Abstract
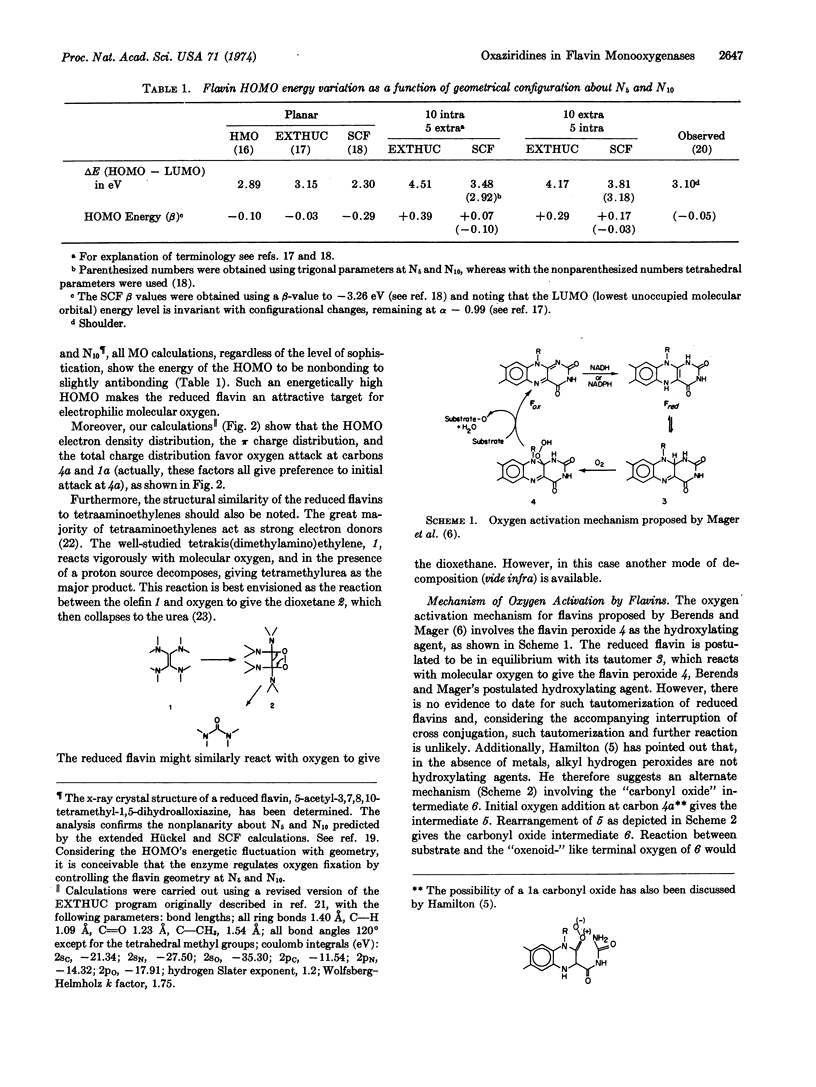
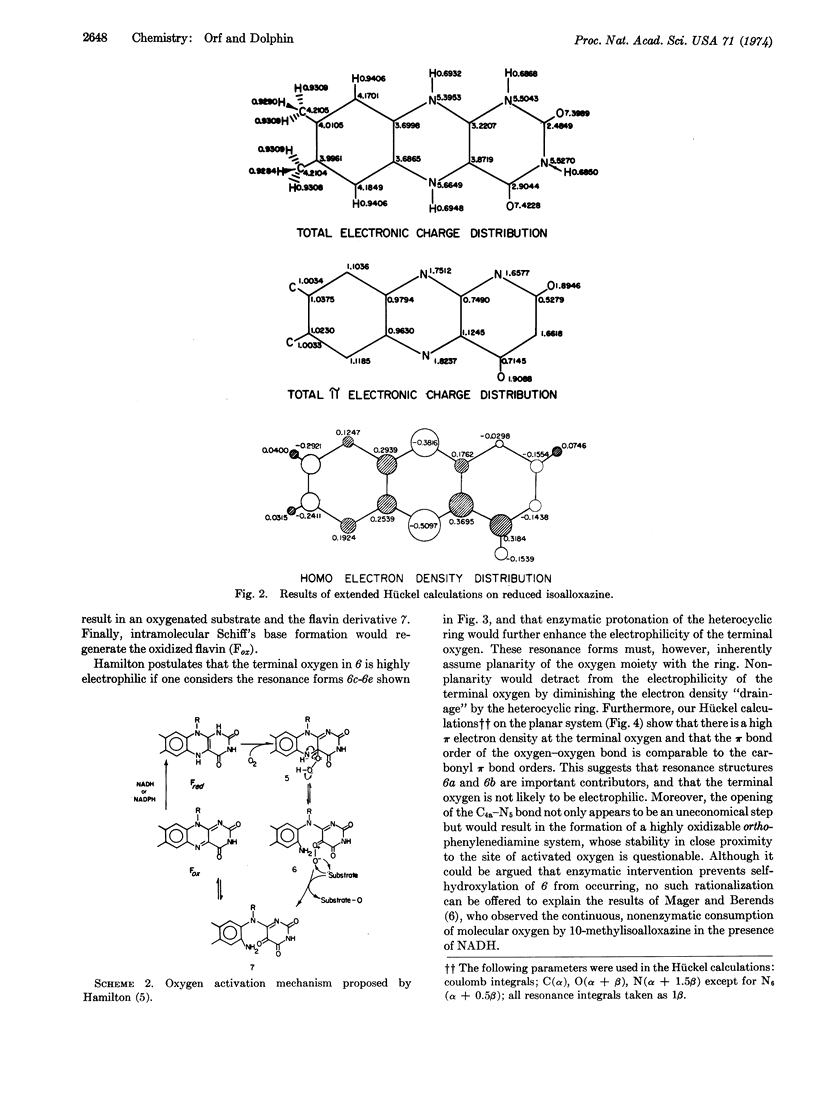
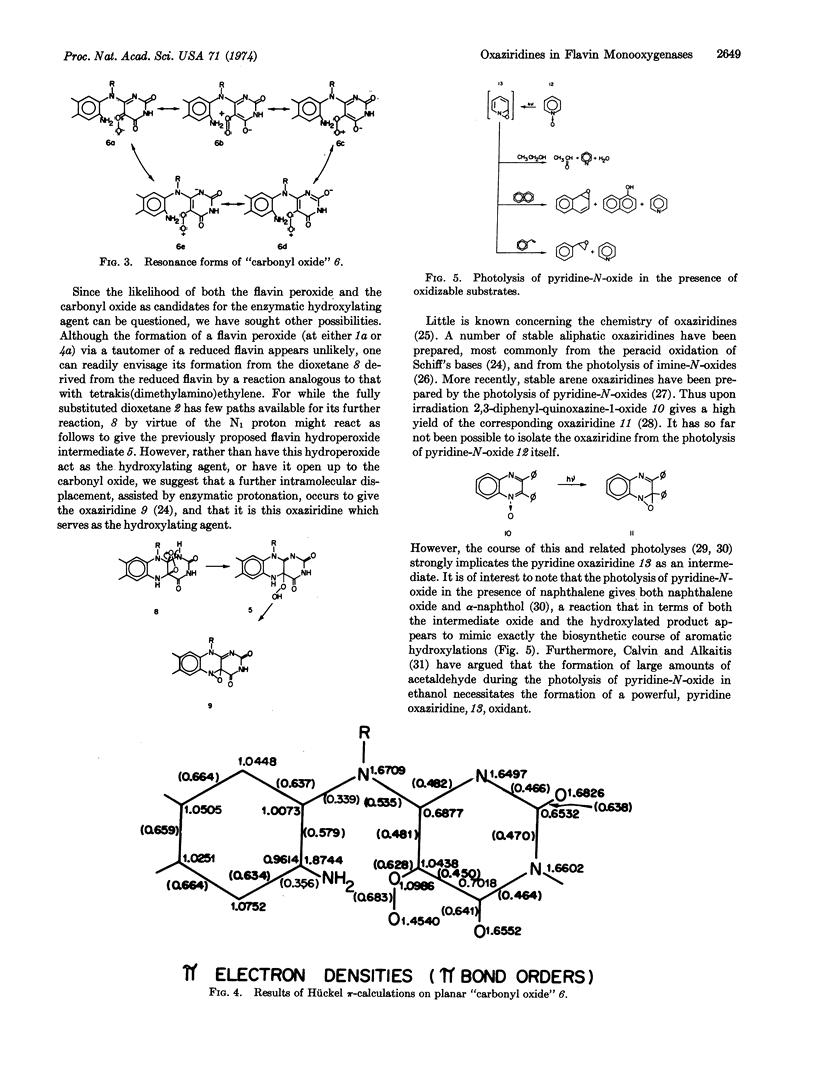
The enzymatic hydroxylation reactions of flavin monooxygenases are suggested to involve an oxaziridine as the active oxygenating agent. The chemistry of these monooxygenases and their nonenzymatic model systems are consistent with an oxaziridine intermediate. Several advantages of the oxaziridine model over the previously proposed “carbonyl oxide” and flavin peroxide models are discussed.
Keywords: oxaziranes, pteridines, molecular orbital calculations
Full text
PDF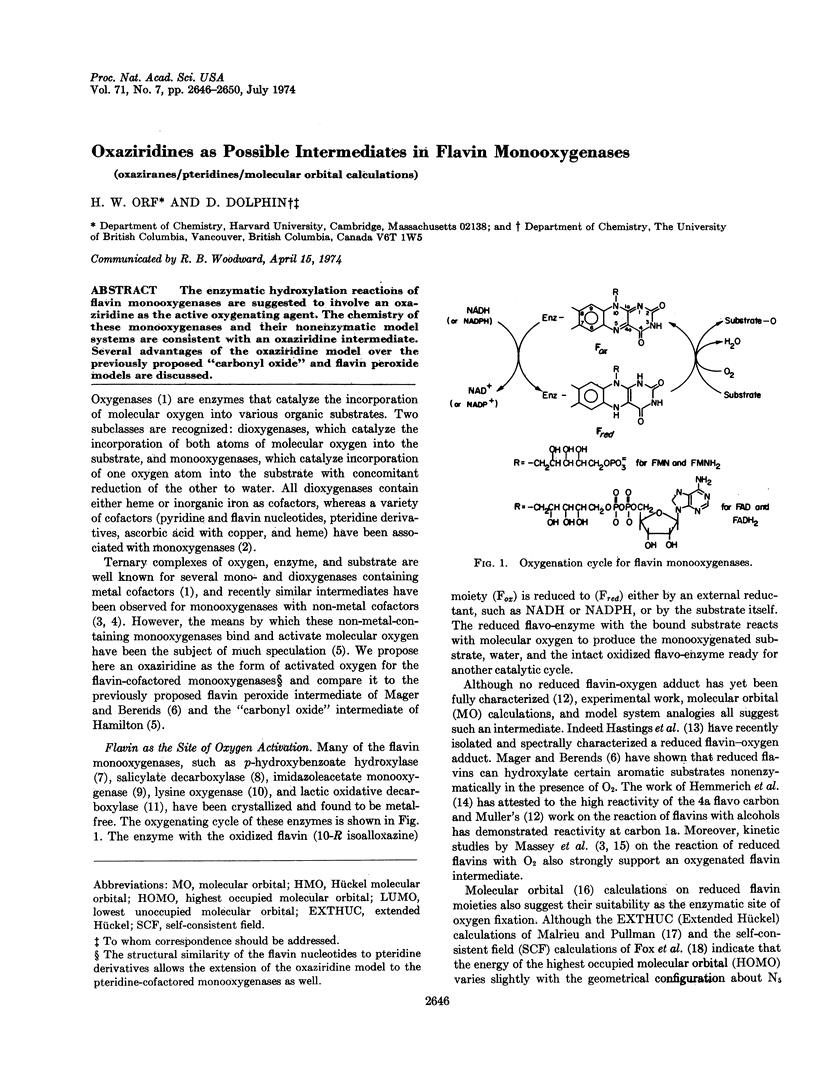

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fox J. L., Laberge S. P., Nishimoto K., Forster L. S. Electronic structures of lumazines and isoalloxazines. Biochim Biophys Acta. 1967 Apr 25;136(3):544–550. doi: 10.1016/0304-4165(67)90012-8. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Balny C., Peuch C. L., Douzou P. Spectral properties of an oxygenated luciferase-flavin intermediate isolated by low-temperature chromatography. Proc Natl Acad Sci U S A. 1973 Dec;70(12 Pt 1-2):3468–3472. doi: 10.1073/pnas.70.12.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O., Nozaki M. Nature and mechanisms of oxygenases. Science. 1969 Apr 25;164(3878):389–396. doi: 10.1126/science.164.3878.389. [DOI] [PubMed] [Google Scholar]
- Hemmerich P., Massey V., Weber G. Photo-induced benzyl substitution of flavins by phenylacetate: a possible model for flavoprotein catalysis. Nature. 1967 Feb 18;213(5077):728–730. doi: 10.1038/213728a0. [DOI] [PubMed] [Google Scholar]
- Hosokawa K., Stanier R. Y. Crystallization and properties of p-hydroxybenzoate hydroxylase from Pseudomonas putida. J Biol Chem. 1966 May 25;241(10):2453–2460. [PubMed] [Google Scholar]
- Maki Y., Yamamoto S., Nozaki M., Hayaishi O. Studies on monooxygenases. II. Crystallization and some properties of imidazole acetate monooxygenase. J Biol Chem. 1969 Jun 10;244(11):2942–2950. [PubMed] [Google Scholar]
- SUTTON W. B. Mechanism of action and crystallization of lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1957 May;226(1):395–405. [PubMed] [Google Scholar]
- Spector T., Massey V. p-Hydroxybenzoate hydroxylase from Pseudomonas fluorescens. Evidence for an oxygenated flavin intermediate. J Biol Chem. 1972 Sep 10;247(17):5632–5636. [PubMed] [Google Scholar]
- Strickland S., Massey V. The mechanism of action of the flavoprotein melilotate hydroxylase. J Biol Chem. 1973 Apr 25;248(8):2953–2962. [PubMed] [Google Scholar]
- Takeda H., Hayaishi O. Crystalline L-lysine oxygenase. J Biol Chem. 1966 Jun 10;241(11):2733–2736. [PubMed] [Google Scholar]
- YAMAMOTO S., KATAGIRI M., MAENO H., HAYAISHI O. SALICYLATE HYDROXYLASE, A MONOOXYGENASE REQUIRING FLAVIN ADENINE DINUCLEOTIDE. I. PURIFICATION AND GENERAL PROPERTIES. J Biol Chem. 1965 Aug;240:3408–3413. [PubMed] [Google Scholar]



