Abstract
Previous evidence has suggested a functional-anatomic dissociation between conscious and nonconscious processing during retrieval where early visual regions BA17/18 are associated with nonconscious processing and late visual regions BA19/37 are associated with conscious processing. However, evidence for this dissociation has only been observed using a limited number of experimental paradigms. In the present functional magnetic resonance imaging (fMRI) study, we tested the hypothesis that conscious processing during retrieval can occur in BA17/18 using memorial paradigms that recruited processing in these early visual regions. During the encoding phase of Experiment 1, abstract shapes with colored and oriented internal lines were presented to the left and right of fixation. During the retrieval phase, old shapes and new shapes were presented at fixation and participants classified each item as “old-left”, “old-right”, or “new”. The contrast of spatial memory-hits > spatial memory-misses (with accurate item memory) produced activity in BA17/18. During the encoding phase of Experiment 2, abstract shapes with colored and oriented internal lines were presented at fixation. During the retrieval phase, old shapes, changed shapes (with the same outline but different colored and oriented internal lines), and new shapes were presented at fixation and participants made an old-new classification during runs with a specific retrieval orientation or a non-specific retrieval orientation. Critically, the contrast of old-hits > old-misses during specific retrieval orientation produced activity in BA17/18. The results of the present experiments support the hypothesis that conscious processing during retrieval can occur in BA17/18.
Keywords: Explicit, Implicit, Nonconscious, Memory, V1, fMRI
Explicit memory involves the conscious retrieval of previous experiences, whereas implicit memory involves nonconsious retrieval (e.g., Schacter, 1987; Lozito & Mulligan, 2010). Neuroimaging studies suggest that conscious and nonconscious retrieval processes are mediated by distinct neural regions (for review see Schacter, Buckner, & Koutstaal, 1998; Schacter, Wig, & Stevens, 2007; Dew & Cabeza, 2011). In two functional magnetic resonance imaging (fMRI) studies, we provided evidence in support of a visual sensory functional-anatomic dissociation between conscious and nonconscious processing (Slotnick & Schacter, 2004, 2006; see also, Kim & Cabeza, 2007; Stark, Okado, & Loftus, 2010). Specifically, early visual regions Brodmann area (BA) 17 and BA18 were associated with nonconscious processing, while later visual regions BA19 and BA37 were associated with conscious processing.
Slotnick and Schacter (2004) presented abstract shapes to the left or the right of a central fixation cross during encoding. During the recognition test, old and new shapes were presented at fixation and participants classified each shape as “old-left”, “old-right”, or “new”. The old-hit versus old-miss comparison was used to isolate conscious processing. Given that this comparison tracks subjective memorial experience (i.e., “old” versus “new” responses) with (old) item type remaining constant, it was assumed that old-hits and old-misses would differ in the degree of conscious retrieval (Wheeler & Buckner, 2003; Schott et al., 2005; Slotnick & Schacter, 2010). This contrast produced activity in BA19/37. The conjunction of old-hit versus new-correct rejection and old-miss versus new-correct rejection was used to isolate nonconscious processing. Given that the latter contrast tracks item type (i.e., old versus new) with response type (“new”) remaining constant, it was assumed to produce nonconscious memory effects (Rugg et al., 1998; see also, Thakral, 2011). This conjunction revealed activity in BA17/18. In a related study, Slotnick and Schacter (2006) provided evidence that the previously observed nonconscious early visual activity was attributable to repetition priming, a form of implicit memory.
Importantly, the previous functional-anatomic dissociation between conscious processing in later visual regions BA19/37 and nonconscious processing in early visual regions BA17/18 was observed under a limited set of experimental conditions. As such, it could be the case that conscious processing can occur in BA17/18 under certain experimental conditions. We conducted two functional magnetic resonance imaging (fMRI) experiments to assess whether conscious processing during retrieval is restricted to BA19/37 or whether conscious processing during retrieval can occur in BA17/18. Both experiments employed memory paradigms that were designed to recruit processing in these early visual regions. Specifically, Experiment 1 evaluated activity associated with retrieval of spatial information (as early visual regions preferentially process retinotopic information; Sereno et al., 1995; Tootell et al., 1997; Slotnick & Yantis, 2003), and Experiment 2 evaluated activity associated with specific (line orientation/color) retrieval orientation (as early visual regions preferentially process line orientation; Hubel & Wiesel, 1974; Tootell et al., 1998; Kamitani & Tong, 2005). To anticipate the present results, conscious processing did occur in BA17/18 in both experiments, which shows that such activity is not restricted to later visual regions but is rather task dependent.
1. Experiment 1
1.1. Materials and methods
1.1.1. Participants
Twelve right-handed participants with normal or corrected-to-normal vision completed the experiment (seven females, 18.4–25.9 years of age). The paradigm was approved by the Massachusetts General Hospital Internal Review Board. Informed consent was obtained from each participant.
1.1.2. Stimuli and task
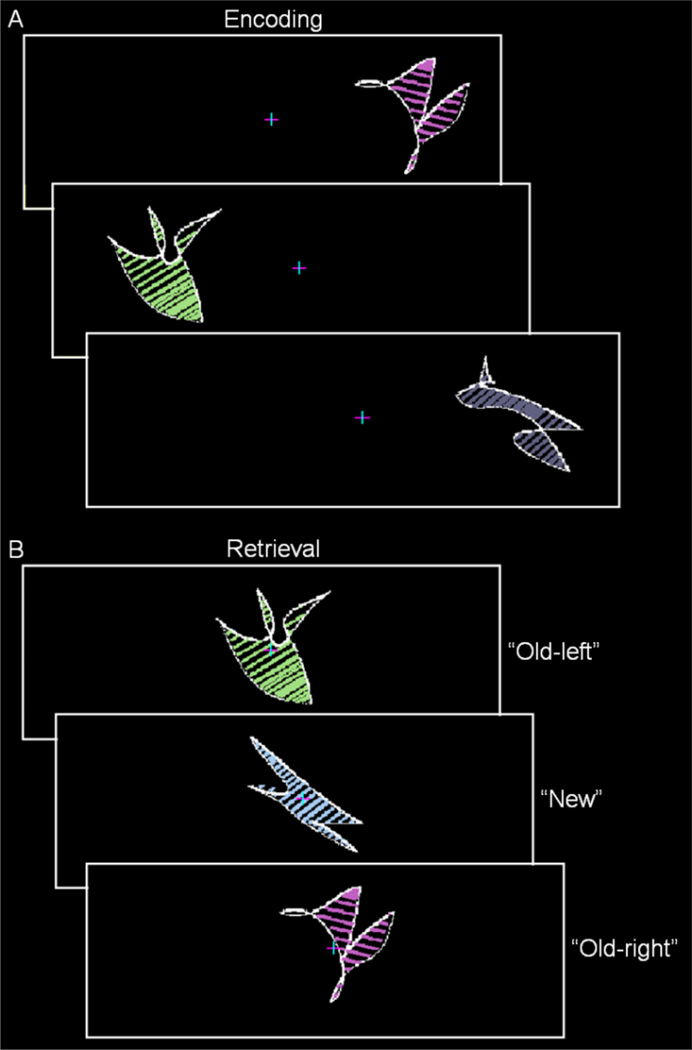
Following one practice run, participants completed six encoding-retrieval runs during fMRI. During encoding, 32 abstract shapes with colored and oriented internal lines were presented to the left and the right of a central fixations cross (Figure 1A; for details on shape construction, see Slotnick & Schacter, 2004). Participants were instructed to remember each shape and its spatial location while maintaining fixation. Each shape was presented in random order for 2.5 s every 3 s and spanned 5.5° of visual angle with its closest point 3° of visual angle from fixation. During retrieval, the 32 old shapes from encoding and 16 new shapes were presented at fixation in random order for 2.5 s every 4–12 s and participants classified each shape as old and on the left (“old-left”), old and on the right (“old-right”), or “new”. Participants also made a “sure”/“unsure” response following each response. Shapes (old-left, old-right, and new) were counterbalanced across participants using a Latin Square, no more than 3 shapes of each type were presented sequentially, and shapes were never repeated across runs.
Figure 1.
Experiment 1 paradigm. A. During encoding, participants were presented abstract shapes with colored and oriented internal lines to the left and right of a central fixation cross. B. During retrieval, participants were presented old and new shapes at fixation and classified each shape as “old-left”, “old-right”, or “new” (correct responses are shown to the right).
1.1.3. Image Acquisition and Analysis
Images were acquired using a 3 Tesla Siemens Allegra scanner. Functional images were acquired using an echo-planar imaging sequence (TR = 2 s, TE = 30 ms, acquisition matrix = 64 × 64, 30 slices, 4.5 mm isotropic resolution). Anatomic images were acquired using a magnetization rapidly acquired gradient echo sequence (1.33 mm × 1 mm × 1 mm resolution).
Imaging analysis was conducted using BrainVoyager QX (Brain Innovation B.V., Maastricht, The Netherlands). Functional data preprocessing included slice-time correction, motion correction (excluding runs with greater than 3 mm of motion), and temporal high pass filtering (removal of linear trends less than or equal to 3 cycles per run length). Functional images were resampled at 3 mm isotropic resolution. All images were transformed into Talaraich space.
A random-effect general linear model analysis was conducted. On an individual participant basis, each event type was modeled as a square wave with corresponding onsets and behavioral responses (event offsets were used when responses were not given). Each square wave was convolved with a hemodynamic response function resulting in a model of the hemodynamic response for each event. Events included accurate retrieval of item information and spatial information (referred to as spatial memory-hits; e.g., responding “old-left” to a shape previously presented on the left), accurate retrieval of item information but inaccurate retrieval of spatial information (referred to as item memory-hits or spatial memory-misses; e.g., responding “old-left” to a shape previously presented on the right), forgotten items (referred to as item memory-misses; responding “new” to a previously presented shape), and new-correct rejections. Shapes at encoding, false-alarms at retrieval, and shapes at retrieval with no responses were also modeled. Each hemodynamic response model was fit to each voxel’s activation timecourse to yield the best-fit model amplitude for each event (i.e., beta-weights). Using a one-tailed t-test, voxels were deemed significant if the difference between beta-weights was significantly positive at an individual voxel threshold of p < 0.001. Activity was corrected for multiple comparisons to p < 0.05 by enforcing a cluster extent threshold of 7 resampled voxels. This extent threshold was computed using a Monte Carlo simulation with 10,000 iterations that incorporated the minimum spatial correlation (full-width-half-maximum) of 4.5 mm in the contrast images (which was selected to avoid inflating the value by spatially autocorrelated memory related activity). An ANOVA was employed to identify activity associated with an interaction between contrasts.
Conscious retrieval of spatial information was isolated by contrasting spatial memory-hits > spatial memory-misses. Of importance, item memory was held constant (accurate) for both of these event types. In an effort to replicate our previous findings (Slotnick & Schacter, 2004, 2006), conscious retrieval of item information was isolated by contrasting item memory-hits > item memory-misses. It was assumed that the comparisons of spatial memory-hits > spatial memory-misses and item memory-hits > item memory-misses would primarily differ in the degree of conscious processing associated with spatial or item information, respectively, as each comparison tracks subjective memorial experience with item type remaining constant.
As retrieval of both item information and spatial information involve the reactivation of the same visual regions involved during encoding (for review see Slotnick, 2004), significant activity during retrieval was restricted to regions of significant visual activity during encoding (identified by contrasting encoding-left > encoding-right and vice versa; see Slotnick, 2009). Significant activity across participants was projected onto the segmented cortical surface of a representative participant (for segmentation procedures, see Slotnick, 2005).
1.2. Results
1.2.1 Behavioral Results
Item memory accuracy independent of spatial location was 67.2 ± 1.6% and spatial location accuracy, contingent on accurate item memory, was 70.0 ± 2.0%.
1.2.2 fMRI Results
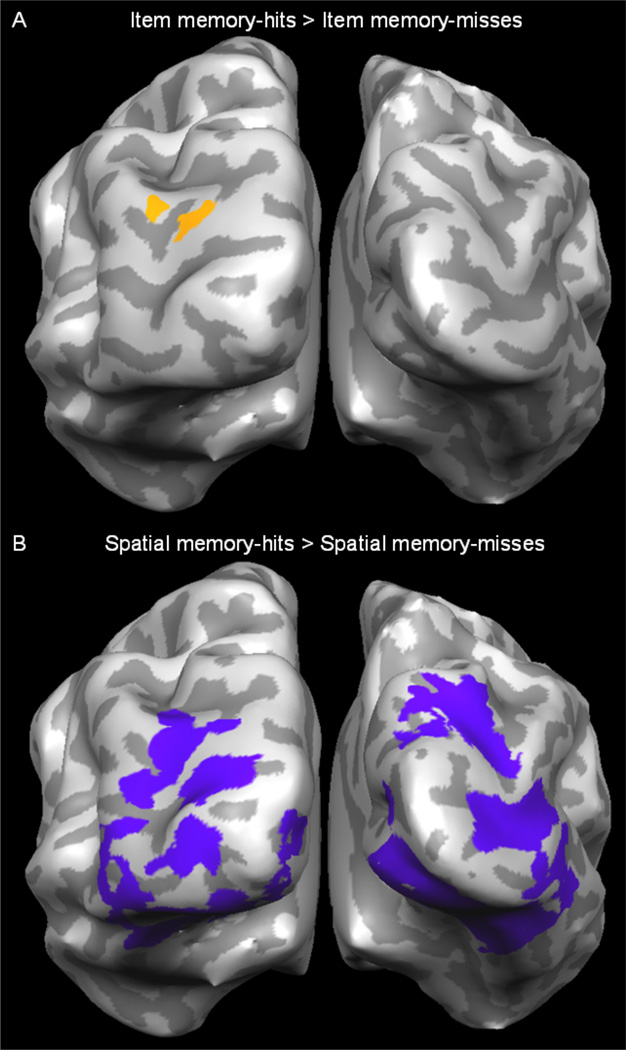
Replicating our previous findings (Slotnick & Schacter, 2004), conscious retrieval of item information (item memory-hits > item memory-misses) produced activity in BA19 (Figure 2A). Of direct relevance to the present investigation, conscious retrieval of spatial information (spatial memory-hits > spatial memory-misses) produced activity in early visual regions BA17/18 as well as BA19/37 (Figure 2B). In contrast to earlier findings (Slotnick & Schacter, 2004, 2006), the conscious memory effects in BA17/18 during accurate spatial memory show that conscious memorial processing can occur in early visual regions. We also contrasted spatial memory-left-hits > spatial memory-left-misses and spatial memory-right-hits > spatial memory-right-misses to assess whether contralateral memory effects would be produced. Both contrasts activated bilateral early and late visual regions BA17/18/19/37.
Figure 2.
Experiment 1 results. A. Visual activity associated with the conscious retrieval of item information was identified by contrasting item memory-hits > item memory-misses (in orange, posterior view). B. Visual activity associated with the conscious retrieval of spatial information identified by contrasting spatial memory-hits > spatial memory-misses (in purple).
In an effort to replicate our previous findings of nonconscious activity in BA17/18 (Slotnick & Schacter, 2004, 2006), we contrasted item memory-misses > new correct rejections (Slotnick & Schacter, 2010; Henson, Hornberger, & Rugg, 2005; Woollams, Taylor, Karayanidis, & Henson, 2008). This contrast was one-tailed as repetition priming effects associated with unfamiliar stimuli, such as abstract shapes, have been consistently associated with increases in visual activity (Schacter et al., 1995; Henson, Shallice, & Dolan, 2000; Slotnick & Schacter, 2006; for review see Henson, 2003). This contrast did not reveal any significant visual activity at the original threshold of p < 0.001. However, at a more lenient threshold of p < 0.01, which is more similar to the thresholds employed in our previous studies (Slotnick & Schacter, 2004, 2006), activity was observed in BA18.
We further evaluated whether conscious processing during retrieval of spatial information in BA17/18 (indexed by spatial memory-hits > spatial memory-misses), our critical finding, produced activity that was significantly greater in magnitude than that produced during nonconscious processing (indexed by item memory-misses > new correct rejections). In support of our previous results, this interaction produced activity in BA17/18 (at p < 0.001, uncorrected; activity in BA17 survived the cluster extent threshold enforced in the primary analysis).
2. Experiment 2
2.1. Materials and methods
2.1.1. Participants
Ten participants with normal or corrected-to-normal vision took part in the study (6 females, 19.5–25.9 years of age). The paradigm was approved by the Massachusetts General Hospital Internal Review Board. Informed consent was obtained from each participant.
2.1.2. Stimuli and task
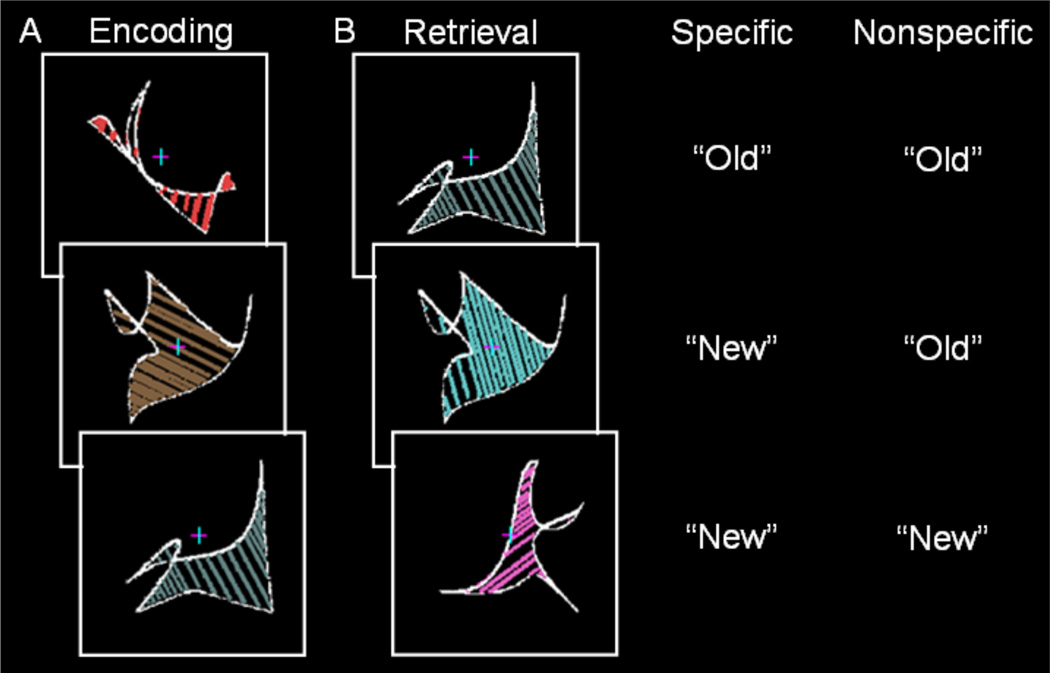
Following one practice run of each type, participants completed six encoding-retrieval runs. During encoding, 30 abstract shapes with colored and oriented internal lines were presented in random order for 2.5 s every 3 s at fixation (Figure 3A). Shapes spanned 5.5° of visual angle. Participants were instructed to remember each shape and its colored and oriented internal lines. During retrieval, participants were shown previously presented shapes with the same colored and oriented internal lines (referred to as old shapes), previously presented shapes with different colored and oriented internal lines (referred to as changed shapes), and new shapes (Figure 3B). Shapes at retrieval were presented at fixation in random order for 2.5 s every 4–12 s. Either specific or nonspecific instructions were presented before each retrieval phase to manipulate retrieval orientation. Specific instructions specified that participants should classify shapes that were identical to those presented during the encoding phase (including shape and internal line color/orientation) as “old” and otherwise respond “new”. Nonspecific instructions specified that participants should classify shapes that were the same as those presented during the encoding as “old” regardless of whether the internal line color and orientation were the same or different and otherwise respond “new”. Of importance, specific retrieval orientation required retrieval of shape and line information, while nonspecific retrieval orientation only required retrieval of shape information. Retrieval instructions were provided at the beginning of each retrieval phase such that encoding processes were identical across the two retrieval orientations. Nonspecific and specific retrieval orientations instructions were given equally often in ABBABA and BAABABA order across participants.
Figure 3.
Experiment 2 paradigm. A. During encoding, participants were presented abstract shapes with colored and oriented internal lines at fixation. B. During retrieval, participants were presented previously presented shapes with the same colored and oriented internal lines (old shapes), previously presented shapes with different colored and oriented internal lines (changed shapes), and new shapes. Either specific instructions or nonspecific instructions were presented before each retrieval phase to manipulate retrieval orientation. During specific retrieval orientation, participants classified old shapes as “old” and otherwise responded “new”. During nonspecific retrieval orientation, participants classified old or changed shapes as “old” (regardless of internal line orientation/color) and otherwise responded “new”. Correct responses for each retrieval orientation are shown to the right.
To avoid response bias differences, each specific orientation retrieval phase consisted of 20 old shapes, 10 changed shapes, and 10 new shapes, while each nonspecific orientation retrieval phase consisted of 20 old shapes, 10 changed shapes, and 30 new shapes (such that perfect accuracy would produce an equal number of “old” and “new” responses for both run types). Old, changed, and new shapes were counterbalanced across participants using a Latin Square, no more than 3 shapes of each type were presented sequentially, and shapes were never repeated across runs.
2.1.3. Image Acquisition and Analysis
Unless otherwise stated, the analysis procedure was identical to Experiment 1. Functional images were acquired using an echo-planar imaging sequence with 35 slices. Of particular relevance, events included accurate retrieval of shape and internal line color and orientation (referred to as old-hits; e.g., responding “old” to a previously presented shape with the same colored and oriented internal lines during both retrieval orientations) and forgotten items (referred to as old-misses; responding “new” to a previously presented shape with the same colored and oriented internal lines). Shapes at encoding in addition to changed and new shapes and their corresponding responses during retrieval were also modeled. The individual voxel threshold was set to p < 0.001, corrected for multiple comparisons to p < 0.05 using a cluster extent threshold of 15 resampled voxels (computed using a full-width-half-maximum spatial autocorrelation of 7.5 mm). Conscious processing during specific retrieval orientation and nonspecific retrieval orientation was isolated by contrasting old-hits > old-misses. Critically, during the specific retrieval orientation, participants were required to retrieve line orientation information to accurately recognize old shapes; therefore, we reasoned that conscious retrieval during this condition would require processing in BA17/18 given that these regions are preferentially involved with processing line orientation (Hubel & Wiesel, 1974; Tootell et al., 1998; Kamitani & Tong, 2005).
2.2. Results
2.2.1 Behavioral Results
Old-new recognition accuracy for the specific retrieval orientation was 64.87 ± 1.7% and old-new recognition accuracy for the nonspecific retrieval orientation was 61.51 ± 2.2%. Participants were able to switch retrieval orientations, as the probability of “old” responses to changed shapes was significantly greater during the nonspecific retrieval orientation as compared to the specific retrieval orientation (t(9) = 4.37, p < 0.001).
2.2.2 fMRI Results
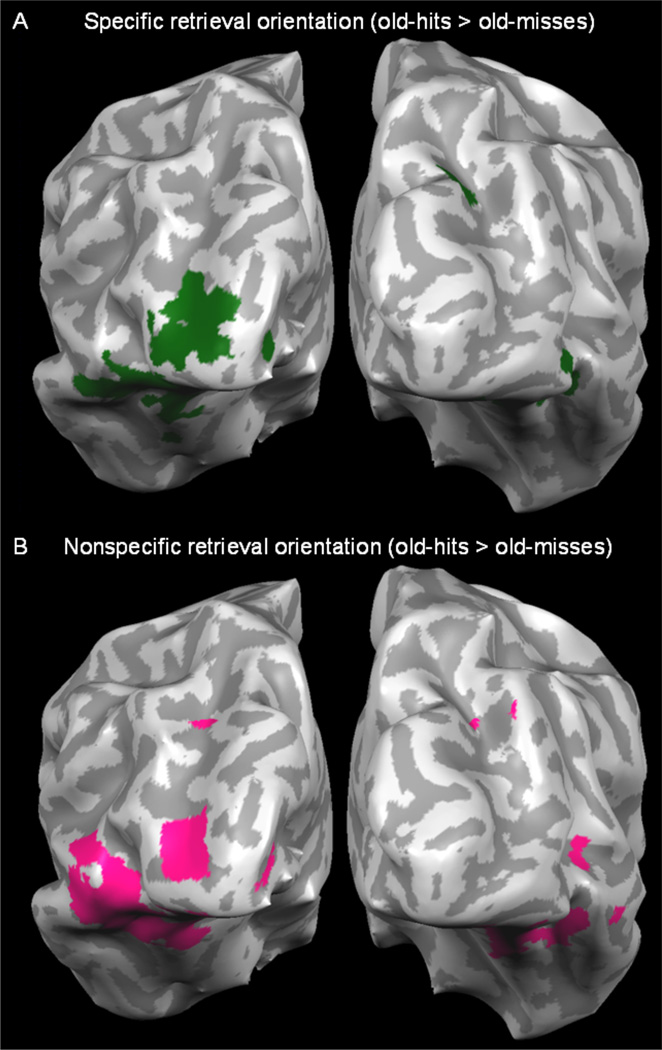
Conscious retrieval during the specific retrieval orientation condition (old-hits > old-misses) produced activity in BA17/18 and BA19/37 (Figure 4A). In addition, conscious retrieval during the nonspecific retrieval orientation condition (old-hits > old-misses) produced activity in BA17/18 and BA19/37 (Figure 4B), which was not predicted given that nonspecific retrieval did not require detailed line orientation processing.
Figure 4.
Experiment 2 results. A. Visual activity associated with the conscious retrieval during specific retrieval orientation was identified by contrasting old-hits > old-misses (in green). B. Visual activity associated with the conscious retrieval during nonspecific retrieval orientation identified by contrasting old-hits > old-misses (in pink).
Given that BA17/18 and BA19/37 were active during both retrieval orientations, we directly compared old-hits for each retrieval orientation to test whether BA17/18 was more active during the specific versus nonspecific retrieval orientation. This contrast produced a null result in BA17/18, which suggests the magnitude of activity in these regions was similar during both retrieval orientations.
3. Discussion
In Experiment 1, conscious processing during the retrieval of spatial information produced activity in BA17/18 (in addition to BA19/37). By contrast, conscious processing during the retrieval of item information produced activity in BA19 but did not produce activity in BA17/18. In Experiment 2, conscious processing during specific retrieval orientation, which required line orientation processing, produced activity in BA17/18 as well as BA19/37. The present findings of activity associated with conscious memory in BA17/18 are in opposition to the functional-anatomic dissociation suggested by previous work (Slotnick & Schacter, 2004, 2006; see also, Kim & Cabeza, 2007; Stark et al., 2010). Taken together, the previous results and present findings suggest that the neural regions associated with conscious processing can be recruited during appropriate memorial tasks.
The present results are relevant to earlier reports of a conscious versus nonconscious processing dissociation in time. Previous event-related potential (ERP) evidence has suggested a visual sensory dissociation at 800 ms, where conscious processing occurs after 800 ms and nonconscious processing occurs before 800 ms (Slotnick & Schacter, 2010). However, using a paradigm similar to that in Experiment 1 of the present study, we recently demonstrated that conscious memory processing can occur during the early epoch (before 200 ms; Thakral & Slotnick, submitted). The present fMRI findings of conscious memory activity in BA17/18 and these ERP findings provide further evidence that spatial and temporal dissociations between conscious and nonconscious processing during retrieval are not fixed but are task-dependant.
In Experiment 2, we found that BA17/18 was similarly active during specific retrieval orientation and nonspecific retrieval orientation. Activity in these regions during nonspecific retrieval orientation was not expected, given that detailed visual processing was not necessary to perform this task. Furthermore, the behavioral results that differed as a function of retrieval orientation were seemingly at odds with the neural results that did not differ as a function of retrieval orientation. One possible explanation is that during nonspecific retrieval orientation participants retrieved internal line orientation/color information, which would produce activity in BA17/18 even though it was irrelevant to the task, but produced their behavioral response based on shape information alone. This idea suggests that our experimental design, which included switching between specific and nonspecific retrieval orientations, produced carry-over effects such that participants were engaged in specific retrieval even when it was not required during nonspecific runs. If correct, this explanation would predict that participants in a paradigm that only consisted of nonspecific retrieval orientation should not activate BA17/18. Regardless of the reason for this effect, Experiment 2 revealed conscious activity during retrieval in BA17/18.
In the current study, conscious activity during retrieval was identified by comparing old-hits > old-misses. It is possible that this activity at retrieval may have been due to differences during encoding, with a lower degree of processing associated with subsequent misses than subsequent hits. To address this, a subsequent memory analysis was conducted for both experiments. Significant activity in BA17/18 was not observed in Experiment 1 when contrasting subsequent spatial memory-hits > subsequent spatial memory-misses. Coupled with the significant findings observed at retrieval, this null finding at encoding does not support the possibility that differences at encoding might account for our retrieval effects. It should be highlighted that this null encoding effect was observed at p < 0.001, the same threshold at which robust retrieval effects were observed, thus the null encoding effect cannot be attributed to the enforcement of a strict statistical threshold. For Experiment 2, activity in BA17/18 was observed when comparing subsequent old-hits > subsequent old-misses during the specific retrieval orientation, which indicates that, in this experiment, differences at encoding might have affected the results at retrieval. Critically, the null encoding results of Experiment 1 show that our conscious memory effects at retrieval cannot be accounted solely by differences in encoding. In addition to this empirical evidence, it is important to consider the cognitive processing associated with each of these event types. Even if old-misses had been processed less at encoding, the key factor is that these items are associated with less conscious memorial experience as compared to old-hits at retrieval. Thus, regardless of whether there were differences at encoding, the contrast of old-hits > old-misses can still be assumed to reflect conscious activity. It is also possible that the old-hit > old-miss contrast may reflect a greater degree of nonconscious processing associated with old-hits than old-misses. However, repeated (old) items, regardless of their retrieval status (hit or miss), produce repetition priming effects, and it is reasonable to assume that such nonconscious effects occur to a similar degree for all old items. This logic was the basis of previous studies that have used the old-hit > old-miss contrast to isolate conscious processing at retrieval (Wheeler & Buckner, 2003; Schott et al., 2005; Slotnick & Schacter, 2004, 2010).
Across both experiments, activity in BA17/18 reflected conscious memorial processing. However, one possibility could be that conscious processing may have occurred in BA19/37 and/or another higher-level cortical region and such processing may have modulated BA17/18 through feedback projections. Of direct relevance to the aim of the present investigation, the current results provide evidence against a strict dissociation between conscious and nonconscious processing in BA19/37 and BA17/18.
The present results suggest that tasks requiring conscious processing of lower-level visual features (such as the spatial location memory task in Experiment 1 or the specific retrieval orientation/line orientation task in Experiment 2) are likely to produce activity in BA17/18, while tasks requiring conscious processing of higher-level visual features (such as the item/shape memory task in Experiment 1) are likely to produce activity in later visual regions. In support of the latter point, we have observed conscious feature-specific memory effects in color processing region V8 (Slotnick, 2009) and motion processing region MT+ (Slotnick & Thakral, 2011). Such task-dependant effects in visual sensory regions should be considered in future studies that aim to investigate conscious memory effects and nonconscious memory effects.
The results of the current experiments provide support for the hypothesis that conscious processing can occur in early visual regions BA17/18 during retrieval. Conscious memorial processing in BA17/18 appears to be task dependent. Future work will be required to further assess which types of stimulus and task conditions are mediated by conscious processing and nonconscious processing in visual sensory regions.
Acknowledgement
This work was supported by NIMH grant MH060941 (to DLS).
References
- Dew ITZ, Cabeza R. The porous boundaries between explicit and implicit memory: Behavioral and neural evidence. Annals of the New York Academy of Science. 2011;1224:174–190. doi: 10.1111/j.1749-6632.2010.05946.x. [DOI] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Hornberger M, Rugg MD. Further dissociating the processes involved in recognition memory: An fMRI study. Journal of Cognitive Neuroscience. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Sequence regularity and geometry of orientation columns in the monkey striate cortex. Journal of Comparative Neurology. 1974;158:267–294. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nature Neuroscience. 2005;5:679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Cabeza R. Differential contributions of prefrontal, medial temporal, and sensory-perceptual regions to true and false memory formation. Cerebral Cortex. 2007;17:2143–2150. doi: 10.1093/cercor/bhl122. [DOI] [PubMed] [Google Scholar]
- Lozito JP, Mulligan NW. Exploring the role of attention during implicit memory retrieval. Journal of Memory and Language. 2010;63:387–399. [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AD, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Implicit memory: History and current status. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Reiman E, Uecker A, Polster MR, Yun LS, Cooper LA. Brain regions associated with retrieval of structurally coherent visual information. Nature. 1995;376:587–590. doi: 10.1038/376587a0. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL, Koutstaal W. Memory, consciousness, and neuroimaging. Philosophical Transactions of the Royal Society Biological Sciences. 1998;353:1861–1878. doi: 10.1098/rstb.1998.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wig G, Stevens WD. Reductions in cortical activity during priming. Current Opinion in Neurobiology. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schott BH, Henson RN, Richardson-Klavehn A, Becker C, Thoma V, Heinze HJ, Düzel E. Redefining implicit and explicit memory: The functional neuroanatomy of priming, remembering, and control of retrieval. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1257–1262. doi: 10.1073/pnas.0409070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. Visual memory and visual perception recruit common neural substrates. Behavioral and Cognitive Neuroscience Reviews. 2004;3:207–221. doi: 10.1177/1534582304274070. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. Spatial working memory specific activity in dorsal prefrontal cortex? Disparate answers from fMRI beta-weight and timecourse analysis. Cognitive Neuropsychology. 2005;22:905–920. doi: 10.1080/02643290442000455. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. Memory for color reactivates color processing region. Neuroreport. 2009;20:1568–1571. doi: 10.1097/WNR.0b013e328332d35e. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. Rapid reactivation during spatial memory. Brain Research. 2009;1268:97–111. doi: 10.1016/j.brainres.2009.02.056. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. A sensory signature that distinguishes true from false memories. Nature Neuroscience. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. The nature of memory related activity in early visual areas. Neuropsychologia. 2006;44:2874–2886. doi: 10.1016/j.neuropsychologia.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. Conscious and nonconscious memory effects are temporally dissociable. Cognitive Neuroscience. 2010;1:8–15. doi: 10.1080/17588920903474263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Thakral PP. Memory for motion and spatial location is mediated by contralateral and ipsilateral motion processing cortex. Neuroimage. 2011;55:794–800. doi: 10.1016/j.neuroimage.2010.11.077. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Yantis S. Efficient acquisition of human retinotopic maps. Human Brain Mapping. 2003;18:22–29. doi: 10.1002/hbm.10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CEL, Okado Y, Loftus EF. Imaging the reconstruction of true and false memories using sensory reactivation and the misinformation paradigms. Learning & Memory. 2010;17:485–488. doi: 10.1101/lm.1845710. [DOI] [PubMed] [Google Scholar]
- Thakral PP. The neural substrates associated with inattentional blindness. Consciousness & Cognition. 2011;20:1768–1775. doi: 10.1016/j.concog.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Thakral PP, Slotnick SD. Distinct conscious and nonconscious sensory timecourses during visual item memory and source memory. doi: 10.1016/j.bbr.2015.04.045. (submitted). [DOI] [PubMed] [Google Scholar]
- Tootell RBH, Hadjikhani NK, Vanduffel W, Liu AK, Mendola JD, Sereno MI, Dale AM. Functional analysis of primary visual cortex (V1) in humans. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:811–817. doi: 10.1073/pnas.95.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RBH, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas J, et al. Functional analysis of V3A and related areas in human visual cortex. Journal of Neuroscience. 1997;17:7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: Control, perceived oldness, and content. The Journal of Neuroscience. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollams AM, Taylor JR, Karayanidis F, Henson RN. Event-related potential associated with masked priming of test cues reveal multiple potential contributions to recognition memory. Journal of Cognitive Neuroscience. 2008;20:1114–1129. doi: 10.1162/jocn.2008.20076. [DOI] [PubMed] [Google Scholar]