Abstract
The effect of ATP on the first step of excision repair of ultraviolet damage in DNA has been studied using toluene-treated E. coli. During postirradiation incubation, five to six times more single-strand breaks are formed in DNA in the presence of exogenous ATP than in its absence. The ATP-dependent as well as the ATP-independent endonucleolytic activities appear to be catalyzed by the same enzyme since both activities are almost completely absent in uvrA and uvrB mutants. An ATP-dependent endonucleolytic activity has been detected in nonirradiated toluene-treated E. coli. It is concluded that ATP is required in vivo for either the incision step of repair or an enzymatic reaction preceding it.
Keywords: permeable cells, incision step of DNA repair, ATP dependent endonuclease
Full text
PDF
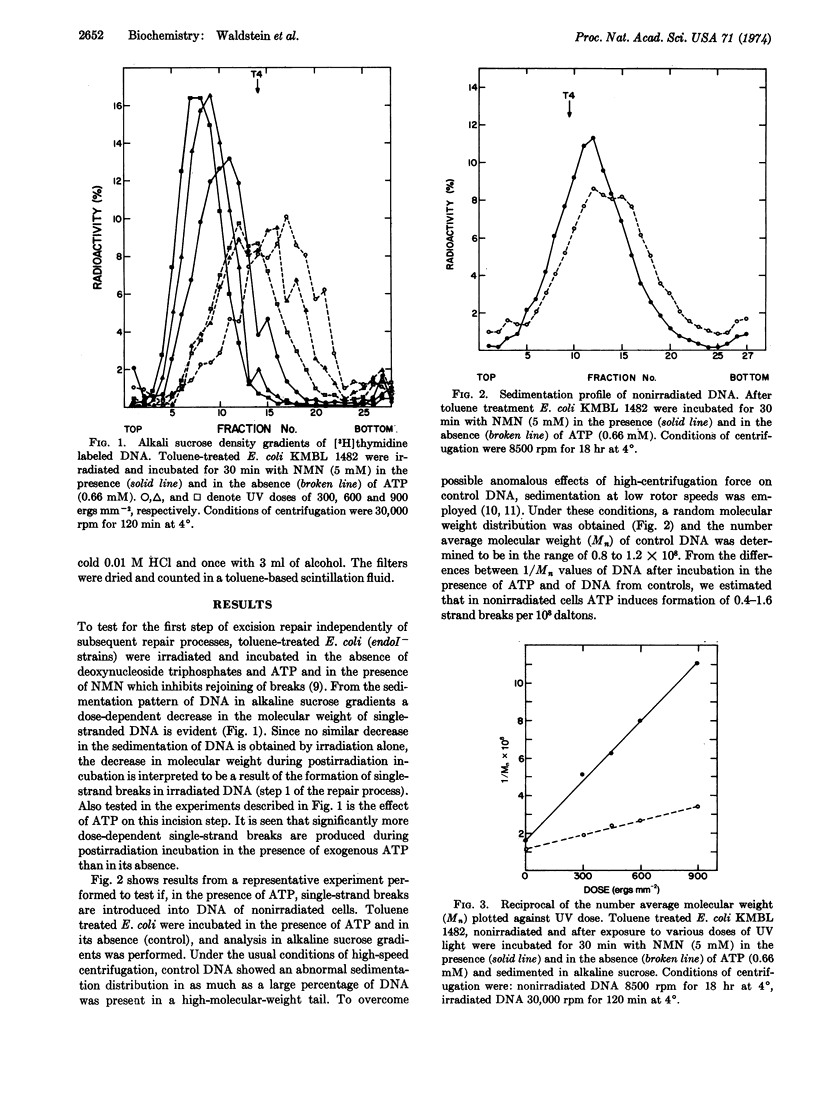
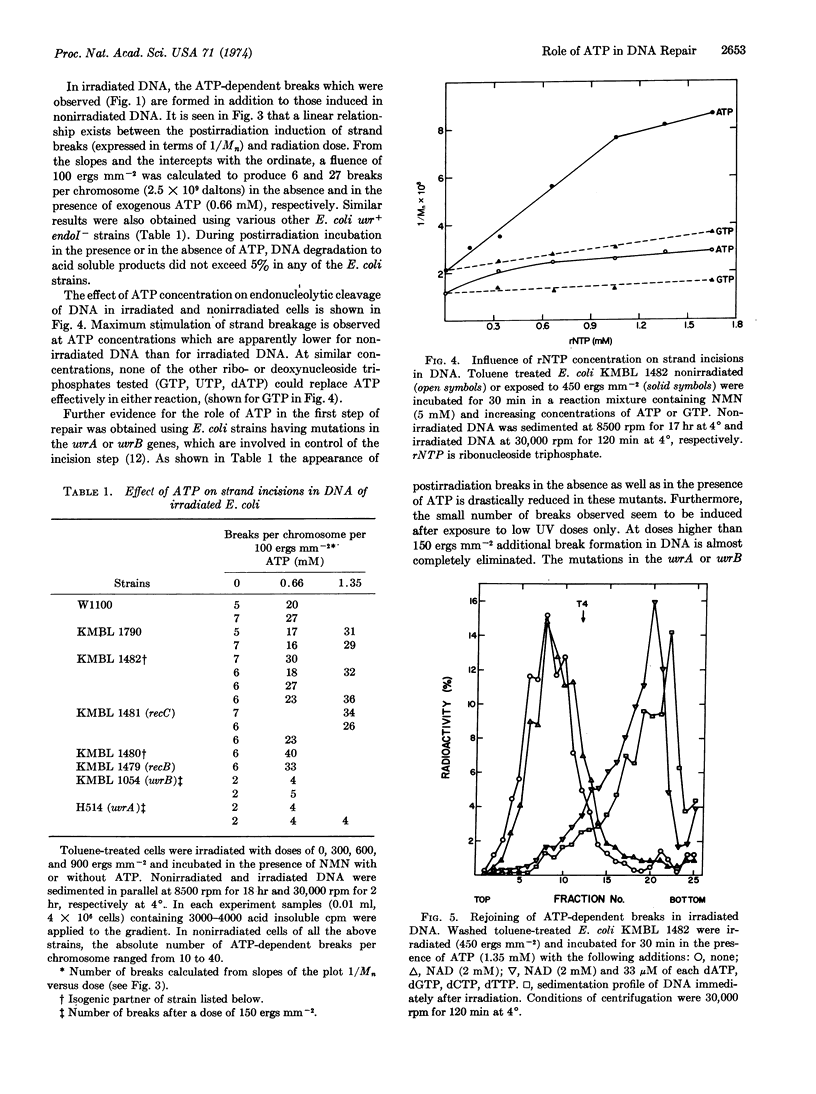

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Setlow R. B. Correlations between host-cell reactivation, ultraviolet reactivation and pyrimidine dimer excision in the DNA of bacteriophage lambda. J Mol Biol. 1970 Jul 14;51(1):131–144. doi: 10.1016/0022-2836(70)90275-5. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Hadden C. T., Billen D., Corrigan K. Stimulation of non-conservative DNA synthesis by ultraviolet light in freeze-treated Bacillus subtilis. Biochim Biophys Acta. 1973 Nov 14;324(4):461–471. doi: 10.1016/0005-2787(73)90205-0. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Kushner S. R., Grossman L. Enzymatic repair of DNA. 3. Properties of the UV-endonuclease and UV-exonuclease. Biochemistry. 1971 Aug 31;10(18):3315–3324. doi: 10.1021/bi00794a001. [DOI] [PubMed] [Google Scholar]
- Kavenoff R. Characterization of the Bacillus subtilis W23 genome by sedimentation. J Mol Biol. 1972 Dec 30;72(3):801–806. doi: 10.1016/0022-2836(72)90192-1. [DOI] [PubMed] [Google Scholar]
- Masker W. E., Hanawalt P. C. Ultraviolet-stimulated DNA synthesis in toluenzied Escherichia coli deficient in DNA polymerase I. Proc Natl Acad Sci U S A. 1973 Jan;70(1):129–133. doi: 10.1073/pnas.70.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Lehman I. R., Wang J. C. Enzymatic joining of polynucleotides. XI. Reversal of Escherichia coli deoxyribonucleic acid ligase reaction. J Biol Chem. 1972 Oct 10;247(19):6370–6372. [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. K., Johnson L. D., Chetty K. G. Effect of relative centrifugal force on the apparent size of DNA from Escherichia coli cells. Can J Biochem. 1973 Apr;51(4):397–406. doi: 10.1139/o73-046. [DOI] [PubMed] [Google Scholar]
- Nakayama H., Okubo S., Takagi Y. Repair of ultraviolet-damaged DNA in Micrococcus lysodeikticus. I. An endonuclease specific for ultraviolet-irradiated DNA. Biochim Biophys Acta. 1971 Jan 1;228(1):67–82. doi: 10.1016/0005-2787(71)90547-8. [DOI] [PubMed] [Google Scholar]
- ROERSCH A., VAN DER KAM P. C., ADEMA J. DARK REACTIVATION OF ULTRAVIOLET IRRADIATED BACTERIOPHAGE DEOXYRIBONUCLEIC ACID IN VITRO. Biochim Biophys Acta. 1964 Feb 17;80:346–348. [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Segev N., Miller C., Sharon R., Ben-Ishai R. Excision repair of ultraviolet radiation damage in toluene treated Escherichia coli. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1241–1245. doi: 10.1016/0006-291x(73)90633-5. [DOI] [PubMed] [Google Scholar]
- Taketo A., Yasuda S., Sekiguchi M. Initial step of excision repair in Escherichia coli: replacement of defective function of uvr mutants by T4 endonuclease V. J Mol Biol. 1972 Sep 14;70(1):1–14. doi: 10.1016/0022-2836(72)90160-x. [DOI] [PubMed] [Google Scholar]
- Val'dshtein E. A., Tomilin N. V. O spetsifichnosti u.-f.-éndonukleazy iz M. lysodeikticus. Deistvie na DNK, obraborannuiu gidroksilaminom. Dokl Akad Nauk SSSR. 1971 Oct;200(6):1459–1462. [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]


