Summary
Epigenetic modifications are important in the normal functioning of the cell, from regulating dynamic expression of essential genes and associated proteins to repressing those that are unneeded. Epigenetic changes are essential for development and functioning of the kidney, and aberrant methylation, histone modifications, and expression of microRNA could lead to chronic kidney disease (CKD). Here, epigenetic modifications modulate transforming growth factor β signaling, inflammation, profibrotic genes, and the epithelial-to-mesenchymal transition, promoting renal fibrosis and progression of CKD. Identification of these epigenetic changes is important because they are potentially reversible and may serve as therapeutic targets in the future to prevent subsequent renal fibrosis and CKD. In this review we discuss the different types of epigenetic control, methods to study epigenetic modifications, and how epigenetics promotes progression of CKD.
Keywords: DNA methylation, microRNAs, chronic kidney disease (CKD), EMT, renal fibrosis
Chronic kidney disease (CKD) is a global epidemic that affects more than 20 million people in the United States alone.1 Despite considerable laboratory-based and epidemiologic research, the underlying risk factors and pathophysiologic pathways for CKD progression remain largely unknown. It is becoming increasingly clear that a better understanding of CKD and its associated outcomes may be achieved by elucidating the genetic contributions to the processes responsible for progressive renal injury leading to end-stage renal disease. Indeed, family aggregation studies, comparisons of incidence rates between different racial or ethnic populations, and segregation analyses suggest that progression of renal disease has a strong genetic component.2–5 However, a great majority of the genome-wide association studies (GWAS)-identified risk alleles for CKD confer only a very small relative risk,6 prompting investigators to look beyond traditional genetics to explain the “missing heritability.”7,8 The recent surge in GWAS certainly has broadened our knowledge of the genetic basis of CKD, often implicating previously unsuspected biological pathways.9–12 The apolipoprotein L, 1 (APOL1) gene is the most successful example of genetic factors influencing predisposition to development of CKD in African Americans, in whom common coding variants confer the largest risk seen to date in CKD genetics.13 However, individual and cumulative effects of other genetic variants are too small to adequately explain the initial estimates of heritability of glomerular filtration rate (GFR). For instance, the Chronic Kidney Disease Genetics (CKDGen) consortium performed a meta-analysis of GWAS data in 67,093 individuals.6 The analyzed 16 variants accounted for only 1.4% of the variation in estimated GFR (eGFR), indicating that other genetic and environmental factors may be related to the risk of CKD. The exploding field of epigenetics increasingly is being explored to explain the gene-environmental interaction in determining complex phenotypes.
In 1942, Waddington14 coined the term epigenetics as the study of the processes by which the genotype gives rise to phenotypes through programmed changes during development. Currently, epigenetics refers to a heritable change in the pattern of gene expression that is mediated by a mechanism specifically not caused by alterations in the primary nucleotide sequence.
GENE–ENVIRONMENT INTERACTION
The epigenome is the interface of genetics and environment, where plasticity of the epigenetic code modifies the rigid genetic code to determine the final phenotype.14,15 Epigenetic changes could modulate disease phenotype by affecting the target gene directly, regardless of sequence variation within the gene. Alternatively, the influence of epigenetic marks on disease phenotype could be through interaction with specific DNA-sequence variants.16,17 Epigenetic states in the cell fluctuate in response to environmental signals and pathologic states, such as diet, exercise, inflammation, oxidative stress, metabolic changes, and toxins.18–20 Because the gene itself is not mutated by methylation and the chromatin is not irreversibly changed, epigenetic changes are potentially reversible.21,22 Most importantly, these modifications are amenable to simple measures such as lifestyle modifications and nutrient supplementation.21,23–25 Thus, the study of epigenetics provides a unique opportunity for implementing personalized medicine.
Epigenetic modifications are important in the pathogenesis of CKD, with a multitude of studies illustrating the role of DNA methylation, histone modifications, and RNA interference in genes important to normal kidney function that can be modified and lead to disease initiation and progression. This review provides an overview of epigenetics modifications important in the progression of CKD.
EPIGENETIC MACHINERY
Dynamic chromatin remodeling processes are required for the initial step in gene expression, which is regulated by epigenetic processes including DNA methylation, histone modifications, and the action of small noncoding RNAs (Fig. 1).
Figure 1. Epigenetic modifications.
(I) DNA methylation: Methylation of the cytosine residues at CpG sites is catalyzed by DNA methyltransferases, which provide a unique epigenetic signature that regulates chromatin organization and gene expression. Methylation of cytosines in CpG dinucleotides is associated with inactive, condensed states of the chromosome. (II) Histone modifications: DNA strands are wrapped around histone cores to form nucleosomes. Nucleosome spacing in an open structure that is accessible to nuclear factors is maintained in part by posttranslational modification of histone tails, including lysine acetylation and specific lysine methylation. (III) miRNA interference: miRNAs are transcribed by RNA polymerase II (PolII) into the primary miRNAs (pri-miRNAs), which are processed by RNase III Drosha with its partner DGCR8 microprocessor complex subunit into the precursor miRNAs (pre-miRNAs). Pre-miRNAs are then exported by the nuclear export factor Exportin 5 into the cytoplasm. In the cytoplasm the pre-miRNAs are further processed by Dicer, another RNase III, into mature miRNAs. The miRNA strand is incorporated into the RISC complex and targets complementary mRNA, either cleaving or translational repressing the mRNA.
Histone Modifications
Chromatin is composed of nucleosomes, which are densely packed DNA bound to an octamer of histones and other proteins. These nucleosomes allow for DNA to be packed tightly into the nucleus and provide much-needed regulation of expression and replication of the DNA. Histones themselves can undergo several different types of modifications, including acetylation, methylation, ubiquitylation, sumoylation, and ribosylation.26 The amino-termini tails of the four core histones, H2A, H2B, H3, and H4, undergo methylation and acetylation, which cause changes to the structure of chromatin and DNA accessibility, controlling gene expression. Generally, DNA is transcriptionally active when histones are acetylated and unmethylated, whereas deacetylated and methylated histones block gene expression.7 The regulation of this histone code controls regulation of expression, and these epigenetic patterns are inherited from one generation to the next.
RNA Interference
The 2006 Nobel Prize in Physiology and Medicine was awarded to Fire and Mello for the discovery of microRNAs (miRNAs).27 miRNAs are small noncoding molecules that affect the expression of genes at the post-translational level. They are 20 to 22 nucleotides in length and silence genes from being expressed into protein by one of two different mechanisms: cleavage and degradation or inhibition of translation.28 Hundreds of discrete regions within the genome are transcribed by RNA polymerase II as pri-miRNA species. The molecules start out as a hairpin double-stranded loop of RNA that is transported out of the nucleus and then cleaved by the RNase III known as Dicer. This remaining double-stranded miRNA binds to the proteins in the RNA-induced silencing complex, which aids the miRNA to find complementary pieces of mRNA.29 Once it locates its mRNA targets, they are either cleaved and degraded, or the complex blocks the translational mechanisms and prevents protein formation, thereby silencing gene expression.
DNA Methylation
Methylation of the DNA occurs at the 5-cystine of CpG dinucleotides. These CpG dinucleotides are found most commonly in CpG islands, which are located in the first exons or near the promoters of genes. There are around 45,000 CpG islands (15% of genomic CpG sites), which account for only 1% of the genome and contain 50% of the unmethylated DNA.30 Methylation stops expression of genes through a number of mechanisms, including altering the arrangement and position of chromatin, blocking transcription factors from accessing the DNA, or recruiting repressors to the site of methylation.7 There is also cross-talk that can occur between histone modifications and DNA methylation. This happens when DNA methylation occurs where an existing histone modification has taken place, strengthening the signal and keeping gene expression repressed.31
DETERMINING EPIGENETIC MODIFICATIONS
A number of techniques are available for the researcher to determine epigenetic changes. DNA methylation is the most commonly studied, using both epigenome-wide and smaller-scope methods. Bisulfite conversion is necessary to prepare the DNA for both whole-genome methylation studies and pyrosequencing. By using this method, unmethylated and methylated cytosines can be distinguished by treating DNA with sodium bisulfite, which converts unmethylated cytosines to uracil.32 During a subsequent DNA amplification, uracil will be replaced with a thymine, therefore methylated cytosines are identified by preservation of cytosine at a particular nucleotide position, whereas unmethylated cytosines appear as substitutions from C to T. Bisulfite treatment is followed by polymerase chain reaction (PCR) amplification to enrich the DNA. Pyrosequencing is often the method chosen for gene-specific methylation, and also can be used for validation of methylation patterns identified from epigenome-wide methylation studies.33 This method measures the ratio of T and C at each CpG position from bisulfite-converted DNA that is PCR-amplified.
The most widely used method for epigenome-wide association studies (EWAS) are array-based technologies, which use bisulfite-converted DNA and allow high coverage of the genome, but sequencing and other types of technologies also can be used (see Rakyan et al34 for an overview). The Illumina 450K Inflnium Methylation BeadChip (San Diego, CA) is the gold standard for EWAS, with more than 450,000 CpG sites analyzed at single base-pair resolution with a high throughput of 12 samples per chip.35 This is an improvement over the HumanMethylation27 (Illumina; San Diego, CA) BeadChip (>27,000) because of its increased coverage and number of samples that can be run at one time.
Novel analytical techniques now also are available, taking into account what is known about effect sizes for methylation, power analysis, and adjusting for confounding factors such as the environment. Methylation odds ratios are a way to determine effect size for DNA methylation in case-control studies and are more effective than methylation rates when determining power and sample size using logistical regression.34 A Bayesian approach to analysis may be more suitable for EWAS than P value ranking because it takes power into account, but little is known to give prior distributions of effect size. In addition to binary analysis methods, profile analysis using tissue-specific methylation may serve as a useful tool to classify methylation of CpG islands through unbiased clustering of attributes of the CpG (ie, evolutionary conservation, structural, and physicochemical properties, and other specific attributes) to identify CpGs that have tissue-specific methylation or that constitutively are methylated or unmethylated, and to identify significant attributes of methylated CpGs that function in control of gene expression.36 However, EWAS studies are still lacking accepted guidelines of analysis in reference to what qualifies as epigenome-wide significance and how to interpret differences in methylation patterns for single cytosine-guanine (CG) dinucleotides versus correlated groups of CGs.37
To identify if nucleosomes contain a particular modified histone, the associated DNA can be enriched using antibodies (chromatin immunoprecipitation [ChIP]). When incubated with fragmented chromatin, antibodies against a given histone modification can bind nucleosomes containing the modification of interest and facilitate detection of the DNA associated with the nucleosomes by quantitative PCR, microarray, or sequencing.38 ChIP followed by high-throughput sequencing is referred to as ChIP-seq, which identifies specific DNA sequences where proteins are bound.20,39 This method has enormous power to characterize histone modifications for genes and associate it to their expression status.38
miRNAs can be identified from a RNA sample using microarray analysis.40 For more specific studies of a few miRNA, quantitative real-time PCR methods can be used to examine expression.41 With the decreasing price of whole-genome studies, global studies of miRNA expression are best performed using high-throughput sequencing. As seen in methylation studies, validation of important miRNAs is necessary and can be accomplished using real-time PCR.
TISSUE SPECIFICITY OF EPIGENETIC MODIFICATION: IMPLICATIONS IN RESEARCH
The type of sample used for methylation association studies for disease has been a source of controversy. Typically, easily accessible samples, such as blood or buccal samples, have been used to obtain a DNA sample in large epigenomic studies. However, it has been suggested that epigenetic changes that cause disease are tissue specific. Differences in methylation patterns within tissues have been identified, and are larger between tissues in the same individual than within the same tissue between different individuals.42 However, another study illustrated that the methylation patterns in 11 somatic tissues studies in the Human Epigenome Project are highly correlated.43 Epidemiologic studies have shown an association between demographic, environmental, and behavioral risk factors with both global and gene-specific DNA methylation in peripheral blood mononuclear cells.44,45 Blood sample composition, taking into consideration the different cell types in blood, did not account for the variation in DNA methylation when comparing individuals. Talens et al46 also showed that DNA methylation measured in blood was a marker for methylation patterns in buccal cells. Although strong circumstantial evidence indicates an association between complex disease phenotypes and epigenetic modifications, currently there is only limited direct evidence linking epigenetic changes in peripheral blood mononuclear cells to complex systemic diseases.47 Thus, this question could be answered only by launching pioneering exploratory studies.
EPIGENETICS OF INFLAMMATION
Inflammation is part of the complex biological response to injury, infection, ischemia, and autoimmune diseases.48 Within physiological limits, the inflammatory response enables removal of the inciting agent and initiates the healing process. Unregulated chronic systemic inflammation is associated with increased morbidity and mortality.49 Proinflammatory cytokines are recognized as promoters of progression of kidney disease.50–52 Indeed, Gupta et al53 reported significant associations between indicators of inflammation and eGFR and cystatin C in Chronic Renal Insufficiency Cohort Study participants. We are beginning to comprehend the complexity of epigenetic programming in determining the acute inflammatory response and its intensity as well as its transition to chronic phase. Epigenetic regulation of cytokines and transcription factors have been shown to be important in directing lineage differentiation of Th1 and Th2, as well as regulatory T cells, which determines the immune response.54,55 Indeed, epigenetic effects seem to allow dividing immune cells to imprint, signaling events that allow the immune cells to mount appropriate immune response.56 Immunity and inflammation are tightly linked and several studies have shown that inflammation levels are controlled by epigenetic mechanisms as well.57–59
One of the first studies in CKD patients showed that those individuals with larger amounts of inflammation have lower ratios of HpaII/MspI, illustrating that inflammation is correlated with global DNA methylation.60 Acute inflammation also is controlled by epigenetic mechanisms, which allows the cell to sense its environment and activate proinflammatory genes, mostly through the activity of Toll-like receptors and nuclear factor-κB (NF-κB) pathway signaling. For example, tumor necrosis factor-α expression is activated by remodeling a nucleosome, via Toll-like receptor signaling, over a NF-κB binding site in its promoter.58 NF-κB also is involved in activating proinflammatory responses by inducing histone acetylation and/or deacetylation.57 Villeneuve et al61 also illustrated that decreased H3 lysine-9 trimethylation in inflammatory gene promoters increased inflammatory gene expression in normal human vascular smooth muscle cells exposed to high glucose levels. Several miRNAs have been implicated in epigenetic control of inflammation, including miRNA (miR)-155 miR-146, mi-150, miR-181a, and miR-223.59 These results illustrate the importance in epigenetic control over immunity and the inflammatory response.
EPIGENETIC MODIFICATIONS AND PROGRESSION OF CKD
It is obvious that CKD shares many features of epigenetics-related diseases such as: (1) a heritability that could not be fully explained by strict genetic inheritance patterns; (2) evidence of the influence of environmental exposure; and (3) an increase in prevalence with aging.62,63 Once a critical proportion of nephrons are lost, nonspecific glomerular and tubulointerstitial scarring result from activation of a common pathway of mechanisms.64 A variety of cytokines, chemokines, and growth factors act in concert to create an imbalance in matrix formation and degradation, which lead to overall accumulation of extracellular matrix and eventually glomerulosclerosis and interstitial fibrosis.64–66 Indeed, emerging findings emphasize that epigenetic changes are key to progression of kidney disease and eventual glomerular and tubulointerstitial fibrosis.67–69
EPITHELIAL MESENCHYMAL TRANSITION
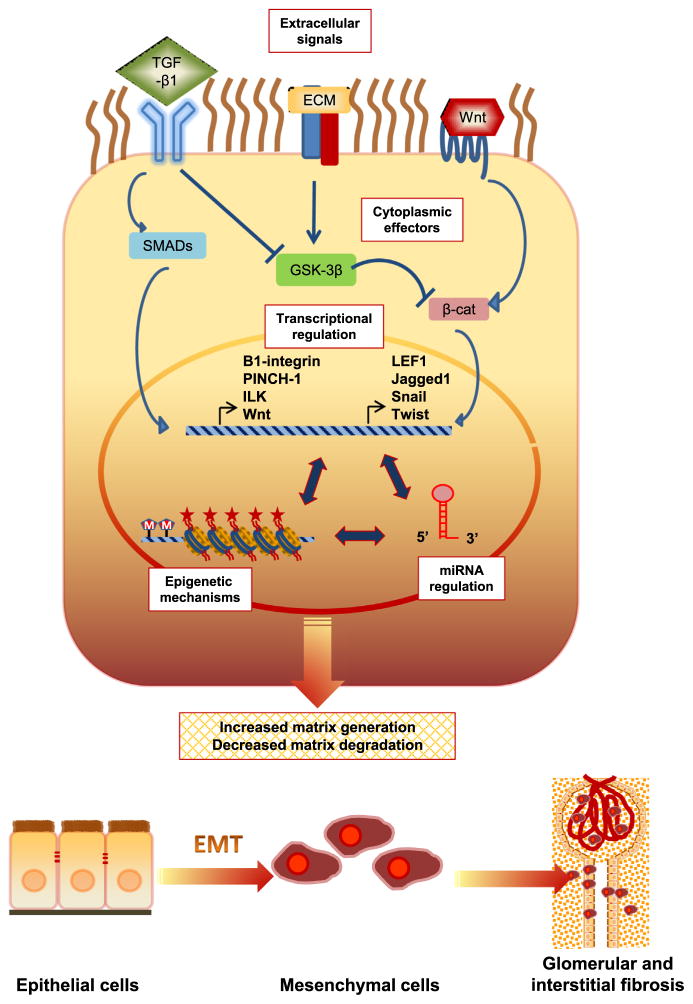
Irrespective of the initial causes, progressive CKD often results in glomerulosclerosis and/or tubulointerstitial fibrosis, characterized by widespread tissue scarring leading to end-stage renal disease. Current evidence indicates that during the disease process more than 30% of the fibroblasts originate from the tubular epithelia at the site of injury through the process of epithelial mesenchymal transition (EMT).70 This molecular reprogramming of the cell allows a fully differentiated epithelial cell to assume the phenotype of matrix-producing fibroblasts and myofibroblasts. Various profibrotic growth factors including transforming growth factor (TGF), fibroblast growth factor-2, connective tissue growth factor, and angiotensin II have been shown to facilitate the process of EMT.71 In response to these autocrine and/or paracrine signals, the tubular cells undergo down-regulation of their epithelial and up-regulation of a mesenchymal genetic program, resulting in loss of epithelial proteins such as E-cadherin, zonula occludens-1, and cytokeratin, and acquisition of mesenchymal markers such as vimentin, matrix metallopeptidase-2, fibroblast-specific protein-1, type I collagen, and fibronectin (Fig. 2).72,73 The cells also show phenotypic changes, loss of epithelial cell adhesion and polarity, actin reorganization and α-smooth muscle actin (SMA) expression, tubular basement membrane disruption, and increased cell migration and invasion.74 This highly complex cellular plasticity is mediated by dynamic control of the transcriptional network (Fig. 3).75
Figure 2. Epithelial Mesenchymal Transition.

The molecular reprogramming of the cell allows a fully differentiated epithelial cell to assume the phenotype of matrix-producing fibroblasts and myofibroblasts. Distinct cell markers characterize these two different phenotypes, as listed below each cell type. Cells that have markers from both epithelial and mesenchymal cells represent an intermediate phenotype as they progress through the the epithelial to mesenchymal transition. Abbreviations: ZO-1, zona occludens 1; MUC1, mucin 1, cell surface associated; miR200, microRNA 200; SIP1, survival of motor neuron protein interacting protein 1; FOXC2, forkhead box C2. Reprinted with permission from Kalluri et al.72
Figure 3. Molecular mechanisms of epithelial-mesenchymal transition during kidney fibrosis.
Numerous mutually reinforcing mechanisms might promote epithelial-mesenchymal transition (EMT). Main intracellular signaling networks such as the transforming growth factor (TGF)-β/Smad, integrin/integrin-linked kinase (ILK), and Wnt/ β-catenin regulate podocyte and tubular EMT during kidney fibrosis by increasing the activity of several transcriptional factors: (1) the repressors in the Twist and Snail families, which repress E cadherin and other epithelial cell adhesion proteins, and (2) inducers of mesenchymal proteins. Epigenetic mechanisms can also promote EMT, whereas certain miRNAs may act to maintain epithelial status. Depicted at the bottom is the transition of epithelial cells into mobile mesenchymal cells which could give rise to fibroblasts and cause glomerular and interstitial fibrosis.
Emerging evidence indicates that dynamic epigenetic modifications orchestrate the process of EMT (Table 1). For instance, in a cell model of TGF-β–mediated EMT, global reduction of the heterochromatin (H3-lys9 dimethylation) marks, increase of the euchromatin (H3-lys4 trimethylation) marks, and increase of the transcriptional (H3-lys36 trimethylation) marks was evident.76 By targeting ZEB1 and ZEB2, two important transcriptional repressors of genes regulating cell adherence and polarity, miR-200 regulates the EMT process.77 We also have shown several genes with differential methylation that have a potential function in EMT when comparing rapid progressors with those with stable kidney function, as measured by an eGFR slope of at least two consecutive measures. This included transcription factor 3 (TCF3), in which suppression of the gene leads TGF-β–controlled EMT changes, such as α-SMA expression78 and IQ motif and Sec7 domain 1 (IQSEC1), which is involved in the regulation of cell adhesion and actin cytoskeleton changes through E-cadherin and α-catenin79 (unpublished data). Thus, transcriptional regulation by epigenetic modifications is key to the cellular phenotypic transformation in EMT.
Table 1.
Significant Epigenetic Modifications Implicated in Chronic Kidney Disease
| Epigenetic Modification | Modification Type | Tissue Type | Description | Reference |
|---|---|---|---|---|
| EMT | ||||
| miR-200 | Methylation | Human | Hypermethylation promotes EMT phenotype through targeting of transcriptional repressors ZEB1 and ZEB2 | Davalos et al,77 2012 |
| H3K9Me2, H3K4Me3, HeK36Me3 | Histone modification | Mouse | EMT mediated by TGF-β caused reduction in H3K9Me2, increase in H3K4Me3, increase in HeK36Me3 | McDonald et al,76 2011 |
| Renal fibrosis | ||||
| miR-192 | MicroRNA | Mouse and rat | Up-regulated in kidney disease models with activation of TGF-β and Smad3 signaling | Kato et al,86 2007; Chung et al,87 2010 |
| miR-21 | MicroRNA | Mouse | Up-regulated in TGF-β and Smad3 signaling with profibrotic genes collagen I, fibronectin, and α-SMA | Zhong et al,88 2011 |
| miR-29 | MicroRNA | Mouse | Down-regulated in kidney disease models; up-regulation inhibits TGF-β activation of collagens I and II | Qin et al,66 2011 |
| RASAL1 | Methylation | Mouse | Hypermethylation activates fibroblasts and fibrogenesis in the kidney | Bechtel et al,67 2010 |
| Histone H3-lysine (H3K4me1, H3K4me2, H3K4me3) | Histone modification | Rat mesangial cells | Methylation increases expression of extracellular matrix genes (Col1a1, CTGF, and PAI-1) | Sun et al,69 2010 |
| Class I HDAC | Histone modification | Mouse | Inhibition through MS-275 prevents TGF-β signaling and renal fibroblast activation | Liu et al,92 2013 |
| CKD | ||||
| Dicer deletion | Dicer deletion | Mouse podocytes | Deletion caused proteinuria, foot process effacement, and glomerular basement membrane abnormalities | Shi et al,95 2008; Harvey et al,93 2008; Ho et al,94 2008 |
| miR-30 family | Dicer deletion | Mouse podocytes | Genes with miR-30 target sequence are up-regulated | Shi et al,95 2008 |
| miR-200a, miR-200b, miR-141, miR-429, miR-205, and miR-192 | MicroRNA | Human kidney biopsies | Up-regulated in hypertensive nephrosclerosis | Wang et al,97 2010 |
| Amino acid transporters | Histone acetylation and methylation | Mouse | DNA methylation controls kidney-specific expression | Kikuchi et al,98 2010 |
| Klotho | Methylation | Human and mouse renal tubular cells | Hypermethylated when exposed to uremic toxins | Azuma et al,100 2012; Sun et al,99 2012 |
| Nephrin and Neph3 | Methylation | Mouse | Down-regulation leads to NF-κB activation and podocyte injury | Ristola et al,101 2012; Hussain et al,102 2009 |
Abbreviations: Col1a1, collagen 1 alpha 1; CTGF, connective tissue growth factor; H3K9Me2, H3 Lys9 dimethylation; H3K4Me3, H3 Lys4 trimethylation; HeK36Me3, H3 Lys36 trimethylation; H3K4me1, H3 Lys4 methylation; H3K4me2, H3 Lys4 dimethylation; HDAC, histone deacetylase; PAI-1, plasminogen activator inhibitor-1; RASAL1, RAS protein activator–like 1 gene.
EPIGENETIC REGULATION OF RENAL FIBROSIS
As noted earlier, the hallmark of progressive CKD is renal fibrosis. Perhaps the most significantly studied cause for this phenotypic change is through the expression and activity of TGF-β.74 This molecule acts through its downstream targets by activating type I TGF-β receptor by binding to type II TGF-β receptor. Type I TGF-β receptor phosphorylates receptor-regulated Smad2 and Smad3, which binds to Smad4 and translocates to the nucleus to bind and activate its target genes.80 Bone morphogenic protein-7 is another member of the TGF family, but it act as an inhibitor of TGF-β signaling and EMT.
MicroRNAs
Multiple miRNAs have been identified that contribute to renal fibrosis (Table 1).81–83 Among these, miR-192 is one of the most studied miRNAs, which has a role in controlling expression of profibrotic genes.84–86 Investigators have shown that miR-192 expression is up-regulated in a mouse model of unilateral ureteral obstruction and a rat model of remnant kidney disease with activation of TGF-β and Smad3 signaling.87 Furthermore, knockdown of miR-192 prevents TGF-β–mediated collagen matrix and collagen I expression, which is involved in renal fibrosis.87 In response to TGF-β and Smad3, an increase in expression of miR-21 and profibrotic genes collagen I, fibronectin, and α-SMA expression was noted.88 To attenuate fibrosis, counterbalancing miRNAs also were activated. Qin et al66 showed that wild-type mice with obstructive nephropathy had reduced expression of miR-29 and progressive renal fibrosis, whereas Smad3 knockout mice had up-regulation of miR-29 without renal fibrosis in mice with obstructive nephropathy. In vitro experiments in renal tubular cells showed that miR-29b overexpression inhibited TGF-β up-regulation of collagens I and III. Smad7 also is involved in controlling the TGF-β/Smad3-regulated miRNAs, and its overexpression protects the kidney from renal fibrosis by suppressing expression of miR-192 and miR-21, and restoring expression of miR-29b.89
DNA Methylation
Bechtel et al67 investigated genes that had differential methylation patterns in fibrosis by comparing the methylation profile of fibroblasts from fibrotic kidneys and nonfibrotic kidneys. They noted that hypermethylation of the RAS protein activator–like 1 gene was associated with activation of fibroblasts and fibrogenesis in the kidney (Table 1). It recently was shown that lack of clusterin expression in unilateral ureteral obstruction mice had increased expression of plasminogen activator inhibitor-1, type I collagen, and fibronectin in conjunction with renal fibrosis (Table 1).90 Clusterin also decreased the activity of Smad3 by inhibiting its phosphorylation and transport into the nucleus, therefore suppressing expression of plasminogen activator inhibitor-1, type I collagen, and fibronectin. Studies have shown that clusterin expression is controlled by DNA methylation in cancer, but this has yet to be shown in kidney disease.91
Histone Modification
Histone modifications also could influence expression and regulation of pathways known to mediate CKD and fibrosis (Table 1). For example, Sun et al69 showed that TGF-β increases histone H3 lysine methylation (H3K4me1, H3K4me2, and H3K4me3), which increased expression of extracellular matrix gene connective tissue growth factor, collagen-1α1, and plasminogen activator inhibitor-1 in mesangial cells. In addition, blocking class I histone deacetylates through a selective class I histone deacetylase inhibitor MS-275 lead to inhibition of TGF-β signaling and blocks renal fibroblast activation.92
EVIDENCE FOR THE ROLE OF DNA METHYLATION IN CKD
The importance of microRNAs in the regulation of kidney function and disease process was shown by three independent investigators, who showed that podocyte-specific genetic ablation of Dicer resulted in proteinuria, foot process effacement, and glomerular basement membrane abnormalities progressing to glomerulosclerosis and tubulointerstitial fibrosis.93–95 Several miRNAs that are important in normal kidney function have been identified, especially the miR-30 family (miR-30c-1, miR-30b, miR-30d, and miR-30c-2).95 The miRNAs that have high expression levels in the kidney include miR-192, miR-194, miR-204, miR-215, and miR-216.96 Studies in kidney disease have shown that miRNA play a role in the development of kidney disease (Table 1). Renal biopsy specimens from hypertensive nephrosclerosis patients have enrichment of miR-200a, miR-200b, miR-141, miR-429, miR-205, and miR-192 expression.97 Expression was correlated with severity of disease, with the highest expression associated with worse kidney disease.
Aberrant methylation or demethylation of specific genes involved in normal kidney function can lead to abnormal functioning and cause progression of CKD (Table 1). One key class of transporters that are expressed in the kidney includes those from the solute carrier family. Kikuchi et al98 investigated more than 282 mouse solute carriers and showed that the expression of amino acid carriers are regulated by epigenetic mechanisms, including histone acetylation and methylation. Klotho is an anti-aging gene involved in renal phosphate handling and resistance to oxidative stress. Sun et al99 explored the regulation of Klotho expression in uninephrectomized B-6 mice and HK2 cells exposed to uremic toxins. The toxins caused up-regulation of DNA methyltransferase and hyper-methylation of Klotho. This gene is expressed in mouse and human renal tubular cells and its expression has been shown to be controlled by methylation.100 Recent findings indicated that nephrin and Neph3 are regulated through methylation.101 Down-regulation of nephrin leads to NF-κB activation and podocyte injury in mice.102
FUTURE DIRECTIONS
We have summarized the literature, which shows that epigenetic mechanisms are involved in the progression of CKD. Studying epigenetic changes in disease is important because these patterns of methylation, histone modifications, and miRNA expression are potentially reversible. For example, blocking TGF-β directly has been unsuccessful. Therefore, identifying downstream targets in the TGF-β and Smad signaling pathways, such as miRNA, is important. Two studies have shown that ultrasound microbubble-mediated gene transfer in mice is an effective way of up-regulating or down-regulating miRNA involved in induction or prevention of renal fibrosis. Zhong et al88 developed a gene transfer via miR-21 knockdown and Tet-repressor–expressing plasmids through ultrasound transducer treatment in mice, which drastically reduced miR-21 expression at first and kept it 40% lower than normal levels for 14 days. This prevented renal injury and renal fibrosis in the mouse model. Another study used ultrasound microbubble-mediated miR-29b gene transfer that resulted in increased expression of this miRNA, preventing subsequent renal injury and fibrosis at the time of induction of unilateral ureteral obstruction nephropathy.66 However, expression of α-SMA did not decrease with miR-29b overexpression. Additional studies blocking expression of these and other miRNAs important in the regulation of renal fibrosis are warranted and may prove to be a potent therapeutic target to stop kidney fibrosis in CKD.
In addition to blocking miRNA expression, epigenetic drugs could be developed in the future that act as inhibitors of DNA methylation or histone modifications in kidney disease. Two DNA methyltransferase inhibitor drugs, azacitidine and decitabine, are available and Food and Drug Administration–approved for treatment in cancer by stopping aberrant DNA methylation.103,104 However, epigenetic drugs available now or that currently are being researched in vitro have major limitations, including lack of specificity and efficacy.22,105
CONCLUSIONS
Completion of the International HapMap Project and the recent advances in genotyping technologies has led to a recent surge in GWAS,106 which certainly has broadened our understanding of the genetic basis of CKD, often implicating previously unsuspected biological pathways.10,11 However, individual and cumulative effects of these genetic variants are too small to adequately explain the initial estimates of heritability of CKD. It is becoming obvious that the malleable and environment-dependent heritability seen in complex traits (such as CKD) cannot be fully explained by highly stable DNA sequences. In this context, the partial stability and plasticity of epigenetic regulation make it a logical functional paradigm.107 It is becoming obvious that epigenetic mechanisms are crucial in the regulation of normal kidney function as well as initiation and progression of CKD. Epigenetic changes represent reversible genomic alterations that are key to progression of renal disease and eventual glomerular and tubulointerstitial fibrosis.67,68 Thus, understanding of epigenetic modifications in the progression of CKD could be of great significance in predicting the pace of disease progression, implementing targeted therapeutic strategies in preventing the progression of CKD, and providing an effective treatment of CKD-related complications.7
Acknowledgments
Financial support: Supported by a National Institutes of Health grant (R01 DK073665-01A1 to D.S.R.), National Heart, Lung and Blood Institute grants (268200900040C and 1R01 HL107241-02 to D.S.R. and M.R.W.), and a National Institute for Diabetes and Digestive and Kidney Diseases National Institutes of Health grant (K01DK082646 to H.S.G.).
Footnotes
Conflict of interest statement: none.
References
- 1.Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 2.Bowden DW. Genetics of kidney disease. Kidney Int Suppl. 2003;83:S8–12. doi: 10.1046/j.1523-1755.63.s83.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Lei HH, Perneger TV, Klag MJ, Whelton PK, Coresh J. Familial aggregation of renal disease in a population-based case-control study. J Am Soc Nephrol. 1998;9:1270–6. doi: 10.1681/ASN.V971270. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–7. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 5.Freedman BI, Spray BJ, Tuttle AB, Buckalew VM., Jr The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis. 1993;21:387–93. doi: 10.1016/s0272-6386(12)80266-6. [DOI] [PubMed] [Google Scholar]
- 6.Kottgen A, Pattaro C, Boger CA, Fuchsberger C, Olden M, Glazer NL, et al. New loci associated with kidney function and chronic kidney disease. Nat Genet. 2010;42:376–84. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwivedi RS, Herman JG, McCaffrey TA, Raj DS. Beyond genetics: epigenetic code in chronic kidney disease. Kidney Int. 2011;79:23–32. doi: 10.1038/ki.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox CS, Yang Q, Cupples LA, Guo CY, Larson MG, Leip EP, et al. Genomewide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: the Framingham Heart Study. J Am Soc Nephrol. 2004;15:2457–61. doi: 10.1097/01.ASN.0000135972.13396.6F. [DOI] [PubMed] [Google Scholar]
- 10.Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41:712–7. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers JC, Zhang W, Lord GM, van der Harst P, Lawlor DA, Sehmi JS, et al. Genetic loci influencing kidney function and chronic kidney disease. Nat Genet. 2010;42:373–5. doi: 10.1038/ng.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman BI, Bowden DW, Rich SS, Xu J, Wagenknecht LE, Ziegler J, et al. Genome-wide linkage scans for renal function and albuminuria in type 2 diabetes mellitus: the Diabetes Heart Study. Diabet Med. 2008;25:268–76. doi: 10.1111/j.1464-5491.2007.02361.x. [DOI] [PubMed] [Google Scholar]
- 13.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African-Americans. Science. 2010;329:841–5. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science. 2010;330:612–6. doi: 10.1126/science.1191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franks PW, Nettleton JA. Invited commentary: gene x lifestyle interactions and complex disease traits–inferring cause and effect from observational data, sine qua non. Am J Epidemiol. 2010;172:992–7. doi: 10.1093/aje/kwq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson HA, Zelle KM, Fawcett GL, Wang B, Pletscher LS, Maxwell TJ, et al. Genetic, epigenetic, and gene-by-diet interaction effects underlie variation in serum lipids in a LG/ JxSM/J murine model. J Lipid Res. 2010;51:2976–84. doi: 10.1194/jlr.M006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg AP. Epigenetics at the epicenter of modern medicine. JAMA. 2008;299:1345–50. doi: 10.1001/jama.299.11.1345. [DOI] [PubMed] [Google Scholar]
- 18.Luch A. Nature and nurture-lessons from chemical carcinogenesis. Nat Rev Cancer. 2005;5:113–25. doi: 10.1038/nrc1546. [DOI] [PubMed] [Google Scholar]
- 19.Perna AF, Ingrosso D, Galletti P, Zappia V, De Santo NG. Membrane protein damage and methylation reactions in chronic renal failure. Kidney Int. 1996;50:358–66. doi: 10.1038/ki.1996.324. [DOI] [PubMed] [Google Scholar]
- 20.Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat Rev Genet. 2000;1:11–9. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- 21.Ingrosso D, Cimmino A, Perna AF, Masella L, De Santo NG, De Bonis ML, et al. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet. 2003;361:1693–9. doi: 10.1016/S0140-6736(03)13372-7. [DOI] [PubMed] [Google Scholar]
- 22.Ptak C, Petronis A. Epigenetics and complex disease: from etiology to new therapeutics. Annu Rev Pharmacol Toxicol. 2008;48:257–76. doi: 10.1146/annurev.pharmtox.48.113006.094731. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Chang X, Lee J, Cho YG, Zhong X, Park IS, et al. Cigarette smoke induced promoter methylation of single-strand DNA-binding protein 2 in human esophageal squamous cell carcinoma. Int J Cancer. 2011;128:2261–73. doi: 10.1002/ijc.25569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima K, Takeoka M, Mori M, Hashimoto S, Sakurai A, Nose H, et al. Exercise effects on methylation of ASC gene. Int J Sports Med. 2010;31:671–5. doi: 10.1055/s-0029-1246140. [DOI] [PubMed] [Google Scholar]
- 25.Oommen AM, Griffin JB, Sarath G, Zempleni J. Roles for nutrients in epigenetic events. J Nutr Biochem. 2005;16:74–7. doi: 10.1016/j.jnutbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–51. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 28.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 29.Li JY, Yong TY, Michael MZ, Gleadle JM. Review: the role of microRNAs in kidney disease. Nephrology (Carlton) 2010;15:599–608. doi: 10.1111/j.1440-1797.2010.01363.x. [DOI] [PubMed] [Google Scholar]
- 30.Antequera F, Bird A. Number of CpG islands and genes in human and mouse. Proc Natl Acad Sci U S A. 1993;90:11995–9. doi: 10.1073/pnas.90.24.11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science. 2010;330:612–6. doi: 10.1126/science.1191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark SJ, Statham A, Stirzaker C, Molloy PL, Frommer M. DNA methylation: bisulphite modification and analysis. Nat Protoc. 2006;1:2353–64. doi: 10.1038/nprot.2006.324. [DOI] [PubMed] [Google Scholar]
- 33.Tost J, Gut IG. DNA methylation analysis by pyrosequencing. Nat Protoc. 2007;2:2265–75. doi: 10.1038/nprot.2007.314. [DOI] [PubMed] [Google Scholar]
- 34.Rakyan VK, Down TA, Balding DJ, Beck S. Epigenome-wide association studies for common human diseases. Nat Rev Genet. 2011;12:529–41. doi: 10.1038/nrg3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisenberger DJ, Van Den Berg D, Laird PW. Technical report. Los Angeles, CA: University of Southern California, Keck School of Medicine, USC/Norris Comprehensive Cancer Center; 2008. Comprehensive DNA methylation analysis on the Illumina® Infinium® assay platform. [Google Scholar]
- 36.Previti C, Harari O, Zwir I, del VC. Profile analysis and prediction of tissue-specific CpG island methylation classes. BMC Bioinformatics. 2009;10:116. doi: 10.1186/1471-2105-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heijmans BT, Mill J. Commentary: the seven plagues of epigenetic epidemiology. Int J Epidemiol. 2012;41:74–8. doi: 10.1093/ije/dyr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10:669–80. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milosavljevic A. Emerging patterns of epigenomic variation. Trends Genet. 2011;27:242–50. doi: 10.1016/j.tig.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson JM, Parker J, Perou CM, Hammond SM. A custom microarray platform for analysis of microRNA gene expression. Nat Methods. 2004;1:47–53. doi: 10.1038/nmeth704. [DOI] [PubMed] [Google Scholar]
- 41.Fiedler SD, Carletti MZ, Christenson LK. Quantitative RT-PCR methods for mature microRNA expression analysis. Methods Mol Biol. 2010;630:49–64. doi: 10.1007/978-1-60761-629-0_4. [DOI] [PubMed] [Google Scholar]
- 42.De BC, Ramos E, Young JM, Tran RK, Menzel U, Langford CF, et al. Tissue-specific variation in DNA methylation levels along human chromosome 1. Epigenetics Chromatin. 2009;2:7. doi: 10.1186/1756-8935-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan S, Zhang X. CpG island methylation pattern in different human tissues and its correlation with gene expression. Biochem Biophys Res Commun. 2009;383:421–5. doi: 10.1016/j.bbrc.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Borghol N, Suderman M, McArdle W, Racine A, Hallett M, Pembrey M, et al. Associations with early-life socio-economic position in adult DNA methylation. Int J Epidemiol. 2012;41:62–74. doi: 10.1093/ije/dyr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGuinness D, McGlynn LM, Johnson PC, MacIntyre A, Batty GD, Burns H, et al. Socio-economic status is associated with epigenetic differences in the pSoBid cohort. Int J Epidemiol. 2012;41:151–60. doi: 10.1093/ije/dyr215. [DOI] [PubMed] [Google Scholar]
- 46.Talens RP, Boomsma DI, Tobi EW, Kremer D, Jukema JW, Willemsen G, et al. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB J. 2010;24:3135–44. doi: 10.1096/fj.09-150490. [DOI] [PubMed] [Google Scholar]
- 47.Talens RP, Jukema JW, Trompet S, Kremer D, Westendorp RG, Lumey LH, et al. Hypermethylation at loci sensitive to the prenatal environment is associated with increased incidence of myocardial infarction. Int J Epidemiol. 2012;41:106–15. doi: 10.1093/ije/dyr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. [erratum appears in N Engl J Med 1999;340:1376] N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 49.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–44. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ortiz A, Gomez-Chiarri M, Alonso J, Bustos C, Gomez-Guerrero C, Lopez-Armada MJ, et al. The potential role of inflammatory and fibrogenic cytokines in the glomerular diseases. J Lipid Mediat Cell Signal. 1994;9:55–74. [PubMed] [Google Scholar]
- 51.Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–8. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- 52.Rao M, Wong C, Kanetsky P, Girndt M, Stenvinkel P, Reilly M, et al. Cytokine gene polymorphism and progression of renal and cardiovascular diseases. Kidney Int. 2007;72:549–56. doi: 10.1038/sj.ki.5002391. [DOI] [PubMed] [Google Scholar]
- 53.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–46. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 55.Janson PC, Winerdal ME, Winqvist O. At the crossroads of T helper lineage commitment-epigenetics points the way. Biochim Biophys Acta. 2009;1790:906–19. doi: 10.1016/j.bbagen.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Reiner SL. Epigenetic control in the immune response. Hum Mol Genet. 2005;14:R41–6. doi: 10.1093/hmg/ddi115. [DOI] [PubMed] [Google Scholar]
- 57.Backdahl L, Bushell A, Beck S. Inflammatory signalling as mediator of epigenetic modulation in tissue-specific chronic inflammation. Int J Biochem Cell Biol. 2009;41:176–84. doi: 10.1016/j.biocel.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 58.McCall CE, El GM, Liu T, Vachharajani V. Epigenetics Yoza B. bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J Leukoc Biol. 2011;90:439–46. doi: 10.1189/jlb.0211075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18:131–40. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Stenvinkel P, Karimi M, Johansson S, Axelsson J, Suliman M, Lindholm B, et al. Impact of inflammation on epigenetic DNA methylation-a novel risk factor for cardiovascular disease? J Intern Med. 2007;261:488–99. doi: 10.1111/j.1365-2796.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- 61.Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105:9047–52. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennis C. Epigenetics and disease: altered states. Nature. 2003;421:686–8. doi: 10.1038/421686a. [DOI] [PubMed] [Google Scholar]
- 63.Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299:2877–83. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baldwin DS. Chronic glomerulonephritis: nonimmunologic mechanisms of progressive glomerular damage. Kidney Int. 1982;21:109–20. doi: 10.1038/ki.1982.17. [DOI] [PubMed] [Google Scholar]
- 65.Wiggins JE, Patel SR, Shedden KA, Goyal M, Wharram BL, Martini S, et al. NFkappaB promotes inflammation, coagulation, and fibrosis in the aging glomerulus. J Am Soc Nephrol. 2010;21:587–97. doi: 10.1681/ASN.2009060663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, et al. TGF-{beta}/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22:1462–74. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bechtel W, McGoohan S, Zeisberg EM, Muller GA, Kalbacher H, Salant DJ, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med. 2010;16:544–50. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uhlenhaut NH, Treier M. Transcriptional regulators in kidney disease: gatekeepers of renal homeostasis. Trends Genet. 2008;24:361–71. doi: 10.1016/j.tig.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R. Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol. 2010;21:2069–80. doi: 10.1681/ASN.2010060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–50. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 75.Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, et al. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci U S A. 2001;98:6686–91. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDonald OG, Wu H, Timp W, Doi A, Feinberg AP. Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition. Nat Struct Mol Biol. 2011;18:867–74. doi: 10.1038/nsmb.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F, et al. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene. 2012;31:2062–74. doi: 10.1038/onc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang KW. Angiotensin II-mediated Nrf2 down-regulation: a potential causing factor for renal fibrosis? Arch Pharm Res. 2011;34:695–7. doi: 10.1007/s12272-011-0500-x. [DOI] [PubMed] [Google Scholar]
- 79.Hiroi T, Someya A, Thompson W, Moss J, Vaughan M. GEP100/BRAG2: activator of ADP-ribosylation factor 6 for regulation of cell adhesion and actin cytoskeleton via E-cadherin and alpha-catenin. Proc Natl Acad Sci U S A. 2006;103:10672–7. doi: 10.1073/pnas.0604091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meng XM, Chung AC, Lan HY. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 2013;124:243–54. doi: 10.1042/CS20120252. [DOI] [PubMed] [Google Scholar]
- 81.Natarajan R, Putta S, Kato M. MicroRNAs and diabetic complications. J Cardiovasc Transl Res. 2012;5:413–22. doi: 10.1007/s12265-012-9368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes. 2011;60:1832–7. doi: 10.2337/db11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vettori S, Gay S, Distler O. Role of MicroRNAs in fibrosis. Open Rheumatol J. 2012;6:130–9. doi: 10.2174/1874312901206010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, et al. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11:881–9. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol. 2012;23:458–69. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A. 2007;104:3432–7. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung AC, Huang XR, Meng X, Lan HY. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol. 2010;21:1317–25. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong X, Chung AC, Chen HY, Meng XM, Lan HY. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol. 2011;22:1668–81. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung AC, Dong Y, Yang W, Zhong X, Li R, Lan HY. Smad7 suppresses renal fibrosis via altering expression of TGF-beta/Smad3-regulated microRNAs. Mol Ther. 2013;21:388–98. doi: 10.1038/mt.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jung GS, Kim MK, Jung YA, Kim HS, Park IS, Min BH, et al. Clusterin attenuates the development of renal fibrosis. J Am Soc Nephrol. 2012;23:73–85. doi: 10.1681/ASN.2011010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serrano A, Redondo M, Tellez T, Castro-Vega I, Roldan MJ, Mendez R, et al. Regulation of clusterin expression in human cancer via DNA methylation. Tumour Biol. 2009;30:286–91. doi: 10.1159/000259912. [DOI] [PubMed] [Google Scholar]
- 92.Liu N, He S, Ma L, Ponnusamy M, Tang J, Tolbert E, et al. Blocking the class i histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling. PLoS One. 2013;8:e54001. doi: 10.1371/journal.pone.0054001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, et al. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol. 2008;19:2150–8. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ho J, Ng KH, Rosen S, Dostal A, Gregory RI, Kreidberg JA. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol. 2008;19:2069–75. doi: 10.1681/ASN.2008020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi S, Yu L, Chiu C, Sun Y, Chen J, Khitrov G, et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol. 2008;19:2159–69. doi: 10.1681/ASN.2008030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun Y, Koo S, White N, Peralta E, Esau C, Dean NM, et al. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 2004;32:e188. doi: 10.1093/nar/gnh186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang G, Kwan BC, Lai FM, Choi PC, Chow KM, Li PK, et al. Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. Am J Hypertens. 2010;23:78–84. doi: 10.1038/ajh.2009.208. [DOI] [PubMed] [Google Scholar]
- 98.Kikuchi R, Yagi S, Kusuhara H, Imai S, Sugiyama Y, Shiota K. Genome-wide analysis of epigenetic signatures for kidney-specific transporters. Kidney Int. 2010;78:569–77. doi: 10.1038/ki.2010.176. [DOI] [PubMed] [Google Scholar]
- 99.Sun CY, Chang SC, Wu MS. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hyper-methylation. Kidney Int. 2012;81:640–50. doi: 10.1038/ki.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Azuma M, Koyama D, Kikuchi J, Yoshizawa H, Thasinas D, Shiizaki K, et al. Promoter methylation confers kidney-specific expression of the Klotho gene. FASEB J. 2012;26:4264–74. doi: 10.1096/fj.12-211631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ristola M, Arpiainen S, Saleem MA, Holthofer H, Lehtonen S. Transcription of nephrin-Neph3 gene pair is synergistically activated by WT1 and NF-kappaB and silenced by DNA methylation. Nephrol Dial Transplant. 2012;27:1737–45. doi: 10.1093/ndt/gfr576. [DOI] [PubMed] [Google Scholar]
- 102.Hussain S, Romio L, Saleem M, Mathieson P, Serrano M, Moscat J, et al. Nephrin deficiency activates NF-kappaB and promotes glomerular injury. J Am Soc Nephrol. 2009;20:1733–43. doi: 10.1681/ASN.2008111219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fahy J, Jeltsch A, Arimondo PB. DNA methyltransferase inhibitors in cancer: a chemical and therapeutic patent overview and selected clinical studies. Expert Opin Ther Pat. 2012;22:1427–42. doi: 10.1517/13543776.2012.729579. [DOI] [PubMed] [Google Scholar]
- 104.Joeckel TE, Lubbert M. Clinical results with the DNA hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in patients with myelodysplastic syndromes: an update. Semin Hematol. 2012;49:330–41. doi: 10.1053/j.seminhematol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 105.Cantley MD, Haynes DR. Epigenetic regulation of inflammation: progressing from broad acting histone deacetylase (HDAC) inhibitors to targeting specific HDACs. Inflammo-pharmacology. 2013 doi: 10.1007/s10787-012-0166-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 106.Kruglyak L. The road to genome-wide association studies. Nat Rev Genet. 2008;9:314–8. doi: 10.1038/nrg2316. [DOI] [PubMed] [Google Scholar]
- 107.Feinberg AP. Methylation meets genomics. Nat Genet. 2001;27:9–10. doi: 10.1038/83825. [DOI] [PubMed] [Google Scholar]