Abstract
We investigated the serum and urine chemokine levels of patients with schistosomal mansoni glomerulonephritis. This cross-sectional study was conducted in the Southeast of Brazil. Overall, 160 subjects were enrolled and divided into five groups: 1) hepatosplenic schistosomiasis with renal disease (N = 12); 2) hepatosplenic schistosomiasis without renal disease (N = 68); 3) hepatointestinal schistosomiasis (N = 27); 4) glomerulopathy caused by other diseases (N = 22); and 5) healthy controls (N = 31). The patients with microalbuminuria > 30 mg in 24 hours were considered to have renal disease. The sera and urine chemokines CCL2, CCL3, CCL5, CCL11, and CXCL8 were measured using an enzyme-linked immunosorbent assay test. A similar profile was observed between the patients with schistosomal glomerulopathy and the patients with glomerulopathy caused by other diseases, with the exception of serum CCL2 ≤ 634.3 pg/mL. In cases with sera CCL2 > 634.3 pg/mL, the diagnosis of schistosomal glomerulopathy should be considered.
Introduction
It has been estimated that 240 million people worldwide are infected by schistosomiasis, and 700 million are at risk of infection. Currently in Brazil, 2–6 million individuals are considered infected.1 Since the start of the Brazilian Schistosomiasis Control Program in 1979, 13 million treatments have been administered.2 Hepatosplenic schistosomiasis, a chronic, severe form of the disease, is the main cause of associated hospital admissions and deaths.3,4 Glomerulonephritis has still been observed in 15% of the patients with hepatosplenic schistosomiasis examined in reference centers for the severe forms of schistosomiasis.5 To our knowledge, there is no recent field work addressing this issue. Even after mass chemotherapy severe conditions, including schistosomal glomerulopathy, have been reported6,7; the high prevalence of glomerulopathy in schistosomiasis and the early detection of renal involvement may interrupt or delay the progression of glomerulopathy to end stage renal disease.8
The chemokine profiles for the early diagnoses of the diseases that evolve with proteinuria and glomerular injury have already been described.9–12 For example, Ibrahim and Rashed13 reported an increase in urine CCL2 in diabetic nephropathy. The CCL2 has also been found in renal tissue and in elevated levels in the sera of patients with a variety of renal diseases.14–16
To our knowledge, no study has addressed the value of chemokine testing in the pathogenesis and/or early diagnosis of schistosomal glomerulopathy. With the aim of identifying the markers of glomerular disease, we investigated the sera and urine chemokine levels in hepatosplenic schistosomiasis that are associated with renal disease.
Materials and Methods
Patients.
This is a cross-sectional analytical study that was conducted from October 2008 to July 2010. Participants were enrolled in the study after signing informed consent forms and submitting to clinical examinations and laboratory tests.
The 160 study participants were divided into 5 groups: 1) hepatosplenic schistosomiasis with schistosomal glomerulopathy (HS + SGN, N = 12); 2) hepatosplenic schistosomiasis mansoni without schistosomal glomerulopathy (HS without SGN, N = 68); 3) hepatointestinal schistosomiasis (HI, N = 27); 4) glomerulopathy of varied causes, without schistosomiasis (GN, N = 22); and 5) the healthy controls (HC, N = 31).
Exclusion from the study.
Patients with visceral leishmaniasis, viral hepatitis C or B, human immunodeficiency virus (HIV) seropositivity, diabetes mellitus, autoimmune diseases, sickle-cell disease, primary cryoglobulinemia, Henoch-Schönlein purpura, visceral abscesses, or neoplasia were excluded from the study.
Sample size.
A pilot study was conducted to estimate the sample size necessary to identify an adequate number of subjects. Using two controls for each case, an α error of 0.05, with the power of the test (1-β error) set at 0.8, 12 patients with schistosomal glomerulopathy were found to be sufficient to reveal the differences in chemokine profiles. In the group with glomerulopathy but without schistosomiasis, only 22 patients were enrolled (1.75 controls per case).
Diagnosis of hepatosplenic schistosomiasis.
The diagnosis was based on the following criteria: epidemiological evidence, contact with stream water from endemic areas, clinical evidence (hepatomegaly and splenomegaly), portal hypertension, esophageal varices diagnosed during upper digestive endoscopy, Schistosoma mansoni eggs in the stools, and ultrasound or magnetic resonance imaging showing characteristic Symmers' fibrosis of the liver and significant portal vein collaterals.17,18 All of the patients had three negative parasitological stool examinations using the Kato-Katz technique,19 and they also reported previous treatment of schistosomiasis ranging 2–5 years before enrollment in the study.
Ultrasound.
An abdominal ultrasound examination was performed by a radiologist who had 8 years of experience in this imaging method using real-time equipment (Aloka SSD 1700, Aloka Co., Japan) with an electronic convex 3.5-MHz transducer, according to the World Health Organization (WHO)17 protocol for ultrasound (US) assessment of schistosomiasis-related morbidity.
Renal function.
After 12 h of oral liquid restriction, an early morning urine sample was collected in a sterile container. Part of the urine was seeded in a culture medium for microbiological study. In another sample, the abnormal elements of the urine were investigated after centrifugation under light microscopy (amplification 400×). One blood cell in the male centrifuged urine and three in the female samples were considered abnormal. The presence of acanthocytes and codocytes (G1 cells) defined the presence of erythrocyte dysmorphism. Dysmorphic cells were investigated by phase-contrast microscopy. Proteinuria was measured in a 24-hour urine sample. Whenever the qualitative test was positive, the sulfosalicylic acid technique or an immunoenzymatic test was performed. Albuminuria > 30 mg/day was considered abnormal. Renal function was evaluated by calculating the glomerular filtration rate (GFR) according to the Modification of Diet in Renal Disease (MDRD) formula: GFR = 170 × serum creatinine-0.999 × age-0.176 × (0.762 if female)20; the serum creatinine used in the formula was measured by a colorimetric method.
Clinical examination.
The clinical examinations were performed by one author (JRL). The arterial blood pressure was measured on two different occasions (1 week apart) by the same physician following the protocol established by Brazilian guidelines for ambulatory monitoring of arterial pressure21; the patients were then instructed on the correct method of collecting a 24-hour urine specimen. In the second visit to the outpatient clinic, 10 mL of venous blood was obtained and stored for laboratory tests.
Laboratory studies.
Routine laboratory tests were performed, including a hemogram (counter Sysmex, E 2100 D), coagulation tests, and liver function (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase, bilirubin, serum albumin) and renal function tests (blood urea nitrogen [BUN], serum creatinine). Antibodies against hepatitis C (anti-HCV) and B (HbsAg and anti-Hbc) were tested. Autoimmune diseases were also investigated; antinuclear antibodies (ANA), protoplasmic-staining anti-neutrophil cytoplasmic antibodies (p-ANCA), and anti-neutrophil cytoplasmic antibodies (c-ANCA) were measured using an indirect immunofluorescent method, and anti-thyroglobulin antibodies were determined. Fasting glycemia and glycohemoglobin levels were measured. The venereal disease research laboratory (VDRL) was measured using a flocculation test. Serum anti-streptolysin O (ASO) and rheumatoid factor were also investigated.
Measurement of chemokine levels in serum and urine.
Peripheral blood and urine were collected from the patients with schistosomiasis and the controls. The blood samples were centrifuged at 1,000 × g for 10 min, and serum was removed and stored at −80°C until the chemokine analysis. The urine was also stored at −70°C until the chemokine analysis. For the chemokine assays, the serum samples were thawed, and the excess proteins were removed using the acid/salt precipitation that is routinely performed in our laboratory. Briefly, equal volumes of serum and 1.2% trifluoroacetic acid/1.35 M NaCl were mixed and left at room temperature for 10 min. The samples were then centrifuged for 5 min at 3,000 × g, and the supernatants were adjusted for salt content (0.14 M sodium chloride and 0.01 M sodium phosphate) and pH (7.4) before determining the chemokine levels. The chemokine concentrations were measured according to the manufacturer's instructions, using sandwich enzyme-linked immunosorbent assay (ELISA) kits for the CCL2, CCL11, CCL3, CXCL8, and CCL5 chemokines (DuoSet, R&D Systems, Minneapolis, MN). The detection limit for these assays was 10 pg/mL. The chemokine concentrations in the urine were measured using the same sandwich ELISA kits. The detection limit for these assays was also 10 pg/mL.
Patients selected for kidney biopsy.
In the hepatosplenic group eight (with nephrotic syndrome) out of 12 patients were submitted to kidney biopsy. In four the diagnosis of glomerular disease was based on microalbuminuria > 30 mg/24 hours. Microalbuminuria has been used as a putative marker of glomerular disease. In such patients biopsy would be important to define the prognosis of the kidney disease. In 22 patients with varied causes of glomerulopathy biopsy was taken from all of them.
Histopathology of kidney fragments.
Three fragments were obtained through a kidney biopsy. One was stored in 10% formaldehyde for light microscopy. Another was frozen in liquid nitrogen for immunofluorescence, and a third fragment was stored in 2.5% glutaraldehyde for electron microscopy. All of the samples were sent to the Department of Pathology of the Triângulo Mineiro Faculty of Medicine for processing and examination.
For light microscopy, the fragments were embedded in paraffin, and 2-μm-thick sections were cut on a microtome and stained with hematoxylin and eosin, Masson's trichrome, silver methenamine and picrosirius. For immunofluorescence, the fragments that were embedded in tissue tech were cut to 2 μm thickness on a cryostat and investigated for deposits of immunoglobulins (IgA, IgG and IgM), kappa and lambda light chains and complement C1q, C3, and fibrinogen. For electron microscopy, the tissue was fixed in Trump's fixative and then routinely processed into resin-embedded blocks; ultrathin sections were stained with uranyl acetate and lead citrate and then examined with a Philips CM100 transmission electron microscope.
Ethical considerations.
This study was approved by the Human Research Ethical Board at the Federal University of Minas Gerais School of Medicine, 369/08.
Statistical analysis.
The questionnaire data were transferred to a databank using EpiData 3.1 (EpiData Association, Odense, Denmark) and analyzed using the Statistical Package for Social Sciences (SPSS) 15.0 (SPSS, IBM Company, Chicago, IL). For multivariate analysis using a logistic regression analysis to compare the five study groups proved impossible.22 Because of multicollinearity, no good convergence was found among the variables. Multicollinearity is a statistical phenomenon in which two or more predictor variables in a multiple regression model are highly correlated. In this situation, the coefficient estimates may change erratically in response to small changes in the model or the data. Because of the observed multicollinearity in our data, a decision tree was used. The decision tree is a non-parametric and predictive regression model and is used as a visual and analytical decision support tool in which the expected values of competing alternatives are calculated23–25; in this study, this model had a good adjustment, with an estimated classification risk of 0.22, meaning that 78% of the data were correctly classified.
Results
Patients.
Of the 160 patients, 31 (55.5%) were male and 87 (54.4%) were non-white. The participants' ages ranged from 29 to 52 years of age (median = 40 years). The systolic and diastolic blood pressure medians were 120 and 80 mm of Hg, respectively. The demographic, clinical, and anthropometric variables for each group are shown in Table 1; the differences observed in Table 1 (bivariate analysis) did not maintain significance in multivariate analysis.
Table 1.
Comparison between the demographic variables of the patients with schistosomal glomerulopathy and the control groups
| Variables | Groups | P† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HS | HS+SGN | HI | GN | HC | |||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Sex | |||||||||||
| Male | 50 | 73.5 | 11 | 91.7 | 12 | 44.4 | 10 | 40.9 | 6 | 19.4 | < 0.001* |
| Female | 18 | 26.5 | 1 | 8.3 | 15 | 55.6 | 12 | 59.1 | 25 | 80.6 | |
| White | 28 | 41.2 | 3 | 25.0 | 0 | 0.0 | 10 | 45.5 | 24 | 77.4 | < 0.001** |
| Mixed race | 36 | 52.9 | 9 | 75.0 | 27 | 100.0 | 12 | 54.5 | 3 | 9.7 | |
| Black | 4 | 5.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 4 | 12.9 | |
| M | IR | M | IR | M | IR | M | IR | M | IR | ||
| Age (years) | 47.5 | 17.7 | 46.5 | 11.5 | 36.0 | 7.0 | 39.0 | 24.3 | 24.0 | 8.0 | < 0.001*** |
| BMI‡ | 23.5 | 4.7 | 23.7 | 3.5 | 23.9 | 5.7 | 24.4 | 9.1 | 22.6 | 4.8 | 0.229*** |
The statistical differences (bivariate analysis) did not maintain significance in multivariate analysis. M = median; IR = interquartile range
Pearson's χ2
Fisher's exact test
Kruskal-Wallis
BMI = body mass index; HS = hepatosplenic; HS + SGN: hepatosplenic with schistosomal glomerulopathy;
GN = glomerulopathy without schistosomiasis; HC = healthy controls.
In the HS+SGN group, the renal biopsy revealed the following diagnoses in 8 of the 12 patients (66.6%): type I membranoproliferative glomerulonephritis (N = 4), mesangioproliferative with segmental sclerosis and deposits of C3 (N = 1), mesangial with deposits of IgA, C1q, and C3 (N = 1), mesangial with segmental sclerosis and deposits of IgM, C3, and C1q (N = 1) and mesangial glomerulonephritis with deposits of IgM, IgG, and IgA (N = 1).
In schistosomiasis the glomerular disease is caused by immune complexes deposited in the basement membrane and/or mesangium (Figures 1 and 2 ).
Figure 1.
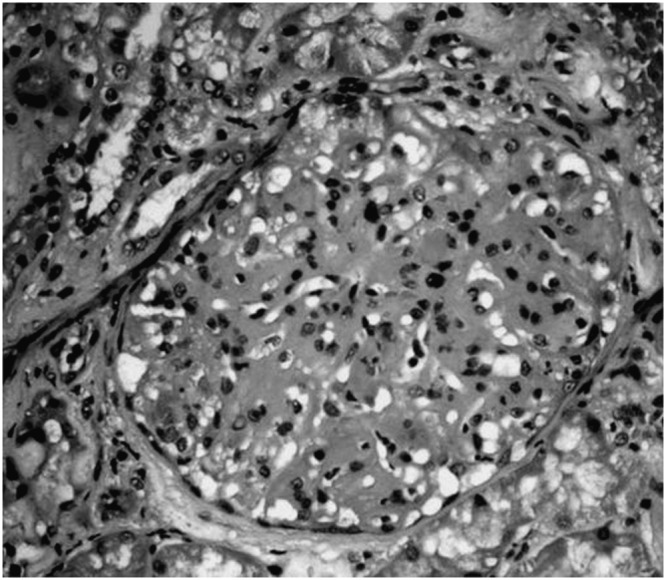
Optic microscopy of a glomerulus stained by hematoxylin-eosin of a patient with type I membranoproliferative glomerulonephritis in hepatosplenic schistosomiasis mansoni.
Figure 2.
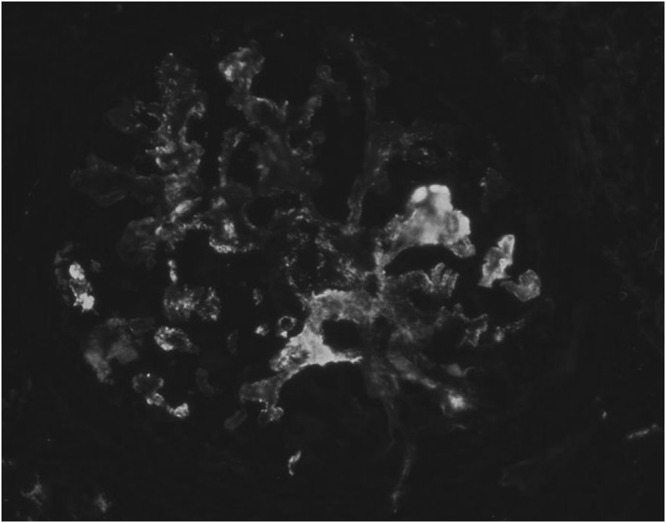
Immunofluorescence showing granular immunoglobulin G (IgG) deposits in the glomerular structure of a patient with type I membranoproliferative glomerulonephritis in hepatosplenic schistosomiasis mansoni.
In the GN group, kidney biopsies revealed the following diagnoses in 22 patients: focal and segmental glomerulosclerosis (N = 7); idiopathic mesangial glomerulopathy (N = 6), diffuse proliferative (N = 2), IgA glomerulopathy (N = 2), systemic lupus erythematosus-associated glomerulopathy (N = 2), diabetes mellitus-associated glomerulopathy (N = 1), minimal change glomerulonephritis (N = 1), and membranous glomerulopathy (N = 1).
Univariate analysis.
Statistically significant differences were observed in all of the analyzed demographic variables (P < 0.05) (Table 1). However, the variables were not identified as confounding because they were not associated with the chemokine profiles or with the study groups. Systemic arterial hypertension was more frequently found in the patients with glomerular disease (HS + SGN group, P < 0.001). No patient in the HC group had systemic arterial hypertension. Microalbuminuria > 30 mg/24 hours was found only in the groups with glomerulopathy. The anthropometric values were not significantly different between the study groups.
Regarding the chemokines, CCL2, CCL11, CCL5, and CCL3 were significantly higher in the urine of the patients with hepatosplenic schistosomiasis and glomerulopathy (HS + SGN). The serum CCL2 and CCL11 were also higher in this group (HS + SGN) (Kruskall-Wallis). The Mann-Whitney U test with a Bonferroni adjustment was used to quantify the difference, the urine CCL2 was shown to be higher in the patients with glomerulopathy (groups HS + SGN and GN) than in the patients from the other three groups (HS without SGN, HI, and HC).
Multivariate analysis.
Decision tree: using this model, the following chemokine profiles were obtained for each group: group 1 (HS + SGN), urine (CCL3 > 14.3 pg/mL, CCL11 > 26.7 pg/mL), and serum (CCL3 > 61.9 pg/mL, CXCL8 ≤ 1030.4 pg/mL, CCL2 > 634.3 pg/mL); group 2 (HS without SGN), urine (CCL3 ≤ 14.2 pg/mL), and serum (CCL2 > 490 pg/mL); group 3 (HI), urine (CCL3 > 14.3 pg/mL) and serum (CCL3 > 61.9 pg/mL and CXCL8 > 40 pg/mL); group 4 (GN), urine (CCL3 > 14.3 pg/mL and CCL11 > 26.7 pg/mL) and serum (CCL3 > 61.9 pg/mL, CXCL8 ≤ 1030.4 pg/mL and CCL2 ≤ 634.3 pg/mL); and group 5 (HC), urine (CCL3 ≤ 14.3 pg/mL) and serum (CCL2 ≤ 490 pg/mL and CCL5 > 11,509.8 pg/mL) (Table 2).
Table 2.
Chemokine profiles obtained using the Decision tree—multivariate analysis
| Groups | |||||
|---|---|---|---|---|---|
| HS + SGN (pg/mL) | HS without SGN, (pg/mL) | HI (pg/mL) | GN (pg/mL) | HC (pg/mL) | |
| Chemokines urinary | |||||
| CCL2 | – | – | – | – | – |
| CCL3 | > 14.3 | ≤ 14.3 | > 14.3 | > 14.3 | ≤ 14.3 |
| CCL5 | – | – | – | – | – |
| CCL11 | > 26.7 | – | – | > 26.7 | – |
| CXCL8 | – | – | – | – | – |
| Chemokines serum | |||||
| CCL2 | > 634.3 | > 490 | – | ≤ 634.3 | ≤ 490 |
| CCL3 | > 61.9 | – | > 61.9 | > 61.9 | – |
| CCL5 | – | – | – | – | > 11,509.8 |
| CCL11 | – | – | – | – | – |
| CXCL8 | ≤ 1030.4 | – | > 40 | ≤ 1030.4 | – |
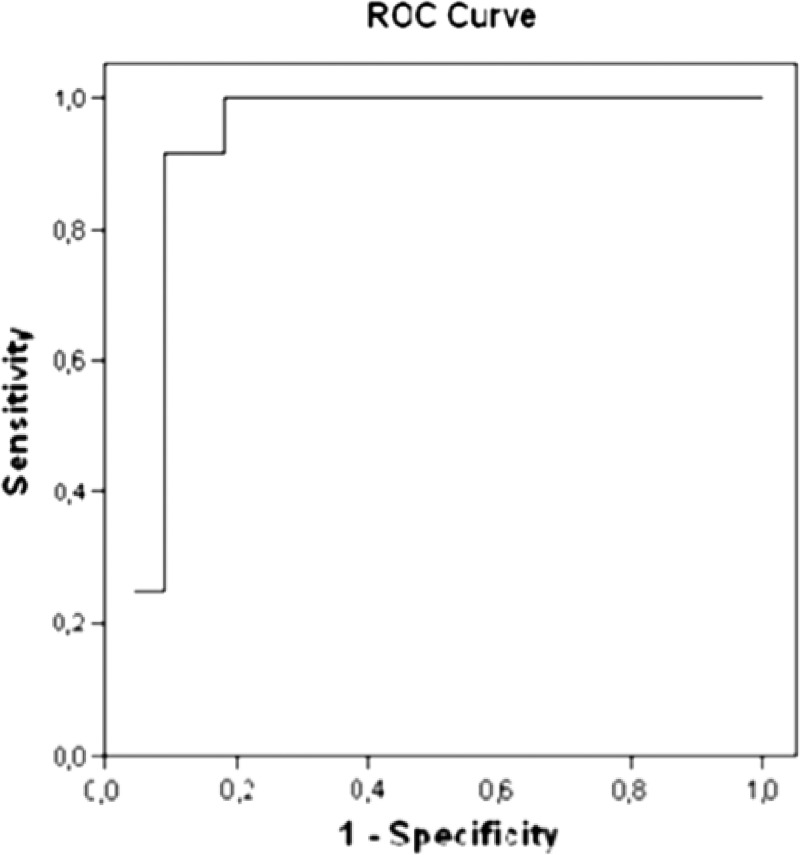
Using the decision tree, a cutoff point of CCL2 > 634.3 pg/mL separated the patients with schistosomal glomerulopathy from other glomerulopathies. To define the sensitivity and specificity of this finding, a receiver operating characteristic curve was built. The curve indicated that schistosomal glomerulopathy occupied an area under the curve of 0.91 (sensitivity and specificity of 90%) (Figure 3).
Figure 3.
Sensitivity and specificity of serum levels of CCL2 > 634.3 pg/mL to diagnosis schistosomal glomerulopathy.
Discussion
Previous chemokine studies have evaluated the roles of isolated chemokines in the pathogenesis and diagnosis of inflammatory diseases, including those associated with renal injury.13,26–28 For a comprehensive view of the inflammatory process, the evaluation of a chemokine profile appears to be more appropriate. This view has been advocated by many authors.29–31
In previous studies, the chemokines were measured but then categorized as low or high; thus, the levels were neither presented (in pg/mL) nor compared with the controls.28,32 Here, in five groups, we compared the urine and serum values of the chemokines (measured in pg/mL). Additionally, the particular cluster of chemokines (CCL2, CCL11, CCL5, CCL3, and CXCL8) that was used in our study has not been evaluated in other glomerulopathies.
The chemokines have never been measured in combination or isolated in schistosomal glomerulopathy. The CCL2 has been shown to have a role in the pathogenesis of clinical and experimental studies on diabetes glomerulopathy.30 The CCL3 has been used as an important marker of the severity of hepatosplenic schistosomiasis.33 The CCL11 has also been implicated in renal diseases.34 CXCL8 was shown to be elevated in the urine in patients with end stage renal disease.35 The CCL5 is a good marker of active schistosomal infection.33
In our study, the patients with hepatosplenic schistosomiasis and schistosomal glomerulopathy (HS + SGN) presented with CCL3 > 14.3 pg/mL and CCL11 > 26.7 pg/mL in the urine and with CCL3 > 61.9 pg/mL, CXCL8 ≤ 1030.4 pg/mL, and CCL2 > 634.3 pg/mL in the sera. A similar profile was observed in the patients with glomerulopathy caused by other diseases, except for the serum CCL2 ≤ 634.3 pg/mL. Further investigations using our approach, particularly in patients with glomerulopathies of different etiologies, would be of interest. Because chemokines have a low molecular weight, they may appear in urine earlier than albumin; this characteristic helps to identify early glomerulonephritis. We have already started another study to confirm the findings described previously.
The chemokines CCL2, CCL3, and CCL5 have been shown to have an important role in macrophage accumulation and renal injury in animal models of non-diabetic and diabetic kidney damage.30 The CCL2 recruits monocytes, memory T-cells, and dendritic cells to the sites of tissue injury, infection, and inflammation.30,36 It has been found that CCL2 levels are increased in the sera of patients with hepatosplenic schistosomiasis.33 In our study, the serum CCL2 level was highest (> 490 pg/mL) in the HS without SGN group, confirming previous studies. In addition, CCL2 serum levels > 634.3 pg/mL were found only in the patients with schistosomal glomerulopathy, not in the patients with glomerulopathies from other causes.
The urine and plasma CCL5 levels in our study were unrelated to glomerular damage (proteinuria), but an alteration in CCL5 was described in adriamycin nephropathy induced in mice or rats, leading to proteinuria and interstitial fibrosis.31,32 During the progression of adriamycin nephropathy, an increase in the expression of chemokines, such as CCL5 and CCL2 and their corresponding receptors, was observed. The induction of autoantibodies against CCL5 and CCL2, achieved through vaccination with naked DNA in rats, resulted in decreased proteinuria, improved creatinine-clearance, conservation of renal morphology, and reduced inflammatory interstitial infiltrates.31 Accordingly, blocking CCR1 (a CCL5 receptor) with a small molecule antagonist decreased the interstitial T-cell and macrophage accumulation and reduced the interstitial fibroblast production and fibrosis.32
In humans, the increase of CCL2 in the kidney tissue has been described as a characteristic of renal injury in diabetic nephropathy. The up-regulation of kidney CCL2 is a feature of human diabetic renal injury that is associated with macrophage recruitment and disease progression; therefore, neutralizing CCL2 activity should be considered an important therapeutic goal in treating diabetic nephropathy37; this same approach may be used to treat schistosomal glomerulopathy. In addition, an alternative strategy may be the use of drugs that block the effect of aldosterone, thereby reducing renal inflammation through CCL2 modulation.
Ibrahim and Rashed13 found a direct correlation between urine CCL2 levels and albuminuria in a study of diabetic nephropathy. In our study, a correlation between the urine levels of CCL2 and microalbuminuria was likewise observed (regression coefficient b = 121.6 and r = 0.294; P = 0.001). For each 1 mg increase in albuminuria, there was a 121.6 pg/mL increase in urine CCL2 (in a linear regression analysis). No correlation with albuminuria was found for the other chemokines (CCL11, CCL5, CCL3, and CXCL8) evaluated in this study.
Souto and others11 showed that in pediatric patients with idiopathic nephrotic syndrome (INS), urinary CXCL8 was 2.9 times higher for the patients with relapsed INS (proteinuria > 100 mg/m2/24 h) than for the patients in remission, and the chemokine levels correlated positively with individual proteinuria values. In our study, an increase in CXCL8 was not found in the serum or urine of patients with schistosomal glomerulopathy.
Using the decision tree as a statistical model, a cutoff point of CCL2 > 634.3 pg/mL distinguished the patients with schistosomal glomerulopathy from those with other glomerulopathies. This is the first time that a cutoff point has been described in the literature to diagnose renal injury.
It is worthwhile to mention that there are residual lesions like schistosomal glomerulopathy that do not regress upon successful treatment of schistosomiasis (based on three negative Kato-Katz stool examinations).
In short, in areas that are endemic for schistosomiasis mansoni, sera CCL2 > 634.3 pg/mL may indicate the presence of schistosomal glomerulopathy.
ACKNOWLEDGMENTS
We thank the nephrologists Sérgio Wyton and Ladeira Rodrigues for helping to select the patients for this study.
Disclaimer: English Editing: Nature Publishing Group.
Footnotes
Financial support: This work has been partially supported by the FAPEMIG and CNPq/Brazil.
Authors' addresses: Alba Otoni, Izabela Voieta, and Vinícius Lins Costa Melo, Universidade Federal de Minas Gerais - Medicina Tropical, Belo Horizonte, Minas Gerais, Brazil, E-mails: albaotoni01@yahoo.com.br, voieta@uol.com.br, and vilinscm@gmail.com. Antônio Lúcio Teixeira, Federal University de Minas Gerais - Internal Medicine and Laboratory of Immunopharmacology, Belo Horizonte, Minas Gerais, E-mails: altexr@gmail.com or altexjr@hotmail.com. Carlos Maurício Antunes, Santa Casa de Misericórdia de Belo Horizonte - Epidemiologia, Belo Horizonte, Minas Gerais, Brazil, E-mail: antunesc@santacasabh.org.br. Sandra Costa Drummond, Secreatria Estadual de Saùde - Helmintologia, Belo Horizonte, Minas Gerais, Brazil, E-mail: sandracdrummond@yahoo.com.br. Valério Ladeira Rodrigues, Universidade Federal de Minas Gerais - Nephrology, Belo Horizonte, Minas Gerais, Brazil, E-mail: varodrigues@uai.com.br. José Roberto Lambertucci, Department of Infectology and Tropical Medicine, Federal University of Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, E-mail: lamber@uai.com.br.
References
- 1.Brasil, Ministério da Saúde.Secretaria de Vigilância em Saúde Situação epidemiológica de Esquistossomose no Brasil. Grupo Técnico das Parasitárias, Sub HA/CGDT/DEVEP/SVS/MS Brasília. 2010. http://portal.saude.gov.br/portal/arquivos/pdf/situacao_esquistossomose_brasil_abril2011.pdf Available at. Acessado em junho de 2013.
- 2.Hoffman DB, Lehman JS, Scott VC, Warren KS, Webbe G. Control of schistosomiasis: report of a workshop. Am J Trop Med Hyg. 1979;28:249–259. doi: 10.4269/ajtmh.1979.28.249. [DOI] [PubMed] [Google Scholar]
- 3.Lambertucci JR. Schistosoma mansoni: pathological and clinical aspects. In: Jordan P, Webbe G, Sturrock RF, editors. Human Schistosomiasis. Wallingford: Cab International; 1993. pp. 195–225. [Google Scholar]
- 4.Lambertucci JR, dos Santos Silva LC, Andrade LM, de Queiroz LC, Carvalho VT, Voieta I, Antunes CM. Imaging techniques in the evaluation of morbidity in schistosomiasis mansoni. Acta Trop. 2008;108:209–217. doi: 10.1016/j.actatropica.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues VL, Otoni A, Voieta I, Antunes CM, Lambertucci JR. Glomerulonephritis in schistosomiasis mansoni: a time to reappraise. Rev Soc Bras Med Trop Nov-Dec. 2010;43:638–642. doi: 10.1590/s0037-86822010000600007. [DOI] [PubMed] [Google Scholar]
- 6.Lambertucci JR, Otoni A, dos Reis MA. Nephrotic syndrome in hepatosplenic schistosomiasis mansoni. Rev Soc Bras Med Trop. 2007;40:492–493. doi: 10.1590/s0037-86822007000400028. [DOI] [PubMed] [Google Scholar]
- 7.Lambertucci JR, Serufo JC, Gerspacher-Lara R, Rayes AA, Teixeira R, Nobre V, Antunes CM. Schistosoma mansoni: assessment of morbidity before and after control. Acta Trop. 2000;77:101–109. doi: 10.1016/s0001-706x(00)00124-8. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins R. New biomarkers of acute kidney injury and the cardio-renal syndrome. Korean J Lab Med. 2011;31:72–80. doi: 10.3343/kjlm.2011.31.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araya CE, Wasserfall CH, Brusko TM, Mu W, Segal MS, Johnson RJ, Garin EH. A case of unfulfilled expectations. Cytokines in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol. 2006;21:603–610. doi: 10.1007/s00467-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 10.Garin EH. Circulating mediators of proteinuria in idiopathic minimal lesion nephrotic syndrome. Pediatric Nephrol. 2000;14:872–878. doi: 10.1007/s004679900269. [DOI] [PubMed] [Google Scholar]
- 11.Souto MF, Teixeira AL, Russo RC, Penido MG, Silveira KD, Teixeira MM, Simões E, Silva AC. Immune mediators in idiopathic nephrotic syndrome: evidence for a relation between interleukin 8 and proteinuria. Pediatr Res. 2008;64:637–642. doi: 10.1203/PDR.0b013e318186ddb2. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, Krolewski AS, Bonventre JV. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Inter. 2011;79:464–470. doi: 10.1038/ki.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim S, Rashed L. Correlation of urinary monocyte chemo-attractant protein-1 with other parameters of renal injury in type-II diabetes mellitus. Saudi J Kidney Dis Transpl. 2008;19:911–917. [PubMed] [Google Scholar]
- 14.Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor. Rev Mar. 1998;9:9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 15.Segerer S. The role of chemokines and chemokine receptors in progressive renal diseases. American J Kidney Dis. 2003;41((Suppl 1)):S15–S18. doi: 10.1053/ajkd.2003.50076. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Yuan S, Xu X. Expression of MCP-1 in renal tissues of patients with IgA nephropathy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34:1023–1028. [PubMed] [Google Scholar]
- 17.Silva LC, Andrade LM, de Paula IB, de Queiroz LC, Antunes CM, Lambertucci JR. Ultrasound and magnetic resonance imaging findings in Schistosomiasis mansoni: expanded gallbladder fossa and fatty hilum signs. Rev Soc Bras Med Trop Jul-Aug. 2012;45:500–504. doi: 10.1590/s0037-86822012005000008. [DOI] [PubMed] [Google Scholar]
- 18.Lambertucci JR, Godoy P, Neves J, Bambirra EA, Ferreira MD. Glomerulonephritis in Salmonella-Schistosoma-mansoni association. Am J Trop Med Hyg. 1988;38:97–102. doi: 10.4269/ajtmh.1988.38.97. [DOI] [PubMed] [Google Scholar]
- 19.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop São Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 20.Nyman HA, Dowling TC, Hudson JQ, Peter WL, Joy MS, Nolin TD. Comparative evaluation of the Cockcroft-Gault Equation and the Modification of Diet in Renal Disease (MDRD) study equation for drug dosing: an opinion of the Nephrology Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2011;31:1130–1144. doi: 10.1592/phco.31.11.1130. [DOI] [PubMed] [Google Scholar]
- 21.Grupo de Trabalho MA. Brazilian guidelines for ambulatory monitoring of arterial pressure and III Brazilian guidelines for home monitoring of blood pressure. J Bras Nefrol Jul-Sep. 2011;33:365–388. doi: 10.1590/s0101-28002011000300013. [DOI] [PubMed] [Google Scholar]
- 22.Bewick V, Cheek L, Ball J. Statistics review 14: logistic regression. Crit Care. 2005;9:112–118. doi: 10.1186/cc3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiglic G, Kocbek S, Pernek I, Kokol P. Comprehensive decision tree models in bioinformatics. PLoS ONE. 2012;7:e33812. doi: 10.1371/journal.pone.0033812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biau G. Analysis of a random forests model. J Mach Learn Res. 2012;13:1063–1095. [Google Scholar]
- 25.Loh WY, Shih YS. Split selection methods for classification trees. Statist Sinica. 1997;7:815–840. [Google Scholar]
- 26.Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;56:2982–2989. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Niño MD, Sanz AB, Ihalmo P, Lassila M, Holthofer H, Mezzano S, Aros C, Groop PH, Saleem MA, Mathieson PW, Langham R, Kretzler M, Nair V, Lemley KV, Nelson RG, Mervaala E, Mattinzoli D, Rastaldi MP, Ruiz-Ortega M, Martin-Ventura JL, Egido J, Ortiz A. The MIF receptor CD74 in diabetic podocyte injury. J Am Soc Nephrol. 2009;20:353–362. doi: 10.1681/ASN.2008020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsing CH, Hsu CC, Chen WY, Chang LY, Hwang JC, Chang MS. Expression of IL-19 correlates with Th2 cytokines in uraemic patients. Nephrol Dial Transplant. 2007;22:2230–2238. doi: 10.1093/ndt/gfm179. [DOI] [PubMed] [Google Scholar]
- 29.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J of Physiol Renal Physiol. 2008;294:F697–F701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 30.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Wang Y, Tay YC, Zheng G, Zhang C, Alexander SI, Harris DC. DNA vaccination with naked DNA encoding MCP-1 and RANTES protects against renal injury in adriamycin nephropathy. Kidney Int. 2005;67:2178–2186. doi: 10.1111/j.1523-1755.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 32.Vielhauer V, Berning E, Eis V, Kretzler M, Segerer S, Strutz F, Horuk R, Gröne HJ, Schlöndorff D, Anders HJ. CCR1 blockade reduces interstitial inflammation and fibrosis in mice with glomerulosclerosis and nephrotic syndrome. Kidney Int. 2004;66:2264–2278. doi: 10.1111/j.1523-1755.2004.66038.x. [DOI] [PubMed] [Google Scholar]
- 33.Sousa-Pereira SR, Teixeira AL, Silva LC, Souza AL, Antunes CM, Teixeira MM, Lambertucci JR. Serum and cerebral spinal fluid levels of chemokines and Th2 cytokines in Schistosoma mansoni myeloradiculopathy. Parasite Immunol. 2006;28:473–478. doi: 10.1111/j.1365-3024.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- 34.Cherney DZ, Scholey JW, Sochett E, Bradley TJ, Reich HN. The acute effect of clamped hyperglycemia on the urinary excretion of inflammatory cytokines/chemokines in uncomplicated type 1 diabetes. Diabetes Care. 2011;34:177–180. doi: 10.2337/dc10-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolkow PP, Niewczas MA, Perkins B, Ficociello LH, Lipinski B, Warram JH, Krolewski AS. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol. 2008;19:789–797. doi: 10.1681/ASN.2007050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takebayashi K, Matsumoto S, Aso Y, Inukai T. Association between circulating monocyte chemoattractant protein-1 and urinary albumin excretion in nonobese Type 2 diabetic patients. J Diabetes Complications. 2006;20:98–104. doi: 10.1016/j.jdiacomp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Amann B, Tinzmann R, Angelkort B. ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care. 2003;26:2421–2425. doi: 10.2337/diacare.26.8.2421. [DOI] [PubMed] [Google Scholar]