Abstract
Salvia macilenta is a member of the genus Salvia (Laminaceae) whose antioxidant activity and neuroprotective effect has been shown previously. The present study aimed to examine the antiglycating and antiapoptotic abilities of methanolic extract of this plant. Moreover, the effect of S. macilenta on neurite outgrowth and complexity after exposure to H2O2 has been studied. Base on our results, S. macilenta has antiglycating activity and protects PC12 cells against oxidative stress-induced apoptotic cell death, as examined by Hoechst staining and Western blot analysis of caspase-3, Bax, Bcl-2 and PARP. We further showed that S. macilenta decreased neurite growth and complexity impairment in differentiated PC12 cells exposed to oxidative stress. It caused a decrease in cell body area, neurite width, and the proportion of bipolar cells, while significantly increasing neurite length, the number of primary neurites per cell and the ratio of nodes to primary neuritis. All around, the mentioned results open a new horizon for future works to use this plant as a potential neuroprotective agent.
Keywords: Antiglycating activity, Neuroprotection, Neurite outgrowth, Salvia, PC12 cells
Introduction
The overproduction of reactive oxygen species (ROS) mediates damage to cell structures, nucleic acids, lipids, and proteins (Valko et al. 2007). These harmful biological effects of ROS are termed oxidative stress. Free radical-mediated oxidative stress leads to pathological conditions and is implicated in a variety of neurodegenerative diseases as well as in the aging process (Bokov et al. 2004; Gibson and Huang 2005; Scott and King 2004; Yu and Chung 2001). Hydrogen peroxide (H2O2), formed as a natural byproduct of enzymatic oxidase action, is an endogenous source of hydroxyl free radicals that contributes to the basal level of cellular oxidative stress (Halliwell 1992; Richardson et al. 1992). Exogenous H2O2 can elevate oxidative stress beyond the protective capacity of endogenous antioxidant defenses and induce apoptotic cell death by initiating mitochondrial dysfunction (Maroto and Perez-Polo 1997; Tong and Perez-Polo 1996), which is associated with changes in the Bcl-2 family of proteins and activation of caspases. Therefore, H2O2 is used extensively as an inducer of neuronal injury to explore the neuroprotective potential of new pharmacotherapies. Moreover, the formation of advanced glycation end products (AGEs) resulting from the non-enzymatic reactions of carbohydrates and oxidized lipids with proteins are stimulated by oxidative stress that is closely linked with the neurodegenerative disorders of the central nervous system, such as Alzheimer’s disease (AD). Given the link between glycation, oxidation and neurodegenerative disorders, we hypothesized that natural compounds with antioxidant and antiglycation properties may be expected to be promising therapeutic means for treatment of these diseases in which oxidative stress is a primary cause (Lim et al. 2001). On the other hand, oxidative stress is a factor which is contributing to neuritic degeneration cascades in AD as well as cell death. So finding natural compounds that support neurite outgrowth against the toxicity of H2O2 is also a key in treating neurodegenerative diseases.
The plant genus Salvia (Laminaceae) includes nearly 900 species spread throughout the world, of which 17 are endemic to Iran (Mozafarian 1996). Plants belonging to this genus are pharmacologically active and have been used in folk medicine all around the world. The phytochemical analysis of Salvia species shows the presence of many compounds that belong mainly to the group of phenolic acids, phenolic glycosides, flavonoids, anthocyanins, coumarins, polysaccharides, sterols, terpenoids and essential oils (Lu and Foo 2002; Ghannadi et al. 1999). Via these compounds, especially phenols and flavonoids, this genus possesses a wide array of biological activities. Many Salvia species and their isolated constituents possess significant antioxidant activities in enzyme-dependent and enzyme-independent systems (Hohmann et al. 1999; Zupkó et al. 2001; Asadi et al. 2011). Also, they have been introduced as antimutagenic, antibacterial, antiviral and anti-inflammatory agents (Senevirathne et al. 2006). Some other members of this genus have revealed, antimicrobial, antithrombotic, antimutagenic and anticarcinogenic activities (Cook and Samman 1996; Cushnie and Lamb 2005).
Salvia macilenta is one member of this large genus whose total phenolic (326 ± 20) and flavonoid (185 ± 32) compounds have been determined. This antioxidant activity and neuroprotective effect of the plant have been reported recently by our laboratory (Asadi et al. 2010). The aim of the present study was to examine its antiglycation activity and anti-apoptotic effect, based on an in vitro model system using nerve growth factor (NGF)-differentiated PC12 cells.
Materials and methods
Plant material
Salvia macilenta was collected from Hajiabad, Bandar Abbas in Iran (June 2001; Voucher herbarium specimen : MPH-56), by Dr. Sonboli (Department of Biology, Medicinal Plants and Drugs Research Institute, Shahid Beheshti University, Tehran, Iran). The plant was air-dried, protected from direct sunlight, and then powdered. Powdered plant (50 g) was extracted four times with methanol at room temperature overnight. The methanolic extract was combined and concentrated under reduced pressure on a rotary evaporator, filtered and then lyophilized.
The plant powder was dissolved in distilled water to use for all assays (Asadi et al. 2010).
Detection of hydroxyl radicals generated by sugar autoxidation
The scavenging capacity for hydroxyl radical was measured according to the method of Hunt et al. (1988). Briefly, different concentrations of S. macilenta extract (10–100 μg/ml) were incubated with sodium benzoate (1 mM; Sigma Aldrich, 18106), potassium phosphate buffer (100 mM, pH 7.2), fructose (100 mM), and CuSO4 (0.1 mM; Sigma Aldrich, 451657) for 4 days at 37 °C. The hydroxylation of benzoate by auto oxidation of fructose (100 mM) in the presence of transition metal (Cu2+) was determined by fluorescence measurement (excitation and emission maxima of 308 and 410 nm, respectively).
Total AGEs and pentosidine fluorescence measurement
In an in vitro system, we produced AGEs by a method previously described (Sharma et al. 2002). Pentosidine is a highly fluorescent AGE product which is a protein crosslink between arginine and lysine residues. Briefly, BSA (10 mg/ml, fatty acid free) was modified in vitro at 37 °C by the reducing sugar, fructose (100 mM). All incubations were carried out in 0.2 M phosphate buffer, pH 7.4, in the absence and presence of different concentrations of S. macilenta extract (10–100 μg/ml) and 1 mM aminoguanidine (AG; Sigma Aldrich, 109266) as a positive control and contained 3 mM sodium azide to prevent bacterial contaminations. Samples were taken at 14 days and dialyzed extensively against phosphate buffer to remove unbound sugar, and any other impurities. To detect total AGEs and pentosidine content, Varian Cary Eclipse spectrofluorometer was used and the fluorescence intensities were measured at the excitation/emission wavelengths of 370/440 and 335/385 nm, respectively.
Determination of fibrillar state with thioflavin T
The fibrillar state of the incubated BSA was determined via (thioflavin T) ThT (Sigma Aldrich, T3516), a reagent that is used for detecting the β-sheet configuration in proteins (Schmitt et al. 2005). ThT interacts with the fibrillar structure of proteins. This interaction intensifies the fluorescence, so the amyloid fibril structures in proteins could be detected. The fluorescence of BSA (0.2 mg/ml) and ThT reagent (10 μM) in phosphate buffer (100 mM, pH 7.4) was measured at 25 °C at the excitation/emission wavelengths of 450/490 nm.
Cell culture and plant extract treatment
Undifferentiated Rat pheochromocytoma (PC12) cells were maintained in high glucose DMEM (Invitrogen, Carlsbad, CA, USA, 52100-039) supplemented with 5 % fetal bovine serum, 10 % horse serum and 1 % antibiotic mixture comprising penicillin–streptomycin (100 U/ml of penicillin and 100 μg/ml of streptomycin) at 37 °C under 95 % O2 and 5 % CO2. PC12 cells (3 × 106 cells) were grown in 75 cm2 culture flasks (Orange Scientific, Braine-l'Alleud, Belgium) and differentiated by using NGF (Sigma Aldrich, N8133; 50 ng/ml) every other day for 6 days. To study the effects of plant extract on differentiated PC12 cells, cells were preincubated with S. macilenta at different concentrations (10, 25, 50 and 100 μg/ml) for 24 h, then H2O2 (150 μM) was added to the medium for 24 h.
Hoechst staining
PC12 cells were stained with Hoechst 33342 dye to evaluate apoptosis (Lotharius et al. 1999). The cells were cultured in a 6-well plate (3 × 105 cells per well) and on the next day plant extract (10, 25, 50 and 100 μg/ml) was added and incubated for 24 h, followed by treatment of H2O2 (150 μM) as an oxidative agent. After 24 h incubation, the cells were harvested and washed three times with PBS and the final suspension (1 × 106 cells/ml) was incubated with Hoechst 33342 (1 μg/ml) (Invitrogen, H3570) for 5 min at room temperature. After incubation, a drop containing cells was splattered on the glass slide and a plastic cover slip was placed over the drop of PBS. Nuclei were visualized using an Olympus microscope.
Western blot analysis
After 24 h exposure to H2O2, the cells were washed with PBS and lysed by homogenizing buffer containing complete protease inhibitor cocktail. Protein concentrations were determined according to Bradford’s method (Bradford 1976). Then, the same concentration of protein in each sample was loaded onto a 12 % gradient gel and was electrophoresed at 120 V. Following electrophoresis, proteins from the gel were transferred to polyvinylidene fluoride membrane (Millipore (Billerica, MA, USA), IPVH00010); membrane was blocked in 5 % powdered nonfat milk solution. The membrane firstly was probed with specific primary antibodies including Bax (Cell Signaling Technologies (Danvers, MA, USA), #2772), Bcl-2 (Cell Signaling, #2876), Caspase-3 (Cell Signaling, #9665), Poly (ADP-ribose) polymerase (PARP; Cell Signaling, #9542) and β-actin (Cell Signaling, #2772; 1:1,000 dilution) for 16 h at 4 °C and then was covered with horseradish peroxidase-conjugated secondary antibody (Cell Signaling, #7074) at room temperature (1:3,000 dilution) for 1.5 h. Finally, the blot was incubated with enhanced ElectroChemiLuminescence (ECL) reagents (Amersham Bioscience (Piscataway, NJ, USA), RPN2132) and expose to X-ray films. Quantification of the results was performed by densitometric scan of films. Data analysis was done by Image.J, measuring integrated density of bands after background subtraction.
Morphological analysis of differentiated PC12 cells
PC12 cells were cultured in a 6-well plate (105 cells per well) and treated by different concentration of plant extract (10, 25, 50 and 100 μg/ml) for 24 h, followed by incubation with H2O2 (150 μM) for 24 h. Three images were taken randomly from each well. To determine the effect of S. macilenta on cell morphology, at least 50 cells were selected and analyzed by using Cell ^ A program. The cells to be analyzed should have 2 characters: the cell body had to be within the field of view and also distinct from the neighboring cell bodies. Their cell body area, average neurite length and average neurite width were measured in μm2 or μm, and number of primary neuritis and bipolar morphology were counted. Cell body area was defined as the area of the cell exclusive of neurite processes. To calculate the neurite length, lengths of the primary process and all associated branches were summed. To establish the average neurite width, the outlines of individual primary neurites were traced, the area calculated and then divided by the length of the neurite. Primary neurites were defined as clear protrusions from the cell body greater than 10 μm length. Cells were considered “bipolar” if they displayed a cell body with one process at either end. To evaluate neurite networks, images were analyzed using the cell counter plugin to score all branching nodes in each image. Nodes were defined as sites at which individual neurites branched or separate neurites contacted each other (Frankel et al. 2009). All measurements expressed as proportions used the number of cells displaying the characteristic as a sub-population of the total number of cells that met the selection criteria described above.
Statistical analysis
All data are represented as the mean ± S.E.M. (Standard Deviation) and processed by commercially available software GraphPad Prism® 5.0. Comparison between groups was made by one-way analysis of variance (ANOVA) followed by an appropriate post hoc test (Newman–Keuls test) to analyze the difference. The statistical significances were achieved when P < 0.05 (* or #P < 0.05, ** or ##P < 0.01 and *** or ###P < 0.001).
Results
Salvia macilenta has hydroxyl radical scavenging capacity
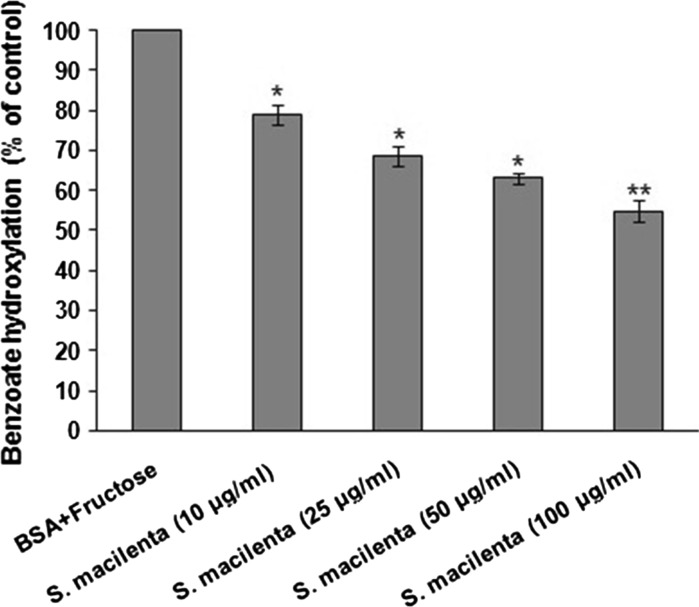
The effect of different concentrations of plant extract (10, 25, 50 and 100 μg/ml) is shown in Fig. 1. The decrease in benzoate hydroxylation correlates with the hydroxyl radical scavenging activity of the extract. An increase in the concentration of extract was associated with lower values of hydroxylated benzoate.
Fig. 1.
Inhibitory effect of S. macilenta extract on hydroxyl radical generation induced by fructose autoxidation in the presence of transition metals. Each value is expressed as mean ± S.E.M (n = 3). *P < 0.05; **P < 0.01 significantly different from BSA + fructose
Salvia macilenta shows antiglycation activity
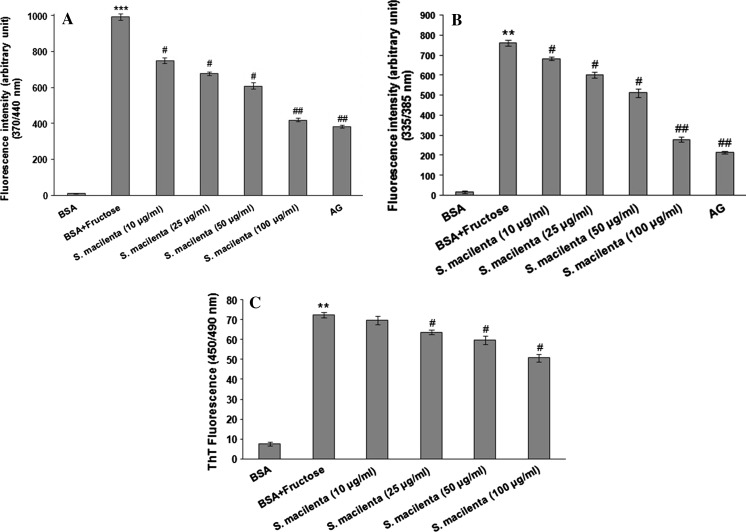
The formation of total AGEs and the inhibitory effect of plant extract were assessed by monitoring the production of fluorescent products. As shown in Fig. 2, the fluorescence intensity of total AGEs highly increased through the incubation of BSA with fructose. As it is evident all different concentrations of S. macilenta have significantly quenched the fluorescence and its activity at 100 μg/ml was comparable to the effect of 1 mM AG, a known inhibitor of the glycation process.
Fig. 2.
Effect of S. macilenta extract (10, 25, 50 and 100 μg/ml) and AG (1 mM) on protein glycosylation and fibrillar structure formation. a Fluorescent AGEs formation. b Fluorescent pentosidine formation. c Inhibitory effect of S. macilenta on fibrillar structure formation. The procedures are described in “Materials and methods”. Each value represents the mean ± S.E.M (n = 3). **P < 0.01 significantly different from BSA control. # P < 0.05; ## P < 0.01 significantly different from BSA + fructose
Figure 2 shows the inhibitory effect of S. macilenta extract on pentosidine formation in BSA incubated with fructose at 37 °C. Plant extract at different concentrations (10, 25, 50 and 100 μg/ml) significantly decreased the fluorescence intensity in a dose-dependent manner and in agreement with the result obtained from AGEs measurements. The inhibitory effect of this plant on pentosidine formation was strong and comparable to the effect of 1 mM AG solution.
To determine the fibrillar state, thioflavin T was used. As shown in Fig. 2C, in the group of fructated BSA, the fibrillar state enhanced compared to BSA group, while S. macilenta extract significantly inhibited fibrillar structure formation at the concentrations more than 10 μg/ml.
Salvia macilenta modulates apoptotic factors in PC12 cells
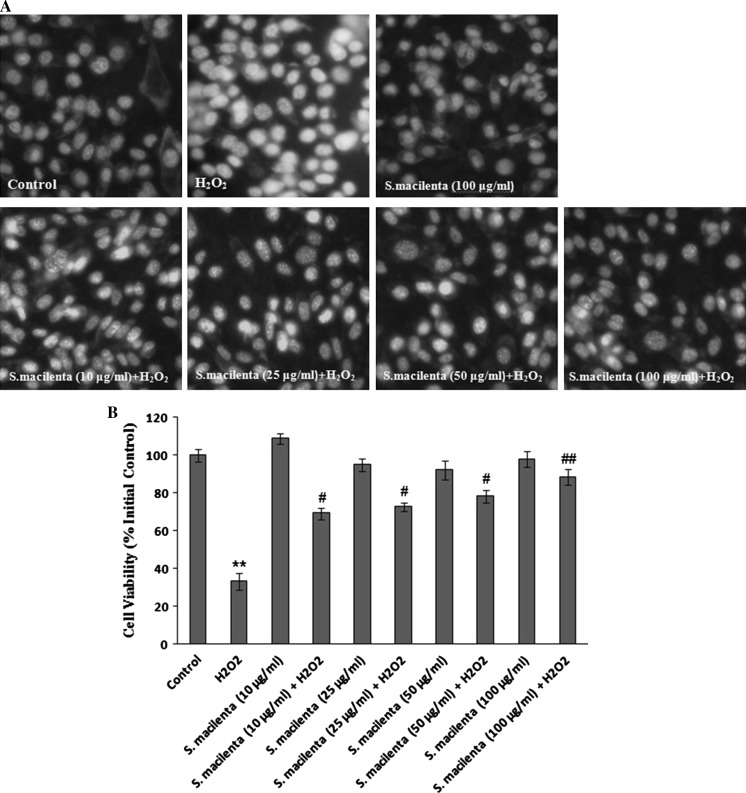
In the previous study, we found that S. macilenta has the neuroprotective effect on H2O2-induced cell death. In this evaluation to determine the type of cell death, Hoechst 33342 was applied to detect apoptotic nuclei. Hoechst staining showed that DNA fragmentation and condensation of chromatin, which cause bright fluorescence, decreased in the presence of different concentrations of plant extract (10, 25, 50 and 100 μg/ml), compared to H2O2-treated cells (Fig. 3a, b). Also, S. macilenta (100 μg/ml) had no toxic effect, when compared to control cells.
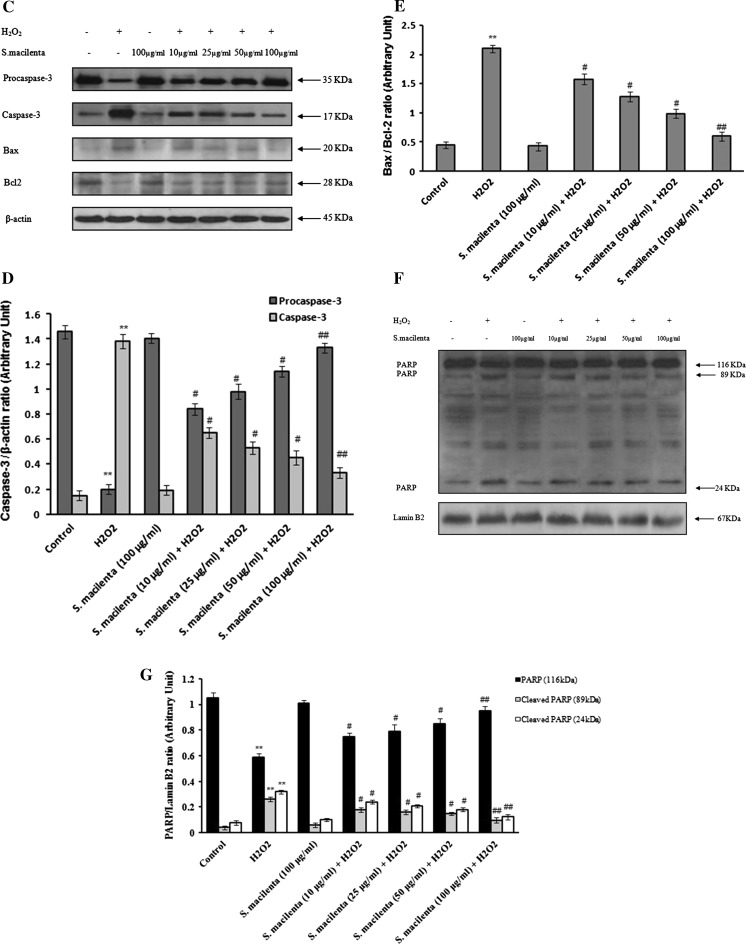
Fig. 3.
The effect of S. macilenta on decrease of apoptotic factors in H2O2-induced cell death in PC12 cells. a, b Hoechst 33342 staining. The cells are exposed to different concentrations of S. macilenta extract for 24 h followed by exposure to 150 μM of H2O2 for 24 h. The number of stained cells is counted in 10 randomly selected fields. Viability is calculated as the percentage of living cells in treated cultures compared to those in control cultures. c, d, e Caspase-3, Bax and Bcl-2 response to different concentrations of S. macilenta extract in PC12 cells pretreated for 24 h and then exposed to H2O2 (150 μM) for 24 h. Twenty microgram proteins are separated on SDS-PAGE, Western blotted, probed with anti-caspase-3, -Bax and -Bcl-2 antibodies and reprobed with anti-β-actin antibody. (One representative Western blot is shown; n = 3). The densities of Procaspase-3, caspase-3 and Bax: Bcl-2 ratio are measured, the ratios to β-actin are calculated. f, g The level of PARP is evaluated in the presence and absence of S. macilenta extract in PC12 cells pretreated for 24 h and then exposed to H2O2 for 24 h. Each value indicates the mean ± S.E.M. from three independent experiments (biological replicates) and three replicates (technical replicates) (n = 3). **P < 0.01 significantly different from control cells. # P < 0.05; ## P < 0.01 significantly different from H2O2-treated cells
Since we wished to investigate the possible antiapoptotic effect of S. macilenta on H2O2-induced cell death, the protein levels of Bax, Bcl-2, cleaved caspase-3 and PARP-1 were determined using western blot analysis. Caspases are major players of apoptotic cell death. Activated caspase-3 is one of the factors responsible for triggering apoptosis. The Bax/Bcl-2 ratio in H2O2-exposed cells that pretreated with S. macilenta, as well as cleavage of caspase-3 (Fig. 3c–e), were reduced compared to the H2O2-treated cells, demonstrating the ability of S. macilenta to suppress the caspase-dependent apoptotic cascade. In addition, the level of cleaved PARP decreased in the presence of S. macilenta (Fig. 3f, g).
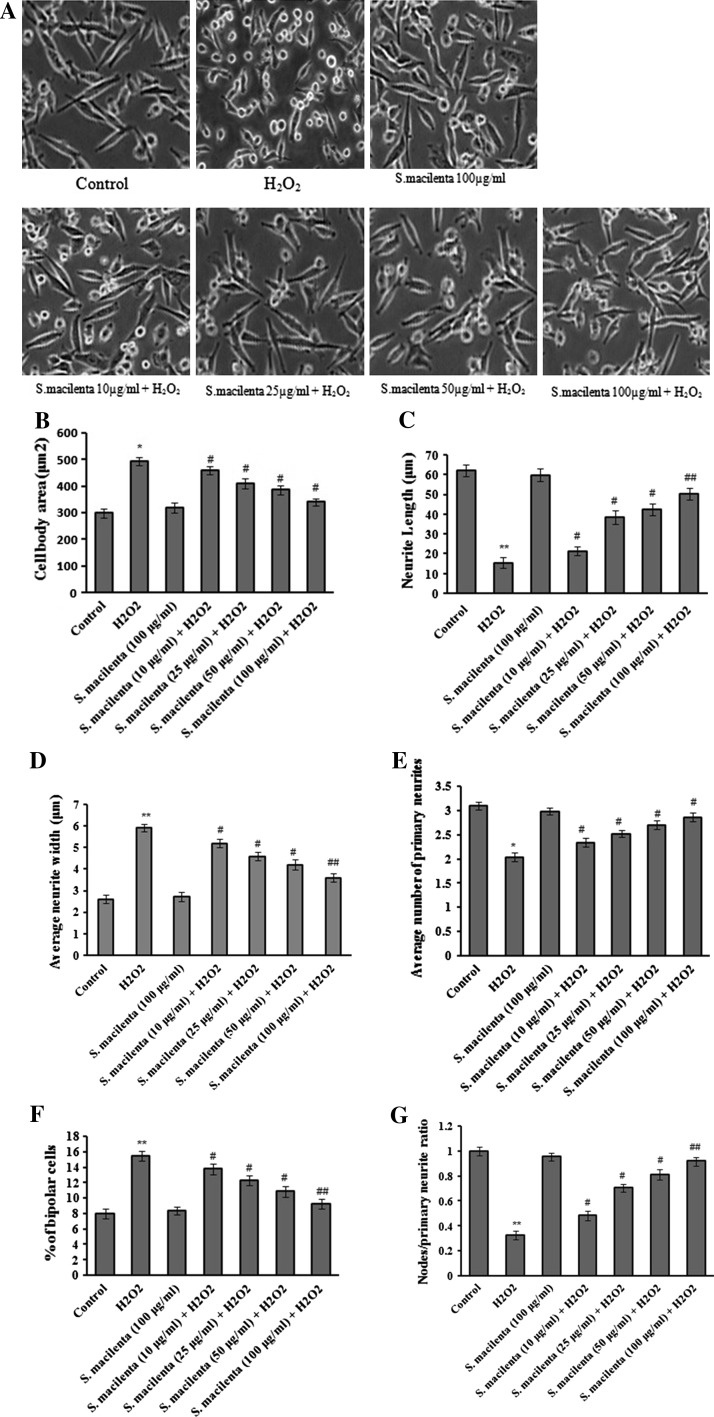
Salvia macilenta attenuates disruption of neurite growth and complexity in differentiated PC12 cells
NGF-differentiated PC12 cells were pretreated with different concentrations of plant extract in the presence of H2O2 (150 μM; Fig. 4a). As shown in Fig. 4b, average cell body area which was defined as the area of the cell exclusive of neurite processes, enhanced in H2O2 exposed cultures, compared to control cultures, whereas pretreatment of cells with S. macilenta significantly caused a decrease in cell body area. Accordingly, as shown in Fig. 4c, S. macilenta significantly increased neurite length that is the summation of the lengths of the primary process and all associated branches. In the same situation neurite width that is the division of the area of individual primary neurite by the length of the neurite decreased (Fig. 4d), compared to H2O2-treated cells.
Fig. 4.
Effect of S. macilenta extract on H2O2-induced disruption of neurite outgrowth in differentiated PC12 cells. a NGF-differentiated PC12 cells are pretreated with different concentrations of S. macilenta extract in the presence of H2O2. Parameters are quantified at 24 h. b Cell body area; c average neurite length; d average neurite width; e primary neurites per cell; f percent of bipolar cells and g the ratio of nodes to primary neurites are calculated. Each value indicates the mean ± S.E.M. from three independent experiments (biological replicates) and three replicates (technical replicates; n = 3). *P < 0.05; **P < 0.01 significantly different from control cells. # P < 0.05; ## P < 0.01 significantly different from H2O2-treated cells
Specific parameters of morphological complexity were measured as well. The number of primary neurites (>10 μm) emanating from individual cell bodies was measured as one of the most important parameters. As shown in Fig. 4e, the number of primary neurites per cell body decreased in H2O2-treated cells. In contrast, the proportion of cells with the very simple bipolar morphology of the cell body and only two neurites increased in H2O2-treated cells, when compared to control cultures (Fig. 4f). Pretreatment of cells with S. macilenta significantly and dose-dependently increased the number of neurites per cell, and thus decreased the proportion of bipolar cells (Fig. 4e, f). As the last parameter, we calculated the ratio of total neurite branching nodes to total number of primary neuritis. As shown in Fig. 4g, the ratio of nodes to primary neuritis decreased in H2O2-treated cells, whereas S. macilenta enhanced this ratio significantly.
Discussion
Oxidative stress cascades, including attack from ROS and deficiency in intracellular antioxidative defense is involved in a variety of human diseases such as AD which may be caused by intracellular oxidative damage to biomolecules via ROS. Minimizing the cellular redox imbalance may be one of the most important approaches for the prevention of this aging-associated disease. So intake of natural antioxidants, that are able to scavenge free radicals, might serve as a feasible method to augment the antioxidant capacity and protect cells from oxidative damage (Bub et al. 2003). Previous studies showed that plants have the ability of free radical scavenging because of containing different groups of phenolic compounds, including simple phenolics, phenolic acids, anthocyanins, hydroxycinnamic acid derivatives and flavonoids (Bandoniene and Murkovic 2002). The genus Salvia (Laminaceae) is one example which includes nearly 900 species spread throughout the world. Many Salvia species have antiapoptotic effects in an in vitro and in vivo model (Shaerzadeh et al. 2011; Khodagholi and Ashabi 2013). Asadi et al. showed in an in vitro model that Salvia extracts increased enzymatic antioxidant defense such as superoxide dismutase and catalase, and improved enzyme-independent systems such as glutathione (Asadi et al. 2011). We have shown previously that S. macilenta which belongs to this genus has antioxidant activity and shows neuroprotective effects against H2O2-induced cell death. In the current study we investigated the antiglycating activity and anti-apoptotic effect of this plant. We further evaluated its effect on H2O2-induced impairment of neurite outgrowth.
Non-enzymatic glycation of proteins which leads to formation of AGE is a process that impairs the function of biomolecules and leads to a variety of endocrine disorders. Halliwell showed that AGE formation is stimulated by free radicals (Halliwell 2001). On another side, large amounts of ROS are produced at many steps of the parallel and sequential glycation cascade (Li et al. 2007). Eventually this may lead to oxidative stress and a variety of degenerative diseases. So finding AGE inhibitors is a way to go against this kind of diseases. Different studies showed that some plant-derived agents have antiglycation activity such as phenolic compounds (Tsuji-Naito et al. 2009; Choudhary et al. 2010). Moreover, phenolics, particularly flavonoids, are responsible for the anti-glycation activity of herbal infusions (Ho et al. 2010). Some reports showed the antiglycating activity of some members of Salvia genus (Shaerzadeh et al. 2011; Asadi et al. 2011) and interestingly, we found S. macilenta as an antiglycating plant via phenolic and flavonoid compounds.
As we mentioned previously (Asadi et al. 2010), this plant protects PC12 cells against H2O2 toxicity. To clarify this ability, we cultured PC12 cells and treated them with H2O2 as an inducer of apoptosis in this cell line (Satoh et al. 1997). Hoechst staining showed thatS. macilenta has neuroprotective effects against H2O2-induced apoptotic cell death in a dose-dependent manner, which was confirmed by western blot analyses of main apoptotic factors as well. Many studies showed H2O2 induces apoptotic cell death by initiating mitochondrial dysfunction (Maroto and Perez-Polo 1997; Tong and Perez-Polo 1996) that is associated with changes in the Bcl-2 family of proteins and activation of caspases. Therefore, we analyzed Bax and Bcl-2, two proteins affecting caspase-dependent apoptotic pathway, and found that S. macilenta could decrease Bax/Bcl-2 ratio significantly. In the following, we focused on caspase-3 cleavage and the caspase-3 substrate PARP and detected that this plant could decrease activated caspase-3 and cleaved PARP.
Besides, S. macilenta not only increases cell viability (Asadi et al. 2010), but also protects the cells against impairment of neurite out growth. Once neurons begin to aggregate into recognizable structures, and sometimes even before, they begin to extend elongated, membrane-enclosed protrusions of cytoplasm that are called “processes” or “neurites”. These neurites grow towards other regions of the nervous system or other structures on which the neurons will eventually form synapses. Since the proper functioning of the nervous system depends on the formation of proper connections, the factors which cause disruption in neurite outgrowth may lead to many problems, including neurodegeneration. Oxidative stress is also the major culprit in neuronal death observed in AD (Choi 1995) which is primarily a disorder of aging with loss of cognitive function. Neurite outgrowth in cultured neurons is considered as a symptom of neuroregenerative potential (Ng et al. 1996). Therefore, finding compounds that support neurite outgrowth against the toxicity of H2O2 is a key element in treating neurodegenerative diseases. In the present study, we found that H2O2 decreased neurite outgrowth and complexity, while S. macilenta improves them. Hence S. macilenta’s potential to aid neurite outgrowth may open a new horizon for more studies.
Taken together, our present study demonstrates the antioxidant and antiglycation effects of S. macilenta. This plant inhibits H2O2-induced apoptotic cell death and attenuates neurite outgrowth and complexity disruption. Whether this neuroprotective effect can be observed in rat model of AD, is still matter of question and is now in progress in our laboratory.
Acknowledgments
The authors thank Dr. Sonboli for providing S. macilenta extract. This work was supported by Shahid Beheshti University of Medical Sciences.
Conflict of interest
The authors have no conflict of interest.
References
- Asadi S, Ahmadiani A, Esmaeili MA, Sonboli A, Ansari N, Khodagholi F. In vitro antioxidant activities and an investigation of neuroprotection by six Salvia species from Iran: a comparative study. Food Chem Toxicol. 2010;48:1341–1349. doi: 10.1016/j.fct.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Asadi S, Khodagholi F, Esmaeili MA, Tusi SK, Ansari N, Shaerzadeh F, Sonboli A, Ghahremanzamaneh M. Chemical composition analysis, antioxidant, antiglycating activities and neuroprotective effects of S. choloroleuca, S. mirzayanii and S. santolinifolia from Iran. Am J Chin Med. 2011;39:615–638. doi: 10.1142/S0192415X1100907X. [DOI] [PubMed] [Google Scholar]
- Bandoniene D, Murkovic M. On-line HPLC-DPPH screening method for evaluation of radical scavenging phenols extracted from apples (Malus domestica L.) J Agric Food Chem. 2002;50:2482–2487. doi: 10.1021/jf011475s. [DOI] [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bub A, Watzl B, Blockhaus M, Briviba K, Liegibel U, Müller H, Pool-Zobel BL, Rechkemmer G. Fruit juice consumption modulates antioxidative status, immune status and DNA damage. J Nutr Biochem. 2003;14:90–98. doi: 10.1016/S0955-2863(02)00255-3. [DOI] [PubMed] [Google Scholar]
- Choi BH. Oxidative stress and Alzheimer’s disease. Neurobiol Aging. 1995;16:675–678. doi: 10.1016/0197-4580(95)00065-M. [DOI] [PubMed] [Google Scholar]
- Choudhary MI, Maher S, Begum A, Abbaskhan A, Ali S, Khan A, Shafique-ur-Rehman, Atta-ur-Rahman (2010) Characterization and antiglycation activity of phenolic constituents from Viscum album (European Mistletoe). Chem Pharm Bull (Tokyo) 58:980–982 [DOI] [PubMed]
- Cook NC, Samman S. Flavonoids—chemistry, metabolism, cardioprotective effects and dietary sources. J Nutr Biochem. 1996;7:66–76. doi: 10.1016/0955-2863(95)00168-9. [DOI] [Google Scholar]
- Cushnie TPT, Lamb A. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel S, Concannon J, Brusky K, Pietrowicz E, Giorgianni S, Thompson WD, Currie DA. Arsenic exposure disrupts neurite growth and complexity in vitro. Neurotoxicology. 2009;30:529–537. doi: 10.1016/j.neuro.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Ghannadi A, Samsam-Shariat SH, Moattar F. Volatile constituents of the flower of Salviahydrangea DC. Ex Benth Daru. 1999;7:23–25. [Google Scholar]
- Gibson GE, Huang HM. Oxidative stress in Alzheimer’s disease. Neurobiol Aging. 2005;26:575–578. doi: 10.1016/j.neurobiolaging.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxygen radicals as key mediators in neurological disease: fact or fiction? Ann Neurol. 1992;32:S10–S15. doi: 10.1002/ana.410320704. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- Ho SC, Wu SP, Lin SM, Tang YL. Comparison of anti-glycation capacities of several herbal infusions with that of green tea. Food Chem. 2010;112:768–774. doi: 10.1016/j.foodchem.2010.03.051. [DOI] [Google Scholar]
- Hohmann J, Zupkó I, Rédei D, Csányi M, Falkay G, Máthé I, Janicsák G. Protective effects of the aerial parts of Salvia officinalis, Melissa officinalis and Lavandula angustifolia and their constituents against enzyme-dependent and enzyme-independent lipid peroxidation. Planta Med. 1999;65:576–578. doi: 10.1055/s-2006-960830. [DOI] [PubMed] [Google Scholar]
- Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988;256:205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodagholi F, Ashabi G. Dietary supplementation with Salvia sahendica attenuates memory deficits, modulates CREB and its down-stream molecules and decreases apoptosis in amyloid beta-injected rats. Behav Brain Res. 2013;241:62–69. doi: 10.1016/j.bbr.2012.11.026. [DOI] [PubMed] [Google Scholar]
- Li SY, Sigmon VK, Babcock SA, Ren J. Advanced glycation endproduct induces ROS accumulation, apoptosis, MAP kinase activation and nuclear O-GlcNAcylation in human cardiac myocytes. Life Sci. 2007;80:1051–1056. doi: 10.1016/j.lfs.2006.11.035. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotharius J, Dugan LL, O’Malley KL. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J Neurosci. 1999;19:1284–1293. doi: 10.1523/JNEUROSCI.19-04-01284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Foo LY. Polyphenolics of Salvia—a review. Phytochemistry. 2002;59:117–140. doi: 10.1016/S0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- Maroto R, Perez-Polo JR. BCL-2-related protein expression in apoptosis: oxidative stress versus serum deprivation in PC12 cells. J Neurochem. 1997;69:514–523. doi: 10.1046/j.1471-4159.1997.69020514.x. [DOI] [PubMed] [Google Scholar]
- Mozafarian V. A dictionary of iranian plant names (Latin English Persian) Tehran: Farhang Mosafer Publication; 1996. [Google Scholar]
- Ng WP, Cartel N, Roder J, Roach A, Lozano A. Human central nervous system myelin inhibits neurite outgrowth. Brain Res. 1996;720:17–24. doi: 10.1016/0006-8993(96)00062-5. [DOI] [PubMed] [Google Scholar]
- Richardson JS, Subbarao KV, Ang LC. On the possible role of iron-induced free radical peroxidation in neural degeneration in Alzheimer’s disease. Ann N Y Acad Sci. 1992;648:326–327. doi: 10.1111/j.1749-6632.1992.tb24570.x. [DOI] [PubMed] [Google Scholar]
- Satoh T, Enokido Y, Aoshima H, Uchiyama Y, Hatanaka H. Changes in mitochondrial membrane potential during oxidative stress-induced apoptosis in PC12 cells. J Neurosci Res. 1997;50:413–420. doi: 10.1002/(SICI)1097-4547(19971101)50:3<413::AID-JNR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Schmitt J, Münch G, Gasic-Milencovic J. Characterization of advanced glycation end products for biochemical studies: side chain modifications and fluorescence characteristics. Anal Biochem. 2005;338:201–215. doi: 10.1016/j.ab.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Scott JA, King GL. Oxidative stress and antioxidant treatment in diabetes. Ann N Y Acad Sci. 2004;1031:204–213. doi: 10.1196/annals.1331.020. [DOI] [PubMed] [Google Scholar]
- Senevirathne M, Kim S, Siriwardhana N, Ha J, Lee K, Jeon Y. Antioxidant potential of Ecklonia cava on reactive oxygen species scavenging metal chelating, reducing power and lipid peroxidation inhibition. Food Sci Technol Int. 2006;12:27–38. doi: 10.1177/1082013206062422. [DOI] [Google Scholar]
- Shaerzadeh F, Ahmadiani A, Esmaeili MA, Ansari N, Asadi S, Tusi SK, Sonboli A, Ghahremanzamaneh M, Khodagholi F. Antioxidant and antiglycating activities of Salvia sahendica and its protective effect against oxidative stress in neuron-like PC12 cells. J Nat Med. 2011;65:455–465. doi: 10.1007/s11418-011-0519-9. [DOI] [PubMed] [Google Scholar]
- Sharma SD, Pandey BN, Mishra KP, Sivakami S. Amadori product and age formation during nonenzymatic glycosylation of bovine serum albumin in vitro. J Biochem Mol Biol Biophys. 2002;6:233–242. doi: 10.1080/10258140290031554. [DOI] [PubMed] [Google Scholar]
- Tong L, Perez-Polo JR. Effect of nerve growth factor on AP-1, NF-kappa B, and Oct DNA binding activity in apoptotic PC12 cells: extrinsic and intrinsic elements. J Neurosci Res. 1996;45:1–12. doi: 10.1002/(SICI)1097-4547(19960701)45:1<1::AID-JNR1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Tsuji-Naito K, Saeki H, Hamano M. Inhibitory effects of Chrysanthemum species extracts on formation of advanced glycation end products. Food Chem. 2009;116:854–859. doi: 10.1016/j.foodchem.2009.03.042. [DOI] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Yu BP, Chung HY. Oxidative stress and vascular aging. Diabetes Res Clin Pract. 2001;54:S73–S80. doi: 10.1016/S0168-8227(01)00338-2. [DOI] [PubMed] [Google Scholar]
- Zupkó I, Hohmann J, Rédei D, Falkay G, Janicsák G, Máthé I. Antioxidant activity of leaves of Salvia species in enzyme-dependent and enzyme-independent systems of lipid peroxidation and their phenolic constituents. Planta Med. 2001;67:366–368. doi: 10.1055/s-2001-14327. [DOI] [PubMed] [Google Scholar]