Abstract
In this study we describe the reproductive phenotypes of a novel mouse model in which Cre-mediated deletion of ERα is regulated by the aP2 (fatty acid binding protein 4) promoter. ERα-floxed mice were crossed with transgenic mice expressing Cre-recombinase under the control of the aP2 promoter to generate aP2-Cre/ERαflox/flox mice. As expected, ERα mRNA levels were reduced in adipose tissue, but in addition we also detected an 80% reduction of ERα levels in the hypothalamus of aP2-Cre/ERαflox/flox mice. Phenotypic analysis revealed that aP2-Cre/ERαflox/flox female mice were infertile. In line with this, aP2-Cre/ERαflox/flox female mice did not cycle and presented 3.8-fold elevated estrogen levels. That elevated estrogen levels were associated with increased estrogen signaling was evidenced by increased mRNA levels of the estrogen-regulated genes lactoferrin and aquaporin 5 in the uterus. Furthermore, aP2-Cre/ERαflox/flox female mice showed an accumulation of intra-uterine fluid, hydrometra, without overt indications for causative anatomical anomalies. However, the vagina and cervix displayed advanced keratosis with abnormal quantities of accumulating squamous epithelial cells suggesting functional obstruction by keratin plugs. Importantly, treatment of aP2-Cre/ERαflox/flox mice with the aromatase inhibitor Letrozole caused regression of the hydrometra phenotype linking increased estrogen levels to the observed phenotype. We propose that in aP2-Cre/ERαflox/flox mice, increased serum estrogen levels cause over-stimulation in the uterus and genital tracts resulting in hydrometra and vaginal obstruction.
Introduction
Estrogen receptor alpha (ERα, NR3A1) and ERβ (NR3A2) are two nuclear receptors that mediate the physiological responses to estrogen [1]. They are ligand-activated transcription factors, encoded by the Esr1 and Esr2 genes, that bind to DNA and regulate transcription in response to their ligands [2]. ERα has important roles in both the regulation of male and female reproduction, and also in the control of metabolism [3]. During the estrous cycle, 17β-estradiol (E2) levels are regulated via feedback mechanisms involving the ovaries, hypothalamus and pituitary gland. At the mid-stage of the estrous cycle, E2 produced within the gonads exerts a stimulatory effect on gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus. The resulting GnRH discharge stimulates the anterior pituitary gland to release luteinizing hormone (LH), which in turn triggers ovulation. At other stages of the estrous cycle, E2 exerts a negative feedback that results in the suppression of GnRH secretion, as reviewed in [4]. Estrogenic signals, via ERα, also control a number of functions in the uterus including early events like hyperemia and water imbibition, and later events such as epithelial cell proliferation and differentiation [5], [6]. Mice lacking ERα, ERα−/− mice, are infertile and have atrophic uteri that do not respond to E2 [7], [8], [9], [10], [11].
In this study, floxed ERα mice were crossed with aP2-Cre transgenic mice to generate aP2-Cre/ERαflox/flox mice. Consistent with the well-described expression of the aP2 promoter in adipocytes, aP2-Cre/ERαflox/flox mice display down-regulation of the ERα transcript in both white and brown adipose tissue (WAT and BAT). However, down-regulation of the ERα transcript is also pronounced in the hypothalamus. Phenotypically, female aP2-Cre/ERαflox/flox mice are infertile and develop hydrometra (fluid-filled distended uteri). We propose that in aP2-Cre/ERαflox/flox mice, increased serum E2 levels cause over-stimulation of the uterus and genital tracts resulting in hydrometra and vaginal obstruction.
Materials and Methods
Ethics Statement
The “Stockholms Södra Djuretiska Nämnd” ethics committees approved all animal experiments (approval numbers: S10/09, S11/09, S17-11, S53/12 and S64/12).
Animals
ERαflox/flox mice (B6.129X1-Esr1tm1Gust) [7] were bred with transgenic mice expressing the Cre enzyme under the control of the aP2/Fabp4 promoter (B6.Cg-Tg(Fabp4-Cre) 1Rev/J) [12] to generate mice with ERα deletion in fat cells. The final breeding step was performed using male aP2-Cre/ERαflox/flox and female ERαflox/flox mice. All mice analyzed in this study were on a congenic C57BL/6J genetic background. Genotyping of the ERα floxed locus was performed using PCR on DNA from ear or tail biopsies as described previously [7]. The presence of the aP2-Cre transgene was detected with primers 5′-GTTTCACTATCCAGGTTACGG and 5′- GTACTCTAAGTCCAGTGATC. Mice were maintained on a 14 h light, 10 h dark cycle and given a continuous supply of food and water.
Fertility Tests
Fertility tests of female aP2-Cre/ERαflox/flox (n = 6) mice were performed using continuous mating with male partners for three months. Mating was started at six weeks of age, and the numbers of litters and litter size were recorded.
Estrous Cycle Stage Determination
Vaginal smears were collected from ERαflox/flox (n = 2) and aP2-Cre/ERαflox/flox (n = 5) mice using 0.9% saline as described elsewhere [13], placed on glass microscopy slides and viewed at 10×magnification.
Measurement of Serum E2 Levels
E2 levels were determined using commercially available RIA kits (Siemens Medical Solutions, CA, USA), according to the manufacturer’s instructions.
RNA Isolation and RT-PCR
Tissues were dissected from 6–9 week-old female mice and immediately frozen on dry ice for storage at −70°C. Total RNA was isolated from frozen tissues using Trizol reagent (Invitrogen) and then purified with RNeasy Plus Mini Kits (Qiagen) as described in [14]. cDNA was synthesized using random primers and either Superscript II (Invitrogen) or TaqMan® Reverse Transcription Reagents (Life Technologies). PCR was performed using RedTaq DNA polymerase (Sigma-Aldrich) and the following primers: ERα exon2F: 5′-CCCTACTACCTGGAGAACGA and ERα exon5R: 5′-TGCCCACTTCGTAACACTTG [7]. ERα expression levels were assessed by semi-quantitative real-time PCR with a 7300 Real-Time PCR System (Applied Biosystems), using TaqMan® Universal PCR Master Mix (Life Technologies) and a TaqMan® Gene Expression Assay (Mm00433148_mH, Life Technologies), according to the manufacturer’s instructions. Gene expression was normalized to 18S (TaqMan® Ribosomal RNA Control Reagents, Life Technologies). The expression of ERα target genes and Cre were analyzed using Power SYBR Green Master Mix (Applied Biosystems), according to the manufacturer’s instructions, and the following primers: Lactoferrin F 5′-CAGCAGGATGTGATAGCCACAA, R 5′-CACTGATCACACTTGCGCTTCT [15], Aqp5 F 5′-TTGTGAAGGCAGTGCAAGCT, R 5′-CACCCCTTTCTGGGATGGT [16]. Cre expression was analyzed with: Cre F 5′-GCCGCGCGAGATATGG and Cre R 5′-AGCTTGCATGATCTGCGGTATT.
Histology
The female genital tract including ovaries, along with liver, kidney, brain, interscapular BAT, visceral (abdominal attached to ovaries and uterus) WAT, and inguinal subcutaneous WAT were collected from 12 mice that were between 6 and 12 weeks old. The tissues were fixed for 24 h in 4% neutral-buffered formaldehyde and stored in 70% ethanol prior to routine processing and embedding in paraffin blocks. Paraffin-embedded tissues were cut to 4 µm thickness, deparaffinized, rehydrated and stained with hematoxylin and eosin (H&E). The resulting slides were microscopically analyzed by a pathologist.
Treatment with Aromatase Inhibitor
Letrozole was purchased from Selleckchem and dissolved at 2 mg/ml in saline containing 0.3% hydroxymethyl cellulose. Letrozole (10 mg/kg body weight) or vehicle control was delivered to mice via daily sc injection.
Results
Female aP2-Cre/ERα Knockout Mice are Infertile and have Increased E2 Serum Levels
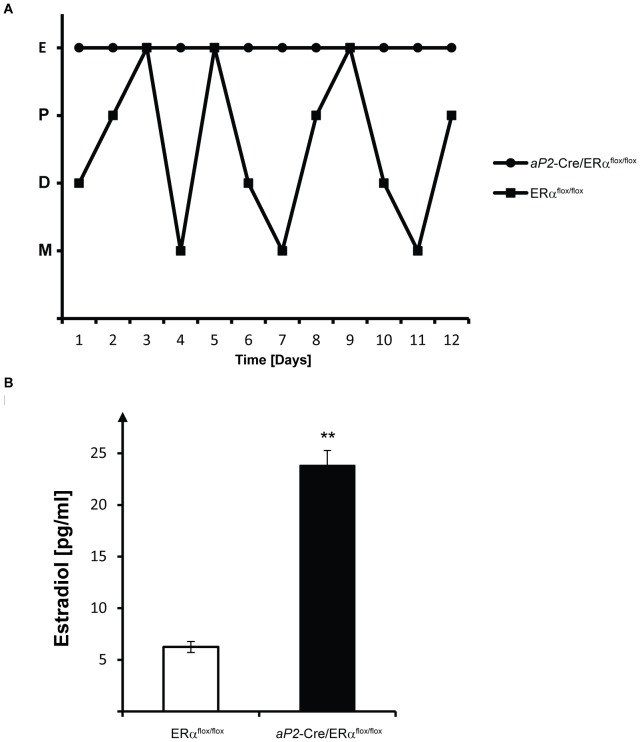
Fertility in female aP2-Cre/ERαflox/flox mice was investigated by continuous mating with fertile males for a three-month period. Since the breeding did not result in any pups (data not shown), we concluded that female aP2-Cre/ERαflox/flox mice are infertile. Vaginal smears from 2 month-old aP2-Cre/ERαflox/flox and ERαflox/flox littermates demonstrated that while ERαflox/flox animals cycled normally, aP2-Cre/ERαflox/flox mice displayed vaginal smears compatible with constant estrus (Fig. 1A). We next analyzed serum E2 concentrations, and found elevated levels in aP2-Cre/ERαflox/flox females compared to control female mice (Fig. 1B). The E2 levels were more than three-fold higher in aP2-Cre/ERαflox/flox females compared to ERαflox/flox female mice (23.8 pg/ml versus 6.25 pg/ml, respectively), and within the same range as our analysis of E2 levels in female mice with a global knockout of ERα (data not shown).
Figure 1. Lack of estrous cycle in aP2-Cre/ERαflox/flox female mice.
Vaginal smear analysis was performed on two month-old ERαflox/flox and aP2-Cre/ERαflox/flox mice on a daily basis for 12 days. (A) ERαflox/flox females cycled, whereas aP2-Cre/ERαflox/flox mice were in constant estrus, as determined by smears which consisted predominantly of cornified squamous epithelial cells. All graphs are representative and show two individuals out of totally seven analyzed. E, estrus; P, proestrus; M, metestrus; D, diestrus. (B) Serum E2 levels in ERαflox/flox (n = 6) and aP2-Cre/ERαflox/flox (n = 10) mice with ages between 10 and 14 weeks. Significance was determined by t tests. Error bars represent SEM, ***P<0.001.
ERα Expression in aP2-Cre/ERα Knockout Mice
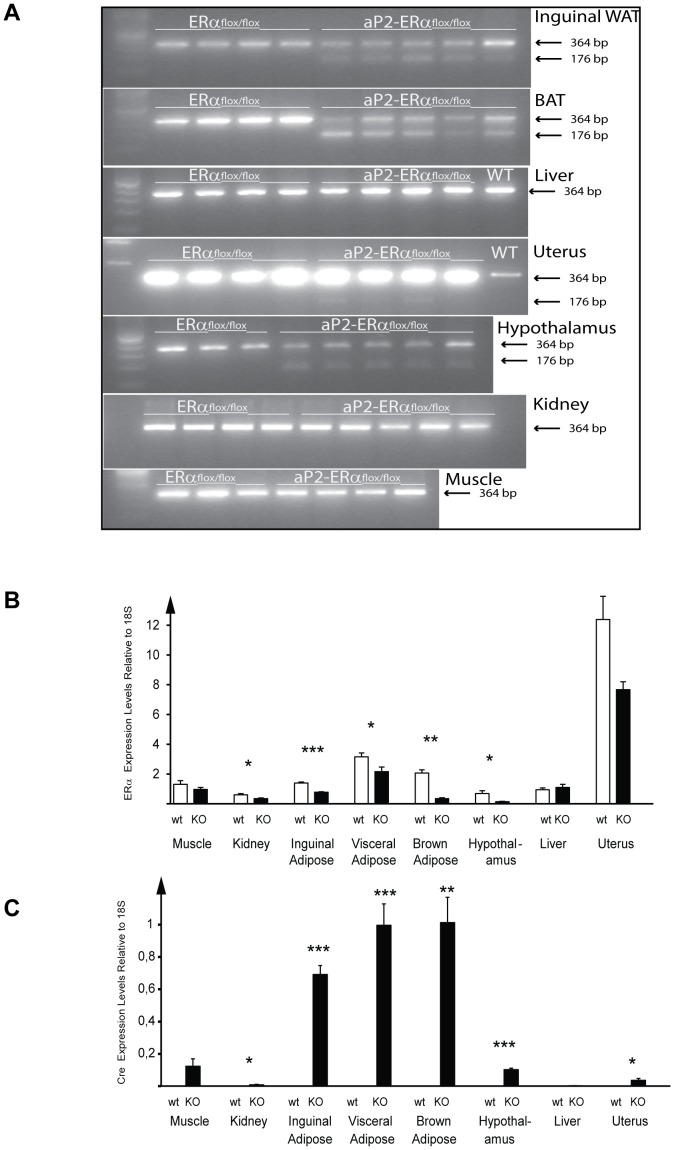
Ablation of the ERα gene in aP2-Cre/ERαflox/flox mice was analyzed by RT-PCR utilizing primers flanking exon 3 of the ERα gene. This analysis generates a 364 bp product from the WT transcript and a 176 bp PCR product from the targeted allele that lacks exon 3. As expected, only the WT transcript was detected in samples from ERαflox/flox mice. A 176 bp product, corresponding to the knockout transcript, was present in WAT and BAT from aP2-Cre/ERαflox/flox mice (Fig. 2A). We also detected mRNA from the knockout allele in hypothalamus. Only the WT transcript was detected in liver and muscle. Traces of the knockout transcripts could also be detected in the uterus and kidney, although their levels are extremely low compared to those of the WT transcript in these tissues. To quantitatively determine the efficiency of knockout in the different tissues, mRNA levels of the WT ERα transcript were assayed using real time PCR. Significant down-regulation of the ERα transcript was observed in aP2-Cre/ERαflox/flox mice, compared to control mice, in inguinal and visceral adipose tissue, brown adipose tissue, hypothalamus, and kidney (reductions of 44%, 31%, 83%, 80% and 42%, respectively), but not in muscle or liver; the reduction in ERα expression levels in the uterus was just below significance (Fig. 2B). To determine if the reduction of ERα mRNA levels in hypothalamus was due to Cre expression we assayed Cre expression using real time PCR. Significant Cre expression was detected in hypothalamus as well as all adipose depots analyzed and to lesser extents in uterus and kidney (Fig. 2C).
Figure 2. Specificity of ERα deletion in aP2-Cre/ERα females.
(A) RT-PCR analysis of total RNA from inguinal adipose tissue, brown adipose tissue (BAT), liver, uterus, hypothalamus, kidney and muscle from ERαflox/flox and aP2-Cre/ERαflox/flox mice. Arrows indicate the WT ERα transcript (364 bp) and the Cre-deleted ERα transcript lacking exon 3 (176 bp). (B) Relative expression levels of ERα in muscle, kidney, inguinal adipose tissue, visceral adipose tissue, brown adipose tissue, hypothalamus, liver and uterus from ERαflox/flox and aP2-Cre/ERαflox/flox mice. (C) Relative expression levels of Cre in muscle, kidney, inguinal adipose tissue, visceral adipose tissue, brown adipose tissue, hypothalamus, liver and uterus from ERαflox/flox and aP2-Cre/ERαflox/flox mice. Values are given as mean ± SEM; *P<0.05, **P<0.01 and ***P<0.001 vs. control mice.
Female aP2-Cre/ERα Knockout Mice Develop Hydrometra
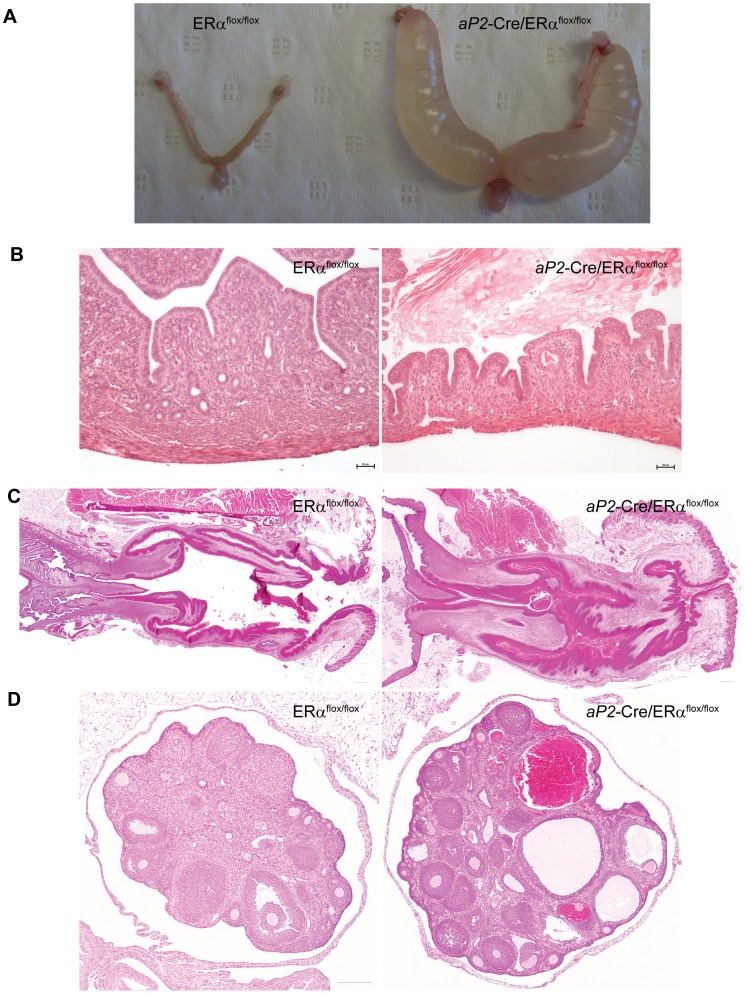
All female aP2-Cre/ERαflox/flox mice had swollen abdomens at 8 weeks of age. Internal anatomical examination revealed that the uteri in these mice were extensively fluid-filled (Fig. 3A). This phenotype was observed in all examined knockout mice but not in any of the control littermates. The accumulated uterine fluid was in most cases a clear and watery liquid characteristic of that seen in hydrometra, but in some of the animals the fluid was cloudy, consistent with an inflammatory response likely resulting from bacterial infection (pyometra).
Figure 3. Defects in uterus and reproductive tract.
(A) Representative images of uteri from five month-old ERαflox/flox and aP2-Cre/ERαflox/flox mice, showing that uteri from aP2-Cre/ERαflox/flox mice are fluid-distended. (B) H&E staining was used to analyze morphological changes in the uteri. aP2-Cre/ERαflox/flox mice have thin uterine walls with uterine distension together with atrophic muscles and glands. (C) Cervix and vagina from aP2-Cre/ERαflox/flox mice have hyperkeratotic epithelium and vaginal debris consisting of accumulated cornified squamous epithelial cells. (D) Ovaries from 6 week-old aP2-Cre/ERαflox/flox mice have hemorrhagic follicles and lack corpora lutea. Scale bar: 50 µm in (B) and 200 µm in (C) and (D).
Histologic analysis of the genital tract revealed that all investigated aP2-Cre/ERαflox/flox mice had distended uteri, usually with watery contents (hydrometra) and thin walls, together with a vastly reduced glandular content and a thin muscular layer (atrophy) (Fig. 3B). All control mice showed normal uterus histology with well-developed muscular walls and glands.
Morphological analysis of the vagina and cervix (Fig. 3C) revealed that aP2-Cre/ERαflox/flox mice had marked epithelial keratosis with abnormal quantities of accumulating cornified squamous epithelial cells in the vaginal lumen. We speculate that the observed hydrometra might have resulted from vaginal keratin plugs which could functionally obstruct the vagina. No other anatomical abnormalities, such as imperforate vagina or cervical/vaginal sagittal septa, were detected. Vaginal keratinization with variable luminal accumulation of cornified squamous epithelial cells was observed in 3 out of 6 normally cycling control mice.
The ovaries of aP2-Cre/ERαflox/flox mice consistently demonstrated hemorrhagic follicles when compared to control mouse ovaries (Fig. 3D). Additionally, aP2-Cre/ERαflox/flox ovaries did not show any signs of luteinisation, compared to control ovaries, which exhibited normal corpora lutea in 2 out of 3 observed specimens. Since antral follicles were abundant in all of the aP2-Cre/ERαflox/flox ovaries examined, aP2-Cre/ERαflox/flox ovaries appear to halt follicle development only at the final stage before ovulation. Indeed, the hemorrhages observed are likely the result of aberrant ovulation or follicle rupture. No marked differences in the numbers of atretic follicles were observed.
Up-regulation of Estrogen Target Genes
To identify genes in the uterus that could be involved in the development of hydrometra we analyzed the expression of the known E2 target genes, lactoferrin and aquaporin 5 [15], [16]. Lactoferrin mRNA levels were almost 10-fold higher, and aquaporin 5 mRNA levels about 7-fold higher, in the uterus of aP2-Cre/ERαflox/flox mice compared to controls (Fig. 4).
Figure 4. Quantitative PCR analysis of selected estrogen target genes.
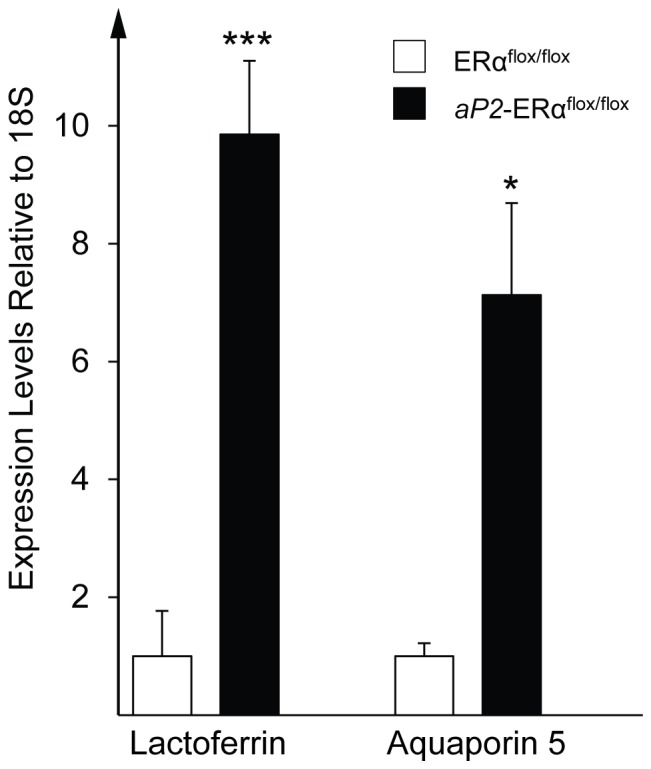
Relative expression levels of lactoferrin and aquaporin 5αflox/flox and aP2-Cre/ERαflox/flox mice. Values are given as mean ± SEM; *P<0.05 and***P<0.001 vs. control mice.
Inhibition of Endogenous Estrogen Synthesis Reduces Hydrometra
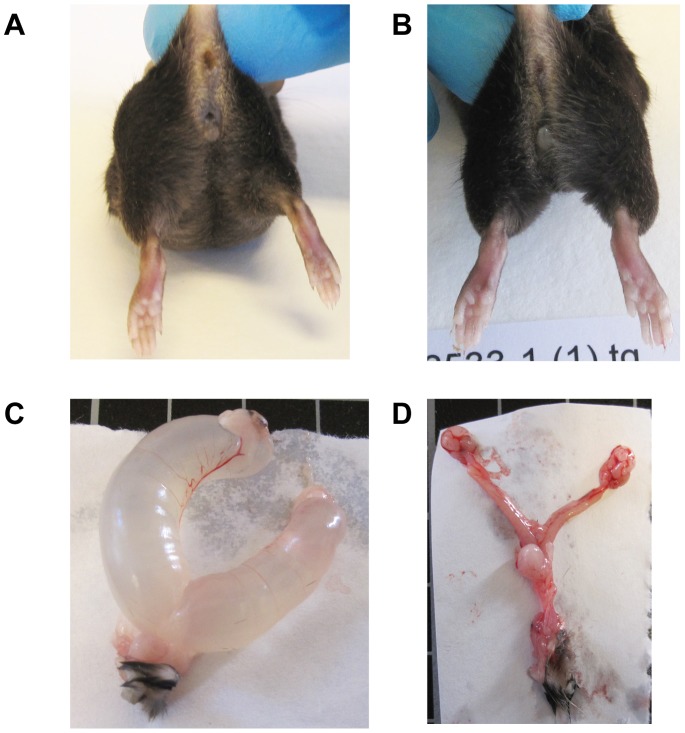
To analyze if inhibition of endogenous estrogen synthesis via the aromatase enzyme would reverse hydrometra, we treated aP2-Cre/ERαflox/flox (n = 5) and ERαflox/flox (n = 5) mice with the aromatase inhibitor Letrozole or vehicle. Mice were 10 weeks old at the start of the treatment, at which stage all aP2-Cre/ERαflox/flox mice displayed clear visual signs of hydrometra. Importantly, Letrozole treatment reversed visual signs of hydrometra in aP2-Cre/ERαflox/flox (n = 3) mice within one week of treatment. When the mice were sacrificed after 17 days of treatment, uteri appeared grossly normal in Letrozole-treated mice while the vehicle-treated mice presented a severe hydrometra phenotype (Fig. 5A, B).
Figure 5. Uterus from mice treated with Letrozole.
(A) 13 weeks old aP2-Cre/ERαflox/flox mice treated with vehicle have swollen abdomen while littermates treated with Letrozole for 17 days looks normal (B). (C) Uterus from vehicle treated aP2−/ERαflox/flox mice with severe hydrometra. (D) Uterus from Letrozole treated aP2-Cre/ERαflox/flox mice looks normal.
Discussion
We generated a novel mouse line in which Cre-mediated deletion of ERα is regulated by the aP2 promoter with the initial aim of targeting adipocyte ERα signaling using the Cre/loxP system. During the generation of this mouse strain, we observed that aP2-Cre-driven deletion of ERα leads to infertility in female mice and an arrest of the estrous cycle with hydrometra and increased serum E2 levels. Expression studies showed that the aP2-Cre transgene directs expression of Cre to adipose tissue as expected. Additionally, the aP2-driven Cre gene is also expressed in the hypothalamus, with a concomitant reduction in ERα levels also in this tissue (Fig. 2). The expression of Cre in the hypothalamus in aP2-Cre mice is consistent with previous observations [17], [18]. Estrogen action in the hypothalamus has drastic effects on the estrous cycle and on regulation of serum E2 levels [19], [20], [21], and it is conceivable that deletion of ERα in this brain region is the dominant cause of the severe reproductive effects observed in female aP2-Cre/ERαflox/flox mice.
We speculate that the increased E2 levels observed in female aP2-Cre/ERαflox/flox mice are related to deletion of ERα in the hypothalamus, resulting in disruption of the E2 feedback loop. Brain-specific ERα deletion using CamKIIα-Cre or nestin-Cre has previously been shown to cause elevated serum E2 levels and infertility [19], [21]. Also deletion of ERα in distinct hypothalamic neurons (SF1-Cre or POMC-Cre) affects fertility [21]. The increased levels of serum E2 in aP2-Cre/ERαflox/flox mice may explain the block in the estrous cycle, since cycling E2 levels control the estrous cycle in mammals, exerting both negative and positive feedback effects [22], [23].
E2 is known to influence both uterine weight and vaginal epithelial cytology, and treatment of mice with E2 stimulates both uterine weight gain and vaginal epithelial proliferation and keratinization. In global ERα knockout mice, E2 treatment does not increase either uterine weight or the abundance of cornified epithelial cells in the vagina, showing that ERα is necessary for both these processes [8]. Long term treatment of WT mice with E2 has been shown to result in hydrometra [24], and we suggest that the hydrometra observed in aP2-Cre/ERαflox/flox mice is a result of continuous E2 stimulation of the uterus, combined with severe vaginal hyperplasia and keratinization, resulting in accumulation of vast numbers of intraluminal keratinized squamous epithelial cells. In support of this, we show that treatment of aP2-Cre/ERαflox/flox mice with the aromatase inhibitor Letrozole reverses hydrometra (Fig 5). Interestingly, short-term E2 treatment of global ERβ knockout mice also results in fluid-filled uteri, and it was speculated that this is a result of increased signaling by ERα due to the loss of ERβ which was suggested to dampen the effects of ERα [25]. A similar uterine phenotype was described by Wintermantel et al. [19] in mice with a CamKIIα-Cre-driven neuron-specific ERα knockout. In contrast, mice with a global deletion of ERα have severely hypoplastic uteri [7], [8], [9], [10], [11], although serum E2 levels are increased [8], [9], [26], [27], [28]. In this case, the uterus cannot respond to the increased E2 levels due to lack of ERα in this organ. Although ERα expression was also reduced in the uteri of aP2-Cre/ERα mice this reduction did not achieve significance, and was presumably insufficient to block overstimulation in this organ in response to elevated E2 levels, followed by atrophy at later stages. In line with this, we still observed marked up-regulation of the known E2 target genes lactoferrin and aquaporin 5 in uteri of aP2-Cre/ERα mice (Fig. 4).
In summary, we have generated a conditional ERα knockout mouse model using aP2-Cre–driven gene deletion, and we here demonstrate that these mice develop hydrometra. Our results are consistent with a mechanism involving reduction of ERα expression in the hypothalamus, which results in disruption of E2 regulation and increased serum E2 levels, leading to a block of the estrous cycle and hyper-stimulation of the uterus. Collectively, these results underscore the roles of E2 and ERα as main players in the development of hydrometra, and also the challenges associated with the use of the aP2-Cre transgene to target adipose gene expression.
Acknowledgments
We thank Annemarie Witte and Tarja Schröder for technical assistance.
Funding Statement
This work is supported by the Swedish Research Council (http://www.vr.se/; The involvement of estrogen receptor signaling in insulin resistance and type 2 diabetes (project # K2009-54X-21122-01-3), Estrogen signaling in metabolic disease; a functional genomics approach (project # K2008-54X-20640-01-3) and Tissue-specific estrogen signaling - new insights in metabolic disease development (project # K2011-54X-20640-04-6)), Cancerfonden (http://www.cancerfonden.se; grant # 080482, 090678, 100404, 110588 and 120435), Novo Nordisk Foundation (http://www.novonordiskfonden.dk/en/; Estrogen signaling in metabolic disease (2006), Molecular mechanisms underlying the regulation of body weight and the anti-diabetic effects of estrogen (2008), Genetic dissection of estrogen signaling in mice (2009), Genetic dissection of estrogen signaling in metabolic disease (2011)), the Strategic Research Area in Diabetes at the Karolinska Institute (http://ki.se/srp-diabetes; SFO/TM Diabetes (2010)), Center for Biosciences (http://ki.se/cb;grant#n/a (2009–2011)), the KI Endomet network (http://researchnetworks.ki.se/converis/; grant details n/a), King Gustaf V and Queen Victoria’s Freemason’s Foundation; Molecular Mechanisms Underlying the Antidiabetic Effects of Estrogen (2008, 2009, 2010, 2011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, et al. (2001) Mechanisms of estrogen action. Physiol Rev 81: 1535–1565. [DOI] [PubMed] [Google Scholar]
- 2. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, et al. (1995) The nuclear receptor superfamily: the second decade. Cell 83: 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barros RP, Gustafsson J-Å (2011) Estrogen receptors and the metabolic network. Cell Metab 14: 289–299. [DOI] [PubMed] [Google Scholar]
- 4. Radovick S, Levine JE, Wolfe A (2012) Estrogenic regulation of the GnRH neuron. Front Endocrinol 3: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, et al. (2003) Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol 17: 2070–2083. [DOI] [PubMed] [Google Scholar]
- 6. Katzenellenbogen BS, Bhakoo HS, Ferguson ER, Lan NC, Tatee T, et al. (1979) Estrogen and antiestrogen action in reproductive tissues and tumors. Recent Prog Horm Res 35: 259–300. [DOI] [PubMed] [Google Scholar]
- 7. Antonson P, Omoto Y, Humire P, Gustafsson J-Å (2012) Generation of ERalpha-floxed and knockout mice using the Cre/LoxP system. Biochem Biophys Res Commun 424: 710–716. [DOI] [PubMed] [Google Scholar]
- 8. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, et al. (1993) Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90: 11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, et al. (2000) Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127: 4277–4291. [DOI] [PubMed] [Google Scholar]
- 10. Chen M, Wolfe A, Wang X, Chang C, Yeh S, et al. (2009) Generation and characterization of a complete null estrogen receptor alpha mouse using Cre/LoxP technology. Mol Cell Biochem 321: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hewitt SC, Kissling GE, Fieselman KE, Jayes FL, Gerrish KE, et al. (2010) Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. Faseb J 24: 4660–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He W, Barak Y, Hevener A, Olson P, Liao D, et al. (2003) Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 100: 15712–15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caligioni CS (2009) Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4. [DOI] [PMC free article] [PubMed]
- 14. Matic M, Bryzgalova G, Gao H, Antonson P, Humire P, et al. (2013) Estrogen signalling and the metabolic syndrome: targeting the hepatic estrogen receptor alpha action. PloS one 8: e57458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hewitt SC, Collins J, Grissom S, Deroo B, Korach KS (2005) Global uterine genomics in vivo: microarray evaluation of the estrogen receptor alpha-growth factor cross-talk mechanism. Molecular endocrinology 19: 657–668. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi M, Takahashi E, Miyagawa S, Watanabe H, Iguchi T (2006) Chromatin immunoprecipitation-mediated target identification proved aquaporin 5 is regulated directly by estrogen in the uterus. Genes to cells : devoted to molecular & cellular mechanisms 11: 1133–1143. [DOI] [PubMed] [Google Scholar]
- 17. Martens K, Bottelbergs A, Baes M (2010) Ectopic recombination in the central and peripheral nervous system by aP2/FABP4-Cre mice: implications for metabolism research. FEBS letters 584: 1054–1058. [DOI] [PubMed] [Google Scholar]
- 18. Mullican SE, Tomaru T, Gaddis CA, Peed LC, Sundaram A, et al. (2013) A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol Endocrinol 27: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, et al. (2006) Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Couse JF, Yates MM, Walker VR, Korach KS (2003) Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol 17: 1039–1053. [DOI] [PubMed] [Google Scholar]
- 21. Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, et al. (2011) Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14: 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herbison AE, Pape JR (2001) New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 22: 292–308. [DOI] [PubMed] [Google Scholar]
- 23. Petersen SL, Ottem EN, Carpenter CD (2003) Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biology of reproduction 69: 1771–1778. [DOI] [PubMed] [Google Scholar]
- 24. Greenman DL, Fullerton FR (1986) Comparison of histological responses of BALB/c and B6C3F1 female mice to estradiol when fed purified or natural-ingredient diets. J Toxicol Environ Health 19: 531–540. [DOI] [PubMed] [Google Scholar]
- 25. Weihua Z, Saji S, Makinen S, Cheng G, Jensen EV, et al. (2000) Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci USA 97: 5936–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, et al. (2008) Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females. Endocrinology 149: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hewitt SC, Couse JF, Korach KS (2000) Estrogen receptor transcription and transactivation: Estrogen receptor knockout mice: what their phenotypes reveal about mechanisms of estrogen action. Breast Cancer Res 2: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, et al. (2002) Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 30: 18–25. [DOI] [PubMed] [Google Scholar]