Abstract
An antiserum was prepared against a highly purified Na+, K+-adenosine triphosphatase (ATP phosphohydrolase, EC 3.6.1.3). Purification and fractionation yielded two globulin components, one of which appears specific for a digitalis glycoside receptor site or binding conformation and the other for a catalytic site or conformation.
Keywords: antibody fractionation, globulin components, digitalis receptor antibody, conformation-specific antibodies
Full text
PDF
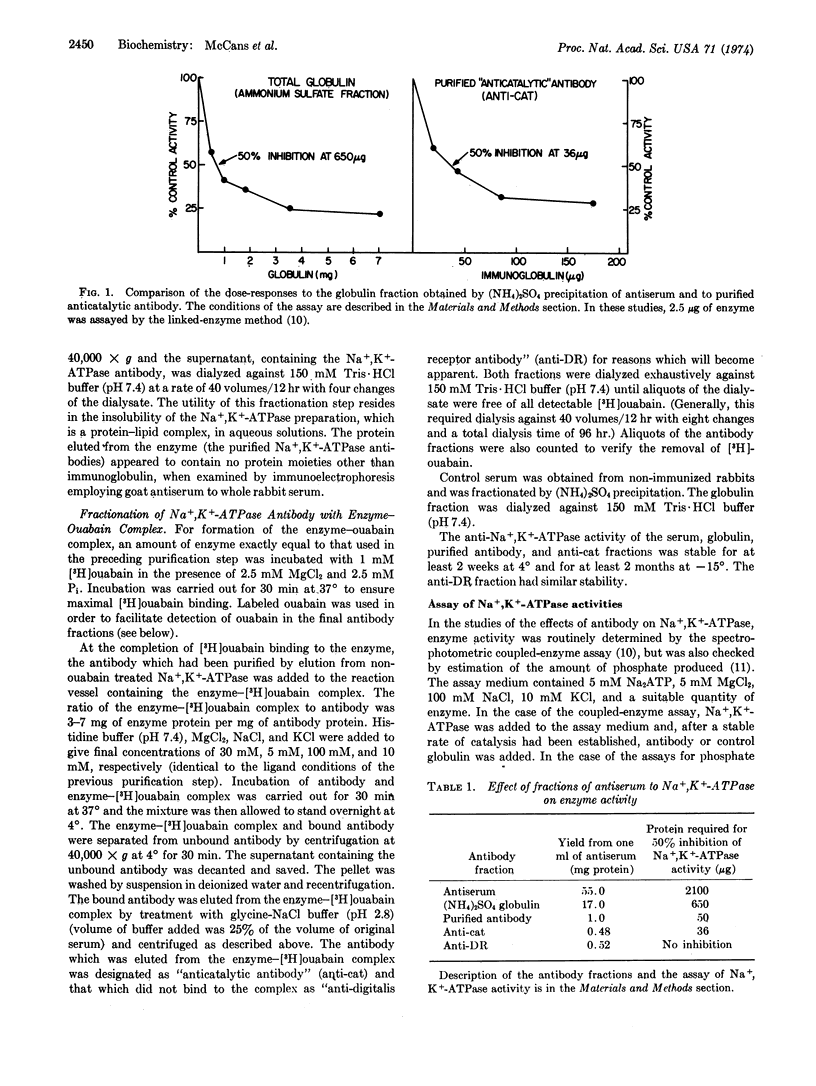
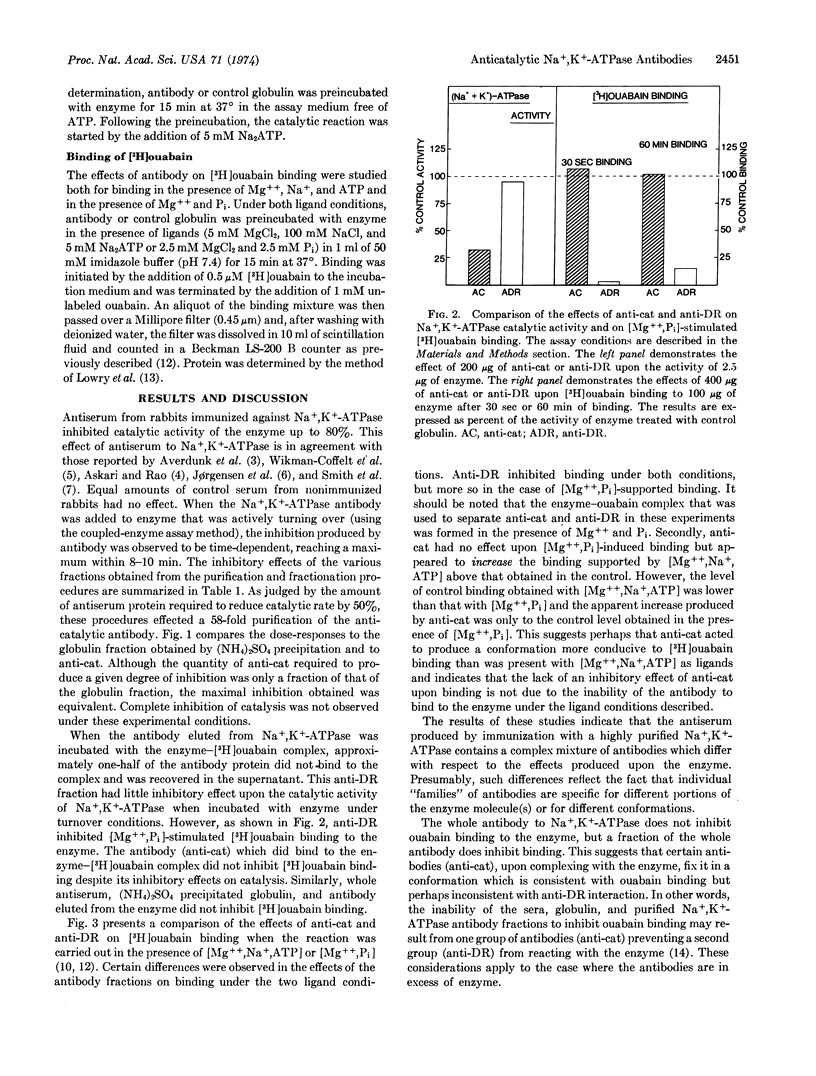
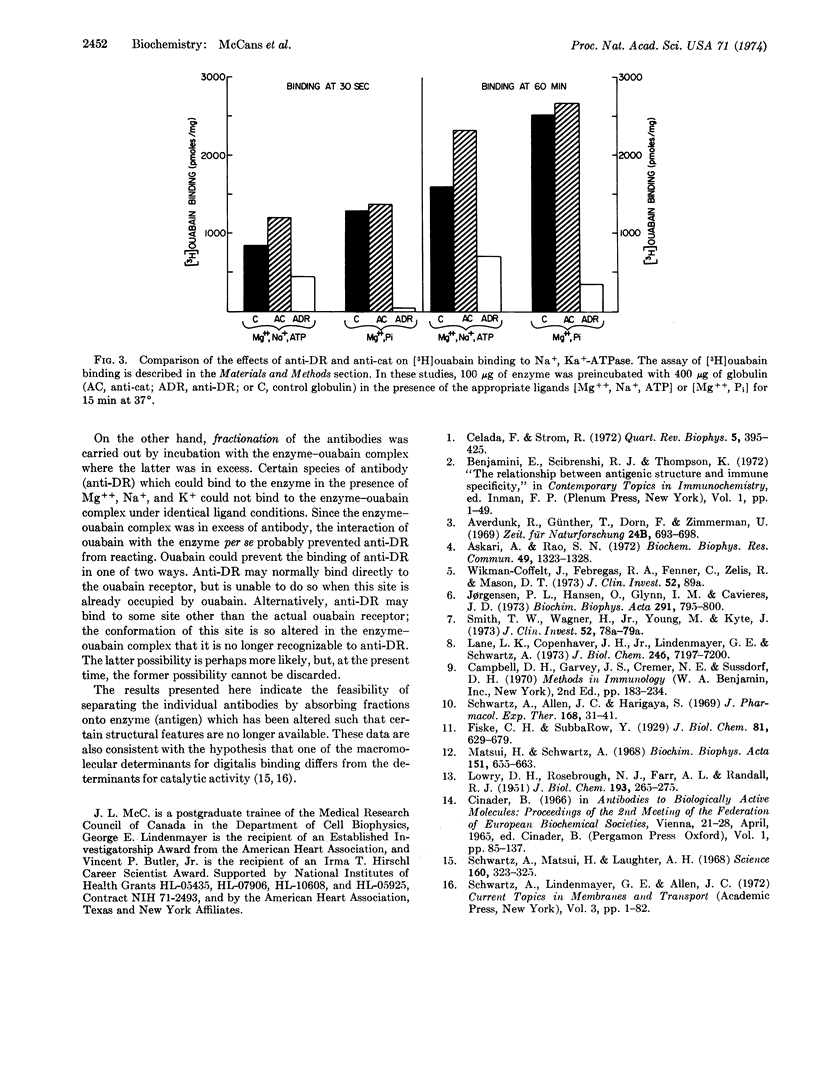
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askari A., Rao S. N. Na + -ATPase complex: effects of anticomplex antibody on the partial reactions catalyzed by the complex. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1323–1328. doi: 10.1016/0006-291x(72)90611-0. [DOI] [PubMed] [Google Scholar]
- Averdunk R., Günther T., Dorn F., Zimmermann U. Uber die Wirkung von Antikörpern auf die ATPase-Aktivität und den aktiven Na-K-Transport von E. coli und Menschen-Erythrozyten. Z Naturforsch B. 1969 Jun;24(6):693–698. [PubMed] [Google Scholar]
- Celada F., Strom R. Antibody-induced conformational changes in proteins. Q Rev Biophys. 1972 Aug;5(3):395–425. doi: 10.1017/s0033583500000998. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L., Hansen O., Glynn I. M., Cavieres J. D. Antibodies to pig kidney (Na + +K + )-ATPase inhibit the Na + pump in human red cells provided they have access to the inner surface of the cell membrane. Biochim Biophys Acta. 1973 Feb 16;291(3):795–800. doi: 10.1016/0005-2736(73)90484-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane L. K., Copenhaver J. H., Jr, Lindenmayer G. E., Schwartz A. Purification and characterization of and (3H)ouabain binding to the transport adenosine triphosphatase from outer medulla of canine kidney. J Biol Chem. 1973 Oct 25;248(20):7197–7200. [PubMed] [Google Scholar]
- Matsui H., Schwartz A. Mechanism of cardiac glycoside inhibition of the (Na+-K+)-dependent ATPase from cardiac tissue. Biochim Biophys Acta. 1968 Mar 25;151(3):655–663. doi: 10.1016/0005-2744(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Allen J. C., Harigaya S. Possible involvement of cardiac Na+, K+-adenosine triphosphatase in the mechanism of action of cardiac glycosides. J Pharmacol Exp Ther. 1969 Jul;168(1):31–41. [PubMed] [Google Scholar]
- Schwartz A., Matsui H., Laughter A. H. Tritiated digoxin binding to (Na+ + K+)-activated adenosine triphosphatase: possible allosteric site. Science. 1968 Apr 19;160(3825):323–325. doi: 10.1126/science.160.3825.323. [DOI] [PubMed] [Google Scholar]


