan M. OMIT: a domain-specific knowledge base for MicroRNA target prediction. Pharmaceutical Research. 2011;28(12):3101–3104. doi: 10.1007/s11095-011-0573-8. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Wei B, Liu H, Li T, Rayner S. miRPara: a SVM-based software tool for prediction of most probable MicroRNA coding regions in genome scale sequences. BMC Bioinformatics. 2011;12, article 107 doi: 10.1186/1471-2105-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarro F, Gutman D, Meire E, et al. miR-34a contributes to megakaryocytic differentiation of K562 cells independently of p53. Blood. 2009;114(10):2181–2192. doi: 10.1182/blood-2009-02-205062. [DOI] [PubMed] [Google Scholar]
- 43.Ichimura A, Ruike Y, Terasawa K, Shimizu K, Tsujimoto G. MicroRNA-34a inhibits cell proliferation by repressing mitogen-activated protein kinase kinase 1 during megakaryocytic differentiation of K562 cells. Molecular Pharmacology. 2010;77(6):1016–1024. doi: 10.1124/mol.109.063321. [DOI] [PubMed] [Google Scholar]
- 44.Romania P, Lulli V, Pelosi E, Biffoni M, Peschle C, Marziali G. MicroRNA 155 modulates megakaryopoiesis at progenitor and precursor level by targeting Ets-1 and Meis1 transcription factors. British Journal of Haematology. 2008;143(4):570–580. doi: 10.1111/j.1365-2141.2008.07382.x. [DOI] [PubMed] [Google Scholar]
- 45.Starczynowski DT, Kuchenbauer F, Argiropoulos B, et al. Identification of miR-145 and miR-146a as mediators of the 5q-syndrome phenotype. Nature Medicine. 2010;16(1):49–58. doi: 10.1038/nm.2054. [DOI] [PubMed] [Google Scholar]
- 46.Opalinska JB, Bersenev A, Zhang Z, et al. MicroRNA expression in maturing murine megakaryocytes. Blood. 2010;116(23):e128–e138. doi: 10.1182/blood-2010-06-292920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu J, Guo S, Ebert BL, et al. MicroRNA-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Developmental Cell. 2008;14(6):843–853. doi: 10.1016/j.devcel.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barroga CF, Pham H, Kaushansky K. Thrombopoietin regulates c-Myb expression by modulating micro RNA 150 expression. Experimental Hematology. 2008;36(12):1585–1592. doi: 10.1016/j.exphem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girardot M, Pecquet C, Boukour S, et al. miR-28 is a thrombopoietin receptor targeting MicroRNA detected in a fraction of myeloproliferative neoplasm patient platelets. Blood. 2010;116(3):437–445. doi: 10.1182/blood-2008-06-165985. [DOI] [PubMed] [Google Scholar]
- 50.Ben-Ami O, Pencovich N, Lotem J, Levanon D, Groner Y. A regulatory interplay between miR-27a and Runx1 during megakaryopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(1):238–243. doi: 10.1073/pnas.0811466106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guimaraes-Sternberg C, Meerson A, Shaked I, Soreq H. MicroRNA modulation of megakaryoblast fate involves cholinergic signaling. Leukemia Research. 2006;30(5):583–595. doi: 10.1016/j.leukres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Klusmann J-H, Li Z, Böhmer K, et al. miR-125b-2 is a potential oncomiR on human chromosome 21 in megakaryoblastic leukemia. Genes and Development. 2010;24(5):478–490. doi: 10.1101/gad.1856210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, Zhang J, Gao L, et al. miR-181 mediates cell differentiation by interrupting the Lin28 and let-7 feedback circuit. Cell Death and Differentiation. 2012;19(3):378–386. doi: 10.1038/cdd.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dore LC, Amigo JD, Dos Santos CO, et al. A GATA-1-regulated MicroRNA locus essential for erythropoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3333–3338. doi: 10.1073/pnas.0712312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rasmussen KD, Simmini S, Abreu-Goodger C, et al. The miR-144/451 locus is required for erythroid homeostasis. Journal of Experimental Medicine. 2010;207(7):1351–1358. doi: 10.1084/jem.20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patrick DM, Zhang CC, Tao Y, et al. Defective erythroid differentiation in miR-451 mutant mice mediated by 14-3-3ζ . Genes and Development. 2010;24(15):1614–1619. doi: 10.1101/gad.1942810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warren AJ, Colledge WH, Carlton MBL, Evans MJ, Smith AJH, Rabbitts TH. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994;78(1):45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 58.Felli N, Pedini F, Romania P, et al. MicroRNA 223-dependent expression of LMO2 regulates normal erythropoiesis. Haematologica. 2009;94(4):479–486. doi: 10.3324/haematol.2008.002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choong ML, Yang HH, McNiece I. MicroRNA expression profiling during human cord blood-derived CD34 cell erythropoiesis. Experimental Hematology. 2007;35(4):551–564. doi: 10.1016/j.exphem.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Chen S-Y, Wang Y, Telen MJ, Chi J-T. The genomic analysis of erythrocyte MicroRNA expression in sickle cell diseases. PLoS ONE. 2008;3(6) doi: 10.1371/journal.pone.0002360.e2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Felli N, Fontana L, Pelosi E, et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(50):18081–18086. doi: 10.1073/pnas.0506216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q, Huang Z, Xue H, et al. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood. 2008;111(2):588–595. doi: 10.1182/blood-2007-05-092718. [DOI] [PubMed] [Google Scholar]
- 63.Zhao H, Kalota A, Jin S, Gewirtz AM. Autoregulatory feedback loop in human hematopoietic cells the c-myb proto-oncogene and MicroRNA-15a comprise an active. Blood. 2009;113(3):505–516. doi: 10.1182/blood-2008-01-136218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z, Lu J, Sun M, et al. Distinct MicroRNA expression profiles in acute myeloid leukemia with common translocations. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(40):15535–15540. doi: 10.1073/pnas.0808266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eyholzer M, Schmid S, Schardt JA, Haefliger S, Mueller BU, Pabst T. Complexity of miR-223 regulation by CEBPA in human AML. Leukemia Research. 2010;34(5):672–676. doi: 10.1016/j.leukres.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 66.Cammarata G, Augugliaro L, Salemi D, et al. Differential expression of specific MicroRNA and their targets in acute myeloid leukemia. The American Journal of Hematology. 2010;85(5):331–339. doi: 10.1002/ajh.21667. [DOI] [PubMed] [Google Scholar]
- 67.Hackanson B, Bennett KL, Brena RM, et al. Epigenetic modification of CCAAT/enhancer binding protein α expression in acute myeloid leukemia. Cancer Research. 2008;68(9):3142–3151. doi: 10.1158/0008-5472.CAN-08-0483. [DOI] [PubMed] [Google Scholar]
- 68.He M, Wang QY, Yin QQ, et al. HIF-1alpha downregulates miR-17/20a directly targeting p21 and STAT3: a role in myeloid leukemic cell differentiation. Cell Death Differentiation. 2013;20(3):408–418. doi: 10.1038/cdd.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garzon R, Liu S, Fabbri M, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113(25):6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coskun E, von der Heide EK, Schlee C, et al. The role of MicroRNA-196a and MicroRNA-196b as ERG regulators in acute myeloid leukemia and acute T-lymphoblastic leukemia. Leukemia Research. 2011;35(2):208–213. doi: 10.1016/j.leukres.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 71.Gao X-N, Lin J, Gao L, Li Y-H, Wang L-L, Yu L. MicroRNA-193b regulates c-Kit proto-oncogene and represses cell proliferation in acute myeloid leukemia. Leukemia Research. 2011;35(9):1226–1232. doi: 10.1016/j.leukres.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 72.Venturini L, Battmer K, Castoldi M, et al. Expression of the miR-17-92 polycistron in chronic myeloid leukemia (CML) CD34+ cells. Blood. 2007;109(10):4399–4405. doi: 10.1182/blood-2006-09-045104. [DOI] [PubMed] [Google Scholar]
- 73.Hu H, Li Y, Gu J, et al. Antisense oligonucleotide against miR-21 inhibits migration and induces apoptosis in leukemic K562 cells. Leukemia and Lymphoma. 2010;51(4):694–701. doi: 10.3109/10428191003596835. [DOI] [PubMed] [Google Scholar]
- 74.Chim CS, Wong KY, Leung CY, et al. Epigenetic inactivation of the hsa-miR-203 in haematological malignancies. Journal of Cellular and Molecular Medicine. 2011;15(12):2760–2767. doi: 10.1111/j.1582-4934.2011.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopotová T, Žáčková M, Klamová H, Moravcová J. MicroRNA-451 in chronic myeloid leukemia: miR-451-BCR-ABL regulatory loop? Leukemia Research. 2011;35(7):974–977. doi: 10.1016/j.leukres.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 76.Li Y, Wang H, Tao K, et al. miR-29b suppresses CML cell proliferation and induces apoptosis via regulation of BCR/ABL1 protein. Experimental Cell Research. 2013;319(8):1094–1101. doi: 10.1016/j.yexcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Xu C, Fu H, Gao L, et al. BCR-ABL/GATA1/miR-138 mini circuitry contributes to the leukemogenesis of chronic myeloid leukemia. Oncogene. 2012 doi: 10.1038/onc.2012.557. [DOI] [PubMed] [Google Scholar]
- 78.Turrini E, Haenisch S, Laechelt S, Diewock T, Bruhn O, Cascorbi I. MicroRNA profiling in K-562 cells under imatinib treatment: influence of miR-212 and miR-328 on ABCG2 expression. Pharmacogenetics and Genomics. 2012;22(3):198–205. doi: 10.1097/FPC.0b013e328350012b. [DOI] [PubMed] [Google Scholar]
- 79.Han J, Lee Y, Yeom K-H, Kim Y-K, Jin H, Kim VN. The Drosha-DGCR8 complex in primary MicroRNA processing. Genes and Development. 2004;18(24):3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruby JG, Jan CH, Bartel DP. Intronic MicroRNA precursors that bypass Drosha processing. Nature. 2007;448(7149):83–86. doi: 10.1038/nature05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of MicroRNA precursors. Science. 2004;303(5654):95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 82.Chendrimada TP, Gregory RI, Kumaraswamy E, et al. TRBP recruits the Dicer complex to Ago2 for MicroRNA processing and gene silencing. Nature. 2005;436(7051):740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haase AD, Jaskiewicz L, Zhang H, et al. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Reports. 2005;6(10):961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh S, Bevan SC, Patil K, Newton DC, Marsden PA. Extensive variation in the 5′-UTR of Dicer mRNAs influences translational efficiency. Biochemical and Biophysical Research Communications. 2005;335(3):643–650. doi: 10.1016/j.bbrc.2005.07.138. [DOI] [PubMed] [Google Scholar]
- 85.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes and Development. 2006;20(14):1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Rooij E. The art of MicroRNA research. Circulation Research. 2011;108(2):219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- 88.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian MicroRNAs uncovers a subset of brain-expressed MicroRNAs with possible roles in murine and human neuronal differentiation. Genome Biology. 2004;5(3, article R13) doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barad O, Meiri E, Avniel A, et al. MicroRNA expression detected by oligonucleotide Microarrays: system establishment and expression profiling in human tissues. Genome Research. 2004;14(12):2486–2494. doi: 10.1101/gr.2845604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen C, Ridzon DA, Broomer AJ, et al. Real-time quantification of MicroRNAs by stem-loop RT-PCR. Nucleic Acids Research. 2005;33(20, article e179) doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes MicroRNA-mRNA interaction maps. Nature. 2009;460(7254):479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of MicroRNAs on protein output. Nature. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vinther J, Hedegaard MM, Gardner PP, Andersen JS, Arctander P. Identification of miRNA targets with stable isotope labeling by amino acids in cell culture. Nucleic Acids Research. 2006;34(16, article e107) doi: 10.1093/nar/gkl590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee K-H, Chen Y-L, Yeh S-D, et al. MicroRNA-330 acts as tumor suppressor and induces apoptosis of prostate cancer cells through E2F1-mediated suppression of Akt phosphorylation. Oncogene. 2009;28(38):3360–3370. doi: 10.1038/onc.2009.192. [DOI] [PubMed] [Google Scholar]
- 96.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Research. 2005;65(14):6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 97.Plaisance V, Abderrahmani A, Perret-Menoud V, Jacquemin P, Lemaigre F, Regazzi R. MicroRNA-9 controls the expression of Granuphilin/Slp4 and the secretory response of insulin-producing cells. Journal of Biological Chemistry. 2006;281(37):26932–26942. doi: 10.1074/jbc.M601225200. [DOI] [PubMed] [Google Scholar]
- 98.Melkman-Zehavi T, Oren R, Kredo-Russo S, et al. miRNAs control insulin content in pancreatic β-cells via downregulation of transcriptional repressors. The EMBO Journal. 2011;30(5):835–845. doi: 10.1038/emboj.2010.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Esau C, Davis S, Murray SF, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metabolism. 2006;3(2):87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Elmén J, Lindow M, Silahtaroglu A, et al. Antagonism of MicroRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Research. 2008;36(4):1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rayner KJ, Sheedy FJ, Esau CC, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. Journal of Clinical Investigation. 2011;121(7):2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rayner KJ, Suárez Y, Dávalos A, et al. miR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328(5985):1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Georgantas RW, III, Hildreth R, Morisot S, et al. CD34+ hematopoietic stem-progenitor cell MicroRNA expression and function: a circuit diagram of differentiation control. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(8):2750–2755. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marson A, Levine SS, Cole MF, et al. Connecting MicroRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134(3):521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen C-Z, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303(5654):83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 106.Koralov SB, Muljo SA, Galler GR, et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132(5):860–874. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 107.Liu X, Zhan Z, Xu L, et al. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIα . The Journal of Immunology. 2010;185(12):7244–7251. doi: 10.4049/jimmunol.1001573. [DOI] [PubMed] [Google Scholar]
- 108.Mao C-P, He L, Tsai Y-C, et al. In vivo MicroRNA-155 expression influences antigen-specific T cell-mediated immune responses generated by DNA vaccination. Cell and Bioscience. 2011;1(1, article 3) doi: 10.1186/2045-3701-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bernstein E, Kim SY, Carmell MA, et al. Dicer is essential for mouse development. Nature Genetics. 2003;35(3):215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 110.Cobb BS, Nesterova TB, Thompson E, et al. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. Journal of Experimental Medicine. 2005;201(9):1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taganov KD, Boldin MP, Chang K-J, Baltimore D. NF-κB-dependent induction of MicroRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Labbaye C, Spinello I, Quaranta MT, et al. A three-step pathway comprising PLZF/miR-146a/CXCR4 controls megakaryopoiesis. Nature Cell Biology. 2008;10(7):788–801. doi: 10.1038/ncb1741. [DOI] [PubMed] [Google Scholar]
- 113.Garzon R, Pichiorri F, Palumbo T, et al. MicroRNA fingerprints during human megakaryocytopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(13):5078–5083. doi: 10.1073/pnas.0600587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Azzouzi I, Moest H, Winkler J, et al. Microrna-96 directly inhibits γ-globin expression in human erythropoiesis. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0022838.e22838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mayoral RJ, Deho L, Rusca N, et al. miR-221 influences effector functions and actin cytoskeleton in mast cells. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0026133.e26133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ishizaki T, Tamiya T, Taniguchi K, et al. miR126 positively regulates mast cell proliferation and cytokine production through suppressing Spred1. Genes to Cells. 2011;16(7):803–814. doi: 10.1111/j.1365-2443.2011.01529.x. [DOI] [PubMed] [Google Scholar]
- 117.Mayoral RJ, Pipkin ME, Pachkov M, van Nimwegen E, Rao A, Monticelli S. MicroRNA-221-222 regulate the cell cycle in mast cells. The Journal of Immunology. 2009;182(1):433–445. doi: 10.4049/jimmunol.182.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Surdziel E, Cabanski M, Dallmann I, et al. Enforced expression of miR-125b affects myelopoiesis by targeting multiple signaling pathways. Blood. 2011;117(16):4338–4348. doi: 10.1182/blood-2010-06-289058. [DOI] [PubMed] [Google Scholar]
- 119.Fazi F, Racanicchi S, Zardo G, et al. Epigenetic silencing of the myelopoiesis regulator MicroRNA-223 by the AML1/ETO oncoprotein. Cancer Cell. 2007;12(5):457–466. doi: 10.1016/j.ccr.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 120.Ventura A, Young AG, Winslow MM, et al. Targeted deletion reveals essential and overlapping functions of the miR-17∼92 family of miRNA clusters. Cell. 2008;132(5):875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Velu CS, Baktula AM, Grimes HL. Gfi1 regulates miR-21 and miR-196b to control myelopoiesis. Blood. 2009;113(19):4720–4728. doi: 10.1182/blood-2008-11-190215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tenedini E, Roncaglia E, Ferrari F, et al. Integrated analysis of MicroRNA and mRNA expression profiles in physiological myelopoiesis: role of hsa-miR-299-5p in CD34+ progenitor cells commitment. Cell Death and Disease. 2010;1(2, article e28) doi: 10.1038/cddis.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuchen S, Resch W, Yamane A, et al. Regulation of MicroRNA expression and abundance during lymphopoiesis. Immunity. 2010;32(6):828–839. doi: 10.1016/j.immuni.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of MicroRNA-126 suppresses the effector function of T H2 cells and the development of allergic airways disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18704–18709. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Collison A, Mattes J, Plank M, Foster PS. Inhibition of house dust mite-induced allergic airways disease by antagonism of MicroRNA-145 is comparable to glucocorticoid treatment. Journal of Allergy and Clinical Immunology. 2011;128(1):160–167. doi: 10.1016/j.jaci.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 126.Allantaz F, Cheng DT, Bergauer T, et al. Expression profiling of human immune cell subsets identifies miRNA-mRNA regulatory relationships correlated with cell type specific expression. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0029979.e29979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wong CK, Lau KM, Chan IHS, et al. MicroRNA-21* regulates the prosurvival effect of GM-CSF on human eosinophils. Immunobiology. 2013;218(2):255–262. doi: 10.1016/j.imbio.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 128.Batliner J, Buehrer E, Federzoni EA, et al. Transcriptional regulation of miR29B by PU.1 (SPI1) and MYC during neutrophil differentiation of acute promyelocytic leukaemia cells. British Journal of Haematology. 2012;157(2):270–274. doi: 10.1111/j.1365-2141.2011.08964.x. [DOI] [PubMed] [Google Scholar]
- 129.Johnnidis JB, Harris MH, Wheeler RT, et al. Regulation of progenitor cell proliferation and granulocyte function by MicroRNA-223. Nature. 2008;451(7182):1125–1129. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- 130.Ward JR, Heath PR, Catto JW, Whyte MKB, Milo M, Renshaw SA. Regulation of neutrophil senescence by MicroRNAs. PLoS ONE. 2011;6(1) doi: 10.1371/journal.pone.0015810.e15810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bazzoni F, Rossato M, Fabbri M, et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(13):5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhong T, Perelman JM, Kolosov VP, Zhou X-D. miR-146a negatively regulates neutrophil elastase-induced MUC5AC secretion from 16HBE human bronchial epithelial cells. Molecular and Cellular Biochemistry. 2011;358(1-2):249–255. doi: 10.1007/s11010-011-0975-2. [DOI] [PubMed] [Google Scholar]
- 133.Izumi B, Nakasa T, Tanaka N, et al. MicroRNA-223 expression in neutrophils in the early phase of secondary damage after spinal cord injury. Neuroscience Letters. 2011;492(2):114–118. doi: 10.1016/j.neulet.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 134.Slezak S, Jin P, Caruccio L, et al. Gene and MicroRNA analysis of neutrophils from patients with polycythemia vera and essential thrombocytosis: down-regulation of MicroRNA-1 and -133a. Journal of Translational Medicine. 2009;7, article 39 doi: 10.1186/1479-5876-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Radom-Aizik S, Zaldivar F, Jr., Oliver S, Galassetti P, Cooper DM. Evidence for MicroRNA involvement in exercise-associated neutrophil gene expression changes. Journal of Applied Physiology. 2010;109(1):252–261. doi: 10.1152/japplphysiol.01291.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schmelzer C, Kitano M, Rimbach G, et al. Effects of ubiquinol-10 on MicroRNA-146a expression in vitro and in vivo. Mediators of Inflammation. 2009;2009:7 pages. doi: 10.1155/2009/415437.415437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Etzrodi M, Cortez-Retamozo V, Newton A, et al. Regulation of monocyte functional heterogeneity by miR-146aand Relb. Cell Reports. 2012;1(4):317–324. doi: 10.1016/j.celrep.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pauley KM, Stewart CM, Gauna AE, et al. Altered miR-146a expression in Sjögren’s syndrome and its functional role in innate immunity. European Journal of Immunology. 2011;41(7):2029–2039. doi: 10.1002/eji.201040757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cocco E, Paladini F, Macino G, Fulci V, Fiorillo MT, Sorrentino R. The expression of vasoactive intestinal peptide receptor 1 is negatively modulated by MicroRNA 525-5p. PLoS ONE. 2010;5(8) doi: 10.1371/journal.pone.0012067.e12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Conrad AT, Dittel BN. Taming of macrophage and Microglial cell activation by MicroRNA-124. Cell Research. 2011;21(2):213–216. doi: 10.1038/cr.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tili E, Michaille J-J, Adair B, et al. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a MicroRNA targeting JunB and JunD. Carcinogenesis. 2010;31(9):1561–1566. doi: 10.1093/carcin/bgq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharbati S, Sharbati J, Hoeke L, Bohmer M, Einspanier R. Quantification and accurate normalisation of small RNAs through new custom RT-qPCR arrays demonstrates Salmonella-induced MicroRNAs in human monocytes. BMC Genomics. 2012;13(1, article 23) doi: 10.1186/1471-2164-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu H, Neilson JR, Kumar P, et al. miRNA profiling of naïve, effector and memory CD8 T cells. PLoS ONE. 2007;2(10) doi: 10.1371/journal.pone.0001020.e1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer MicroRNA target sites. Science. 2008;320(5883):1643–1647. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cobb BS, Hertweck A, Smith J, et al. A role for Dicer in immune regulation. Journal of Experimental Medicine. 2006;203(11):2519–2527. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liston A, Lu L-F, O’Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent MicroRNA pathway safeguards regulatory T cell function. Journal of Experimental Medicine. 2008;205(9):1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Laufer TM. T-cell sensitivity: a MicroRNA regulates the sensitivity of the T-cell receptor. Immunology and Cell Biology. 2007;85(5):346–347. doi: 10.1038/sj.icb.7100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Virts EL, Thoman ML. Age-associated changes in miRNA expression profiles in thymopoiesis. Mechanisms of Ageing and Development. 2010;131(11-12):743–748. doi: 10.1016/j.mad.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ohyashiki M, Ohyashiki JH, Hirota A, Kobayashi C, Ohyashiki K. Age-related decrease of miRNA-92a levels in human CD8+ T-cells correlates with a reduction of naïve T lymphocytes. Immunity & Ageing. 2011;8, article 11 doi: 10.1186/1742-4933-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li J, Wan Y, Guo Q, et al. Altered MicroRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Research & Therapy. 2010;12(3, article R81) doi: 10.1186/ar3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Almanza G, Fernandez A, Volinia S, Cortez-Gonzalez X, Croce CM, Zanetti M. Selected MicroRNAs define cell fate determination of murine central memory CD8 T cells. PLoS ONE. 2010;5(6) doi: 10.1371/journal.pone.0011243.e11243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tan LP, Wang M, Robertus J-L, et al. miRNA profiling of B-cell subsets: specific miRNA profile for germinal center B cells with variation between centroblasts and centrocytes. Laboratory Investigation. 2009;89(6):708–716. doi: 10.1038/labinvest.2009.26. [DOI] [PubMed] [Google Scholar]
- 153.Xiao C, Calado DP, Galler G, et al. miR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131(1):146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 154.Vigorito E, Perks KL, Abreu-Goodger C, et al. MicroRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27(6):847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of Micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cancer Facts & Figures 2012. 2012, http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts.
- 157.Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Annals of Internal Medicine. 1999;131(3):207–219. doi: 10.7326/0003-4819-131-3-199908030-00008. [DOI] [PubMed] [Google Scholar]
- 158.Rokah OH, Granot G, Ovcharenko A, et al. Downregulation of miR-31, miR-155, and miR-564 in chronic myeloid leukemia cells. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035501.e35501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Machova Polakova K, Lopotova T, Klamova H, et al. Expression patterns of MicroRNAs associated with CML phases and their disease related targets. Molecular Cancer. 2011;10, article 41 doi: 10.1186/1476-4598-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fei J, Li Y, Zhu X, Luo X. miR-181a post-transcriptionally downregulates oncogenic rala and contributes to growth inhibition and apoptosis in chronic myelogenous leukemia (CML) PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0032834.e32834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Suresh S, McCallum L, Lu W, Lazar N, Perbal B, Irvine AE. MicroRNAs 130a/b are regulated by BCR-ABL and downregulate expression of CCN3 in CML. Journal of Cell Communication and Signaling. 2011;5(3):183–191. doi: 10.1007/s12079-011-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bommert K, Bargou RC, Stühmer T. Signalling and survival pathways in multiple myeloma. European Journal of Cancer. 2006;42(11):1574–1580. doi: 10.1016/j.ejca.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 163.Mahindra A, Hideshima T, Anderson KC. Multiple myeloma: biology of the disease. Blood Reviews. 2010;24(supplement 1):S5–S11. doi: 10.1016/S0268-960X(10)70003-5. [DOI] [PubMed] [Google Scholar]
- 164.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Corthals SL, Sun SM, Kuiper R, et al. MicroRNA signatures characterize multiple myeloma patients. Leukemia. 2011;25(11):1784–1789. doi: 10.1038/leu.2011.147. [DOI] [PubMed] [Google Scholar]
- 166.Jones CI, Zabolotskaya MV, King AJ, et al. Identification of circulating MicroRNAs as diagnostic biomarkers for use in multiple myeloma. British Journal of Cancer. 2012;107(12):1987–1996. doi: 10.1038/bjc.2012.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou Y, Chen L, Barlogie B, et al. High-risk myeloma is associated with global elevation of miRNAs and overexpression of EIF2C2/AGO2. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(17):7904–7909. doi: 10.1073/pnas.0908441107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roccaro AM, Sacco A, Thompson B, et al. MicroRNAs 15a and 16 regulate tumor proliferation in multiple myeloma. Blood. 2009;113(26):6669–6680. doi: 10.1182/blood-2009-01-198408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Corthals SL, Jongen-Lavrencic M, de Knegt Y, et al. Micro-RNA-15a and Micro-RNA-16 expression and chromosome 13 deletions in multiple myeloma. Leukemia Research. 2010;34(5):677–681. doi: 10.1016/j.leukres.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 170.Chen L, Li C, Zhang R, et al. miR-17-92 cluster MicroRNAs confers tumorigenicity in multiple myeloma. Cancer Letters. 2011;309(1):62–70. doi: 10.1016/j.canlet.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 171.Zhang Y-K, Wang H, Leng Y, et al. Overexpression of MicroRNA-29b induces apoptosis of multiple myeloma cells through down regulating Mcl-1. Biochemical and Biophysical Research Communications. 2011;414(1):233–239. doi: 10.1016/j.bbrc.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 172.Unno K, Zhou Y, Zimmerman T, Platanias LC, Wickrema A. Identification of a novel MicroRNA cluster miR-193b-365 in multiple myeloma. Leukemia and Lymphoma. 2009;50(11):1865–1871. doi: 10.3109/10428190903221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Adamia S, Avet-Loiseau H, Amin SB, et al. Clinical and biological significance of MicroRNA profiling in patients with myeloma. Journal of Clinical Oncology. 2009;27(supplement 15):p. 8539. [Google Scholar]
- 174.Gao X, Zhang R, Qu X, et al. miR-15a, miR-16-1 and miR-17-92 cluster expression are linked to poor prognosis in multiple myeloma. Leukemia Research. 2012;36(12):1505–1509. doi: 10.1016/j.leukres.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 175.Chi J, Ballabio E, Chen X-H, et al. MicroRNA expression in multiple myeloma is associated with genetic subtype, isotype and survival. Biology Direct. 2011;6, article 23 doi: 10.1186/1745-6150-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]


