Abstract
Objective: Patients diagnosed with a psychotic disorder and their first-degree relatives display increased reactivity to stress. Theory predicts that experience of psychosocial stress is associated both with ventromedial prefrontal and mesolimbic dopamine neurotransmission. However, while there is evidence of aberrant striatal dopamine processing in psychotic disorder, the role of the prefrontal cortex remains under-researched. This study aimed at investigating stress-induced in vivo dopamine release in ventromedial prefrontal cortex (vmPFC) of individuals at familial risk for psychosis. Method: Fourteen healthy first-degree relatives of patients with a diagnosis of psychotic disorder and 10 control subjects underwent a single dynamic positron emission tomography (PET) scanning session after intravenous administration of 183.2 (SD = 7.6) MBq [18F]fallypride. Psychosocial stress was initiated at 100min postinjection using a computerized mental arithmetic task with social evaluative threat components. PET data were analyzed using the linearized simplified reference region model. Regression analyses were performed to compare the spatial extent of task-related ligand displacement between control subjects and relatives and to find how it related to self-rated experiences of psychosocial stress and psychosis. Results: First-degree relatives displayed hyporeactive dopamine signaling in the vmPFC in response to stress. Increased levels of subjectively rated stress were associated with increased intensity of psychotic experiences. This effect was particularly pronounced in first-degree relatives. Conclusion: Although previous studies have hypothesized a role for prefrontal dopamine dysfunction in psychosis, this study, to our knowledge, is the first in vivo human imaging study showing attenuated (ie, hyporeactive) dopamine stress neuromodulation in vmPFC of individuals at familial risk for psychosis.
Key words: schizophrenia, positron emission tomography, neuromodulation, relatives, mesolimbic, salience
Introduction
Patients diagnosed with a psychotic disorder, as well as their unaffected first-degree relatives, display increased reactivity to stress, responding to minor daily stressors with increased intensity of psychotic experiences.1 Given the suggested involvement of dopaminergic neurotransmission in the human stress response2,3 and the central role for dopamine dysfunction in patients with a psychotic disorder (see Howes and Kapur4 for review of the literature), observations of increased stress reactivity in psychosis-prone individuals may represent a functional state of underlying anomalies in dopamine stress signaling.5 However, in vivo human imaging studies investigating stress-induced changes in dopamine levels are scarce and rarely include assessment of the context of psychosis.3,6 Although the experience of psychosocial stress is suggested to involve both frontocortical and mesolimbic neurotransmissions,7 these studies have focused solely on striatal dopamine release.3,6
Rodent studies, however, suggest a cardinal role for prefrontal dopamine in stress processing because exposure to social stress is associated with increased extracellular dopamine levels predominantly in PFC, and to a lesser extent in mesolimbic areas.8 Moreover, depletion of dopamine in rodent PFC increases dopamine reactivity to stress in mesolimbic brain regions, suggesting that PFC dopamine transmission plays a role in the attenuation of mesolimbic dopamine release, exerting a “brake-like” function that may play a vital role in diminishing the adverse effects of stress on the brain.7 Individuals vulnerable to psychosis, alike PFC dopamine-depleted rodents, display abnormally low PFC activity (ie, “hypofrontality”)9 and increased reactivity of mesolimbic dopamine neurons.10 These data suggest underlying alterations resulting in failure of the prefrontal dopamine system to exert control over stress-induced mesolimbic dopaminergic neurotransmission, the behavioral expression of which is increased psychotic reactivity to stress.1 However, direct in vivo evidence for an impaired stress-attenuating function of the prefrontal dopamine system in psychosis is currently lacking. This is mainly due to methodological restriction, a lack of high-affinity D2/3 ligands limiting positron emission tomography (PET) exploration of dopaminergic transmission to brain regions with high D2 receptor density, primarily the striatum. Recent development of high-affinity D2/3 radioligands, among which [18F]fallypride, has offered possibilities for exploration of extrastriatal dopamine sites such as the PFC2,11 where D2/3 receptor density is an order of magnitude lower than in striatal regions.12 In a previous study,2 we used [18F]fallypride to detect in vivo prefrontal dopamine release in healthy human subjects in response to the Montreal Imaging Stress Task (MIST), a psychosocial stress challenge developed and used previously by Pruessner et al.3 Subjectively rated experiences of psychosocial stress were positively associated with the spatial extent of dopaminergic activity in ventromedial PFC (vmPFC), implicating an important role for vmPFC dopamine neurotransmission in human stress processing, in line with abnormal stress responsivity in neurological patients with medial PFC damage.13 This study aimed at extending this work to the domain of psychosis, utilizing the same [18F]fallypride PET stress paradigm2,14 in a sample of healthy subjects and first-degree relatives of patients with a psychotic disorder to examine to what degree stress-induced changes in [18F]fallypride ligand displacement in the vmPFC mediate the stress reactivity endophenotype of psychosis.
It was hypothesized that first-degree relatives would display impaired prefrontal regulation of stress-induced mesolimbic dopamine release, reflected by attenuated stress-induced vmPFC dopamine signaling associated with psychotic reactivity to the MIST laboratory stressor. Based on the available evidence of increased behavioral stress sensitivity in relatives,1 attenuated dopamine signaling in vmPFC was hypothesized to index trait sensitivity to stress rather than represent a marker of illness. Thus, both siblings, parents, and children of patients diagnosed with a psychotic disorder were selected as carriers of stable trait sensitivity, regardless of probability of transition to illness.
Method
Subjects
The sample consisted of 14 healthy first-degree relatives of individuals with a psychotic disorder and 12 healthy control subjects with no family history of psychotic illness (control sample has been described in previous work).2 Subjects were recruited through pamphlets, advertisements in local newspapers, and random mailing procedures in the local area. Relatives were additionally recruited by contacting local family organizations for relatives of patients with a psychotic disorder.
Inclusion criteria were (1) age 18–65 years and (2) sufficient command of the Dutch language to understand instructions and give informed consent. Exclusion criteria were (1) known diagnosis of intellectual disability according to the explicit diagnostic criteria of the DSM-IV-TR19; (2) head trauma with loss of consciousness or central neurological disorder; (3) endocrine disorder; (4) cardiovascular disorder; (5) presence or history of psychiatric illness according to the explicit diagnostic criteria of the DSM-IV-TR, generated with the OPCRIT computer program15; (6) positive family history of psychosis (controls only); (7) current use of psychotropic medication; (8) current or previous use of illicit drugs; (9) use of alcohol in excess of 5 standard units per day; (10) presence of metal elements in the body; (11) previous experience of claustrophobia; and (12) pregnancy or lactation.
The study was approved by the standing medical ethics committee and was consistent with the ethical principles for medical research involving human subjects, as formulated in the Helsinki declaration.16 All subjects signed informed consent after description of the study. Drug and medication use were assessed at the day of scanning by urinalysis (MultiTest 1990–2010 SureScreen Diagnostics Ltd) in order to ensure that subjects were drug free at the moment of scanning. In addition, pregnancy tests (Clearblue 2008 Swiss Precision Diagnostics) were carried out, in order to exclude pregnancy at the moment of scanning.
Assessment of Subclinical Psychopathology
All subjects were assessed for frequency of subclinical depressive, negative, and positive psychotic symptoms at study entry with the Community Assessment of Psychic Experiences (CAPE; Dutch version), a 42-item self-report questionnaire (van Os, Verdoux & Hanssen, 1999).17,18 Frequency scores were measured on a 4-point scale ranging from never (=1), sometimes (=2), often (=3) to nearly always (=4). The CAPE depressive, negative, and positive psychotic dimensions encompass 8, 14, and 20 items, respectively. A total score per dimension is provided by adding up the respective frequency scores. Consistent with previous work,17,18 CAPE scores were weighted for the number of valid answers per dimension.
Global functioning was furthermore assessed with the Global Assessment of Functioning (GAF) scale,19 a reliable and valid measure of overall psychological disturbance.20
Psychosocial Stress Task
Psychosocial stress was induced in the PET-scanner using the Montreal Imaging Stress Task (MIST), the psychosocial stress paradigm developed by Pruessner et al.3 The experiment consisted of a control and a stress condition. In the control condition, subjects performed 6-min blocks of mental arithmetic on a computer screen with no time constraints or performance feedback, resulting in an average performance of 90% correct responses. In the stress condition, subjects performed similar mental arithmetic as in the control condition, with the addition of information about the total number of errors, expected average number of errors, time spent on the current problem, and performance feedback (correct, incorrect, and timeout) displayed on the computer screen. In addition, a time constraint, slightly below the time needed according to the subject’s ability, was set for solving each problem. The constraint was automatically adjusted by the computer algorithm for each individual subject, based on the average time needed to solve a problem during a practice session before start of the PET scan. A tone rising in frequency indicated the remaining time for the subject to solve each problem.
Subjects were informed that an average performance of 80%–90% correct answers was expected. However, due to the manipulation of the time constraint, subjects achieved, on average, only 20%–30% correct answers. Psychosocial stress was additionally induced through negative verbal feedback from a confederate investigator, who commented upon subjects’ performance, emphasizing that they needed to achieve at least minimal performance requirements. An extensive debriefing session took place at the end of the experiment, in which subjects were told that the task was specifically designed to be out of reach of their mental capacity and that in reality it did not assess their ability to perform mental arithmetic.
Behavioral Measures
Self-rated experiences of stress and psychosis were assessed every 12min, throughout the experiment, which consisted 6 stress and 7 psychosis items, rated on 7-point Likert scales (rating from not at all [=1] to very [=7]), adapted from Experience Sampling Methodology.1 All scores were recoded such that an increase in scores corresponded to increased intensity of subjective stress or psychotic experiences. Averages of the (recoded) scores on the items “I feel relaxed,” “I feel pressured,” “I feel comfortable among these people,” “I feel judged by these people,” and “I do not live up to expectations” constituted the subjective stress scale (Cronbach’s alpha =.69). The psychosis scale consisted of averages of the (recoded) scores on the items “I feel suspicious,” “I feel unreal,” “My thoughts are being influenced by others,” “I can’t get rid of my thoughts,” “I see things that aren’t really there,” “I hear voices,” and “I’m afraid I’ll lose control” (Cronbach’s alpha =.73).
PET Acquisition and Data Reduction
The highly selective dopamine D2/3 PET radioligand [18F]fallypride was used to measure in vivo dopamine release in the PFC2,11 in response to the MIST psychosocial stress challenge.2,3 Details regarding radiotracer preparation, PET acquisition, and data reduction have been described in detail previously,2 and are enclosed in the Appendix. To detect and map PFC dopamine release, we used the linearized simplified reference region method (LSSRM),21 a method that makes use of the endogenous competition between a neurotransmitter and the radioligand for occupancy of its receptor; hereby an increase in the rate of [18F]fallypride displacement induced by the stimulus indicates increased dopamine release.2,11,12,14 The LSSRM permits to detect the presence of voxel-wise transient changes, with the advantage to investigate also regions with low radiotracer uptake such as the PFC.2,11,12
Main Analysis
Linear regression analyses were conducted with group (control [=0] vs relative [=1]), task-induced changes in scores on the subjective stress scale (quantified as the difference between mean scores on the scale under stress vs control conditions) or task-induced changes in scores on the psychosis scale (quantified as the difference between mean scores on the scale under stress vs control conditions), as well as their interaction (ie, group x subjective stress and group x psychosis) as independent variable, and task-induced changes in the spatial extent of [18F]fallypride ligand displacement in left and right vmPFC (quantified as the percentage of voxels exceeding the false discovery rate [FDR]22 corrected significance threshold of P(α(FDR) = 5%) < .05; see Lataster et al.)2 as dependent variable.
Second, linear regression analysis was performed with group (control [=0] vs relative [=1]), task-induced changes in scores on the subjective stress scale (quantified as the difference between mean scores on the scale under stress vs control conditions), as well as their interaction (ie, group x subjective stress) as independent variable, and task-induced changes in scores on the psychosis scale (quantified as the difference between mean scores on the scale under stress vs control conditions) as dependent variable.
Significance tests were performed in STATA release 10.0.23 When interactions were detected, simple effects were calculated using the LINCOM command in STATA. In absence of interactions, main effects were calculated. All analyses were a priori corrected for age, gender, nicotine use (continuous: number of cigarettes/day), and alcohol consumption (continuous: grams of alcohol/week).24
Results
Subjects
Of the 26 subjects who entered the study, 1 control subject was excluded because of excessive stress during the control condition of the experimental PET paradigm, attributable to uncomfortable placement of the catheter for bolus injection. For another control subject, data on the subjective stress scale and psychosis scale were lost due to a computer failure. Consequently, final analyses were performed in a sample of 10 control subjects and 14 first-degree relatives (7 siblings, 6 parents, and 1 child of a patient diagnosed with psychotic disorder).
Subject Characteristics
No large or significant sociodemographic differences, nor differences in subclinical psychopathology were observed between groups (see table 1). Task-induced changes in subjective stress, psychotic experiences, and [18F]fallypride ligand displacement, in addition to baseline nondisplaceable binding potential (BPND), are summarized in table 2. Mean levels of vmPFC baseline BPND, task-induced stress, psychosis, and spatial extent of [18F]fallypride ligand displacement in vmPFC were similar between groups (see table 2). The stress condition was experienced as significantly more stressful compared with the control condition, reflected by higher scores on the subjective stress scale (Mean(control) = 2.86 [SD = 0.56]; Mean(stress) = 4.03 [SD = 0.78]; t(23) = −7.98; P = .0001).
Table 1.
Sample Characteristics
| Control Subjects (n = 10) | First-Degree Relatives (n = 14) | Test Statistics | P-Values | |
|---|---|---|---|---|
| Age (years), mean (SD) | 36.6 (15.3) ranges 23–60 | 40.6 (15.0) ranges 19–63 | −0.65a | .525 |
| Gender (n, %) | 2.24b | .134c | ||
| Male | 8 (80) | 7 (50) | — | — |
| Female | 2 (20) | 7 (50) | — | — |
| Relative status (n, %) | ||||
| Sibling | — | 7 (50) | — | — |
| Parent | — | 6 (43) | — | — |
| Child | — | 1 (7) | — | — |
| Level of education (n, %)d | — | — | 0.64b | .727 |
| Secondary education | 1 (10) | 3 (21) | — | — |
| Bachelor degree | 6 (60) | 8 (57) | — | — |
| Master degree | 3 (30) | 3 (21) | — | — |
| Paternal education level (n, %)d | — | — | 1.78b | .619 |
| Primary education | 0 | 2 (14) | — | — |
| Secondary education | 7 (70) | 8 (57) | — | — |
| Bachelor degree | 2 (20) | 2 (14) | — | — |
| Master degree | 1 (10) | 2 (14) | — | — |
| Maternal education level (n, %)d, e | — | — | 3.75b | .290 |
| Primary education | 0 | 4 (29) | — | — |
| Secondary education | 7 (70) | 6 (43) | — | — |
| Bachelor degree | 2 (20) | 2 (14) | — | — |
| Master degree | 1 (10) | 1 (7) | — | — |
| Work situation (n, %)d | — | — | 1.92b | .750 |
| Household | 0 | 2 (14) | — | — |
| School/education | 2 (20) | 3 (21) | — | — |
| Regular job (full-time) | 6 (60) | 6 (43) | — | — |
| Regular job (part-time) | 1 (10) | 2 (14) | — | — |
| Other activities | 1 (10) | 1 (7) | — | — |
| Marital status (n, %) | — | — | 1.05b | .590 |
| Married or cohabitating | 5 (50) | 8 (57) | — | — |
| Divorced | 0 | 1 (7) | — | — |
| Never married | 5 (50) | 5 (36) | — | — |
| Nicotine use (cigarettes/day) (n, %) | 0.24a | .810 | ||
| 0 | 8 (80) | 12 (86) | — | — |
| 1–10 | 1 (10) | 1 (7) | — | — |
| 10–20 | 1 (10) | 1 (7) | — | — |
| Alcohol consumption (grams/week) (n, %)f | — | — | 0.72a | .476 |
| 0–50 | 5 (50) | 8 (57) | — | — |
| 50–100 | 1 (10) | 2 (14) | — | — |
| 100–150 | 4 (40) | 4 (29) | — | — |
| CAPE, weighted mean (SD) | ||||
| CAPE-depressive symptoms | 1.5 (.23) | 1.6 (.27) | −0.77a | .448 |
| CAPE-negative symptoms | 1.5 (.27) | 1.5 (.19) | 0.48a | .639 |
| CAPE-psychotic symptoms | 1.1 (.19) | 1.1 (.13) | 0.54a | .593 |
| CAPE total | 1.3 (.14) | 1.3 (.13) | 0.29a | .773 |
| GAF, mean (SD) | ||||
| GAF symptoms | 87.2 (7.6) | 81.4 (8.4) | 1.72a | .100 |
| GAF handicap | 87.0 (8.6) | 84.3 (6.9) | 0.86a | .400 |
a t-value (comparing group means; 2-sided P-value presented).
bPearson’s χ2 (comparing categorical distributions between groups).
cFisher’s exact P-value = .210.
dPercentages do not total 100 because of rounding.
eData on maternal education level missing for one of the participants in the relative group.
fStandard drink/unit size in the Netherlands contains 9.9 grams of ethanol.
Table 2.
Mean (SD) Values and t-Test Statistics of Baseline Nondisplaceable Binding Potential and Task-Induced Stress, Psychotic Experiences, and [18F]fallypride Ligand Displacement for Control Subjects and First-Degree Relatives
| Control Subjects (n = 10) | First-Degree Relatives (n = 14) | ta | P-Values | |
|---|---|---|---|---|
| Nondisplaceable binding potential (BPND) | ||||
| vmPFC (left) | 0.19 (0.24) | 0.15 (0.20) | 0.51 | .616 |
| vmPFC (right) | 0.22 (0.22) | 0.18 (0.22) | 0.39 | .698 |
| ΔSubjective stressb | 0.98 (0.68) | 1.31 (0.74) | −1.13 | .270 |
| ΔSubjective psychosisc | 0.09 (0.22) | 0.03 (0.30) | 0.53 | .601 |
| [18F]fallypride ligand displacement (%)d | ||||
| vmPFC(left) | 54.1 (24.0) | 41.6 (27.0) | 1.16 | .258 |
| vmPFC (right) | 48.7 (22.0) | 38.1 (28.2) | 0.99 | .333 |
a t-value (comparing group means; 2-sided P-value presented).
bDifference between mean scores on the subjective stress scale under stress vs control conditions of the MIST psychosocial stress task.
cDifference between mean scores on the psychosis scale under stress vs control conditions of the MIST psychosocial stress task.
dPercentage of voxels exceeding the FDR corrected significance threshold of P(α(FDR) = 5%) < 0.05, reflecting spatial extent of task-induced ligand displacement. Percentages reflect the number of significant voxels relative to the total number of voxels within the mask of the respective brain region.
Main Results
Effect of Familial Liability to Psychosis on the Association Between Task-Induced Changes in Subjective Stress and [18F]fallypride Ligand Displacement.
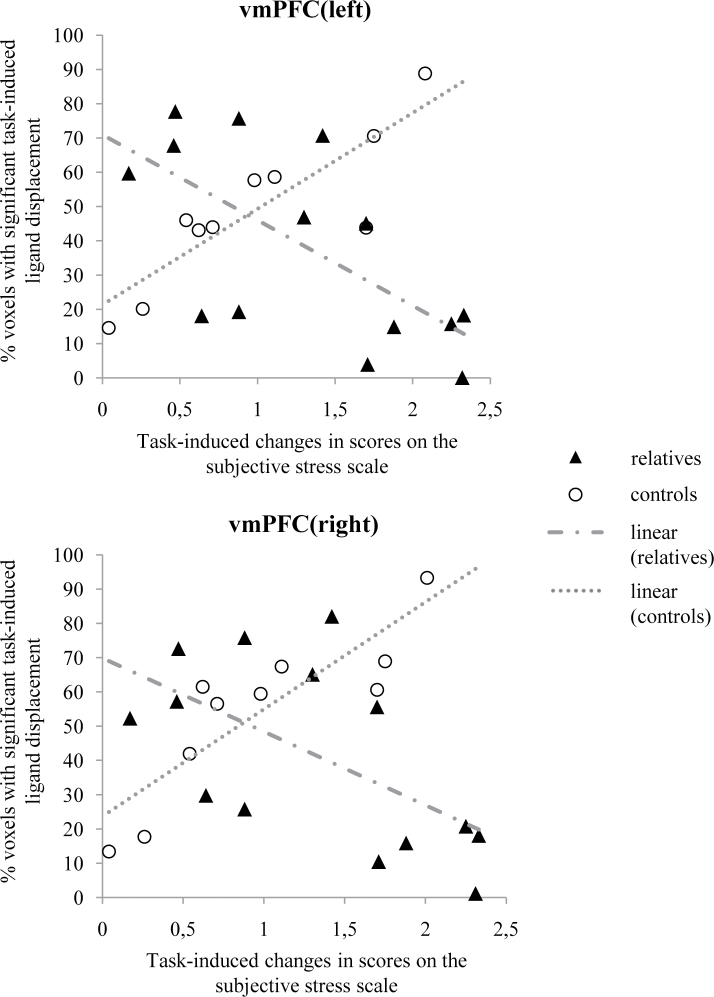
Linear regression analyses showed significant interactions between group (control vs relative) and subjective, task-induced stress in the models of task-induced [18F]fallypride ligand displacement in left and right vmPFC (left: F(1,16) = 19.12; β = −2.04; 95% CI [−3.03; −1.05]; P = .0005; right: F(1,16) = 12.32; β = −1.92; 95% CI [−3.09; −.76]; P = .003; figure 1), indicating a differential association between the experience of stress and task-induced dopamine activity in these brain regions in controls vs relatives.
Fig. 1.
Associations between task-induced feelings of subjective stress and task-induced [18F]fallypride ligand displacement in left and right ventromedial prefrontal cortex of healthy control subjects and healthy first-degree relatives of patients diagnosed with a psychotic disorder. Increased feelings of subjective stress were associated with increased ligand displacement in controls vs decreased ligand displacement in first-degree relatives.
In relatives, higher levels of subjective stress were associated with decreased spatial extent of [18F]fallypride ligand displacement in bilateral vmPFC (left: β = −1.02; t(16) = −4.09; 95% CI [−1.54; −0.49]; P = .001; right: β = −0.85; t(16) = −2.92; 95% CI [−1.47; −.23]; P = .010), suggesting decreased dopaminergic activity in these brain regions. Conversely, in controls, higher levels of subjective stress were associated with increased spatial extent of [18F]fallypride ligand displacement in bilateral vmPFC (left: β = 1.02; t(16) = 2.91; 95% CI [0.28; 1.77]; P = .010; right: β = 1.07; t(16) = 2.59; 95% CI [0.19; 1.95]; P = .020), suggestive of increased dopaminergic activity.
Post Hoc Analyses Exploring Nonlinear Associations Between Task-Induced Changes in Subjective Stress and [18F]fallypride Ligand Displacement in First-Degree Relatives.
Although linear regression analyses suggested linear associations between task-induced changes in subjective stress and vmPFC [18F]fallypride ligand displacement, observation of the data from the first-degree relatives hinted toward nonlinear associations between task-induced changes in subjective stress and vmPFC [18F]fallypride ligand displacement. Therefore, a post hoc likelihood ratio analysis was performed—corrected for age, gender, nicotine use, and alcohol consumption—testing whether model fit for observations of task-induced subjective stress and ligand displacement improved with inclusion of a quadratic term (ie, subjective stress) in the model.
Inclusion of a quadratic term, indeed, increased model fit for observations of task-induced subjective stress and ligand displacement in the left (χ2 = 4.13; P = .042; adjusted R2[linear] = .50 vs adjusted R2[quadratic] = 0.57; Root-mean-square-error (RMSE)[linear] = .76 vs RMSE[quadratic] = 0.70) and right vmPFC (χ2 = 8.83; P = .003; adjusted R2[linear] = 0.15 vs adjusted R2[quadratic] = 0.48; RMSE[linear] = 0.98 vs RMSE[quadratic] = 0.76) of first-degree relatives. Moreover, β-coefficients for the linear terms in the quadratic models were positive (vmPFC[left]: β = 1.42; 95% CI [−2.32; 5.15]; vmPFC[right]: β = 3.29; 95% CI [−0.75; 7.33]), whereas negative for the quadratic terms (vmPFC[left]: β = −0.90; 95% CI [−2.28; 0.48]; vmPFC[right]: β = −1.56; 95% CI [−3.06; −0.73]), indicating a positive association between subjective stress and [18F]fallypride ligand displacement in left and right vmPFC until a turning point is reached, beyond which subjective stress is negatively associated with [18F]fallypride ligand displacement.
Effect of Familial Liability to Psychosis on the Association Between Task-Induced Changes in Self-Rated Psychotic Experiences and [18F]fallypride Ligand Displacement.
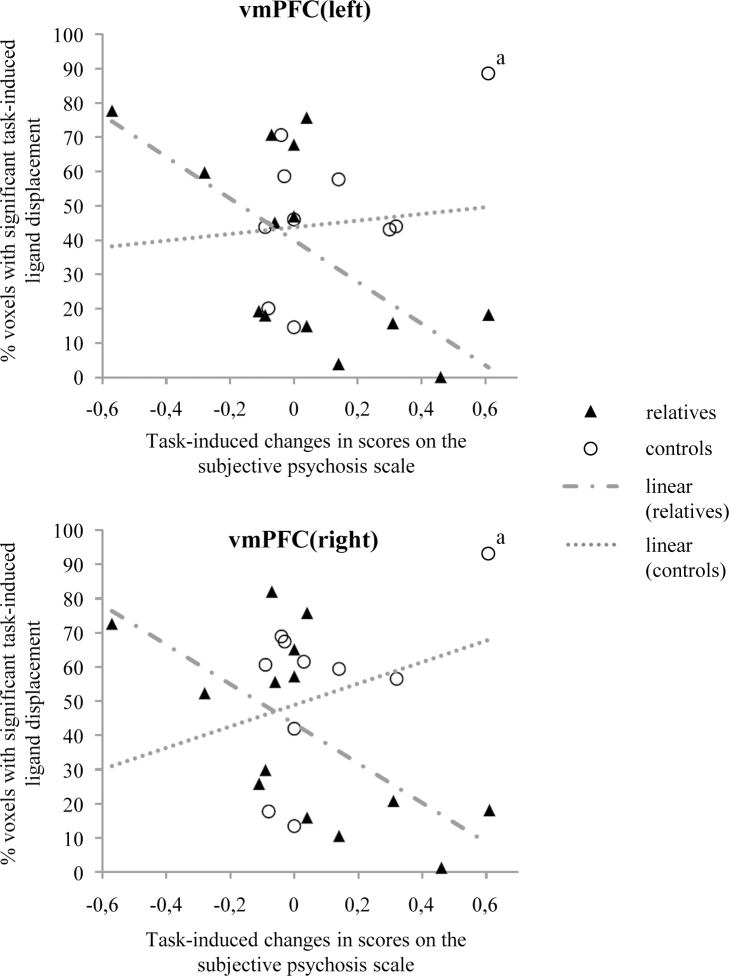
Linear regression analyses showed significant interactions between group (control vs relative) and task-induced psychotic experiences in the model of task-induced [18F]fallypride ligand displacement in left and right vmPFC (left: F(1,16) = 9.03; β = −4.20; 95% CI [−7.16; −1.24]; P = .008; right: F(1,16) = 6.89; β = −4.03; 95% CI [−7.29; −0.78]; P = .018; figure 2), indicating a differential association between task-induced psychotic experiences and task-induced dopamine release in controls vs relatives.
Fig. 2.
Associations between task-induced psychotic experiences and task-induced [18F]fallypride ligand displacement in left and right ventromedial prefrontal cortex of healthy control subjects and healthy first-degree relatives of patients diagnosed with a psychotic disorder. Interactions between group (control vs relative) and task-induced psychotic experiences in the model of task-induced [18F]fallypride ligand displacement in left and right vmPFC did not reach statistical significance. aOutlier based on Cook’s distance test for influential cases25 and not included in final analyses.
Visual inspection of the data, however, suggested these findings to be influenced by 1 outlying subject in the control group (figure 2), which was statistically confirmed by performance of a Cook’s distance test for influential cases25 (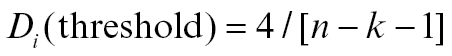 ).26 Interaction analyses were therefore repeated with exclusion of this subject, yielding nonsignificant results for both left (F(1,15) = 1.69; β = −3.06; 95% CI [−8.08; 1.96]; P = .213) and right vmPFC (F(1,15) = 1.45; β = −3.13; 95% CI [−8.69; 2.42]; P = .248). The main effect of task-induced psychotic experiences on [18F]fallypride ligand displacement was negative and significant, however (left vmPFC: β = −1.83; t(17)= −2.57; 95% CI [−3.33; −0.33]; P = .020; right vmPFC: β = −1.58; t(17) = −2.02; 95% CI [−3.23;.07]; P = .060), albeit most pronounced in left vmPFC. Increased intensity of subjective psychotic experiences was associated with decreased dopaminergic activity. This effect was largely due to strong negative associations between task-induced psychotic experiences and [18F]fallypride ligand displacement in relatives (left vmPFC: β = −2.20; t(15) = −2.92; 95% CI [−3.80; −.594]; P = .011; right vmPFC: β = −1.96; t(15) = −2.35; 95% CI [−3.73; −0.18]; P = .033) as opposed to controls, for which associations between task-induced psychotic experiences and [18F]fallypride ligand displacement were positive and did not reach significance (left vmPFC: β = 0.86; t(15) = 0.39; 95% CI [−3.83; 5.55]; P = .700; right vmPFC: β = 1.18; t(15) = 0.48; 95% CI [−4.01; 6.37]; P = .635).
).26 Interaction analyses were therefore repeated with exclusion of this subject, yielding nonsignificant results for both left (F(1,15) = 1.69; β = −3.06; 95% CI [−8.08; 1.96]; P = .213) and right vmPFC (F(1,15) = 1.45; β = −3.13; 95% CI [−8.69; 2.42]; P = .248). The main effect of task-induced psychotic experiences on [18F]fallypride ligand displacement was negative and significant, however (left vmPFC: β = −1.83; t(17)= −2.57; 95% CI [−3.33; −0.33]; P = .020; right vmPFC: β = −1.58; t(17) = −2.02; 95% CI [−3.23;.07]; P = .060), albeit most pronounced in left vmPFC. Increased intensity of subjective psychotic experiences was associated with decreased dopaminergic activity. This effect was largely due to strong negative associations between task-induced psychotic experiences and [18F]fallypride ligand displacement in relatives (left vmPFC: β = −2.20; t(15) = −2.92; 95% CI [−3.80; −.594]; P = .011; right vmPFC: β = −1.96; t(15) = −2.35; 95% CI [−3.73; −0.18]; P = .033) as opposed to controls, for which associations between task-induced psychotic experiences and [18F]fallypride ligand displacement were positive and did not reach significance (left vmPFC: β = 0.86; t(15) = 0.39; 95% CI [−3.83; 5.55]; P = .700; right vmPFC: β = 1.18; t(15) = 0.48; 95% CI [−4.01; 6.37]; P = .635).
Effect of Familial Liability to Psychosis on the Association Between Task-Induced Changes in Subjective Stress and Task-Induced Changes in Subjective Psychotic Experiences.
Linear regression analyses revealed no significant interaction effect between group (control vs relative) and task-induced subjective stress on subjectively rated psychotic experiences (F(1,18) = 1.15; β = 0.58; 95% CI [−0.56; 1.71]; P = .299; corrected for age and gender only). The main effect of task-induced subjective stress on subjectively rated psychotic experiences was significant; however (β = 0.90; t(20) = 3.86; 95% CI [0.41; 1.38]; P = .001; corrected for age and gender only), higher levels of subjective stress were associated with increased intensity of psychotic experiences. This effect was largely due to strong associations between task-induced subjective stress on subjectively rated psychotic experiences in relatives (β = 1.19; t(18) = 4.06; 95% CI [0.57; 1.81]; P = .001; corrected for age and gender only), and to a much lesser and nonsignificant extent in controls (β = 0.61; t(18) = 1.46; 95% CI [−0.27; 1.50]; P = .161; corrected for age and gender only).
Discussion
Results from this study indicate that individuals at familial risk for psychosis display attenuated dopamine stress processing in ventromedial prefrontal cortex (vmPFC), a brain region previously identified to play a key role in human dopaminergic stress regulation.2 Furthermore, increased levels of subjectively rated stress were associated with increased intensity of psychotic experiences. This effect was particularly pronounced in first-degree relatives, fitting previously reported associations between abnormal dopamine reactivity and increased stress reactivity in the daily life of subjects at familial risk of developing psychosis.5 Although previous studies have suggested a role for prefrontal dysfunctions in psychosis,9 this is, to our knowledge, the first in vivo human imaging study showing familial liability to psychosis to be associated with alterations in dopamine stress processing in PFC.
Ventromedial Prefrontal Cortex Involvement in Stress Regulation
These findings underline a regulatory role for vmPFC dopamine neurotransmission in the human stress response,2 given that individuals at familial risk for psychosis, characterized by impairments in stress regulation,1 display alterations in vmPFC dopamine stress transmission. These findings are in line with reports of medial prefrontal cortex damage being associated with heightened self-reported stress responses to the Trier Social Stress Test.13
The vmPFC strongly connects to the amygdala, ventral striatum, and hypothalamus27,28 and is thought to be involved in emotion regulation,29 impulse and self-control,30 and fear extinction.31 Furthermore, the loss of top-down control by the vmPFC is implicated in mood and anxiety disorders,32,33 supporting a role for the vmPFC as emotion control centre, further emphasized by observations of vmPFC damage being associated with deficits in emotion regulation.29
Importantly, the human stress response is uniquely determined by the interaction between a stressor and the individual’s appraisal thereof, which may be incongruous with the actual threat of the stressful situation.34 The vmPFC is implicated in the neural processing of these appraisals,35 in turn activating subcortical brain regions involved in the initiation of the physiological stress response.28 Alike patients with a psychotic disorder,36 vmPFC-damaged patients display difficulty appraising social and emotional cues.37 Inappropriate psychological (eg, psychotic) and physiological reactions to social stress, as observed in individuals at risk of psychosis,1 may therefore result from impaired appreciation of the stressful nature of a situation, mediated by alterations in vmPFC functioning.
A Role for Prefrontal-Mesolimbic Dopamine Asymmetry in Psychotic Stress Processing
Although previous studies suggest a role for asymmetry of prefrontal-mesolimbic dopamine networks in psychotic disorders, implicating “hypoactivity” of prefrontal and “hyperactivity” of mesolimbic dopamine structures,9 a scarcity of in vivo human studies investigating stress-induced changes in prefrontal dopamine levels limits our understanding of the role of this neurochemical asymmetry in the stress reactivity endophenotype of psychosis. Rodent studies indicating that depletion of dopamine in the PFC increases stress-induced dopamine release in the mesolimbic system7 have advanced the hypothesis that prefrontal dopamine transmission attenuates stress-induced mesolimbic dopamine release. A possible function of this mechanism could be to protect the organism for the adverse consequences of stress on the brain, supported by the observation that stress-related psychopathologies are characterized by disturbed mesolimbic dopamine transmission38 and prefrontal diminution.39 Our previous finding of healthy subjects responding to psychosocial stress with increased levels of endogenous dopamine in the PFC,2 whereas a similar stressor in previous studies did not necessarily induce dopamine release in mesolimbic brain regions,3,6 further supports the hypothesis that the organism may benefit from such a stress-reducing mechanism, with a suggested regulatory role of the prefrontal dopamine system. Findings from this study, showing hyporeactive dopamine signaling in the vmPFC of first-degree relatives in response to stress, suggest familial risk for psychosis is mediated, among others, by impaired attenuating control of the prefrontal dopamine system, and hence increased stress reactivity of mesolimbic dopamine neurons. Given the proposed mediating role of mesolimbic dopamine in salience attribution,40 a stress-induced hyperactive state of the mesolimbic dopamine system may result in “aberrant salience” (the assignment of salience to otherwise unimportant stimuli), and fuel psychotic experiences,40 supported by observations of stress-induced psychotic experiences in individuals at risk of psychosis.1
Animal studies suggest that excessive stimulation of prefrontal dopamine neurons (eg, by stress) can put the prefrontal cortex “off-line,”41 disrupting the attenuating control over subcortical brain regions, rendering these regions hyperreactive, and enabling them to dominate behavior. This mechanism may have survival value because it enables the organism to automatically switch from complex, slow, prefrontally regulated behaviors to instinctive, fast (eg, “fight”–“flight”) behaviors guided by subcortical brain regions, when exposed to severe stress.42 However, incorrect tuning of this mechanism may render the organism hyper- or hyporesponsive to stress. These findings may point toward a proneness to stress-induced “overload” of the vmPFC dopamine system in individuals at familial risk for psychosis, reflected by observations of inverse dopamine stress reactivity in vmPFC compared with healthy control subjects. Post hoc likelihood ratio analyses suggested observations of task-induced subjective stress and ligand displacement in vmPFC of healthy first-degree relatives to be best represented by a quadratic, inverted U-shaped, rather than linear function. Hence, feelings of subjectively experienced stress appear positively related to vmPFC dopamine activity until a “turning point” is reached, beyond which increased stress levels are associated with decreased vmPFC dopamine signaling. Consequently, attenuating control of the vmPFC dopamine system over the stress-induced mesolimbic dopamine response may be lost, increasing likelihood of stress-induced psychotic experiences.40 However, further studies are needed to clarify mechanisms of pathophysiological dopamine communication between cortical and subcortical brain regions in the context of psychosis.
Strengths and Limitations
The findings reported in this study result from significant advances made in the field of neuroimaging and show, for the first time, aberrant in vivo dopamine stress signaling (ie, aberrant stress-induced changes in the spatial extent of [18F]fallypride ligand displacement) in the PFC of individuals at familial risk for psychosis. Further strengths of the study include the use of a laboratory stressor that attempts to emulate real-world social interactions and succeeds in eliciting stress not only at a neural level, but also at the level of subjective experience. Some limitations require consideration. PET measurement of alterations in dopamine concentration in response to a pharmacological manipulation or during a behavioral task can be obtained by calculating the percentage change in dopamine D2/3 receptor binding potential BPND (ΔBPND), measured under dual scanning conditions (control and activation condition).43,44 This design has the advantage that the quantitative index of dopamine release, BPND, is obtained by directly applying standard techniques such as the simplified reference tissue model (SSRM).45 However, BPND measurement in the activated condition assumes that the subject is in steady state during activation. In addition, the need for 2 separate BPND measurements and possibly noisy subtraction of 2 low BPND values in extrastriatal regions may reduce the sensitivity of the design21 and may have influenced studies that failed to detect significant amphetamine-induced ΔBPND decreases in cortical regions.44 Simulations furthermore demonstrated that ΔBPND has an inherent sensitivity to timing of dopamine perturbations and could lead to incorrect inferences of the relative amounts of dopamine released during conditions.46 In this study, we implemented a common variant of the SSRM, the LSSRM,21 which has several practical advantages, such a single scanning session to avoid possible session effects. Fundamentally, it is based on a kinetic model of the stimulus-induced physiological phenomenon involved, where nonsteady-state effects are considered by making parameters time dependent. The presence of significant dopamine-induced transient changes in ligand displacement after the stimulus initiation is estimated by fitting the model to data from individual subjects, therefore facilitating the detection of relatively small differences in dopamine release, which is of particular interest for areas with low signal-to-noise ratio such as the PFC. Moreover, because the model generates voxel-wise parametric calculations of the time-dependent parameters, it allows direct comparisons of spatial extent of dopamine release between subject populations within a specific region of interest.46
On the other hand, in the LSSRM approach, possible alterations in regional cerebral blood flow (rCBF) are not fully accounted for. However, as argued by Christian and colleagues,14 using a single injection protocol in combination with the in vivo kinetics of [18F]fallypride minimizes the possible confounds of changing rCBF under psychological task paradigm conditions.
Consequential to the 1-day scanning protocol utilized in this study, the stress and control conditions of the MIST were consistently administered in the same sequence, ie, the control condition preceded the stress condition for each subject. Although this increases risk of carry-over effects from the control condition into the stress condition, these effects were minimized by separating the 2 conditions by a baseline condition. Most importantly, a 2-scan protocol, with counterbalanced administration of stress and control conditions, would have meant that the extensive and important debriefing session, in which subjects were told that the task was specifically designed to be out of reach of their mental capacity and that it did not assess their ability to perform mental arithmetic, could only take place after the second scan, which was considered to be ethically problematic. Furthermore, a 1-day scan protocol has the advantage of avoiding session effects.
Studies with a small sample size may be particularly susceptible to Type-II error47 although power calculations are rarely reported. Adequate power in respect of a given effect size has been defined at ≥0.80 or 80% (ie, β ≤ 0.20).48 Post hoc power estimates (G*Power 3.1.5)49 for detecting the reported interactions in this study sample ranged from 0.13 to 0.67 for the analyses yielding negative results, suggestive of Type-II error.50 The behavioral phenotype of increased psychotic reactivity to stress in first-degree relatives of patients with a psychotic disorder has been established through larger sample studies, using fine-grained ecological monitoring techniques that employ repeated measurements in daily-life context,1 and are therefore equipped with more sensitivity for detecting such phenotypes at the behavioral level. Therefore, a lack of potential to experimentally induce variation in subclinical psychotic phenomena using this experimental stress paradigm and/or a lack of sensitivity to detect subtle fluctuations in stress-induced subclinical psychotic phenomena in the laboratory possibly underlies the negative findings in this study. However, the power to detect the anticipated and significant interactions between group (control vs relative) and subjective, task-induced stress in the models of task-induced [18F]fallypride ligand displacement in left and right vmPFC was shown to be of adequate magnitude (0.80 and 0.88, respectively). Nonetheless, the issue of statistical power in relation to Type-II error should be prioritized in future replication studies, and care should be taken in the interpretation of underpowered study findings.
Generalizability of these findings to other groups at increased genetic risk for psychosis is hampered by the fact that the first-degree relatives included in this study were all healthy and, in most cases, beyond the age of risk for the onset of psychotic illness. Therefore, the nature and extent of dopamine abnormalities observed in these relatives may differ from those displayed by at-risk and ultra-high risk adolescent samples and may in fact represent an expression of biological resilience, rather than risk, in the context of psychosis vulnerability. However, this is in part contradicted by our finding of “hyporeactivity” being associated with increased, rather than decreased, intensity of subjective psychotic experiences. In addition, regardless of biological risk or resilience factors, healthy relatives of patients diagnosed with a psychotic disorder may manage to cope better with stress and/or have other resilience factors protecting them from psychotic disorder. Future investigations of unmedicated patients and medication-naive subjects with at-risk mental states are therefore required to further clarify the role of attenuated prefrontal dopamine signaling in the etiology of psychosis. Finally, it should be noted that our results do not imply causality, and the specific function of each brain region in the human stress response remains an important subject for further investigation.
Conclusion
Although previous studies have hypothesized a role for prefrontal dopamine dysfunction in psychosis,9 this study, to our knowledge, is the first in vivo human imaging study showing attenuated (ie, hyporeactive) dopamine stress neuromodulation in vmPFC of individuals at familial risk for psychosis. Development of high-affinity D2/3 radioligands, like [18F]fallypride,12 and advances in PET-methodology21 offer possibilities for further exploration of extrastriatal dopamine sites suggested to be involved in the pathophysiology of psychosis.2,11 The work presented in this study is at an early stage, and there is an urgent need for further human in vivo studies aimed at clarifying mechanisms of pathophysiological dopamine communication between cortical and subcortical brain regions in the context of psychosis.
Funding
National Alliance for Research on Schizophrenia and Depression (the Brain & Behavior Research Foundation) Young Investigator Award 2006 (to Dr Myin-Germeys); Dutch Medical Research Council (No 917.76.341 to Dr Myin-Germeys); European Community’s Seventh Framework Program (No HEALTH-F2-2009–241909).
Supplementary Material
Acknowledgments
All authors report no financial relationships with commercial interests. We thank Wendy Beuken, Rufa Diederen, Stijn Dirix, Truda Driesen, Bernice Gulpers, Kwinten Porters, and Mieke Steukers for their assistance in collecting data, and Ron Mengelers for technical support. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry. 2001; 58: 1137–1144 [DOI] [PubMed] [Google Scholar]
- 2. Lataster J, Collip D, Ceccarini J, et al. Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: a positron emission tomography study using [(18)F]fallypride. Neuroimage. 2011;58:1081–1089 [DOI] [PubMed] [Google Scholar]
- 3. Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004; 24: 2825–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009; 35: 549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Myin-Germeys I, Marcelis M, Krabbendam L, Delespaul P, van Os J. Subtle fluctuations in psychotic phenomena as functional states of abnormal dopamine reactivity in individuals at risk. Biol Psychiatry. 2005; 58: 105–110 [DOI] [PubMed] [Google Scholar]
- 6. Soliman A, O’Driscoll GA, Pruessner J, et al. Stress-induced dopamine release in humans at risk of psychosis: a [11C]raclopride PET study. Neuropsychopharmacology. 2008; 33: 2033–2041 [DOI] [PubMed] [Google Scholar]
- 7. Deutch AY, Clark WA, Roth RH. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Res. 1990; 521: 311–315 [DOI] [PubMed] [Google Scholar]
- 8. Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996; 721: 140–149 [DOI] [PubMed] [Google Scholar]
- 9. Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991; 148: 1474–1486 [DOI] [PubMed] [Google Scholar]
- 10. Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996; 93: 9235–9240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vrieze E, Ceccarini J, Pizzagalli DA, et al. Measuring extrastriatal dopamine release during a reward learning task. Hum Brain Mapp. 2011;. 10.1002/hbm.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mukherjee J, Christian BT, Dunigan KA, et al. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002; 46: 170–188 [DOI] [PubMed] [Google Scholar]
- 13. Buchanan TW, Driscoll D, Mowrer SM, et al. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010; 35: 56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christian BT, Lehrer DS, Shi B, et al. Measuring dopamine neuromodulation in the thalamus: using [F-18]fallypride PET to study dopamine release during a spatial attention task. Neuroimage. 2006; 31: 139–152 [DOI] [PubMed] [Google Scholar]
- 15. McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991; 48: 764–770 [DOI] [PubMed] [Google Scholar]
- 16. WMA Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian Med Assoc. 2009; 107: 403–405 [PubMed] [Google Scholar]
- 17. Konings M, Bak M, Hanssen M, van Os J, Krabbendam L. Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr Scand. 2006; 114: 55–61 [DOI] [PubMed] [Google Scholar]
- 18. Stefanis NC, Hanssen M, Smirnis NK, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002; 32: 347–358 [DOI] [PubMed] [Google Scholar]
- 19. The American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4thed. Washington DC: Text Revision; 2000. [Google Scholar]
- 20. Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). Br J Psychiatry. 1995; 166: 654–659 [DOI] [PubMed] [Google Scholar]
- 21. Alpert NM, Badgaiyan RD, Livni E, Fischman AJ. A novel method for noninvasive detection of neuromodulatory changes in specific neurotransmitter systems. Neuroimage. 2003; 19: 1049–1060 [DOI] [PubMed] [Google Scholar]
- 22. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995; 57: 289–300 [Google Scholar]
- 23. Stata/MP 10.0 for Windows © 1985–2007 StataCorp. LP [computer program]
- 24. Fadda F, Mosca E, Meloni R, Gessa GL. Ethanol-stress interaction on dopamine metabolism in the medial prefrontal cortex. Alcohol Drug Res. 1985; 6: 449–454 [PubMed] [Google Scholar]
- 25. Cook R. Detection of influential observations in linear regression. Technometrics. 1977; 19: 15–18 [Google Scholar]
- 26. Belsley DA, Kuh E, Welsch RE. Regression Diagnostics. Identifying Influential Data and Sources of Collinearity. New York: John Wiley; 1980. [Google Scholar]
- 27. Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002; 115: 1261–1279 [DOI] [PubMed] [Google Scholar]
- 28. Ongür D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998; 401: 480–505 [PubMed] [Google Scholar]
- 29. Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. J Int Neuropsychol Soc. 2006; 12: 224–235 [DOI] [PubMed] [Google Scholar]
- 30. Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008; 63: 927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993; 163: 109–113 [DOI] [PubMed] [Google Scholar]
- 32. Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003; 37: 8–25 [PubMed] [Google Scholar]
- 33. Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001; 11: 240–249 [DOI] [PubMed] [Google Scholar]
- 34. Lazarus R, Folkman S. Stress, Appraisal, and Coping. New York: Springer Publishing Company; 1984. [Google Scholar]
- 35. Rudebeck PH, Bannerman DM, Rushworth MF. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci. 2008; 8: 485–497 [DOI] [PubMed] [Google Scholar]
- 36. Tremeau F. A review of emotion deficits in schizophrenia. Dialogues Clin Neurosci. 2006; 8: 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heberlein AS, Padon AA, Gillihan SJ, Farah MJ, Fellows LK. Ventromedial frontal lobe plays a critical role in facial emotion recognition. J Cogn Neurosci. 2008; 20: 721–733 [DOI] [PubMed] [Google Scholar]
- 38. Nikolaus S, Antke C, Beu M, Müller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders–results from in vivo imaging studies. Rev Neurosci. 2010; 21: 119–139 [DOI] [PubMed] [Google Scholar]
- 39. Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008; 10: 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003; 160: 13–23 [DOI] [PubMed] [Google Scholar]
- 41. Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997; 17: 8528–8535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998; 55: 362–368 [DOI] [PubMed] [Google Scholar]
- 43. Koepp MJ, Gunn RN, Lawrence AD, et al. Evidence for striatal dopamine release during a video game. Nature. 1998; 393: 266–268 [DOI] [PubMed] [Google Scholar]
- 44. Slifstein M, Kegeles LS, Xu X, et al. Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse. 2010; 64: 350–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996; 4: 153–158 [DOI] [PubMed] [Google Scholar]
- 46. Yoder KK, Wang C, Morris ED. Change in binding potential as a quantitative index of neurotransmitter release is highly sensitive to relative timing and kinetics of the tracer and the endogenous ligand. J Nucl Med. 2004; 45: 903–911 [PubMed] [Google Scholar]
- 47. Rosner B. Fundamentals of Biostatistics. 4thed. Belmont, CA: Wadsworth Publishing Company; 1995. [Google Scholar]
- 48. Cohen J. Statistical Power Analysis for the Behavioural Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- 49. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009; 41: 1149–1160 [DOI] [PubMed] [Google Scholar]
- 50. Ioannidis JP. Why most discovered true associations are inflated. Epidemiology. 2008; 19: 640–648 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.