Abstract
Background: Recent advances in schizophrenia genetics are shedding new light on etiopathogenesis, but issues germane to translation of findings into clinical practice are relatively understudied. We assessed the need for, and efficacy of, a contemporary genetic counseling protocol for individuals with schizophrenia. Methods: After characterizing rare copy number variation in a cohort of adults with schizophrenia, we recruited subjects from the majority of individuals who had no clinically relevant structural genetic variant. We used a pre-post study design with longitudinal follow-up to assess both the profile of need and the impact of general genetic counseling on key knowledge-based and psychological factors. Results: Thirty-nine (60.0%) of 65 patients approached actively expressed an interest in the study. At baseline, participants (n = 25) tended to overestimate the risk of familial recurrence of schizophrenia, express considerable concern related to this perceived risk, endorse myths about schizophrenia etiology, and blame themselves for their illness. Postcounseling, there was a significant improvement in understanding of the empiric recurrence risk (P = .0090), accompanied by a decrease in associated concern (P = .0020). There were also significant gains in subjective (P = .0007) and objective (P = .0103) knowledge, and reductions in internalized stigma (P = .0111) and self-blame (P = .0401). Satisfaction with genetic counseling, including endorsement of the need for such counseling (86.4%), was high. Conclusions: These results provide initial evidence of need for, and efficacy of, genetic counseling for individuals with schizophrenia. The findings may help facilitate development of a contemporary genetic counseling process that could optimize outcomes in the nascent field of evidence-based psychiatric genetic counseling.
Key words: schizophrenia, genetics, genetic counseling, genetic predisposition to disease, copy number variation, stigma
Introduction
Schizophrenia is a complex neuropsychiatric disease with evidence for genetic heterogeneity and neurodevelopmental origins.1 Genetic variants of clinical interest are currently limited to chromosome 22q11.2 microdeletions and select other individually rare copy number variants (CNVs) that underlie emerging genetic subtypes of the illness.1,2 For the vast majority of patients (>90%–95%),2 no major causal factor can yet be identified and genetic counseling (Box 1) would be informed primarily by nonspecific genetic epidemiology and basic empiric recurrence risk estimates.1,3–6 Nonetheless, up to 72% of individuals with schizophrenia express a hypothetical interest in such general genetic counseling7–9 and a longstanding theoretical interest exists in the literature.1,3–6,10,11 The obvious next questions relate to the uptake and outcomes of counseling actually provided to patients with schizophrenia.
Box 1. What is genetic counseling?
“Genetic counseling is the process of helping people understand and adapt to the medical, psychological, and familial implications of genetic contributions to disease. This process integrates the following:
Interpretation of family and medical histories to assess the chance of disease occurrence or recurrence.
Education about inheritance, testing, management, prevention, resources, and research.
Counseling to promote informed choices and adaptation to the risk or condition.”
Source: Resta R, Biesecker BB, Bennett RL, et al. A new definition of Genetic Counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns 2006;15:77–83.
A recent systematic review exposed the surprising paucity of empirical research,12 including a lack of outcome studies of genetic counseling for patients with schizophrenia. This is in stark contrast to other common complex diseases like cancer,13–15 albeit where there are fewer features that may interfere with provision of standard genetic counseling (table 1). Data that are available show low referral rates and access to counseling.8,16–18 There is evidence of self-reported deficiencies in knowledge regarding schizophrenia etiology and risk factors among clinicians,16,17 and of widespread stigma and misconceptions,19 even among genetic counselors (table 1).20,21 In addition, there is speculation that attribution of schizophrenia to genetic factors could actually increase stigma22,23 and even decrease confidence in the extent to which pharmacotherapy will be helpful (“therapeutic nihilism”).11
Table 1.
Common Features of Schizophrenia and Potential Negative Consequences for Genetic Counseling Provided by a Medical Genetics and/or Mental Health Care Professional
| Common Features of Schizophrenia | Potential Consequences for Genetic Counseling |
|---|---|
| Individual patient characteristics | • Rapport building is challenging |
| “Positive” symptoms | • Patient is unwilling to believe information provided |
| Fixed false beliefs (delusions), false perceptions like hearing voices (hallucinations), suspiciousness, unusual thought form and content | • Patient gives false impression of disinterest during counseling |
| “Negative” symptoms | • Patient displays poor retention of information provided |
| Lack of drive or motivation (avolition), flat affect, lack of spontaneous speech (alogia), loss of interest in pleasurable activities (anhedonia), social withdrawal | • Patient is an unreliable historian |
| Cognitive impairments | • Patient is less likely to actively seek out a referral |
| Attention and/or memory problems | |
| Poor insight | |
| Limited awareness that they have a mental illness | |
| External factor | • False belief on the part of counselor and/or patient that etiology is entirely uncertain |
| Clinical and genetic heterogeneity | • False belief on the part of counselor and/or patient that diagnosis is entirely uncertain |
| Clinical diagnosis only, variable signs and symptoms, lack of universal genetic testing, no common genetic variants or environmental factors of notable effect size, limited opportunity to individualize recurrence risk statistics | • Diagnostic uncertainty in family history taking |
| Stigma | • Counselor and patient frustration with perceived lack of knowledge and relatively fewer opportunities for personalized information |
| Stereotypes, myths about etiology and risk factors, belief that schizophrenia is somehow fundamentally different from other diseases, desire for social distance | • Provision of misinformation by counselor |
| Knowledge gaps of key professionals | • Patient is less likely to be referred or offered counseling |
| Limited training of medical genetics professionals in psychiatric illness and of mental health care professionals in genetics |
In the current study, we aimed to test for the first time the effectiveness of a standardized protocol for genetic counseling for patients with schizophrenia, as delivered by a nongenetics professional in a community setting. We strove to make this genetic counseling as close as possible to that which a genetics professional would provide in the same circumstances. We were able to exclude the minority of patients with clinically relevant genetic diagnoses, for whom this basic research-based counseling would have been inappropriate. Among patients who expressed interest in counseling, we anticipated a profile of need (distorted recurrence risk perception, associated concern, and deficiencies in knowledge) similar to that observed in family members.12 Based on results for relatives,12 and research for other complex diseases,13–15 we further hypothesized that this brief genetic counseling session would result in (1) an improved understanding of the empiric recurrence risk, (2) less recurrence-related concern, (3) measureable gains in knowledge of schizophrenia etiology and risk factors, (4) decreased stigma and self-blame, and (5) high satisfaction.
Methods
Participants
Potential study participants were adults (age ≥18 years) with DSM-IV diagnoses of schizophrenia or schizoaffective disorder (collectively termed “schizophrenia”)2,12,24 originally recruited through a single community outpatient clinic as part of a genetic case-control study.2 The process of (1) ascertainment; (2) confirmation of diagnosis; (3) acquisition of detailed medical and family histories, including review of lifetime medical records and interview of family members for collateral information where possible; (4) clinical screening; and (5) molecular characterization of rare CNVs using the Affymetrix® Genome-Wide Human SNP Array 6.0 for this case-control study is described elsewhere.2,24 As recommended,1,3–5 patients with karyotypic abnormalities, 22q11.2 deletions, or other clinically confirmed pathogenic CNVs likely to be associated with schizophrenia2,12,24 were counseled on a clinical basis by a medical genetics professional. We then approached a randomly selected subset of the remaining unrelated psychiatrically stable adult outpatients about participating in this study of genetic counseling (figure S1). The study was approved by local research ethics boards. After complete description of the study, written informed consent was obtained. No patients who expressed an interest in the study were subsequently unable to provide informed consent, or deemed too ill to receive genetic counseling on the basis of their current state of functioning.
Counseling
We developed and standardized a genetic counseling protocol for adults with schizophrenia. This was based on tested protocols for other complex diseases (Esplen et al.13 and unpublished), the existing literature for schizophrenia,1,3–6,10 and input from medical genetics and mental health care professionals, patients, and family members. A workflow algorithm for use in clinical practice is presented elsewhere.12 The core educational content was drawn from previously published comprehensive reviews of the existing schizophrenia literature,1,4,5,25 and is described elsewhere.12 The first four patients to consent were involved in the pilot testing of the counseling intervention and outcomes measures—a period also used for training the counselor (figure S1). Changes were subsequently made to improve clarity and decrease response burden. The responses of these four patients are not included in this report but were noted to be consistent with the overall results.
As part of the research protocol, all tenets of genetic counseling (Box 1) were observed. In particular, as in our previous study,12 we provided individualized recurrence risk estimates based on family history and the best available empiric risk data5,10 and personalized each discussion surrounding medical and family history factors that may qualitatively affect recurrence risk.26,27 All counseling (mean 46.4 [SD 11.1] minutes, not including the family history assessment that had been recently performed by a nongenetics professional) was delivered on an individual basis by a graduate student (G.C.), with oversight and supervision provided by experienced clinicians with expertise in this area (A.S.B., M.J.E.). Each patient participated in a single genetic counseling session. Sessions were audio recorded and transcribed, where possible (n = 19 [67.9%] of 28), to (1) ensure adherence to the protocol and (2) allow for a thematic analysis of the thoughts, feelings, and questions of participants and the process of counseling. Detailed field notes were kept for nonrecorded sessions. Qualitative data were supportive of the quantitative findings and will be published separately.
Measures
Each participant completed a battery of self-administered psychological and knowledge-based questionnaires at three time points, as described below. Baseline (T0) measures were completed in person, and follow-up measures at one (T1) and seven (T2) weeks postcounseling were returned using stamped, preaddressed envelopes. To the extent possible, given literacy and attention issues in this study population, the measures used were directly comparable to those employed in our recent study of schizophrenia genetic counseling for family members.12 Two participants received counseling but were unable to complete paper-based measures and so only qualitative data were collected (figure S1). Another participant failed to complete either set of follow-up measures, and so his baseline quantitative responses were excluded (figure S1). Thus we present quantitative data from 25 unrelated adults with schizophrenia, all of whom completed at least one set of follow-up measures.
Recurrence Risk Perception and Associated Concern.
We used standard methods in genetic counseling outcome research to assess risk perception.12–15 A visual analog scale (0%–100%) and a five-point (“Very low” to “Very high”) Likert-scale item were used to assess, respectively, perceived quantitative and qualitative lifetime morbid risk for schizophrenia to a specific family member. For each participant, we defined a quantitative risk estimate ≥10% different from the best individualized risk assessment for their specified family member as inaccurate.14 Concern about perceived genetic risk and familial recurrence was queried using a visual analog scale (0% = “Not at all concerned” to 100% = “Extremely concerned”).
Knowledge and Etiological Attribution.
Perceived (subjective) knowledge of schizophrenia etiology was assessed using a visual analog scale (0% = “Don’t understand at all” to 100% = “Understand very clearly”). Participants also completed a questionnaire developed for the study comprising 14 true or false items (table S1). Etiological attribution of their schizophrenic illness was queried in an open-ended fashion, but we included a list of 18 possible factors adapted from the revised Illness Perception Questionnaire.28 To address the question of therapeutic nihilism, at follow-up a five-point (“Strongly disagree” to “Strongly agree”) Likert-scale was used in conjunction with the statement, “Understanding more about schizophrenia means I’m more likely to take my medications every day.”
Psychological Functioning.
We used the Internalized Stigma of Mental Illness (ISMI) scale, a well-validated measure of the subjective experience of stigma.29 The ISMI contains 29 four-point (“Strongly disagree” to “Strongly agree”) Likert-scale items and is comprised of five subscales.29 Visual analog scales (0% = “Not at all” to 100% = “Completely”) concerning both personal contribution to, and self-blame surrounding, the participants’ schizophrenia and four-point (“Strongly disagree” to “Strongly agree”) Likert-scale items about whether they or anyone else was to blame were also included. At T1 and T2, satisfaction with genetic counseling was determined using 5 five-point Likert-scale items (table S2).
Analyses
We tested for within-participant differences in responses provided at T0 and T1, and to assess retention of any change at T2.12 Visual inspection and the Cramér-von Mises criterion were used to test the normality of each difference distribution, with subsequent use of the paired Student’s t test (t) or the nonparametric paired Wilcoxon signed-rank test (S) as appropriate. Coefficients of determination (R 2) were calculated from single-variable linear regression models. Missing values in follow-up measures, where necessary for a cumulative score, were replaced with baseline responses to bias away from change (all rates of missing data were <2%). Where appropriate, we compared patient responses at T0 with family member responses from our previous study12 using standard tests. All analyses were two tailed and performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC), with statistical significance defined as P < .05.
Results
Thirty-nine (60.0%) of 65 patients approached actively expressed an interest in the study (figure S1), with no significant differences between those who did and did not express an interest (table S3). Basic demographic and clinical characteristics of final study participants (table 2) were representative of the patient population in this region2,24 and measures of illness severity and premorbid and current functioning (table 2 and data not shown) suggest this study cohort is comparable to other general schizophrenia populations.30 No participant had previously received genetic counseling.
Table 2.
Demographic and Clinical Features of Unrelated Adults With Schizophrenia Participating in a Study of Genetic Counseling
| Participants With Schizophrenia (n = 25a) | ||
|---|---|---|
| Mean | (SD) | |
| Age at time of counseling (years) | 46.8 | (12.6) |
| Age at onset of psychosisb (years) | 22.6 | (4.7) |
| NARTc-estimated full scale premorbid IQ | 102.8 | (9.6) |
| Number of liveborn offspring | 1.0 | (1.4) |
| n | (%) | |
| Female sex | 10 | (40.0) |
| European ancestry | 24 | (96.0) |
| Family history of psychosisd | 7 | (28.0) |
| Ever marriede | 11 | (44.0) |
| Childless | 14 | (56.0) |
| Education | ||
| Did not complete high school | 7 | (28.0) |
| Completed high school (only) | 8 | (32.0) |
| Completed some postsecondary education | 10 | (40.0) |
| Primary source of concern about recurrence | ||
| Niece/nephew | 10 | (40.0) |
| Offspringf | 10 | (40.0) |
| Grandchild | 3 | (12.0) |
| Sibling | 1 | (4.0) |
| No person of concern | 1 | (4.0) |
aNot including n = 4 involved in pilot testing, n = 2 unable to complete paper-based measures, and n = 1 who failed to complete either set of follow-up measures (see text and figure S1 for details).
bDefined as age at first treatment for psychosis; none with childhood onset schizophrenia.
cNational Adult Reading Test30.
dDefined as having a first degree relative with a psychotic illness.
eIncludes marriage or a common-law arrangement of one year or more.
fNone had two parents with schizophrenia; includes potential future offspring.
Recurrence Risk Perception and Associated Concern
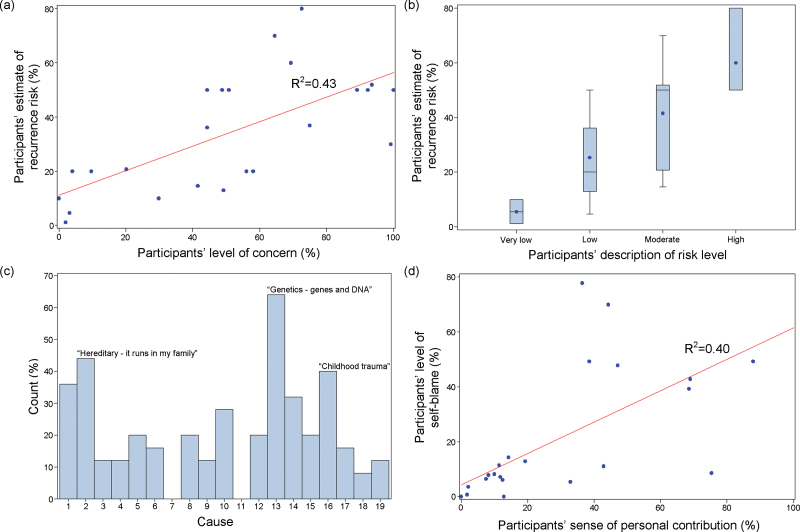
Participants initially reported high levels of concern about familial recurrence, usually in relation to a niece/nephew (unmodified mean empiric recurrence risk estimate ~3%)5,10 or offspring (unmodified mean empiric recurrence risk estimate ~13%)5,10 (table 2). Fifteen (62.5%) of 24 participants gave inaccurate estimates of the recurrence risk (all overestimates). Concern and quantitative estimates of recurrence risk were well-correlated (F[1,22] = 18.5, P = .0003; adjusted R 2 = 0.43) (figure 1a). We also observed a correlation between quantitative and qualitative estimates of risk (F[1,22] = 18.1, P = .0003; adjusted R 2 = 0.43) (figure 1b). Responses regarding risk and concern, and their correlations, were strikingly similar to those of family members of individuals with schizophrenia (table S4; figure S2).12
Fig. 1.
Responses of individuals with schizophrenia to selected items prior to genetic counseling. (a) Estimated risk of recurrence of schizophrenia to a specific family member vs concern about familial recurrence. (b) Estimated risk of recurrence by qualitative description of risk. (c) All perceived possible causes of their schizophrenic illness. (d) Self-blame vs perceived personal contribution to their schizophrenic illness. See Methods section for item details. Cause coding key: 1 = Stress of worry, 2 = Hereditary—it runs in my family, 3 = A germ or virus, 4 =Diet or eating habits, 5 = Chance or bad luck, 6 = Bad parenting, 7 = Pollution in the environment, 8 = Own behavior, 9 = Negative thinking, 10 = Family problems, 11 = Overwork, 12 = Alcohol, 13 = Genetics—genes and DNA, 14 = Drug use, 15 = Bad life choices, 16 = Childhood trauma, 17 = Head injury, 18 = Something that happened during pregnancy or at birth, 19 = Other.
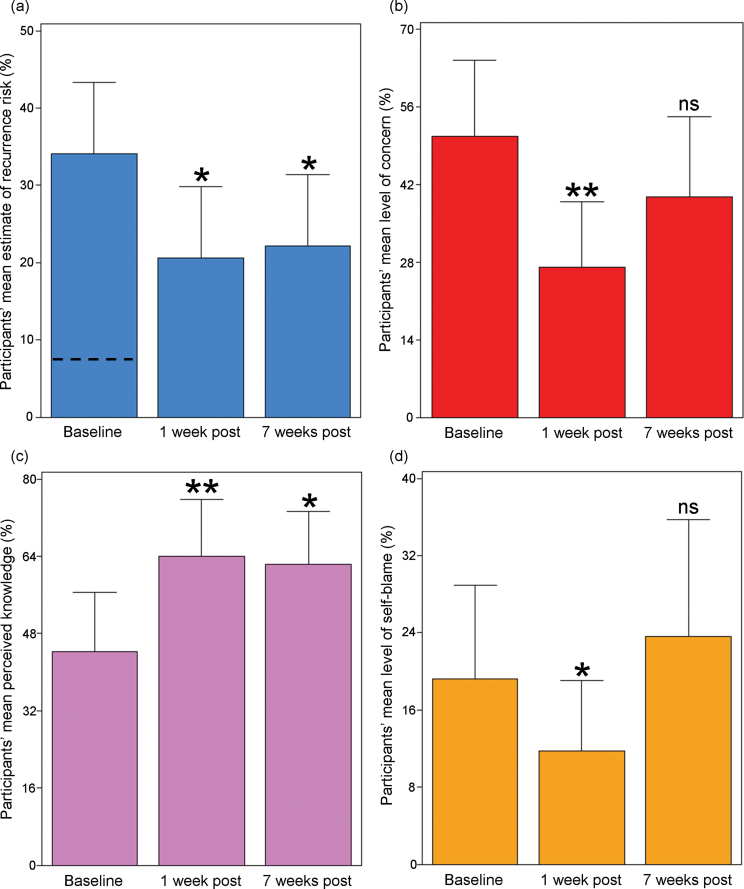
One week postcounseling (T1), the mean recurrence risk estimate had decreased by two-fifths (figure 2a), with a concomitant decrease in mean recurrence-related concern of almost half (figure 2b) and in the number of subjects with inaccurate overestimates of the risk (n = 8). Risk was also qualitatively described as lower at T1 than at T0 (S = 50, df = 21, P = .0072). However, at T2 the mean level of concern and the mean qualitative risk description were no longer significantly different from T0 (figure 2b; table S4).
Fig. 2.
Selected significant benefits of genetic counseling for individuals with schizophrenia. (a) Participants’ estimated risk of familial recurrence of schizophrenia to a specific family member (dashed line indicates mean of personalized recurrence risk assessments quoted to participants). (b) Participants’ concern related to familial recurrence. (c) Participants’ perceived knowledge about the illness. (d) Participants’ level of self-blame. In (d), the nonsignificantly greater mean at 7 weeks postcounseling was driven primarily by a single respondent whose results returned to his baseline subsequently. See Methods section for item details. *P < .05, **P < .005, and ns = nonsignificant. All summary data and P values are presented in table S4. Error bars represent upper 95% confidence intervals.
Knowledge and Etiological Attribution
Although a common etiological attribution of schizophrenia was “genetics,” participants endorsed diverse factors including perceived abuse in childhood (figure 1c). Significant and lasting improvements were observed postcounseling in mean perceived knowledge (figure 2c) and in mean knowledge quiz score (T1: t = 2.8, df = 21, P = .0103; T2: t = 3.7, df = 20, P = .0013) (table S4). Responses by item are displayed in table S1. A majority of respondents (T1: 90.9%) also agreed with a statement, suggesting that their improved understanding of schizophrenia etiology might improve their compliance with pharmacotherapy.
Psychological Functioning
At baseline, perceived personal contribution to the schizophrenic illness and self-blame were well-correlated (F[1,23] = 16.7, P = .0005; adjusted R 2 = 0.40) (figure 1d). As expected, visual analog and Likert scale measures of self-blame were also correlated (F[1,23] = 10.9, P = .0031; adjusted R 2 = 0.29). Compared with relatives,12 levels of guilt and self-blame were significantly greater on average (table S4). Many participants reported having blamed themselves or others even more in the past than they did at the time of counseling. At T1, measures of self-blame (table S4) and the mean total ISMI score (t = 2.8, df = 21, P = .0111; table S5) were significantly lower. However, differences were no longer significant at T2 relative to T0 (tables S4,S5).
Satisfaction
Satisfaction with genetic counseling was high, with most participants endorsing that they “felt better” after the counseling (86.4%), that the counseling was valuable to them (90.9%), and that genetic counseling should be offered to all adults with schizophrenia (86.4%) (table S2). Levels of satisfaction were similar to those observed in our previous study of family members.12 The sole individual who consistently expressed dissatisfaction stated, “I don’t think the origin of my Illness lies in Genomes. I believe every person is born with their own series of Genes and what is made of them makes them their own person [sic].” He believed his illness to have been caused by, “…poor diet as a child, [and] very little interaction with other children,” and had not wanted to consider other possibilities.
Targeted Follow-Up
Upon noting the transient nature of some immediate benefits, we added a post-hoc experiment to investigate the potential effects of repetition of counseling. Twelve of the first 17 participants approached were available for a second iteration of genetic counseling 16 weeks after their first counseling session. The study protocol was repeated with the counselor unchanged and follow-up assessments again at 1 and 7 weeks postcounseling, but with relatively less emphasis in the genetic counseling session placed on knowledge provision and more on understanding feelings of self-blame. Ten of 12 counseling sessions were audio recorded and transcribed. In general, further lasting gains in understanding of the recurrence risk and schizophrenia etiology, and a decrease in recurrence-associated concern, were observed, and satisfaction was again high (table S6). We made an initial attempt to assess the potential impact of a simple acquiescence bias associated with the sole item about treatment compliance by, on repeat testing, reversing its wording. We observed no change in the strength of the response, with 8 of 10 respondents at T1 and T2 who completed this section now disagreeing with the negative statement. However, as before, no lasting change in either the mean level of self-blame (T0: 15.6%, T2: 14.9%; S = 4, df = 10, P = .6406) or the mean total ISMI score (T0: 2.0, T2: 1.9; t = 0.1, df = 9, P = .9011) was observed at seven weeks postcounseling.
Discussion
To our knowledge, this is the first study of genetic counseling for individuals with schizophrenia.1,3,12 The counseling needs of patients, with respect to distorted recurrence risk perception, associated concern, and deficiencies in knowledge, were broadly consistent with those of family members,12 but patients tended to have higher levels of self-blame. These preliminary data suggest that, as hypothesized, genetic counseling for schizophrenia may improve understanding of the recurrence risk, psychological functioning, and knowledge about the illness, with outcomes similar to but not as lasting as those observed in family members.12 Thus the presence of a psychotic disorder (table 1), and the absence of a clinically relevant genetic variant, do not appear to be absolute contraindications to providing helpful genetic counseling.
Evidence-Based Counseling
Taking an evidence-based approach is recognized as essential for all clinical interventions including genetic counseling.13–15 This may be particularly important in the context of a disease like schizophrenia that is fraught with misconceptions and stigma. How, therefore, is it possible that this is the first study to ever investigate the efficacy of genetic counseling for individuals with schizophrenia? We speculate that the illusion of data generated by the many reviews and commentaries on this topic may inadvertently give a false impression about the existing evidence.12 Others may feel that studying genetic counseling necessitates molecular genetic diagnoses or more individualized recurrence risk estimates as afforded, eg, by the Gail model for breast cancer, or would be of no value to individuals with cognitive impairments who may not reproduce (table 1). The results of this initial study may help dispel such misconceptions, and also emphasize the need for further study in this area. Our findings are in line with similar studies of the efficacy of genetic counseling in other multifactorial diseases15 and established practice in medical genetics.31 There are immediate and long-term implications for clinical practice and for private and public financial support for genetics services.
Recurrence Risk Perception and Associated Concern
Optimization of risk perception accuracy is a common challenge in clinical practice,32 but the results for individuals with schizophrenia were comparable to those for a group of family members.12 Varying descriptions of risk (eg, “low” or “high”) associated with similar numerical estimates (figure 1b) emphasize the need to frame risks for patients in different, complementary ways. The perceived recurrence risk was an incomplete predictor of concern, however, and the significant reduction in the latter was not lasting. Pharmacological and other advances that decrease the burden of illness associated with schizophrenia may also facilitate the alleviation of concerns about familial recurrence.
Recurrence risk estimates quoted to patients participating in this study were generally modest (≤13%). We note, however, that the number of offspring born to two affected parents, where the recurrence risk approaches that of an autosomal dominant condition,5,10 may be increasing.33 An accurate knowledge of the recurrence risk, even if higher than initially anticipated, may benefit the patient even in the absence of specific preventative measures. In the context of reassurance about the inability to predict or control the future, it may be helpful to emphasize that early diagnosis and effective management can improve outcomes.4
Knowledge and Etiological Attribution
Incomplete knowledge of the genetic origins of any complex disease is the rule, and is rarely seen as an impediment to providing helpful genetic counseling.31 In this study, relaying details about the current state of knowledge helped to dispel myths about schizophrenia etiology and risk factors, including the myth that there exist specific independent nongenetic factors (eg, childhood trauma, including perceived abuse) of moderate or greater effect.25 Providing genetic counseling also seemed to engender an improved sense of confidence in that knowledge. Our results are consistent with those of a recent study involving parents of children with autism, where respondents valued hearing about genetic discoveries even if not directly relevant to their own family.34 For example, a failure to distinguish between genetic and inherited was common.12,34 Conveying recent molecular genetic evidence that confirms a long-standing hypothesis about the importance of de novo mutations1 may have helped to decrease self-blame and reconcile the presence of a genetic disease with an absence of a family history, the most common situation for individuals with schizophrenia.10 It is also important to be transparent about the limits of our knowledge.34 We strove to temper hype surrounding psychiatric genetics by, eg, explaining the known genetic heterogeneity of schizophrenia and the probabilitistic nature of all risk variants identified.1
Stigma and Self-Blame
The results suggest that individuals with schizophrenia may on average endorse greater self-blame regarding their illness than do their mothers and other family members.12 In this sense, they demonstrate an even greater need for genetic counseling than do unaffected relatives. We found no evidence that attribution of schizophrenia to genetic factors increases stigma or blame.22,23 Indeed, typical genetic counseling may even affect a lasting reduction in these features in a subset of individuals. Expanding the scope of lasting benefit could therefore be a key goal of future research. However, it may be that while for some self-blame is malleable and the result of underlying misconceptions about the illness that can be remedied with accurate information, for others self-blame may reflect delusional thinking more amenable to improved treatment of the underlying schizophrenic illness (table 1). Notably, none of the participants had molecular genetic diagnoses. Initial data regarding 22q11.2 deletions in schizophrenia,21,35 and pathogenic CNVs in autism,34 suggest that having a clinically relevant genetic anomaly could further alleviate guilt and self-blame and decrease stigma by raising awareness.
Opportunities for Further Gains
Some positive effects of genetic counseling appeared short-lived, consistent with studies of psychoeducational approaches in schizophrenia,36 where repetition is often needed to a degree unfamiliar to most genetic counselors and standard genetic counseling approaches (table 1). For example, our results and post-hoc additional counseling sessions suggest that additional counseling sessions, different approaches, and/or extended support may help more patients achieve a lasting reduction in self-blame or stigma. Clearly, additional studies will be needed.
Implementation
These results indicate that, in the absence of a clinically relevant genetic variant or other complicating factors, a trained clinician may be able to deliver a standardized genetic counseling-type intervention with measurable benefit to the patient with schizophrenia.12 Taking a family history is standard practice in psychiatry, as in the rest of medicine. Many psychiatrists already see genetic counseling as within their potential scope of practice, and demand is likely to increase over time.16,17 Our results provide initial evidence of patient interest, feasibility, and efficacy related to such an approach. While counseling provided by a genetic counselor with expertise in psychiatric genetics would be the ideal, this may not be realistic for general genetic counseling for such a common disease. The challenge, however, remains for physicians to triage patients appropriately for genetic counseling.12 In addition to concerns about the genomic literacy of psychiatrists and psychiatric residents, there may be low awareness of the roles that genetic counselors can play on a clinical team.37 Expanding knowledge and application of genetic counseling in psychiatry will likely require both additional genetics training for psychiatrists and increased training in psychiatric disease for medical genetics professionals.16,17,20,21,37 The former would help to improve the genomic literacy of psychiatrists,16,17,37 while the latter may help to reduce the self-reported personal discomfort in discussing psychiatric illness and limited knowledge about clinical features and treatment.20,21
Advantages and Limitations
Major strengths of this study include the detailed molecular and clinical characterization of the patients, use of an evidence-based manual, and assessments at multiple time points using standard measures of genetic counseling outcomes. A particular advance of the genetic counseling protocol used was to incorporate information about 21st century molecular genetic discoveries into the content,1,12 a feature not included in preexisting descriptions of general psychiatric genetic counseling.12 Where possible, we used measures previously employed in our study of schizophrenia genetic counseling for family members,12 allowing for direct between-group comparisons. The provision of counseling by a nongenetics professional using a standardized protocol was a novel approach to addressing the problems resulting from the well-recognized dearth of medical geneticists and genetic counselors.38
Anecdotal evidence from our clinical practice4,5 and others3,6,10 supports our interpretations of the primary results. Trends related to retention of benefits that did not reach statistical significance in our study may have done so with a larger group. Nonetheless, more data are needed to both replicate these findings and examine specific contextual factors that may modulate interest and efficacy. Improved training of the counselor may result in even better outcomes although genetic counseling provided by board-certified genetic counselors, eg, is likely not scalable to large clinical trials or current clinical practice environments.
Arguably, in this study we did not conduct a “typical” genetic counseling assessment or session. As one would expect for an initial research study of this kind, our assessment and counseling protocol was more detailed, provided more information, and had a novel focus on conveying, even to individuals where these do not directly apply, general information about recent genetic findings of actual clinical relevance. Today, indications for clinical genetic testing in schizophrenia using chromosomal microarrays include only comorbid intellectual disability or autism spectrum disorder, multiple congenital anomalies, or an increased index of suspicion for 22q11.2 deletion syndrome.1 These existing criteria appear insufficient to identify many of the patients harboring clinically pathogenic CNVs,2 and thus most clinicians will currently be unable to replicate our optimal molecular characterization protocol in their clinical practice. This should however not preclude the provision of general genetic counseling as provided in this study.
All patients in this study had broadly defined schizophrenia. Counseling of individuals with psychotic mood and other disorders may present different chal lenges, and require a different approach.39 Participants were recruited from a typical community mental health clinic; we did not recruit from university health clinics or otherwise preferentially enrol higher functioning patients.2 Nevertheless, participants were all psychiatrically stable outpatients who had previously agreed to participate in our genetic case-control study.2 While these factors may have had an impact on the participants’ receptiveness to genetic counseling, these patients would also presumably be representative of those most likely to seek out this service. Lastly, nonrandomized, uncontrolled trials come with well-known caveats but often represent essential first steps toward more sophisticated study designs.12 Best practices in genetic counseling for schizophrenia remain to be demonstrated empirically via, eg, randomized control trials.
Conclusions
This study of genetic counseling for individuals with schizophrenia provides initial evidence for (1) needs for counseling similar to those observed in family members; (2) feasibility, with provision of such general counseling by a nongenetics professional; and, (3) immediate benefits accrued postcounseling. The results may help to optimize the process and thus outcomes in the nascent field of evidence-based psychiatric genetic counseling. While these data support efficacy in the absence of individual genetic test results, they also have implications for communicating clinically relevant genetic findings to patients—a growing consideration in genome-wide microarray and next-generation sequencing studies of schizophrenia where such results may be discovered.1,2,40 Further studies of providing clinical genetics services for schizophrenia may help to reduce stigma associated with the illness and to ensure that this population benefits from genomic research to the same extent as those with other common complex diseases.
Funding
MindCare New Brunswick grant; Canadian Institutes of Health Research grants (MOP-111238, MOP-53216); a Vanier Canada Graduate Scholarship (G.C.); a Canada Research Chair in Schizophrenia Genetics and Genomic Disorders (A.S.B.).
Supplementary Material
Acknowledgments
There are no actual or potential conflicts of interest to disclose. The authors thank the patients for their participation, and staff at the Saint John Community Mental Health Centre and Saint John Regional Hospital. Kathryn Russell deserves special thanks.
References
- 1. Costain G, Bassett AS. Clinical applications of schizophrenia genetics: genetic diagnosis, risk, and counseling in the molecular era. Appl Clin Genet. 2012; 5: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bassett AS, Lionel AC, Costain G, et al. The burden of rare copy number variants in community-based schizophrenia suggests a potential role for clinical microarrays. 2012;. In review [DOI] [PMC free article] [PubMed]
- 3. Austin JC, Honer WG. The genomic era and serious mental illness: a potential application for psychiatric genetic counseling. Psychiatr Serv. 2007; 58: 254–261 [DOI] [PubMed] [Google Scholar]
- 4. Bassett AS, Chow EWC, Hodgkinson KA. Genetics of schizophrenia and psychotic disorders. In: Smoller JW, Rosen Sheidley B, Tsuang MT, eds. Psychiatric Genetics: Applications in Clinical Practice. Arlington, VA: American Psychiatric Publishing, Inc.; 2008; 99–130 [Google Scholar]
- 5. Hodgkinson KA, Murphy J, O’Neill S, Brzustowicz L, Bassett AS. Genetic counselling for schizophrenia in the era of molecular genetics. Can J Psychiatry. 2001; 46: 123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsuang MT. Genetic counseling for psychiatric patients and their families. Am J Psychiatry. 1978; 135: 1465–1475 [DOI] [PubMed] [Google Scholar]
- 7. DeLisi LE, Bertisch H. A preliminary comparison of the hopes of researchers, clinicians, and families for the future ethical use of genetic findings on schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006; 141B: 110–115 [DOI] [PubMed] [Google Scholar]
- 8. Lyus VL. The importance of genetic counseling for individuals with schizophrenia and their relatives: potential clients’ opinions and experiences. Am J Med Genet B Neuropsychiatr Genet. 2007; 144B: 1014–1021 [DOI] [PubMed] [Google Scholar]
- 9. Schulz PM, Schulz SC, Dibble E, Targum SD, van Kammen DP, Gershon ES. Patient and family attitudes about schizophrenia: implications for genetic counseling. Schizophr Bull. 1982; 8: 504–513 [DOI] [PubMed] [Google Scholar]
- 10. Gottesman II, Wolfgram DL. Schizophrenia Genesis: The Origins of Madness. New York, NY: Freeman; 1991. [Google Scholar]
- 11. Reed SC. Counseling in Medical Genetics. 3rded. New York, NY: A. R. Liss; 1980. [Google Scholar]
- 12. Costain G, Esplen MJ, Toner B, Hodgkinson KA, Bassett AS. Evaluating genetic counseling for family members of individuals with schizophrenia in the molecular age. Schizophr Bull. 2012;. 10.1093/schbul/sbs124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esplen MJ, Hunter J, Leszcz M, et al. A multicenter study of supportive-expressive group therapy for women with BRCA1/BRCA2 mutations. Cancer. 2004; 101: 2327–2340 [DOI] [PubMed] [Google Scholar]
- 14. Lerman C, Lustbader E, Rimer B, et al. Effects of individualized breast cancer risk counseling: a randomized trial. J Natl Cancer Inst. 1995; 87: 286–292 [DOI] [PubMed] [Google Scholar]
- 15. Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008; 299: 1320–1334 [DOI] [PubMed] [Google Scholar]
- 16. Finn CT, Wilcox MA, Korf BR, et al. Psychiatric genetics: a survey of psychiatrists’ knowledge, opinions, and practice patterns. J Clin Psychiatry. 2005; 66: 821–830 [DOI] [PubMed] [Google Scholar]
- 17. Hoop JG, Roberts LW, Green Hammond KA, Cox NJ. Psychiatrists’ attitudes regarding genetic testing and patient safeguards: a preliminary study. Genet Test. 2008; 12: 245–252 [DOI] [PubMed] [Google Scholar]
- 18. Hunter MJ, Hippman C, Honer WG, Austin JC. Genetic counseling for schizophrenia: a review of referrals to a provincial medical genetics program from 1968 to 2007. Am J Med Genet A. 2010; 152A: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bassett AS, Chow EW, Waterworth DM, Brzustowicz L. Genetic insights into schizophrenia. Can J Psychiatry. 2001; 46: 131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feret H, Conway L, Austin JC. Genetic counselors’ attitudes towards individuals with schizophrenia: desire for social distance and endorsement of stereotypes. Patient Educ Couns. 2011; 82: 69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin N, Mikhaelian M, Cytrynbaum C, et al. 22q11.2 Deletion Syndrome: attitudes towards disclosing the risk of psychiatric illness. J Genet Counsel. 2012;. 10.1007/s10897-012-9517-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennett L, Thirlaway K, Murray AJ. The stigmatising implications of presenting schizophrenia as a genetic disease. J Genet Couns. 2008; 17: 550–559 [DOI] [PubMed] [Google Scholar]
- 23. Pescosolido BA, Martin JK, Long JS, Medina TR, Phelan JC, Link BG. “A disease like any other”? A decade of change in public reactions to schizophrenia, depression, and alcohol dependence. Am J Psychiatry. 2010; 167: 1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bassett AS, Costain G, Fung WL, et al. Clinically detectable copy number variations in a Canadian catchment population of schizophrenia. J Psychiatr Res. 2010; 44: 1005–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matheson SL, Shepherd AM, Laurens KR, Carr VJ. A systematic meta-review grading the evidence for non-genetic risk factors and putative antecedents of schizophrenia. Schizophr Res. 2011; 133: 133–142 [DOI] [PubMed] [Google Scholar]
- 26. Austin JC, Peay HL. Applications and limitations of empiric data in provision of recurrence risks for schizophrenia: a practical review for healthcare professionals providing clinical psychiatric genetics consultations. Clin Genet. 2006; 70: 177–187 [DOI] [PubMed] [Google Scholar]
- 27. Austin JC, Palmer CG, Rosen-Sheidley B, Veach PM, Gettig E, Peay HL. Psychiatric disorders in clinical genetics II: Individualizing recurrence risks. J Genet Couns. 2008; 17: 18–29 [DOI] [PubMed] [Google Scholar]
- 28. Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (R-IPQ). Psychol Health. 2002; 17: 1–16 [Google Scholar]
- 29. Ritsher JB, Otilingam PG, Grajales M. Internalized stigma of mental illness: psychometric properties of a new measure. Psychiatry Res. 2003; 121: 31–49 [DOI] [PubMed] [Google Scholar]
- 30. Kondel TK, Mortimer AM, Leeson VC, Laws KR, Hirsch SR. Intellectual differences between schizophrenic patients and normal controls across the adult lifespan. J Clin Exp Neuropsychol. 2003; 25: 1045–1056 [DOI] [PubMed] [Google Scholar]
- 31. Harper PS. Practical Genetic Counselling. 7thed. London, UK: Hodder Arnold; 2010. [Google Scholar]
- 32. Smerecnik CM, Mesters I, Verweij E, de Vries NK, de Vries H. A systematic review of the impact of genetic counseling on risk perception accuracy. J Genet Couns. 2009; 18: 217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lichtenstein P, Björk C, Hultman CM, Scolnick E, Sklar P, Sullivan PF. Recurrence risks for schizophrenia in a Swedish national cohort. Psychol Med. 2006; 36: 1417–1425 [DOI] [PubMed] [Google Scholar]
- 34. Trottier M, Roberts W, Drmic I, et al. Parents’ Perspectives on Participating in Genetic Research in Autism. J Autism Dev Disord. 2012;. 10.1007/s10803-012-1592-y [DOI] [PubMed] [Google Scholar]
- 35. Costain G, Chow EW, Ray PN, Bassett AS. Caregiver and adult patient perspectives on the importance of a diagnosis of 22q11.2 deletion syndrome. J Intellect Disabil Res. 2012; 56: 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xia J, Merinder LB, Belgamwar MR. Psychoeducation for schizophrenia. Cochrane Database Syst Rev. 2011; CD002831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winner JG, Goebert D, Matsu C, Mrazek DA. Training in psychiatric genomics during residency: a new challenge. Acad Psychiatry. 2010; 34: 115–118 [DOI] [PubMed] [Google Scholar]
- 38. Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med. 2005; 7: 439–443 [DOI] [PubMed] [Google Scholar]
- 39. Peay HL, Hooker GW, Kassem L, Biesecker BB. Family risk and related education and counseling needs: perceptions of adults with bipolar disorder and siblings of adults with bipolar disorder. Am J Med Genet A. 2009; 149A: 364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Need AC, McEvoy JP, Gennarelli M, et al. Exome sequencing followed by large-scale genotyping suggests a limited role for moderately rare risk factors of strong effect in schizophrenia. Am J Hum Genet. 2012; 91: 303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.