Abstract
Objective
Humanin (HN) is a cytoprotective peptide derived from endogenous mitochondria, expressed in the endothelial layer of human vessels, but its role in atherogenesis in vivo is not known. In vitro study, however, HN reduced oxidized low-density lipoprotein induced formation of reactive oxygen species and apoptosis. The present study tested the hypothesis that long term treatment with HN will have a protective role against endothelial dysfunction and progression of atherosclerosis in vivo.
Methods and results
Daily intraperitonial injection of the HN analogue HNGF6A for 16 weeks prevented endothelial dysfunction and decreased atherosclerotic plaque size in the proximal aorta of ApoE-deficient mice fed on a high cholesterol diet, without showing direct vasoactive effects or cholesterol-reducing effects. HN was expressed in the endothelial layer on the aortic plaques. HNGF6A treatment reduced apoptosis and nitrotyrosine immunoreactivity in the aortic plaques without affecting the systemic cytokine profile. HNGF6A also preserved expression of endothelial nitric oxide synthase in aorta.
Conclusions
HN may have a protective effect on endothelial function and progression of atherosclerosis by modulating oxidative stress and apoptosis in the developing plaque.
Keywords: Humanin, Endothelial dysfunction, Atherosclerosis, ApoE-deficient mice
1. Introduction
Endothelial dysfunction is an important step in the development of atherosclerosis and plaque progression [1,2]. Imbalance between endothelium-dependent vasodilators and endothelium-derived contraction factors leads to endothelial dysfunction [3]. Increased vascular oxidative stress is one of the major mechanisms of development and progression of atherosclerosis through functional and structural impairment of vascular wall [4]. Endothelial cells, smooth muscle cells, and macrophages produce reactive oxygen species (ROS) in the subendothelial space. ROS oxidize low-density lipoprotein (LDL) to form oxidized-LDL (Ox-LDL). Ox-LDL stimulates ROS generation in endothelial cells and smooth muscle cells, which results in arrest in all phases of cell cycle and subsequently leads to apoptosis [5,6]. Apoptosis of vascular smooth muscle cells, endothelial cells, and macrophages may promote plaque growth and pro-coagulation and may induce rupture, the major mortal consequence of atherosclerosis [7].
Humanin (HN) is a 24 amino acid peptide originally isolated from a cDNA library of surviving neurons of familial Alzheimer’s disease (AD)[8] and is expressed from an open reading frame within the mitochondrial 16S ribosomal RNA [9]. HN is known as a rescue factor against neuronal cell death associated with AD [8,10]. The cytoprotective effect of HN has also been demonstrated in human cerebrovascular smooth muscle cells [11]. We have previously demonstrated that infusion of HN into the third ventricle of rats improves both hepatic and peripheral insulin sensitivity, thus indirectly proving that HN plays an important role as a central regulator of systemic insulin action [12]. We have also shown that systemic HN administration delayed and ameliorated diabetes in non-obese diabetic mice, by preventing beta cell apoptosis and inhibiting islet inflammation [13].
In a recent study, we have demonstrated that HN was expressed in the endothelial layer of human internal mammary arteries, atherosclerotic coronary arteries, and greater saphenous vein. Pretreatment of HN analogue HNGF6A to human aortic endothelial cells (HAECs) reduced Ox-LDL-induced apoptosis and formation of ceramides involved in the apoptotic signaling cascade. In addition to apoptosis, pretreatment of HNGF6A to HAECs in vitro also reduced Ox-LDL-induced formation of ROS, one of the most important mechanisms of atherosclerosis [14]. Thus, it may be speculated that HN may play a protective role in the development of endothelial dysfunction and atherosclerosis in vivo. The current study was designed to test the hypothesis that chronic administration of HNGF6A might prevent the development and progression of endothelial dysfunction and atherosclerosis in vivo. For this purpose, we investigated the effect of HN on aortic endothelial function and morphology in hypercholesterolemic apolipoprotein E (ApoE)-deficient mice.
2. Materials and methods
Detailed information is provided in online Supplementary material.
2.1. Animals
The study was approved by the Institutional Animal Care and Use Committee of Mayo Clinic and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Female C57BL/6 mice and ApoE-deficient mice were obtained at the age of 4 weeks from Jackson Laboratory. Depending on the study group, the mice were treated with a normal diet (regular chow) or high cholesterol diet (HC, 0.15% cholesterol and 42% milk fat by weight, TD88137; Harlan Teklad) and received intraperitoneal injection (IP) of saline or HNGF6A (glycine variant of HN [8,14], 0.4 mg/kg/day, Peptide International, Louisville, KY) for 16 weeks. HNGF6A is 1000 times more potent than normal HN [8]. The effect of HNGF6A on mice was studied by 4 different animal groups (n = 12 per group) after 16 weeks injection of HNGF6A; C57BL/6 + normal diet + IP saline (Group 1), C57BL/6 + normal diet + IP HNGF6A (Group 2), ApoE-deficient + HC + IP saline (Group 3), and ApoE-deficient + HC + IP HNGF6A (Group 4).
2.2. Measurement of plasma lipids and cytokines
Plasma total cholesterol (T.Chol), high density lipoprotein-cholesterol (HDL-Chol), and triglyceride (TG) were measured using Cobas c311 analyzer (Roche Diagnotics, Indianapolis, IN). Plasma interleukin (IL)-6, monocyte chemotactic protein (MCP)-1, tumor necrosis factor (TNF)-α, vascular endothelial growth factor (VEGF), insulin, leptin, resistin, and tissue plasminogen activator inhibitor (tPAI)-1 were measured by the Inflammation Core Laboratories of the Diabetes and Endocrinology Research Center at the University of California, Los Angeles: by the LINCOplex assay for Mouse Cytokines and Adipokines.
2.3. Measurement of vascular function
The entire thoracic aorta was placed into ice cold (4°C) Krebs Ringer bicarbonate solution and cut into rings (3–4 mm long). The first aortic ring from just distal to left subclavian artery was defined as proximal aorta. Rings were suspended in organ chambers and relaxation to acetylcholine (10−9 to 10−5 mol/L, Sigma–Aldrich), calcium ionophore A23187 (10−10 to 3 × 10−6 mol/L, Enzo Life Sciences, Plymouth Meeting, PA), and sodium nitroprusside (10−9 to 10−5 mol/L, Sigma–Aldrich) were recorded.
To find out whether HNGF6A had direct vasoactive effects, excess internal mammary artery (IMA) segments were collected from patients undergoing coronary artery bypass surgery and relaxation to 105 mol/L HNGF6A or saline were recorded.
2.4. Histological and morphometric analyses
Morphometric analyses on hematoxylin and eosin-stained slides were performed for the measurement of plaque size using a digital image system (Nikon DXM 1200). Plaque sizes were analyzed in 4 different cross sections of each proximal aorta with MetaImaging series 6.1; Metamorph (Universal Imaging Corporation, Downingtown, PA).
2.5. Immunohistochemistry
IHC was performed with the primary Ab of HN [15] (titer 1:500), nitrotyrosine (NT, titer 1:80, Cayman Chemical, Ann Arbor, MI), or endothelial nitric oxide synthase (eNOS, titer 1:50, Novus Biologicals, Littleton, CO) were applied and incubated at 4°C overnight.
For the quantification, NT was expressed as percentage of staining of total plaque area and eNOS was expressed as percentage of staining of total tissue area.
2.6. Apoptosis
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was performed using the in situ Apoptosis Detection Kit (S7100-KIT; Chemicon). The procedure was performed following the manufacturers’ protocol. The Number of positive cells in atherosclerotic plaque was counted using 400× magnification. Results are presented as the number of cells divided by plaque area.
2.7. Statistical analysis
Data was expressed as mean±SEM. Comparison of different groups was performed by one-way ANOVA followed by post hoc tests for parametric and nonparametric distribution. Comparison between the 2 groups was made by Student’s t-test or Mann–Whitney rank sum test. A value of P < 0.05 was considered significant.
3. Results
3.1. Mice characteristics
After 16 weeks of diet and daily IP, both Group 3 and Group 4 were heavier than Group 1 and Group 2 (P <0.01, Table 1). There were no significant difference of body weight between Group 3 and Group 4.
Table 1.
Mice characteristics according to experimental groups.
| Characteristics | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| Bwt (g) | 21.7 ± 0.5 | 21.0 ± 0.3 | 24.3 ± 0.5* | 24.9 ± 0.5* |
| T.Chol (mg/dL) | 64.8 ± 3.1 | 68.4 ± 3.1 | 1150.4 ± 95.4* | 1120.0 ± 87.7* |
| HDL-Chol (mg/dL) | 45.4 ± 2.4 | 46.4 ± 3.0 | 201.6 ± 19.4* | 208.6 ± 19.7* |
| TG (mg/dL) | 65.2 ± 8.0 | 65.0 ± 5.5 | 88 ± 8.0 | 96.8 ± 17.7 |
Bwt: body weight, T.Chol: total cholesterol, HDL-Chol: high density lipoprotein-cholesterol, TG: triglyceride, HC: high cholesterol diet, HN: humanin.
P <0.01 vs. Group 1.
3.2. Plasma lipid levels
Plasma levels of T. Chol and HDL-Chol were markedly elevated in ApoE-deficient mice fed on a HC, compared with Group 1 and Group 2. However, Group 3 and Group 4 did not show significant difference. TG was not different among the 4 groups (Table 1).
3.3. Vascular relaxations
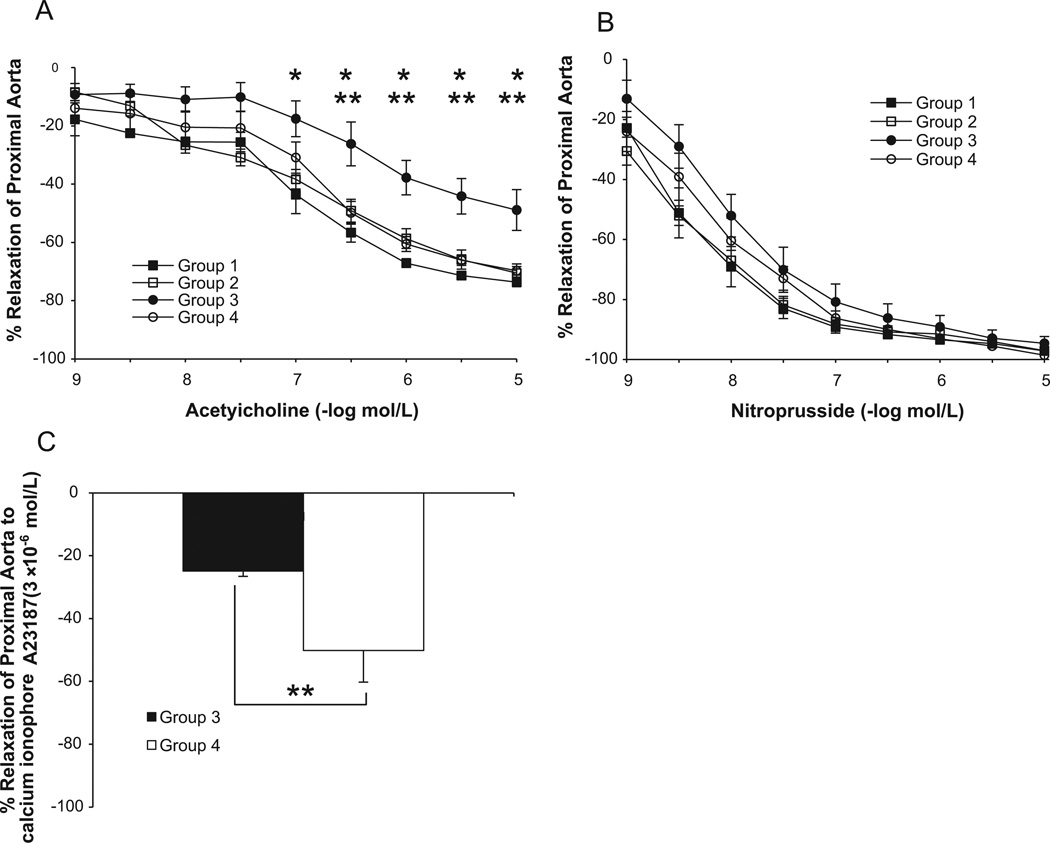
After 16 weeks of the high cholesterol diet, endothelium-dependent relaxation of proximal aorta to acetylcholine was impaired in Group 3 compared with Group 1 and Group 2 (P <0.05; Fig. 1A). Aortic rings from the Group 4 demonstrated significantly improved response to acetylcholine compared with Group 3 (P <0.05; Fig. 1A). Maximum relaxations to Ca ionophore A23187 of proximal aorta of Group 3 were also significantly reduced than those of Group 4 (−32.7 ± 7.0% vs. −58.6 ± 7.7%, respectively, P <0.05, Fig. 1C). Sodium nitroprusside-induced endothelium-independent relaxations of aorta were not different among the four groups (Fig. 1B).
Fig. 1.
Relaxation to acetylcholine, sodium nitroprusside, and calcium ionophore A23187 of proximal aorta in Group 1, Group 2, Group 3, and Group 4. ApoE-deficient with HC (Group 3; n=8) showed attenuated relaxations to acetylcholine compared with C57BL/6 with normal diet (Group 1, n=4 and Group 2, n=11). HNGF6A treatment to ApoE-deficient with HC (Group 4, n=9) restored relaxations to acetylcholine (A). No differences in relaxations to sodium nitroprusside were found between each group (B). HNGF6A treatment to ApoE-deficient with HC (Group 4) significantly increased maximum relaxation to calcium ionophore A23187 compared with ApoE-deficient with HC (Group 3) (C). *P <0.05 Group 3 vs. Group 1; **P <0.05 Group 3 vs. Group 4.
No significant effect of HNGF6A on vasorelaxation response was observed in human IMAs (data not shown), which argues against a direct vasorelaxation effect of HN.
3.4. Histological and morphometric analyses
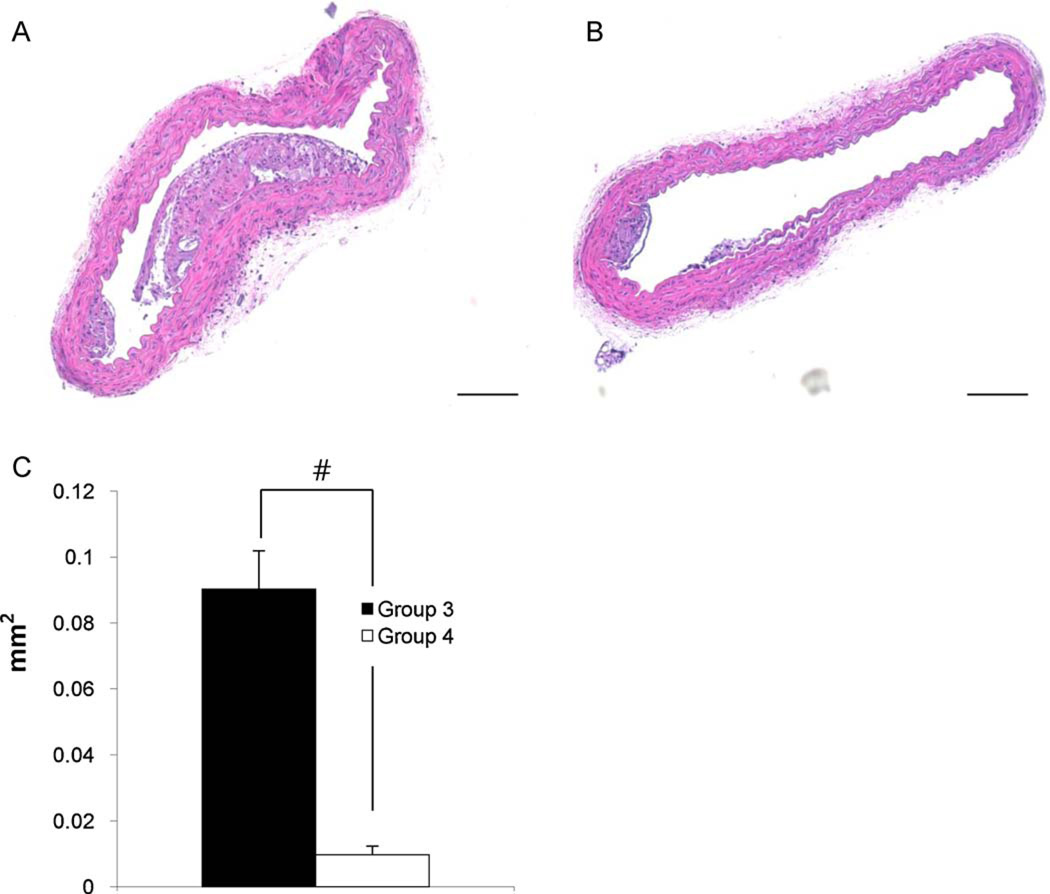
Proximal aorta cross sections showed the presence of atherosclerotic plaques in both Groups 3 and 4. There was no plaque in Groups 1 and 2. Plaque size of Group 3 (0.09 ± 0.01 mm2) was significantly larger than that of Group 4 (0.01 ± 0.003 mm2) (Fig. 2, P < 0.01).
Fig. 2.
Morphological studies of proximal aorta. Representative photographs of Group 3 (A) n = 8 and Group 4 (B) n = 6. Quantification of plaque size showed that HNGF6A treatment to ApoE-deficient with HC (Group 4) had significantly decreased plaque size compared with ApoE-deficient with HC (Group 3) (C). Bar = 50 µm. #P <0.01.
3.5. Immunohistochemistry (IHC)
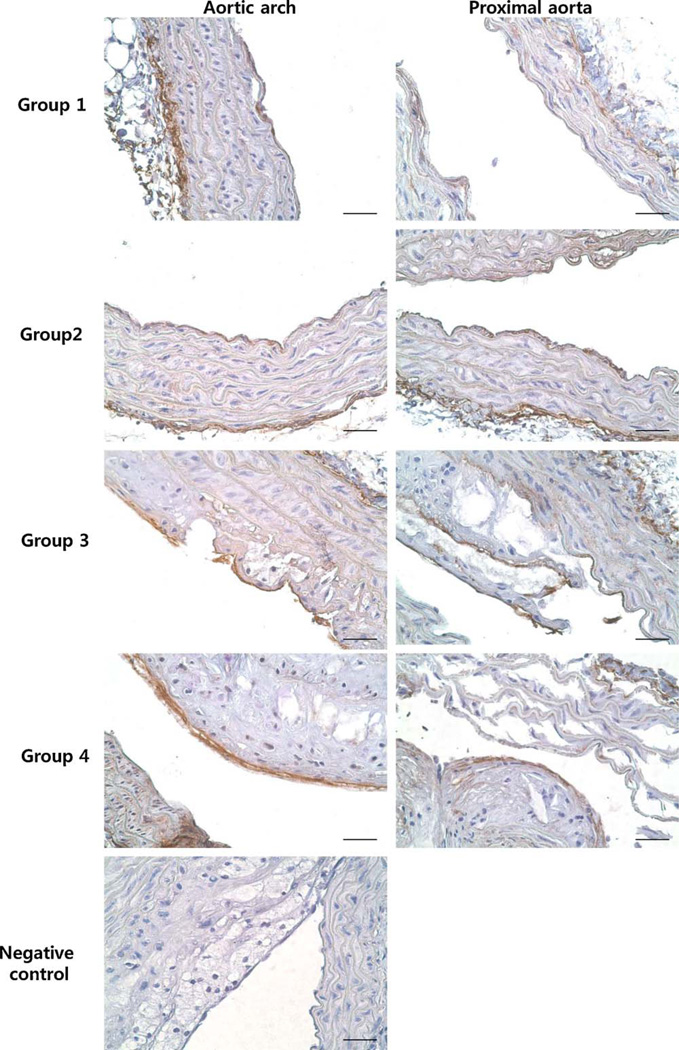
There were weak HN signals in the endothelial layer of aortic arches and proximal aortas of Groups 1 and 2, and positive signals were detected in aortic arches of Groups 3 and 4. In the proximal aorta, HN was expressed in the plaques of Group 3. In Group 4, only 2 small plaques were observed on IHC and only weak positive staining was found on one plaque. Positive signals were mainly found in the endothelial layer of the plaques (Fig. 3).
Fig. 3.
Immunohistochemistry of HN in aortic arch and proximal aorta from Group 1, Group 2, Group 3, and Group 4. There were weak signals in endothelial layer of aortic arch and proximal aorta of Group 1 and Group 2. HN was expressed in the endothelial layer on the plaques of aortic arch from Group 3 and Group 4. In proximal aorta of Group 3 showed positive HN staining in the endothelial layer on the plaques. Proximal aorta of Group 4 showed weak positive HN staining in the endothelial layer on the plaques. Bar = 200 µm.
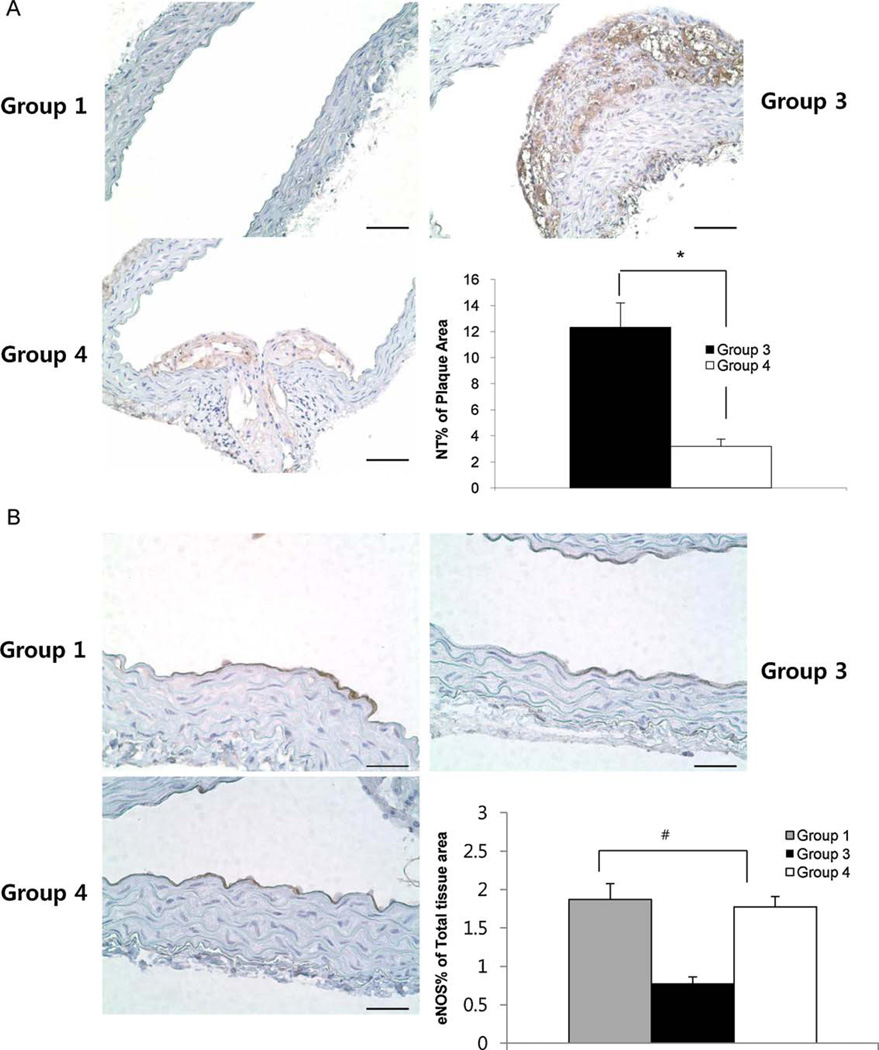
In order to investigate whether the antioxidative stress effect of HN is involved in its protective effect on endothelial dysfunction and atherosclerosis in vivo, we carried out IHC of NT. There was no NT expression in proximal aorta of Group 1. In the proximal aorta, 6 of 7 specimens of Group 3 included plaques and NT was positive in all of them. In Group 4 (12.3 ± 1.9% of plaque area), only 2 of 6 aortas had plaques and the NT signals were weaker (3.2 ± 0.6% of plaque area) than Group 3 (12.3 ± 1.9% of plaque area) (Fig. 4A, P <0.05).
Fig. 4.
Immunohistochemistry of NT and eNOS in proximal aorta from Group 1, Group 3, and Group 4. There was no positive NT staining in Group 1. HNGF6A treatment to ApoE-deficient with HC (Group 4) showed weaker NT staining in the plaques than ApoE-deficient with HC (Group 3). (A) Bar=100µm. *P <0.05. eNOS was expressed in endothelial layer of Group 1, Group 3, and Group 4. eNOS staining in ApoE-deficient with HC (Group 3) was weaker than C57BL/6 with normal diet (Group 1). HNGF6A treatment to ApoE-deficient with HC (Group 4) restored the eNOS staining. (B) Bar = 200 µm. #P < 0.01 between 3 groups.
eNOS expression was decreased in the endothelial layer of proximal aortas of Group 3 (0.7 ± 0.01% of total tissue area) compared with that in Group 1 (1.9 ± 0.2% of total tissue area). In Group 4 (1.8 ± 0.1% of total tissue area), eNOS expression was more increased than in Group 3 and signals were similar with those of Group 1 (Fig. 4B, P <0.01 between 3 groups).
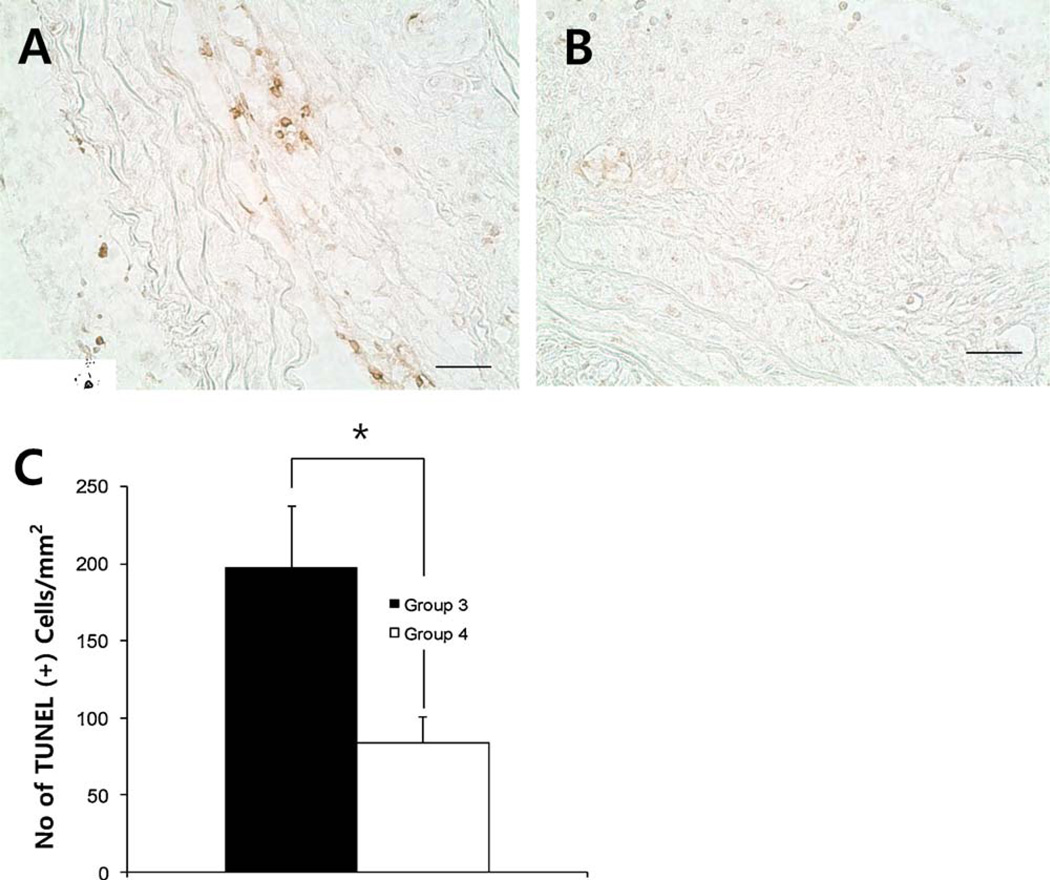
3.6. Apoptosis
Group 3 showed a significantly larger number of apoptotic cells (197 ± 41 cells/mm2) than Group 4 (84 ± 17 cells/mm2) in plaques of aortic arch (Fig. 5, P <0.05).
Fig. 5.
TUNEL staining in aortic arch from Group 3 (A) and Group 4(B). HNGF6A treatment to ApoE-deficient with HC (Group 4) showed significantly decreased numbers of apoptotic cells in the plaques compared with ApoE-deficient with HC (Group 3) (C). Bar = 200 µm. *P <0.05.
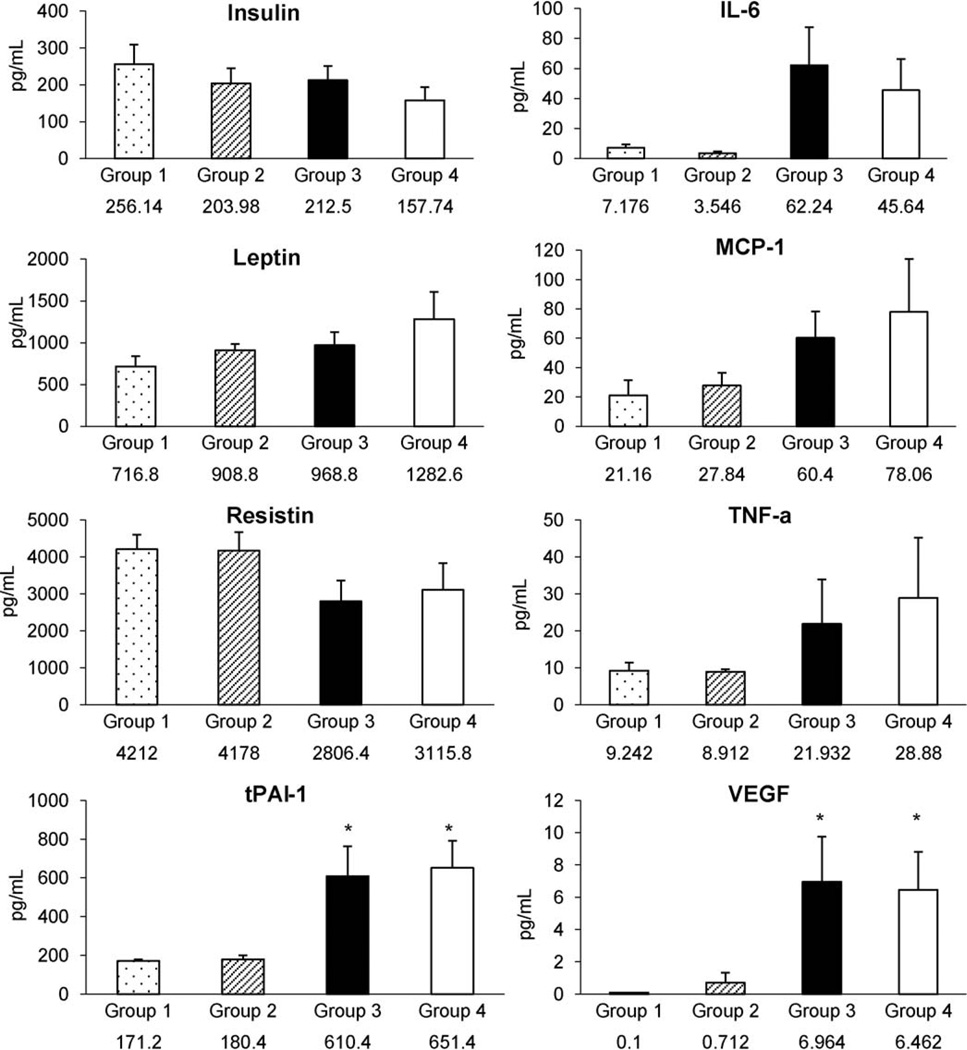
3.7. Plasma cytokines and adipokines level
Plasma level of tPA-1 and VEGF were significantly higher in Groups 3 and 4 than in Group 1, but unaffected by HNGF6A treatment (Fig. 6).
Fig. 6.
Plasma adipokines and cytokines level of each group of mice. tPA-1 and VEGF showed statistically significant difference between Group 1 and ApoE-deficient mice fed on a HC (Group 3 and Group 4). However, there were no differences of cytokines and adipokines between Group 3 and Group 4 *P <0.05 vs. Group 1.
4. Discussion
The current study demonstrates for the first time that chronic administration of HNGF6A to ApoE deficient mice on HC diet preserves endothelial function and prevents the progression of the atherosclerotic plaques. These effects were associated with a reduction of markers of oxidative stress and apoptosis by IHC. HNGF6A also preserved expression of eNOS. The current study supports a potential role for HN in the protection against the development of endothelial dysfunction and early atherosclerosis.
Endothelial dysfunction is the marker of atherosclerosis process [3] and is accompanied by a proinflammatory, proliferative, and procoagulatory milieu [16]. Increased oxidative stress is one of the important mechanisms of endothelial dysfunction [4]. The current study extends our previous in vitro observation that demonstrated that HN has a protective role on human endothelial cell from ox-LDL induced apoptosis and formation of ROS [14].
The mechanism by which long term HNGF6A administration prevented endothelial dysfunction and atherosclerotic plaque formation may be speculated to involve the decreases in oxidative stress and apoptosis. The current study also presents that HN preserves endothelial NO bioavailability in endothelium.
The apoptosis of endothelial cells, smooth muscle cells, and macrophages in atherosclerotic plaques is known to lead to loss of collagen type I and results in unstable plaques that are prone to rupture [17]. Shed membrane microparticles of apoptotic cells act as potent procoagulant substrates both locally and systemically [18]. Therefore, apoptosis is one of the prominent detrimental mechanisms that advance atherosclerosis.
Bcl-2 family proteins display either proapoptotic or antiapoptotic function by affecting mitochondria function. One of the key proapoptotic proteins is Bax, which, in the presence of a death signal, translocates from cytosol to mitochondria [19] followed by oligomerization [20], insertion into the outer membrane of mitochondria, and integration into the mitochondrial membrane [20] in that order. This leads to Cytochrome-c release from mitochondria. Cytochrome-c binds to the apoptosis-inducing factor and activates caspase-9 [21]. HN, expressed from the mitochondrial chromosome, prevents apoptosis through several mechanisms, including the inhibition of the translocation of Bax from the cytosol to the mitochondria [22].
Another potential mechanism is by binding to the insulinlike growth factor-binding protein-3(IGFBP-3). IGFBP-3 induces apoptosis through both IGF-dependent and-independent mechanisms. IGFBP-3 translocates to the nucleus and interacts with nuclear receptors such as retinoid X receptor [23] and regulates both intrinsic and extrinsic apoptosis pathways [24]. IGFBP-3 is also up-regulated by proapoptotic signal transduction pathways, such as tumor necrosis factor α, transforming growth factor β, and the tumor suppressor p53 [25]. HN and IGFBP-3 bind to each other and have opposing effects on the cell death in glioblostoma cells and other cells [15]. This also occurs in vivo where HN attenuates germ cell apoptosis induced by intratesticular hormone deprivation involving IGFBP-3 [26]. We have previously shown that the HN analogue HNGF6A is not attenuated by endogenous IGFBP-3 in diabetic rats and therefore acts as an ultra-potent peptide [12]. This peptide was used in this study to achieve optimal outcome.
We demonstrated that there were fewer apoptotic cells in the plaques of Group 4. This finding suggests that HN has antiapoptotic effect in vivo model of atherosclerosis. A recent in vivo study also demonstrates that HN provides cardioprotection through alteration of proapoptotic signals in a mouse model of myocardial ischemia and reperfusion [27].
In a previous in vitro study, we found that another cytoprotective mechanism of HNGF6A is inhibition of ROS production during oxidative stress [14]. Under oxidative stress in atherosclerosis, superoxide radical anion can react with nitric oxide radical to form peroxynitrite (ONOO−), which is involved in protein-bound NT formation [28]. NT is detected in atherosclerotic lesions of human coronary arteries. The presence of NT indicates that superoxide anions are generated in human atherosclerosis and may be involved in its pathogenesis [29]. In the current study, we observed reduced NT staining in the plaques of Group 4 compared with those of Group 3 in proximal aortas. The result suggests that decreased oxidative stress may be a key mechanism for the protective effect of HNGF6A on the endothelium.
NO is a potent vasodilator and decreases endothelial expression of the cell adhesion molecules [30]. Regulation of eNOS seems to play an important role in the level of NO in the vessel. The current study demonstrated that chronic HNGF6A treatment increases endothelial eNOS, the constitutive enzyme of synthesis of NO in the vessel. It is one of the mechanisms that preserved endothelial function in Group 4.
The current study demonstrated that HN is expressed in the endothelial layer of mice aorta, especially in the endothelial layer on the atherosclerotic plaques of both saline and HNGF6A treated groups. In accordance with our previous study demonstrating that HAECs produce HN [14], the presence of HN in the endothelial layer of Group 3 likely represent endogenously produced HN from endothelial cells of atherosclerotic plaques in response to increased oxidative stress underscoring an attempt to compensate for the emerging atherosclerotic process. However, endothelial dysfunction and atherosclerotic plaques were progressed in this group, suggesting the endogenous production may be insufficient. Notably, we measured plasma cytokines and adipokines as well as lipids to explore a potential systemic effect of HNGF6A, but none was observed. These results suggest that HNGF6A mediates a protective effect through local endothelial mechanisms rather than as a systemic effect.
In the current study, protective effects of HNGF6A were studied in the aorta, but vasoactive changes occur mainly at the level of resistance vessels. To find out whether HN had direct vasoactive effects, relaxation of human IMAs to HNGF6A were studied. But, no significant effect of HNGF6A on vasorelaxation response was observed in human IMAs.
The current study has several limitations. Although we suggested that anti-oxidative stress, antiapoptosis, and preservation of eNOS are the mechanisms of protective effect of HNGF6A in atherosclerosis, we did not discover all the potential mechanisms of HNGF6A. Further studies are needed to explore more mechanisms by which mitochondrial and plasma HN play a role in atherosclerosis development.
In summary, this is the first demonstration that chronic administration of HNGF6A prevents endothelial dysfunction and potently delays progression of atherosclerosis in vivo in ApoE-deficient mice fed on a high cholesterol diet. These effects are associated with an antiapoptotic, antioxidative stress, and increased eNOS expression in vivo.
Supplementary Material
Acknowledgments
Funding
This study was partly supported by grant numbers DK73608, HL77131, HL085307, HL92954, AG 31750, R01AG034430, R01GM090311, and P30DK063491 from the NIH and the Mayo Foundation.
Footnotes
Conflict of interest
None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.atherosclerosis.2011.06.038.
References
- 1.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–116. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Kinlay S, Ganz P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am J Cardiol. 1997;80:11I–16I. doi: 10.1016/s0002-9149(97)00793-5. [DOI] [PubMed] [Google Scholar]
- 3.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 4.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 5.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 6.Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis. 2006;185:219–226. doi: 10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Littlewood TD, Bennett MR. Apoptotic cell death in atherosclerosis. Curr Opin Lipidol. 2003;14:469–475. doi: 10.1097/00041433-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto Y, Niikura T, Tajima H, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and aβ. Proc Natl Acad Sci U S A. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tajima H, Niikura T, Hashimoto Y, et al. Evidence for in vivo production of humanin peptide, a neuroprotective factor against Alzheimer’s disease-related insults. Neurosci Lett. 2002;324:227–231. doi: 10.1016/s0304-3940(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto Y, Niikura T, Ito Y, et al. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer’s disease-relevant insults. J Neurosci. 2001;21:9235–9245. doi: 10.1523/JNEUROSCI.21-23-09235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonia SJ, William EVN. Humanin rescues human cerebrovascular smooth muscle cells from aβ-induced toxicity. J Neurochem. 2003;84:266–272. doi: 10.1046/j.1471-4159.2003.01524.x. [DOI] [PubMed] [Google Scholar]
- 12.Muzumdar RH, Huffman DM, Atzmon G, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoang PT, Park P, Cobb LJ, et al. The neurosurvival factor humanin inhibits β-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59:343–349. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachar AR, Scheffer L, Schroeder AS, et al. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88:360–366. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikonen M, Liu B, Hashimoto Y, et al. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci U S A. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson TJ. Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol. 1999;34:631–638. doi: 10.1016/s0735-1097(99)00259-4. [DOI] [PubMed] [Google Scholar]
- 17.Kockx MM, Herman AG. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovascular Res. 2000;45:736–746. doi: 10.1016/s0008-6363(99)00235-7. [DOI] [PubMed] [Google Scholar]
- 18.Mallat Z, Hugel B, Ohan J, et al. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- 19.Hsu Y-T, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of bax and bcl-xl during apoptosis. Proc Natl Acad Sci U S A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskes R, Desagher S, Antonsson B, Martinou J-C. Bid induces the oligomerization and insertion of bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desagher S, Martinou J-C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 22.Guo B, Zhai D, Cabezas E, et al. Humanin peptide suppresses apoptosis by interfering with bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 23.Liu B, Lee H-Y, Weinzimer SA, et al. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid x receptor-α regulate transcriptional signaling and apoptosis. J Biol Chem. 2000;275:33607–33613. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- 24.Cohen P. Insulin-like growth factor binding protein-3: insulin-like growth factor independence comes of age. Endocrinology. 2006;147:2109–2111. doi: 10.1210/en.2006-0195. [DOI] [PubMed] [Google Scholar]
- 25.Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000;183:1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lue Y, Swerdloff R, Liu Q, et al. Opposing roles of insulin-like growth factor binding protein 3 and humanin in the regulation of testicular germ cell apoptosis. Endocrinology. 2010;151:350–357. doi: 10.1210/en.2009-0577. [DOI] [PubMed] [Google Scholar]
- 27.Muzumdar RH, Huffman DM, Calvert JW, et al. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol. 2010;30:1940–1948. doi: 10.1161/ATVBAHA.110.205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upmacis RK, Crabtree MJ, Deeb RS, et al. Profound biopterin oxidation and protein tyrosine nitration in tissues of ApoE-null mice on an atherogenic diet: Contribution of inducible nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2007;293:H2878–H2887. doi: 10.1152/ajpheart.01144.2006. [DOI] [PubMed] [Google Scholar]
- 29.Beckmann JS, Ye YZ, Anderson PG, et al. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 30.Adams MR, Jessup W, Hailstones D, Celermajer DS. L-Arginine reduces human monocyte adhesion to vascular endothelium and endothelial expression of cell adhesion molecules. Circulation. 1997;95:662–668. doi: 10.1161/01.cir.95.3.662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.