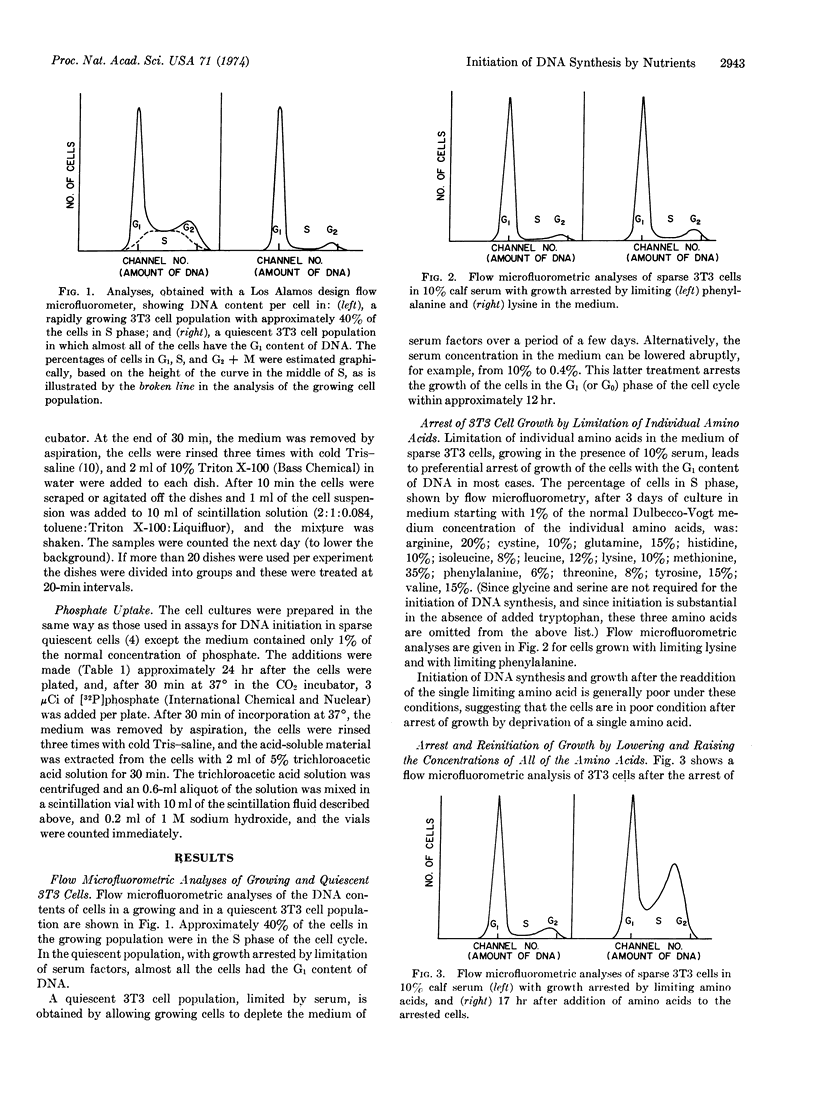
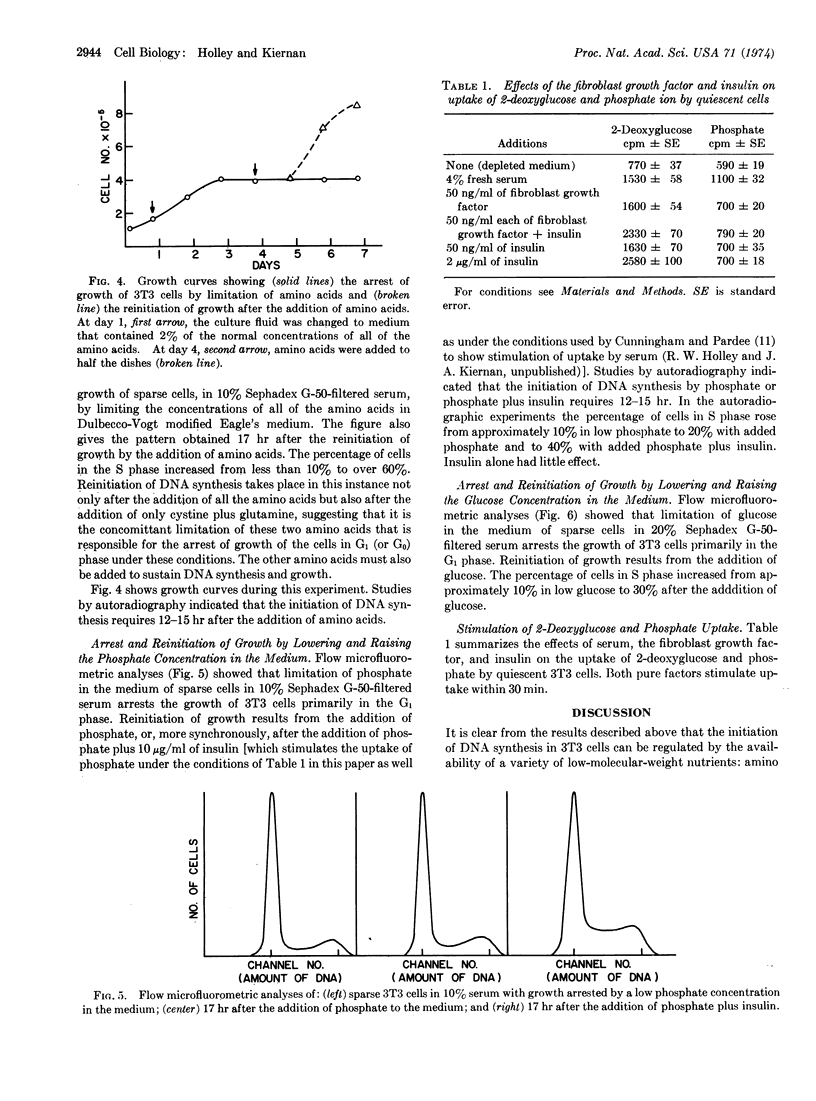
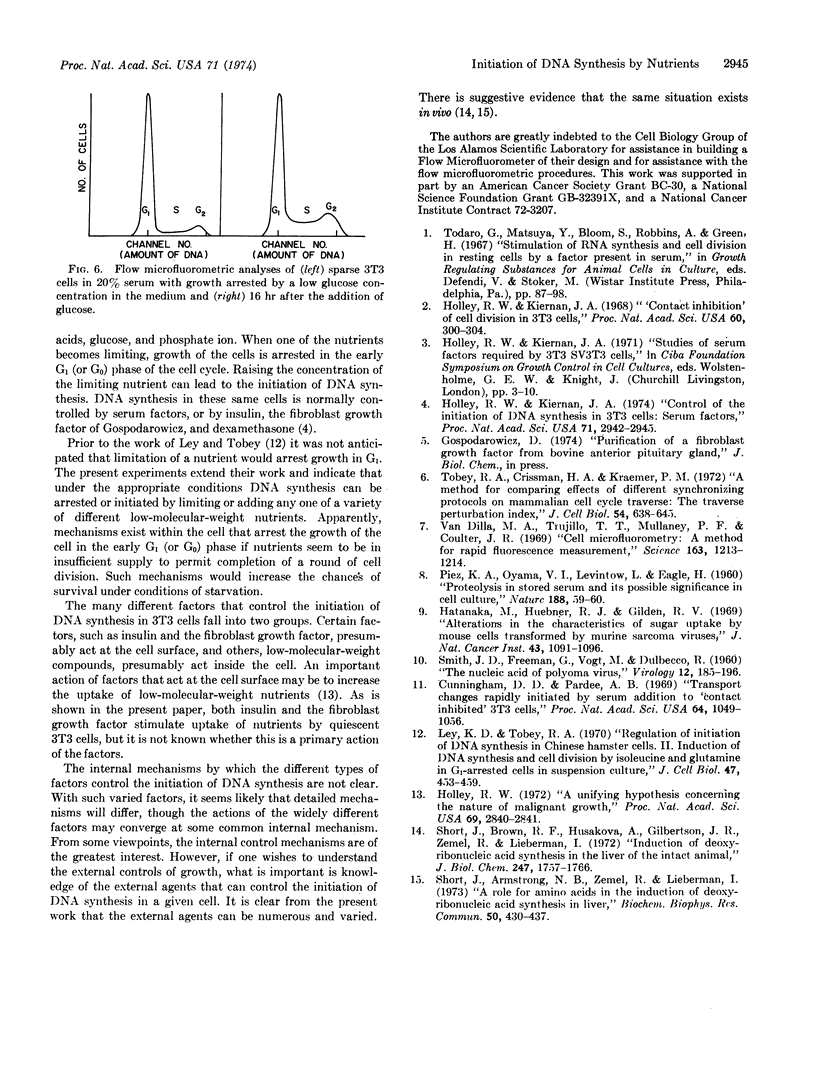
Abstract
Sparse 3T3 cells, in excess serum, become quiescent in the early G1 (or G0) phase of the cell cycle if the cells are cultured in low concentrations of amino acids, glucose, or phosphate ion. These quiescent cells then initiate DNA synthesis if the concentration of the limiting nutrient is increased. In normal medium, DNA synthesis in the same 3T3 cell line is controlled by serum factors. The results demonstrate the complexity of the control by external agents of DNA synthesis in these “normal,” density-dependent cells.
Keywords: amino acids, glucose, phosphate, cell cycle
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cunningham D. D., Pardee A. B. Transport changes rapidly initiated by serum addition to "contact inhibited" 3T3 cells. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1049–1056. doi: 10.1073/pnas.64.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. Alterations in the characteristics of sugar uptake by mouse cells transformed by murine sarcoma viruses. J Natl Cancer Inst. 1969 Nov;43(5):1091–1096. [PubMed] [Google Scholar]
- Holley R. W. A unifying hypothesis concerning the nature of malignant growth. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2840–2841. doi: 10.1073/pnas.69.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIEZ K. A., OYAMA V. I., LEVINTOW L., EAGLE H. Proteolysis in stored serum and its possible significance in cell culture. Nature. 1960 Oct 1;188:59–60. doi: 10.1038/188059a0. [DOI] [PubMed] [Google Scholar]
- Short J., Armstrong N. B., Zemel R., Lieberman I. A role for amino acids in the induction of deoxyribonucleic acid synthesis in liver. Biochem Biophys Res Commun. 1973 Jan 23;50(2):430–437. doi: 10.1016/0006-291x(73)90858-9. [DOI] [PubMed] [Google Scholar]
- Short J., Brown R. F., Husakova A., Gilbertson J. R., Zemel R., Lieberman I. Induction of deoxyribonucleic acid synthesis in the liver of the intact animal. J Biol Chem. 1972 Mar 25;247(6):1757–1766. [PubMed] [Google Scholar]
- Tobey R. A., Crissman H. A., Kraemer P. M. A method for comparing effects of different synchronizing protocols on mammalian cell cycle traverse. The traverse perturbation index. J Cell Biol. 1972 Sep;54(3):638–645. doi: 10.1083/jcb.54.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dilla M. A., Trujillo T. T., Mullaney P. F., Coulter J. R. Cell microfluorometry: a method for rapid fluorescence measurement. Science. 1969 Mar 14;163(3872):1213–1214. doi: 10.1126/science.163.3872.1213. [DOI] [PubMed] [Google Scholar]


