Abstract
Multiple human population studies have established the concentration of high density lipoprotein (HDL) cholesterol as an independent, inverse predictor of the risk of having a cardiovascular event. Furthermore, HDLs have several well-documented functions with the potential to protect against cardiovascular disease. These include an ability to promote the efflux of cholesterol from macrophages in the artery wall, inhibit the oxidative modification of low density lipoproteins (LDLs), inhibit vascular inflammation, inhibit thrombosis, promote endothelial repair, promote angiogenesis, enhance endothelial function, improve diabetic control, and inhibit hematopoietic stem cell proliferation. There are undoubtedly other beneficial functions of HDLs yet to be identified. The HDL fraction in human plasma is heterogeneous, consisting of several subpopulations of particles of varying size, density, and composition. The functions of the different HDL subpopulations remain largely unknown. Given that therapies that increase the concentration of HDL cholesterol have varying effects on the levels of specific HDL subpopulations, it is of great importance to understand how distribution of different HDL subpopulations contribute to the potentially cardioprotective functions of this lipoprotein fraction. This review summarizes current understanding of the relationship of HDL subpopulations to their cardioprotective properties and highlights the gaps in current knowledge regarding this important aspect of HDL biology.
Keywords: cholesterol efflux, vascular inflammation, pancreatic beta cell function, oxidation, thrombosis, endothelial repair, endothelial function, angiogenesis, apoptosis
The proposition that high density lipoproteins (HDLs) protect against the development of cardiovascular diseases is based on a number of robust and consistent observations: i) numerous human population studies have shown that the plasma concentrations of both HDL cholesterol and the major HDL apolipoprotein, apoA-I, are independent, inverse predictors of the risk of having a cardiovascular event (1–5); moreover, a low concentration of HDL cholesterol remains predictive of increased cardiovascular risk, even when low density lipoprotein (LDL) cholesterol has been reduced to very low levels by treatment with statins (6); ii) HDLs have several well-documented functions with the potential to protect against cardiovascular disease (7, 8); iii) interventions that increase the concentration of HDLs inhibit the development and progression of atherosclerosis in several animal models (9–12); and iv) in “proof-of-concept” studies in humans, intravenous infusions of reconstituted HDLs (rHDLs) consisting of apoA-I complexed with phospholipids promote regression of coronary atheroma as assessed by intravascular ultrasound (13, 14).
However, interventions that increase the concentration of HDL cholesterol in humans have not yet been shown to translate into a reduction in clinical cardiovascular events. Indeed, recent human clinical trials investigating the effects of raising the level of HDL cholesterol by treatment with cholesteryl ester transfer protein (CETP) inhibitors or with niacin failed to demonstrate any clinical cardiovascular benefit (15–17) (http://www.thrivestudy.org), and in one case, the treatment caused harm (15). A reasonable assumption that has been made from these studies is that the cholesterol content of HDLs is not the factor that protects. Thus, if HDLs do have direct cardioprotective properties, it follows from the human population studies that, while the concentration of HDL cholesterol is generally an excellent marker of the HDL functions that do protect, it does not invariably reflect their cardioprotective functions. This is consistent with the finding in a recent Mendelian randomization study that some genetic mechanisms that raise the concentration of HDL cholesterol appear not to lower the risk of myocardial infarction (18). The authors of this analysis concluded that, while this result challenges the concept that raising the concentration of HDL cholesterol will translate into a reduction in risk of myocardial infarction, it did not address the possibility that an enhancement of HDL function would be protective.
For example, in some clinical circumstances, a high concentration of HDL cholesterol is not accompanied by a decrease in cardiovascular risk (19). This may reflect the fact that an intervention that raises the level of HDL cholesterol is not necessarily accompanied by an increase in the functionality of the HDL fraction. This leads to an obvious question, Given the robust evidence from animal and human population studies that HDLs are antiatherogenic, why have recent clinical trials using agents that increase the level of HDL cholesterol failed to demonstrate a reduction in clinical cardiovascular events?
There are several potential explanations for this. It is possible that the inverse relationship between the concentration of HDL cholesterol and cardiovascular risk observed in human population studies is an epiphenomenon rather than a direct cardioprotective effect. However, this is not supported by many animal studies showing that atherosclerosis is inhibited by interventions that increase the level of HDL cholesterol (9–12). It is also possible, but unlikely, that the ability of HDLs to inhibit the development of atherosclerosis in animals and to promote regression of atherosclerosis in humans does not necessarily translate into an ability of HDLs to reduce clinical cardiovascular events in humans. There is also evidence that some agents that increase HDL cholesterol levels have adverse off-target effects that may negate the benefit conferred by the HDL elevation. This may well have been the case with the CETP inhibitor torcetrapib (15). It is also possible that a niacin-induced insulin resistance (20) may partially negate the effects of this agent on HDLs.
Furthermore, it has been suggested that increasing HDL cholesterol levels may be ineffective in statin-treated patients in whom the concentration of LDL cholesterol is very low (21), although there is little evidence to support this. It is also possible that in some studies the ability of HDL cholesterol-raising agents to reduce cardiovascular events was tested in people in whom HDL function was compromised. This may well have been the case in the population of acute coronary syndrome patients studied in the Dal-OUTCOME trial, in which even in the placebo group, there was no evidence of a relationship between HDL cholesterol levels and cardiovascular events (17). This observation raised the possibility, as has been reported in ex vivo studies (22), that the cardioprotective properties of the HDLs are compromised after an acute coronary syndrome. If this is true, it follows that increasing endogenous HDL levels in these individuals may not lead to a reduction in cardiovascular events.
These observations raise several questions and highlight major gaps in current knowledge about the relationship between HDL and cardiovascular disease. For example, it is not known which HDL functions are clinically important, nor is it known whether some functions are more cardioprotective than others. Moreover, we know little about the functionality of individual HDL subpopulations. Nor do we know whether therapeutic interventions that increase the concentration of HDL cholesterol are necessarily accompanied by an enhancement of HDL function.
This review is concerned with what is currently known about the potentially cardioprotective functions of HDL, and it highlights the gaps in current knowledge regarding this important aspect of HDL biology. In the interest of keeping the size of the review manageable, it has not been possible to cite all of the references that would otherwise be worthy of inclusion.
OVERVIEW OF HDL FUNCTIONS THAT HAVE THE POTENTIAL TO REDUCE CARDIOVASCULAR RISK
Several well-documented functions of HDLs and apoA-I have the potential to protect against cardiovascular disease. The most extensively studied of these relates to the ability of HDLs to promote efflux of cholesterol from macrophages in the artery wall (23). HDLs also inhibit vascular inflammation (8, 24) and has antioxidant (8) and antithrombotic (25) properties. They enhance endothelial function (26), promote endothelial repair (27, 28), increase angiogenesis (29), suppress the production and mobilization of monocytes and neutrophils from bone marrow (30), and have recently been reported to have antidiabetic properties (31, 32). Which of these functions of HDLs are clinically important is not known. Nor is it known which HDL component(s) or subpopulations are responsible for these potentially cardioprotective properties. This lack of insight is largely due to the fact that HDLs consist of multiple subpopulations of particles that are continually being interconverted from one to another by a range of plasma factors (33), as well as by the fact that it is currently not feasible to isolate specific HDL subpopulations in amounts that are sufficient to investigate their functionality in a systematic manner.
HETEROGENEITY OF THE HDL FRACTION IN HUMAN PLASMA
The HDLs in human plasma are heterogeneous in terms of particle density, size, shape, surface charge, and composition (34). When isolated on the basis of density, human HDLs can be resolved into two major subfractions: HDL2 which comprises large, less dense particles and HDL3, which consists of smaller, denser particles. Separation of the total HDL fraction on the basis of particle size by nondenaturing polyacrylamide gradient gel electrophoresis has identified at least five distinct HDL subpopulations (35).
HDLs also vary in surface charge and can be resolved on this basis by agarose gel electrophoresis into several subpopulations. According to this approach, α-migrating particles equate with the spherical HDL particles that predominate in human plasma, while pre-β-migrating particles comprise a single molecule of lipid-poor apoA-I, a single molecule of apoA-I complexed with a small number of phospholipid molecules, or discoidal particles that contain two or three molecules of apoA-I complexed with multiple phospholipid molecules plus a small amount of unesterified cholesterol (36).
Discrete HDL subpopulations have also been identified on the basis of their apolipoprotein composition: those that contain apoA-I but are deficient in apoA-II, the second most abundant HDL apolipoprotein (A-I HDLs), and those that contain both apoA-I and apoA-II (A-I/A-II HDLs) (37). A minor subpopulation of apoE-containing, γ-migrating HDL particles has also been reported (38).
It has been suggested that A-I HDLs may be superior to A-I/A-II HDLs in their ability to protect against atherosclerosis (39), although evidence for this is associative and requires confirmation in large prospective population studies. It has also been suggested that particles in the HDL2 subfraction may be more cardioprotective than those in the HDL3 subfraction (40), although other studies have concluded that a very high concentration of large, cholesterol-rich HDL2 particles, when not accompanied by a correspondingly high level of apoA-I-containing HDL, may be associated with increased rather than decreased cardiovascular risk (19). Thus, the evidence linking cardioprotection to specific HDL subpopulations in humans is both limited and conflicting.
There is compelling evidence from human studies that interventions that raise the level of HDL cholesterol have varying effects on specific HDL subpopulations. For example, statins (41, 42), niacin (43), and CETP inhibitors (44) tend to increase the concentration of large, apoA-I-containing HDLs. Fibrates, on the other hand, tend to increase levels of smaller HDL particles that contain both apoA-I and apoA-II (45). The clinical implications of these differences are not known. There is also evidence from animal studies that different approaches to raising the concentration of HDL cholesterol may vary markedly in terms of their ability to inhibit atherosclerosis. For example, there is robust and consistent evidence that overexpression of apoA-I in mice (10, 46) and rabbits (11), as well as intravenous infusions of native HDLs or rHDLs into these animals (9, 47), inhibits the development of atherosclerosis. There is also consistent evidence that increasing endogenous HDL cholesterol levels by inhibiting CETP (48, 49) or by overexpressing LCAT in cholesterol-fed rabbits (50) is antiatherogenic. However, there are situations in some genetically engineered mouse models where an increase in HDL cholesterol level is not accompanied by an inhibition of atherosclerosis. These include mice that overexpress LCAT (51) or lack endothelial lipase (52).
Until we know which HDL components and subpopulations relate to specific, potentially cardioprotective HDL functions and until we have much more information about the effects of HDL-raising therapies on HDL composition, HDL subpopulation distribution, and HDL function, it will be difficult to predict how any specific HDL-targeted therapy will impact on human cardiovascular risk. The following sections summarize what is currently known about the potentially cardioprotective functions of HDLs, how they relate to specific HDL lipid and apolipoprotein constituents and, wherever possible, the relationship of specific HDL functions to specific HDL subpopulations.
HDLs AND CHOLESTEROL EFFLUX
The efflux of cholesterol from a variety of cell types, including macrophages, to HDLs in the extracellular space is mediated by four distinct processes (53). These are i) the efflux of cholesterol to apoA-I from cells expressing the ATP binding cassette transporter A1 (ABCA1) (54, 55); ii) the efflux of cholesterol to HDLs from cells expressing ABCG1 (56, 57); iii) the bidirectional exchange of cholesterol between HDLs and cell membranes expressing scavenger receptor-B1 (SR-B1) (58, 59); and iv) passive aqueous diffusion of cholesterol from cell membranes to HDLs (58).
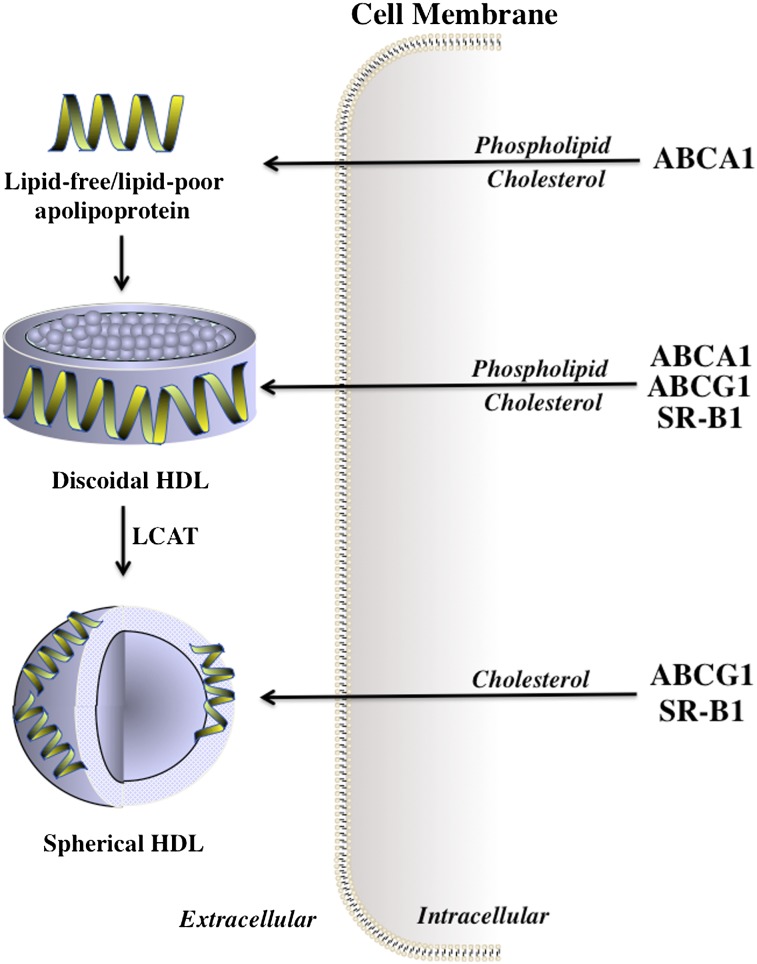
ABCA1 translocates phospholipids and cholesterol from the inner to the outer leaflets of cell membranes, from where they are exported to lipid-free or lipid-poor apolipoproteins in the extracellular space (60). This progressively lipidates the apolipoproteins, leading to the generation of discoidal HDL particles that can also act as acceptors of the cholesterol that effluxes from ABCA1-containing cells (Fig. 1). Discoidal HDLs are also excellent substrates for lecithin:cholesterol acyltransferase (LCAT), the plasma enzyme that esterifies the newly acquired cholesterol. The resulting cholesteryl esters are hydrophobic and are sequestered into the center of the particles in a process that converts discoidal HDLs into the spherical HDL particles that predominate in human plasma. Both discoidal and spherical HDL particles act as acceptors of the cholesterol that is exported from cells by ABCG1 (Fig. 1).
Fig. 1.
Efflux of cholesterol and phospholipids from cells to HDL. Phospholipids and cholesterol are exported from cell membranes via ABCA1 to lipid-free or lipid-poor apolipoproteins, generating discoidal HDL that accepts cholesterol from ABCA1, ABCG1, and SR-B1. The cholesterol in discoidal HDL is esterified by LCAT, which generates spherical HDL that accepts cholesterol from ABCG1 and SR-B1.
The third cholesterol efflux mechanism is mediated by the binding of both discoidal and spherical HDLs to SR-B1. As the rate of SR-B1-mediated cholesterol efflux from cells is positively correlated with the concentration of HDL cholesterol (59), it follows that the contribution of this process to total efflux may increase when HDL levels are increased by pharmacological inhibition of CETP, by infusions of rHDLs, or by other means. Evidence in support of this notion comes from a recent report showing that SR-B1 accounts, at least in part, for the rapid mobilization of cellular cholesterol into the plasma compartment in mice infused with discoidal rHDLs (61).
Passive aqueous diffusion of cholesterol from the cell surface to HDL particles in the extracellular space reflects the movement of cholesterol down the concentration gradient that is formed when LCAT depletes the HDL surface of cholesterol by generating cholesteryl esters that move into the center of the particle. This LCAT-generated cholesterol concentration gradient enables HDL particles to acquire additional cholesterol from cell membranes as well as from other lipoproteins.
The contributions of the various efflux pathways in cholesterol-normal and cholesterol-enriched cells were evaluated by Adorni et al. (62). They reported that ABCA1 had little effect on total efflux when cell cholesterol levels were normal. However, efflux was decreased by 50% in macrophages from ABCA1-deficient mice, whereas studies of macrophages from ABCG1-deficient mice indicated that approximately 20% of the efflux from cholesterol-loaded monocyte-derived macrophages was attributable to ABCG1. In this study, SR-BI was shown to make only a minor contribution to the total cholesterol efflux. In contrast, aqueous diffusion was found to be a major contributor to efflux, particularly in cells with normal cholesterol levels.
Despite significant efforts in recent years, the relative contributions of these four processes to the net efflux of cholesterol from macrophages in the artery wall remain poorly defined. The effects of variations in HDL composition and subpopulation distribution on the regulation of cholesterol efflux are also not well understood.
The importance of understanding such factors is highlighted by the recent observation that HDLs isolated from different human subjects vary in their ability to promote cholesterol efflux from macrophages ex vivo (63). Furthermore, the cholesterol efflux promoted by HDLs isolated from different individuals correlates inversely with measures of atherosclerosis, independent of the plasma concentration of HDL cholesterol (63). So the question arises, how does HDL composition and HDL subpopulation distribution impact the ability of HDL to promote the efflux of cholesterol from cells?
Roles of specific HDL constituents and specific HDL subpopulations as mediators of cellular cholesterol efflux
Lipid-free apoA-I, lipid-poor apoA-I, and discoidal HDL particles, all of which migrate to a pre-β position when subjected to agarose gel electrophoresis, are the preferred acceptors of the cholesterol that effluxes from cells that express ABCA1 (60, 64). There is evidence that, in addition to apoA-I, other HDL apolipoproteins that contain multiple amphipathic α-helical domains (apoA-II, apoA-IV, apoC-I, apoC-II, apoC-III, and apoE) can accept the cholesterol and phospholipids released from cells that express ABCA1 (65). The relative contributions of these acceptors to ABCA1-mediated cholesterol efflux in vivo are completely unknown. The fact that all of these apolipoproteins contain multiple amphipathic α-helical motifs suggests that there is likely a similarity in the mechanism by which they mediate efflux. It is tempting to speculate that their capacity to remove cholesterol from cells may simply be a reflection of their relative abundance in plasma.
Larger, spherical HDL particles, whether containing apoA-I without apoA-II or containing both apoA-I and apoA-II, are the preferred acceptors of the cholesterol released from cells by ABCG1 (56) and by SR-B1 (53). It should be noted, however, that the physiological importance of the ABCG1 and SR-B1 pathways is unknown, as is the role of large, spherical HDL particles as acceptors of cell cholesterol in vivo. It is also noteworthy that the efflux of cholesterol mediated by both ABCG1 and SR-B1 is regulated by the phospholipid content and size of the acceptor particles (66, 67).
The results of in vitro measures of cholesterol efflux need to be interpreted with care. Many of these studies involve quantifying the export of radiolabeled cholesterol from cells. This provides a reliable estimate of efflux only when the donor cells are cholesterol loaded (68). Under circumstances where donor cells are not cholesterol loaded, an accurate estimation of cholesterol efflux depends on the quantification of cholesterol mass in the medium and the donor cells (68).
ANTIOXIDANT ACTIVITY OF HDLs
HDLs possess antioxidant properties that have the capacity to inhibit the proatherogenic oxidative modification of LDL (69). The mechanism by which HDLs inhibit oxidation is uncertain. The most extensively studied aspect of this process involves activity of paraoxonase-1 (PON-1), which is transported in the plasma as a component of the HDL fraction (70). However, as outlined below, HDL apolipoproteins also have intrinsic antioxidant effects that are independent of PON-1.
Roles of specific HDL constituents and subpopulations as mediators of the antioxidant properties of HDL
ApoA-I (71), apoA-II (71), apoA-IV (72, 73), and apoE (74) all have antioxidant properties in vitro. Which of these apolipoproteins, if any, is also antioxidant in vivo is not known. While the PON-1 that is transported as a component of HDL particles is widely considered to be a major contributing factor to the antioxidant potential of HDLs, there is also evidence that HDLs have intrinsic antioxidant properties that are PON-1 independent (71, 75). According to these reports, the lipid hydroperoxides that are associated with HDLs are reduced to less deleterious lipid hydroxides via a process that is accompanied by the concomitant oxidation of apoA-I methionine residues (71, 75). These events, which are initiated by the transfer of lipid hydroperoxides from LDLs to HDLs by CETP (76), represent a PON-1-independent antioxidant property of HDLs. There is evidence that the antioxidant activity of HDL3 is superior to that of HDL2 (77). It has also been reported that phospholipids modulate the antioxidant properties of HDLs (75).
ANTI-INFLAMMATORY ACTIVITY OF HDLs
Inflammation plays a pivotal role in both the genesis and the instability of atherosclerotic plaques. HDLs have the capacity to inhibit this inflammation by multiple mechanisms. They inhibit the binding of monocytes to cultured endothelial cells in vitro (69). They also reduce the cytokine-induced expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and E-selectin in cultured endothelial cells in a concentration-dependent manner (24). The ability of HDLs to inhibit inflammation in endothelial cells in vitro varies according to the phospholipid composition of the particles (78), with their constituent apolipoproteins exhibiting much lower specificity (79).
HDLs also inhibit inflammation in vivo. For example, intravenous infusions of apoA-I-containing rHDLs reduce endothelial adhesion molecule expression in cholesterol-fed, apoE-knockout mice in which vascular inflammation has been induced by insertion of a periarterial carotid cuff (80). Studies of apoE-knockout mice transgenic for human apoA-I have established that increasing the concentration of HDLs reduces ex vivo leukocyte adhesion to endothelial cells (81). In a study conducted in rabbits, in which aortic atherosclerosis was induced by endothelial denudation of the abdominal aorta followed by 17 weeks of intervention with a high-cholesterol diet, intravenous infusions of relatively small amounts of HDLs given only during the last week of the study significantly reduced inflammation and decreased the macrophage/smooth muscle cell ratio in the aortic wall (12).
HDLs also have vascular anti-inflammatory properties in vivo in the absence of atherosclerosis, with intravenous infusions of rHDLs inhibiting the development of a local inflammatory infiltrate following the subcutaneous administration of interleukin-1 in a porcine model (82). In studies of experimental stroke in rats, pretreatment with rHDLs has also been shown to substantially reduce the brain necrotic area (83).
In other studies conducted in normocholesterolemic rabbits, in which arterial inflammation was induced by placing a nonocclusive silastic collar around the common carotid artery, intravenous infusions of small amounts of rHDLs containing apoA-I markedly inhibited the infiltration of neutrophils into the intima/media of the artery wall, the collar-induced reactive oxygen species formation, and the collar-induced endothelial expression of adhesion molecules and chemokines (84). It was subsequently shown that this inhibition of periarterial collar-induced acute vascular inflammation in rabbits could be achieved with a single injection of remarkably small amounts (2 mg/kg) of apoA-I, which were not sufficient to increase circulating levels of HDL cholesterol (85).
The latter observation suggests that other events, such as increased expression of key genes that prevent inflammation, are likely to be important for mediating the anti-inflammatory effects of HDLs and apoA-I. This notion is further supported by the observation that the ability of rHDLs to inhibit VCAM-1 expression in tumor necrosis factor-α (TNF-α)-activated endothelial cells persists, even when the rHDLs were removed from the culture medium several hours prior to the cytokine activation (86).
These findings led to the hypothesis that exposure of endothelial cells to rHDLs alters the expression of proteins that have the potential to protect against a subsequent inflammatory insult. One such protein that is induced both in vitro (87) and in vivo (88) by rHDLs is the antioxidant protein 3β-hydroxysteroid-Δ24 reductase (also known as 24-dehydrocholesterol reductase or DHCR24). Silencing DHCR24 expression in endothelial cells not only increases nuclear factor κB cells (NF-κB) activation and VCAM-1 protein levels in both nonactivated and TNF-α-activated endothelial cells, but it is also associated with a loss of the anti-inflammatory effects of rHDLs (87). The rHDL-induced expression of DHCR24 in cultured endothelial cells is dependent on SR-B1, but not on ABCA1 or ABCG1 (87). This effect appears not to be related to the ability of SR-B1, ABCA1, or ABCG1 to maintain intracellular cholesterol homeostasis but, rather, is associated with the activation of intracellular signal transduction pathways that are involved in inflammation (89–91).
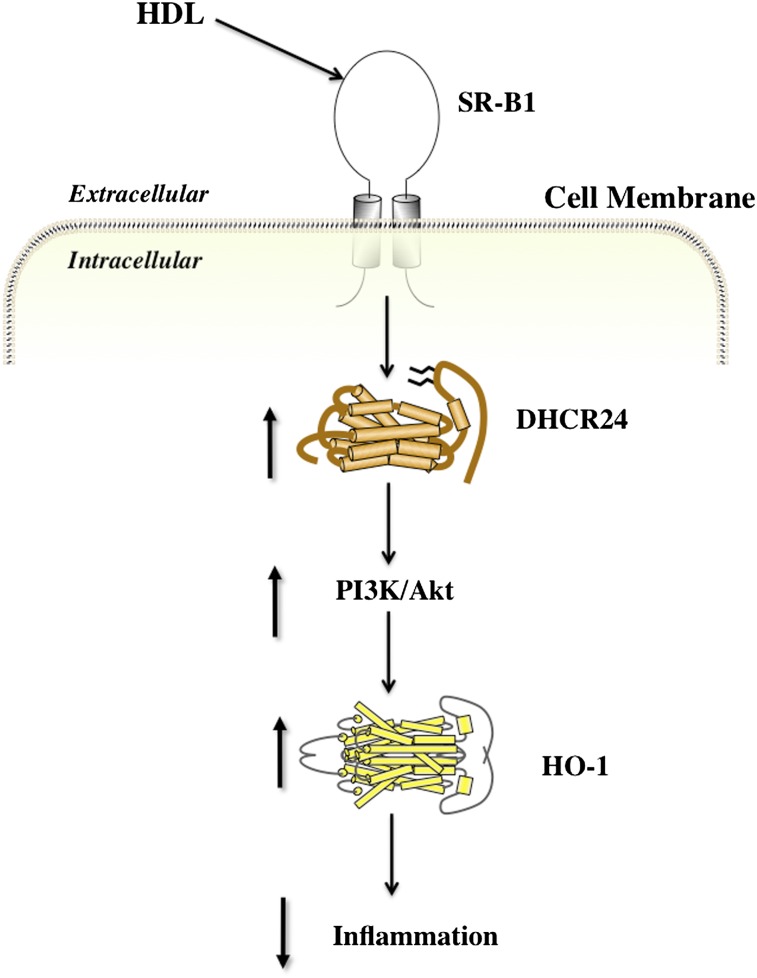
Expression of DHCR24 can also be induced in rabbits with acute vascular inflammation by administering a single intravenous infusion of lipid-free apoA-I 24 h prior to inserting a nonocclusive periarterial silastic collar (88). The results from that study also established that apoA-I-containing rHDLs inhibit vascular inflammation in vivo and adhesion molecule expression in TNF-α-activated endothelial cells in vitro by increasing DHCR24 expression and that this mediated downstream activation of the PI3K/Akt signal transduction pathway, with subsequent induction of the cardioprotective enzyme heme oxygenase-1 (HO-1) (88) (Fig. 2).
Fig. 2.
Inhibition of inflammation by HDLs. Reconstituted HDLs inhibit inflammation in endothelial cells by increasing DHCR24 expression. This leads to activation of the PI3K/Akt signal transduction pathway and induction of the cardioprotective enzyme HO-1 (adapted from Ref. 88).
Roles of specific HDL constituents and subpopulations as mediators of the anti-inflammatory properties of HDLs
It has been reported that the HDL3 subfraction, which predominantly consists of A-I/A-II HDLs, inhibits TNF-α-induced inflammation in endothelial cells more effectively than the HDL2 subfraction, which is mostly A-I HDLs (79). The HDL constituents that are responsible for this difference have yet to be identified. Existing evidence suggests that this observation is unlikely to be a consequence of variations in HDL apolipoprotein content as HDL preparations that contain apoA-I or apoA-II as the sole protein appear to be equally effective at inhibiting the TNF-α-induced increase in adhesion molecule expression in cultured endothelial cells (79).
Phosphatidylcholine vesicles that do not contain apolipoproteins also inhibit adhesion molecule expression in vitro in TNF-α-activated endothelial cells (78). Of interest, lipid-free apoA-I is unable to inhibit endothelial cell inflammation in vitro (92). In vivo, by contrast, intravenous infusions of lipid-free apoA-I (84) inhibit the acute inflammation induced by inserting nonocclusive carotid collars into rabbits as effectively as the corresponding rHDL preparations. The explanation for the difference between these in vitro and in vivo findings is not known, but it may reflect the fact that intravenously injected lipid-free apoA-I rapidly acquires phospholipids and is incorporated into the endogenous HDL fraction as discoidal HDL particles that resemble the rHDLs that effectively inhibits endothelial inflammation in vitro.
INHIBITION OF THROMBOSIS BY HDLs
HDLs inhibit thrombosis by attenuating expression of tissue factor and selectins, by downregulating thrombin generation via the protein C pathway, and by directly and indirectly blunting platelet activation (25). Inhibition of platelet activation is also dependent on SR-B1 (93, 94). SR-B1- and endothelial nitric oxide synthase (eNOS)-knockout mice, which have increased susceptibility to deep vein thrombosis, have also been used to establish that activation of the SR-B1/eNOS signaling pathway by apoA-I is important for inhibiting thrombosis (95). HDLs also have the potential to protect against arterial and venous thrombosis by activating prostacyclin synthesis (25) and can reduce the risk of recurrent venous thrombosis (96).
Roles of specific HDL constituents and subpopulations as mediators of the antithrombotic properties of HDLs
The HDL constituents and subpopulations that inhibit thrombosis have not been investigated extensively. There is, however, one report of venous thrombosis being associated with low apoA-I levels and a reduction in HDL size (97).
HDLs AND ENDOTHELIAL REPAIR
HDLs enhance endothelial repair by two distinct mechanisms. They stimulate endothelial cell migration from healthy to damaged endothelium via the SR-B1-mediated activation of Rac GTPase in a process involving activation of Src kinases, PI3K, and p44/42 mitogen-activated protein kinases (27). In addition, HDLs can promote endothelial repair in vivo by enhancing the engraftment of endothelial progenitor cells into areas of damaged endothelium (28). These topics are covered in detail in the thematic review article from this series by Riwanto and Landmesser (126).
Roles of specific HDL components and subpopulations in promoting endothelial repair
There is little information regarding which HDL components and subpopulations are responsible for enhancing endothelial repair, other than that an adenoviral-mediated increase in apoA-I expression increases circulating endothelial progenitor cell numbers and facilitates their incorporation into the endothelium of apoE-deficient mice (98). There is also evidence that the involvement of SR-B1, Src kinases, PI3K, and p44/42 mitogen-activated protein kinases in endothelial repair is dependent on apoA-I (27).
HDLs AND ENDOTHELIAL FUNCTION
HDLs are known to play an important and beneficial role in endothelial function. A single intravenous infusion of rHDLs into human subjects with hypercholesterolemia (99) or with low levels of HDLs secondary to partial deficiency of ABCA1 (26) normalizes endothelial function by a mechanism that involves the restoration of endothelial nitric oxide production. Ex vivo studies of endothelial cells incubated with HDLs isolated from healthy subjects have also indicated that they can induce eNOS expression, stimulate endothelial cell nitric oxide production, and reduce endothelial oxidant stress (100). Of interest, these potentially beneficial effects of HDLs are not observed in preparations of HDLs isolated from patients with type 2 diabetes (101) or from patients with coronary artery disease (22). The ability of HDLs to induce expression of eNOS in endothelial cells is partly dependent on SR-BI (100). Binding of apoA-I to SR-B1 in endothelial cells initiates a signaling cascade that involves PDZK1-dependent activation of the Src family kinases PI3K and AKt, which leads to phosphorylation of eNOS and a subsequent increase in enzyme activity (102).
Roles of specific HDL components and subpopulations in enhancing endothelial function
The ability of HDLs to enhance endothelial function in humans in vivo has been observed with apoA-I-containing rHDLs, but it has not been investigated with other HDL preparations. The ability of HDLs to enhance synthesis of nitric oxide in vitro has been reported in incubations of endothelial cells with HDLs isolated from human subjects (100), but as far as we are aware, there are no reports of this effect being specifically due to apoA-I-containing HDLs.
HDLs AND ANGIOGENESIS
Intravenous injections of rHDLs have been shown to significantly augment blood flow recovery and to increase capillary density in a murine ischemic hind-limb model of angiogenesis (29). In this model, treatment with rHDL increased the number of bone marrow-derived cells that were incorporated into newly formed capillaries in ischemic muscle and promoted the differentiation of peripheral mononuclear cells into endothelial progenitor cells in a dose-dependent manner. The effect of rHDLs on endothelial progenitor cell differentiation is abrogated by coadministration of LY294002, an inhibitor of PI3K (29, 103). It is noteworthy that rHDLs are unable to promote angiogenesis in endothelial nitric oxide-deficient mice (29).
Low concentrations of HDLs isolated from healthy donors, but not from donors with cardiovascular disease, have also been shown to enhance endothelial progenitor cell-mediated tubulogenesis by processes that involve PI3K/Akt and eNOS, whereas higher, more physiologically relevant HDL concentrations from both types of subjects do not (104). The mechanistic basis for this difference is not clear at present. There is also evidence from in vitro studies that the angiogenesis mediated by HDLs involves Ras/MAP kinase activation (105).
Roles of specific HDL constituents and subpopulations in promoting angiogenesis
The contributions of specific HDL lipid and apolipoprotein constituents to angiogenesis have not been investigated systematically. Published reports have focused on the ability of apoA-I-containing rHDLs to stimulate angiogenesis (29). Studies of the HDL2 and HDL3 subfractions have indicated that they both mediate tubulogenesis to an extent comparable to that of the total HDL fraction (104), which suggests that there is unlikely to be specificity in terms of the apolipoprotein content of HDLs. The effects of HDL lipids on this function have not been investigated.
EFFECTS OF HDLs ON HEMATOPOIETIC STEM CELL PROLIFERATION
Increased blood levels of monocytes and neutrophils are known to predict the development of atherosclerosis (106), with evidence indicating that they play a causative role in lesion development (30). Studies conducted in mice have shown that suppression of the cholesterol efflux promoted by ABCA1 and ABCG1 in bone marrow cells leads to hematopoietic stem cell proliferation and mobilization (30). This increases monocyte and neutrophil production in bone marrow, as well as the number of circulating leukocytes, which infiltrate into multiple organs. This deleterious effect can be reduced by transgenic overexpression of apoA-I in LDL receptor-deficient mice transplanted with bone marrow from ABCA1/ABCG1-deficient mice, with the end result of reduced leukocytosis, reduced myeloid cell infiltration into organs, and reduced diet-induced atherosclerosis (30).
Effects of specific HDL constituents and subpopulations on hematopoietic stem cell proliferation
In addition to the original report of the beneficial effects of transgenic overexpression of apoA-I on leukocytosis in mice transplanted with ABCA1/ABCG1-deficient bone marrow, a later report from the same group established that apoE-deficient mice have leukocytosis, which is significantly decreased by a single intravenous infusion of apoA-I-containing rHDLs (107). It is also noteworthy that global deficiency of apoE, but not apoA-I, is sufficient to induce leukocytosis in mice (107). Other HDL subpopulations that may be involved in these events have yet to be identified. These observations are relevant to the development of atherosclerosis in humans, with a strong association of leukocytosis and excessive morbidity and mortality in cardiovascular disease being reported (106).
ANTIDIABETIC EFFECTS OF HDLs
HDLs have recently been recognized as having a potentially important role in glucose metabolism. Increasing HDL levels with infusions of apoA-I-containing rHDLs increases plasma insulin levels and reduces plasma glucose levels in people with type 2 diabetes (31). Increasing HDL cholesterol levels by inhibiting activity of CETP has also been shown to improve glycemic control in people with type 2 diabetes (108). The outcomes of these studies suggest that HDL cholesterol-raising therapies currently being assessed in humans in large-scale cardiovascular clinical outcome trials may also be useful for maintaining glycemic control in people with type 2 diabetes. It should be noted, however, that not all HDL-raising therapies are antidiabetic, with clear evidence that treatment with niacin is prodiabetic rather than antidiabetic (109).
Effects of specific HDL constituents and subpopulations in diabetes
To date, apoA-I and apoA-II in the lipid-free form and as constituents of rHDLs have both been shown to increase insulin synthesis and secretion in the clonal MIN6 mouse β-cell line, as well as in isolated islets from Sprague-Dawley rats via processes that are dependent on ABCA1, ABCG1, and SR-B1 (110). HDLs and apoA-I also protect β-cells from apoptosis in vitro (111), while treatment with the apoA-I mimetic peptide L-4F has been reported to decrease insulin resistance in obese mice (112). The C-terminal domain of apoA-I has also been shown to increase glucose uptake in vitro into cultured L6 skeletal muscle myotubes (113). These findings are consistent with reports of mice deficient in apoA-I and apoA-II having compromised glycemic control (62, 114) and mice overexpressing apoA-I having improved insulin sensitivity (115).
INHIBITION OF APOPTOSIS BY HDLs
Endothelial cell apoptosis may play a role in the development of atherosclerosis. HDLs protect both macrophages (116) and endothelial cells (117) from apoptosis, in part by promoting the ABCG1-mediated efflux of 7-ketocholesterol and related oxysterols from the cells. It has also been found that small, dense, lipid-poor HDL3 particles protect endothelial cells from apoptosis induced by oxidized LDL, with evidence that apoA-I plays a key role in such protection (118). The antiapoptotic properties of HDLs appear to be lost in patients with coronary artery disease, with evidence that HDLs isolated from these patients may even be proapoptotic (119). In other studies, HDLs have been shown to inhibit apoptosis in pancreatic β-cells in a process that has the capacity to decrease the progression of type 2 diabetes mellitus (111, 120).
Effects of specific HDL constituents and subpopulations on apoptosis
There is evidence that apoA-I (117) and the lysophospholipid sphingosine-1-phosphate (S1P) (121–125) have antiapoptotic effects comparable to those of intact HDLs. The observation that S1P enhances endothelial cell survival with effects comparable to those of native HDLs suggests that signaling by lysophospholipid components of HDLs may be important for the inhibition of apoptosis (122).
UNANSWERED QUESTIONS AND FUTURE CHALLENGES
There has been a recent move toward the view that an accurate assessment of HDL function is likely to provide a more informative assessment of cardiovascular risk than simply measuring HDL cholesterol levels. This has led to considerable progress in recent years in understanding known and identifying new cardioprotective functions of HDLs.
Despite these advances, many important unanswered questions remain. For example, we do not understand i) which, if any, of the known functions of HDLs are clinically important; ii) which HDL constituents and subpopulations are responsible for which functions; iii) whether specific HDL subpopulations and functions reduce cardiovascular risk better than others; or iv) how HDL-targeted interventions affect HDL subpopulation levels and function. The answers to these questions are likely to guide future HDL-raising strategies with the potential to reduce cardiovascular risk.
CONCLUSIONS
The results from human population and preclinical animal studies demonstrating that HDLs protect against atherosclerotic cardiovascular disease is compelling. Despite this, there is still no evidence in humans that increasing HDL cholesterol levels translates into a reduction in clinical cardiovascular events. Given that interventions that increase the concentration of HDL cholesterol may not necessarily be accompanied by an enhancement of HDL function, it is clear that we need to understand much more about how HDL function relates to HDL subpopulation distribution and cardiovascular risk in order to develop rational, HDL-based therapeutic strategies for preventing atherosclerosis.
Footnotes
Abbreviations:
- CETP
- cholesteryl ester transfer protein
- HO-1
- heme oxygenase-1
- PON-1
- paraoxonase-1
- rHDL
- reconstituted HDL
- SR-B1
- scavenger receptor-B1
- VCAM-1
- vascular cell adhesion molecule-1
This work was supported by National Health and Medical Research Council of Australia Grant RG-124089.
REFERENCES
- 1.Gordon D. J., Knoke J., Probstfield J. L., Superko R., Tyroler H. A. 1986. High-density lipoprotein cholesterol and coronary heart disease in hypercholesterolemic men: the Lipid Research Clinics Coronary Primary Prevention Trial. Circulation. 74: 1217–1225 [DOI] [PubMed] [Google Scholar]
- 2.Miller N. E., Thelle D. S., Forde O. H., Mjos O. D. 1977. The Tromso heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet. 1: 965–968 [DOI] [PubMed] [Google Scholar]
- 3.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62: 707–714 [DOI] [PubMed] [Google Scholar]
- 4.Miller M., Seidler A., Kwiterovich P. O., Pearson T. A. 1992. Long-term predictors of subsequent cardiovascular events with coronary artery disease and ‘desirable’ levels of plasma total cholesterol. Circulation. 86: 1165–1170 [DOI] [PubMed] [Google Scholar]
- 5.Di Angelantonio E., Sarwar N., Perry P., Kaptoge S., Ray K. K., Thompson A., Wood A. M., Lewington S., Sattar N., Packard C. J., et al. 2009. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 302: 1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barter P., Gotto A. M., LaRosa J. C., Maroni J., Szarek M., Grundy S. M., Kastelein J. J., Bittner V., Fruchart J. C. 2007. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 357: 1301–1310 [DOI] [PubMed] [Google Scholar]
- 7.Rye K. A., Bursill C. A., Lambert G., Tabet F., Barter P. J. 2009. The metabolism and anti-atherogenic properties of HDL. J. Lipid Res. 50(Suppl.): S195–S200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barter P. J., Nicholls S., Rye K. A., Anantharamaiah G. M., Navab M., Fogelman A. M. 2004. Antiinflammatory properties of HDL. Circ. Res. 95: 764–772 [DOI] [PubMed] [Google Scholar]
- 9.Badimon J. J., Badimon L., Fuster V. 1990. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 85: 1234–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin E. M., Krauss R. M., Spangler E. A., Verstuyft J. G., Clift S. M. 1991. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 353: 265–267 [DOI] [PubMed] [Google Scholar]
- 11.Duverger N., Kruth H., Emmanuel F., Caillaud J. M., Viglietta C., Castro G., Tailleux A., Fievet C., Fruchart J. C., Houdebine L. M., et al. 1996. Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein A-I-transgenic rabbits. Circulation. 94: 713–717 [DOI] [PubMed] [Google Scholar]
- 12.Nicholls S. J., Cutri B., Worthley S. G., Kee P., Rye K. A., Bao S., Barter P. J. 2005. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler. Thromb. Vasc. Biol. 25: 2416–2421 [DOI] [PubMed] [Google Scholar]
- 13.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290: 2292–2300 [DOI] [PubMed] [Google Scholar]
- 14.Tardif J. C., Gregoire J., L'Allier P. L., Ibrahim R., Lesperance J., Heinonen T. M., Kouz S., Berry C., Basser R., Lavoie M. A., et al. 2007. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 297: 1675–1682 [DOI] [PubMed] [Google Scholar]
- 15.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122 [DOI] [PubMed] [Google Scholar]
- 16.Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267 [DOI] [PubMed] [Google Scholar]
- 17.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099 [DOI] [PubMed] [Google Scholar]
- 18.Voight B. F., Peloso G. M., Orho-Melander M., Frikke-Schmidt R., Barbalic M., Jensen M. K., Hindy G., Holm H., Ding E. L., Johnson T., et al. 2012. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 380: 572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Steeg W. A., Holme I., Boekholdt S. M., Larsen M. L., Lindahl C., Stroes E. S., Tikkanen M. J., Wareham N. J., Faergeman O., Olsson A. G., et al. 2008. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J. Am. Coll. Cardiol. 51: 634–642 [DOI] [PubMed] [Google Scholar]
- 20.Poynten A. M., Gan S. K., Kriketos A. D., O'Sullivan A., Kelly J. J., Ellis B. A., Chisholm D. J., Campbell L. V. 2003. Nicotinic acid-induced insulin resistance is related to increased circulating fatty acids and fat oxidation but not muscle lipid content. Metabolism. 52: 699–704 [DOI] [PubMed] [Google Scholar]
- 21.Ridker P. M., Genest J., Boekholdt S. M., Libby P., Gotto A. M., Nordestgaard B. G., Mora S., MacFadyen J. G., Glynn R. J., Kastelein J. J. 2010. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet. 376: 333–339 [DOI] [PubMed] [Google Scholar]
- 22.Besler C., Heinrich K., Rohrer L., Doerries C., Riwanto M., Shih D. M., Chroni A., Yonekawa K., Stein S., Schaefer N., et al. 2011. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Invest. 121: 2693–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy D., Rader D. J. 2006. Emerging therapies targeting high-density lipoprotein metabolism and reverse cholesterol transport. Circulation. 113: 1140–1150 [DOI] [PubMed] [Google Scholar]
- 24.Cockerill G. W., Rye K. A., Gamble J. R., Vadas M. A., Barter P. J. 1995. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 15: 1987–1994 [DOI] [PubMed] [Google Scholar]
- 25.Mineo C., Deguchi H., Griffin J. H., Shaul P. W. 2006. Endothelial and antithrombotic actions of HDL. Circ. Res. 98: 1352–1364 [DOI] [PubMed] [Google Scholar]
- 26.Bisoendial R. J., Hovingh G. K., Levels J. H., Lerch P. G., Andresen I., Hayden M. R., Kastelein J. J., Stroes E. S. 2003. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 107: 2944–2948 [DOI] [PubMed] [Google Scholar]
- 27.Seetharam D., Mineo C., Gormley A. K., Gibson L. L., Vongpatanasin W., Chambliss K. L., Hahner L. D., Cummings M. L., Kitchens R. L., Marcel Y. L., et al. 2006. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ. Res. 98: 63–72 [DOI] [PubMed] [Google Scholar]
- 28.Tso C., Martinic G., Fan W. H., Rogers C., Rye K. A., Barter P. J. 2006. High-density lipoproteins enhance progenitor-mediated endothelium repair in mice. Arterioscler. Thromb. Vasc. Biol. 26: 1144–1149 [DOI] [PubMed] [Google Scholar]
- 29.Sumi M., Sata M., Miura S., Rye K. A., Toya N., Kanaoka Y., Yanaga K., Ohki T., Saku K., Nagai R. 2007. Reconstituted high-density lipoprotein stimulates differentiation of endothelial progenitor cells and enhances ischemia-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol. 27: 813–818 [DOI] [PubMed] [Google Scholar]
- 30.Yvan-Charvet L., Pagler T., Gautier E. L., Avagyan S., Siry R. L., Han S., Welch C. L., Wang N., Randolph G. J., Snoeck H. W., et al. 2010. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 328: 1689–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drew B. G., Duffy S. J., Formosa M. F., Natoli A. K., Henstridge D. C., Penfold S. A., Thomas W. G., Mukhamedova N., de Courten B., Forbes J. M., et al. 2009. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 119: 2103–2111 [DOI] [PubMed] [Google Scholar]
- 32.Drew B. G., Rye K. A., Duffy S. J., Barter P., Kingwell B. A. 2012. The emerging role of HDL in glucose metabolism. Nat. Rev. Endocrinol. 8: 237–245 [DOI] [PubMed] [Google Scholar]
- 33.Rye K. A., Barter P. J. 2004. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 24: 421–428 [DOI] [PubMed] [Google Scholar]
- 34.Rye K. A., Clay M. A., Barter P. J. 1999. Remodelling of high density lipoproteins by plasma factors. Atherosclerosis. 145: 227–238 [DOI] [PubMed] [Google Scholar]
- 35.Blanche P. J., Gong E. L., Forte T. M., Nichols A. V. 1981. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim. Biophys. Acta. 665: 408–419 [DOI] [PubMed] [Google Scholar]
- 36.Castro G. R., Fielding C. J. 1988. Early incorporation of cell-derived cholesterol into pre-beta-migrating high-density lipoprotein. Biochemistry. 27: 25–29 [DOI] [PubMed] [Google Scholar]
- 37.Cheung M. C., Albers J. J. 1984. Characterization of lipoprotein particles isolated by immunoaffinity chromatography. Particles containing A-I and A-II and particles containing A-I but no A-II. J. Biol. Chem. 259: 12201–12209 [PubMed] [Google Scholar]
- 38.Huang Y., von Eckardstein A., Wu S., Maeda N., Assmann G. 1994. A plasma lipoprotein containing only apolipoprotein E and with gamma mobility on electrophoresis releases cholesterol from cells. Proc. Natl. Acad. Sci. USA. 91: 1834–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amouyel P., Isorez D., Bard J. M., Goldman M., Lebel P., Zylberberg G., Fruchart J. C. 1993. Parental history of early myocardial infarction is associated with decreased levels of lipoparticle AI in adolescents. Arterioscler. Thromb. 13: 1640–1644 [DOI] [PubMed] [Google Scholar]
- 40.Miller N. E. 1987. Associations of high-density lipoprotein subclasses and apolipoproteins with ischemic heart disease and coronary atherosclerosis. Am. Heart J. 113: 589–597 [DOI] [PubMed] [Google Scholar]
- 41.Johansson J., Molgaard J., Olsson A. G. 1991. Plasma high density lipoprotein particle size alteration by simvastatin treatment in patients with hypercholesterolaemia. Atherosclerosis. 91: 175–184 [DOI] [PubMed] [Google Scholar]
- 42.Neuman M. P., Neuman H. R., Neuman J. 1991. Significant increase of high-density lipoprotein2-cholesterol under prolonged simvastatin treatment. Atherosclerosis. 91(Suppl.): S11–S19 [DOI] [PubMed] [Google Scholar]
- 43.Kuvin J. T., Dave D. M., Sliney K. A., Mooney P., Patel A. R., Kimmelstiel C. D., Karas R. H. 2006. Effects of extended-release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease. Am. J. Cardiol. 98: 743–745 [DOI] [PubMed] [Google Scholar]
- 44.Brousseau M. E., Schaefer E. J., Wolfe M. L., Bloedon L. T., Digenio A. G., Clark R. W., Mancuso J. P., Rader D. J. 2004. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N. Engl. J. Med. 350: 1505–1515 [DOI] [PubMed] [Google Scholar]
- 45.Bard J. M., Parra H. J., Camare R., Luc G., Ziegler O., Dachet C., Bruckert E., Douste-Blazy P., Drouin P., Jacotot B., et al. 1992. A multicenter comparison of the effects of simvastatin and fenofibrate therapy in severe primary hypercholesterolemia, with particular emphasis on lipoproteins defined by their apolipoprotein composition. Metabolism. 41: 498–503 [DOI] [PubMed] [Google Scholar]
- 46.Plump A. S., Scott C. J., Breslow J. L. 1994. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc. Natl. Acad. Sci. USA. 91: 9607–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiesa G., Monteggia E., Marchesi M., Lorenzon P., Laucello M., Lorusso V., Di Mario C., Karvouni E., Newton R. S., Bisgaier C. L., et al. 2002. Recombinant apolipoprotein A-I(Milano) infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ. Res. 90: 974–980 [DOI] [PubMed] [Google Scholar]
- 48.Okamoto H., Yonemori F., Wakitani K., Minowa T., Maeda K., Shinkai H. 2000. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 406: 203–207 [DOI] [PubMed] [Google Scholar]
- 49.Morehouse L. A., Sugarman E. D., Bourassa P. A., Sand T. M., Zimetti F., Gao F., Rothblat G. H., Milici A. J. 2007. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J. Lipid Res. 48: 1263–1272 [DOI] [PubMed] [Google Scholar]
- 50.Brousseau M. E., Kauffman R. D., Herderick E. E., Demosky S. J., Jr, Evans W., Marcovina S., Santamarina-Fojo S., Brewer H. B., Jr, Hoeg J. M. 2000. LCAT modulates atherogenic plasma lipoproteins and the extent of atherosclerosis only in the presence of normal LDL receptors in transgenic rabbits. Arterioscler. Thromb. Vasc. Biol. 20: 450–458 [DOI] [PubMed] [Google Scholar]
- 51.Berard A. M., Foger B., Remaley A., Shamburek R., Vaisman B. L., Talley G., Paigen B., Hoyt R. F., Jr, Marcovina S., Brewer H. B., Jr, et al. 1997. High plasma HDL concentrations associated with enhanced atherosclerosis in transgenic mice overexpressing lecithin-cholesteryl acyltransferase. Nat. Med. 3: 744–749 [DOI] [PubMed] [Google Scholar]
- 52.Ko K. W., Paul A., Ma K., Li L., Chan L. 2005. Endothelial lipase modulates HDL but has no effect on atherosclerosis development in apoE-/- and LDLR-/- mice. J. Lipid Res. 46: 2586–2594 [DOI] [PubMed] [Google Scholar]
- 53.Jessup W., Gelissen I. C., Gaus K., Kritharides L. 2006. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr. Opin. Lipidol. 17: 247–257 [DOI] [PubMed] [Google Scholar]
- 54.Oram J. F., Lawn R. M., Garvin M. R., Wade D. P. 2000. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 275: 34508–34511 [DOI] [PubMed] [Google Scholar]
- 55.Santamarina-Fojo S., Peterson K., Knapper C., Qiu Y., Freeman L., Cheng J. F., Osorio J., Remaley A., Yang X. P., Haudenschild C., et al. 2000. Complete genomic sequence of the human ABCA1 gene: analysis of the human and mouse ATP-binding cassette A promoter. Proc. Natl. Acad. Sci. USA. 97: 7987–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang N., Lan D., Chen W., Matsuura F., Tall A. R. 2004. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA. 101: 9774–9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura K., Kennedy M. A., Baldan A., Bojanic D. D., Lyons K., Edwards P. A. 2004. Expression and regulation of multiple murine ATP-binding cassette transporter G1 mRNAs/isoforms that stimulate cellular cholesterol efflux to high density lipoprotein. J. Biol. Chem. 279: 45980–45989 [DOI] [PubMed] [Google Scholar]
- 58.Yancey P. G., Bortnick A. E., Kellner-Weibel G., de la Llera-Moya M., Phillips M. C., Rothblat G. H. 2003. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23: 712–719 [DOI] [PubMed] [Google Scholar]
- 59.Ji Y., Jian B., Wang N., Sun Y., Moya M. L., Phillips M. C., Rothblat G. H., Swaney J. B., Tall A. R. 1997. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J. Biol. Chem. 272: 20982–20985 [DOI] [PubMed] [Google Scholar]
- 60.Yokoyama S. 2006. ABCA1 and biogenesis of HDL. J. Atheroscler. Thromb. 13: 1–15 [DOI] [PubMed] [Google Scholar]
- 61.Cuchel M., Lund-Katz S., de la Llera-Moya M., Millar J. S., Chang D., Fuki I., Rothblat G. H., Phillips M. C., Rader D. J. 2010. Pathways by which reconstituted high-density lipoprotein mobilizes free cholesterol from whole body and from macrophages. Arterioscler. Thromb. Vasc. Biol. 30: 526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adorni M. P., Zimetti F., Billheimer J. T., Wang N., Rader D. J., Phillips M. C., Rothblat G. H. 2007. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 48: 2453–2462 [DOI] [PubMed] [Google Scholar]
- 63.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Favari E., Calabresi L., Adorni M. P., Jessup W., Simonelli S., Franceschini G., Bernini F. 2009. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 48: 11067–11074 [DOI] [PubMed] [Google Scholar]
- 65.Remaley A. T., Stonik J. A., Demosky S. J., Neufeld E. B., Bocharov A. V., Vishnyakova T. G., Eggerman T. L., Patterson A. P., Duverger N. J., Santamarina-Fojo S., et al. 2001. Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem. Biophys. Res. Commun. 280: 818–823 [DOI] [PubMed] [Google Scholar]
- 66.Sankaranarayanan S., Oram J. F., Asztalos B. F., Vaughan A. M., Lund-Katz S., Adorni M. P., Phillips M. C., Rothblat G. H. 2009. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 50: 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yancey P. G., de la Llera-Moya M., Swarnakar S., Monzo P., Klein S. M., Connelly M. A., Johnson W. J., Williams D. L., Rothblat G. H. 2000. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 275: 36596–36604 [DOI] [PubMed] [Google Scholar]
- 68.Sankaranarayanan S., de la Llera-Moya M., Drazul-Schrader D., Asztalos B. F., Weibel G. L., Rothblat G. H. 2010. Importance of macrophage cholesterol content on the flux of cholesterol mass. J. Lipid Res. 51: 3243–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H., et al. 1991. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 88: 2039–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mackness M. I., Durrington P. N., Mackness B. 2004. The role of paraoxonase 1 activity in cardiovascular disease: potential for therapeutic intervention. Am. J. Cardiovasc. Drugs. 4: 211–217 [DOI] [PubMed] [Google Scholar]
- 71.Garner B., Witting P. K., Waldeck A. R., Christison J. K., Raftery M., Stocker R. 1998. Oxidation of high density lipoproteins. I. Formation of methionine sulfoxide in apolipoproteins AI and AII is an early event that accompanies lipid peroxidation and can be enhanced by alpha-tocopherol. J. Biol. Chem. 273: 6080–6087 [DOI] [PubMed] [Google Scholar]
- 72.Wong W. M., Gerry A. B., Putt W., Roberts J. L., Weinberg R. B., Humphries S. E., Leake D. S., Talmud P. J. 2007. Common variants of apolipoprotein A-IV differ in their ability to inhibit low density lipoprotein oxidation. Atherosclerosis. 192: 266–274 [DOI] [PubMed] [Google Scholar]
- 73.Ostos M. A., Conconi M., Vergnes L., Baroukh N., Ribalta J., Girona J., Caillaud J. M., Ochoa A., Zakin M. M. 2001. Antioxidative and antiatherosclerotic effects of human apolipoprotein A-IV in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21: 1023–1028 [DOI] [PubMed] [Google Scholar]
- 74.Tarnus E., Wassef H., Carmel J. F., Rondeau P., Roche M., Davignon J., Bernier L., Bourdon E. 2009. Apolipoprotein E limits oxidative stress-induced cell dysfunctions in human adipocytes. FEBS Lett. 583: 2042–2048 [DOI] [PubMed] [Google Scholar]
- 75.Zerrad-Saadi A., Therond P., Chantepie S., Couturier M., Rye K. A., Chapman M. J., Kontush A. 2009. HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity: relevance to inflammation and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 29: 2169–2175 [DOI] [PubMed] [Google Scholar]
- 76.Christison J. K., Rye K. A., Stocker R. 1995. Exchange of oxidized cholesteryl linoleate between LDL and HDL mediated by cholesteryl ester transfer protein. J. Lipid Res. 36: 2017–2026 [PubMed] [Google Scholar]
- 77.Camont L., Chapman M. J., Kontush A. 2011. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 17: 594–603 [DOI] [PubMed] [Google Scholar]
- 78.Baker P. W., Rye K. A., Gamble J. R., Vadas M. A., Barter P. J. 2000. Phospholipid composition of reconstituted high density lipoproteins influences their ability to inhibit endothelial cell adhesion molecule expression. J. Lipid Res. 41: 1261–1267 [PubMed] [Google Scholar]
- 79.Ashby D. T., Rye K. A., Clay M. A., Vadas M. A., Gamble J. R., Barter P. J. 1998. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 18: 1450–1455 [DOI] [PubMed] [Google Scholar]
- 80.Dimayuga P., Zhu J., Oguchi S., Chyu K. Y., Xu X. O., Yano J., Shah P. K., Nilsson J., Cercek B. 1999. Reconstituted HDL containing human apolipoprotein A-1 reduces VCAM-1 expression and neointima formation following periadventitial cuff-induced carotid injury in apoE null mice. Biochem. Biophys. Res. Commun. 264: 465–468 [DOI] [PubMed] [Google Scholar]
- 81.Rong J. X., Li J., Reis E. D., Choudhury R. P., Dansky H. M., Elmalem V. I., Fallon J. T., Breslow J. L., Fisher E. A. 2001. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 104: 2447–2452 [DOI] [PubMed] [Google Scholar]
- 82.Cockerill G. W., Huehns T. Y., Weerasinghe A., Stocker C., Lerch P. G., Miller N. E., Haskard D. O. 2001. Elevation of plasma high-density lipoprotein concentration reduces interleukin-1-induced expression of E-selectin in an in vivo model of acute inflammation. Circulation. 103: 108–112 [DOI] [PubMed] [Google Scholar]
- 83.Paterno R., Ruocco A., Postiglione A., Hubsch A., Andresen I., Lang M. G. 2004. Reconstituted high-density lipoprotein exhibits neuroprotection in two rat models of stroke. Cerebrovasc. Dis. 17: 204–211 [DOI] [PubMed] [Google Scholar]
- 84.Nicholls S. J., Dusting G. J., Cutri B., Bao S., Drummond G. R., Rye K. A., Barter P. J. 2005. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 111: 1543–1550 [DOI] [PubMed] [Google Scholar]
- 85.Puranik R., Bao S., Nobecourt E., Nicholls S. J., Dusting G. J., Barter P. J., Celermajer D. S., Rye K. A. 2008. Low dose apolipoprotein A-I rescues carotid arteries from inflammation in vivo. Atherosclerosis. 196: 240–247 [DOI] [PubMed] [Google Scholar]
- 86.Clay M. A., Pyle D. H., Rye K. A., Vadas M. A., Gamble J. R., Barter P. J. 2001. Time sequence of the inhibition of endothelial adhesion molecule expression by reconstituted high density lipoproteins. Atherosclerosis. 157: 23–29 [DOI] [PubMed] [Google Scholar]
- 87.McGrath K. C., Li X. H., Puranik R., Liong E. C., Tan J. T., Dy V. M., DiBartolo B. A., Barter P. J., Rye K. A., Heather A. K. 2009. Role of 3beta-hydroxysteroid-delta 24 reductase in mediating antiinflammatory effects of high-density lipoproteins in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 29: 877–882 [DOI] [PubMed] [Google Scholar]
- 88.Wu B. J., Chen K., Shrestha S., Ong K. L., Barter P. J., Rye K. A. 2013. High-density lipoproteins inhibit vascular endothelial inflammation by increasing 3beta-hydroxysteroid-Delta24 reductase expression and inducing heme oxygenase-1. Circ. Res. 112: 278–288 [DOI] [PubMed] [Google Scholar]
- 89.Liu Y., Tang C. 2012. Regulation of ABCA1 functions by signaling pathways. Biochim. Biophys. Acta. 1821: 522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saddar S., Mineo C., Shaul P. W. 2010. Signaling by the high-affinity HDL receptor scavenger receptor B type I. Arterioscler. Thromb. Vasc. Biol. 30: 144–150 [DOI] [PubMed] [Google Scholar]
- 91.Whetzel A. M., Sturek J. M., Nagelin M. H., Bolick D. T., Gebre A. K., Parks J. S., Bruce A. C., Skaflen M. D., Hedrick C. C. 2010. ABCG1 deficiency in mice promotes endothelial activation and monocyte-endothelial interactions. Arterioscler. Thromb. Vasc. Biol. 30: 809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baker P. W., Rye K. A., Gamble J. R., Vadas M. A., Barter P. J. 1999. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J. Lipid Res. 40: 345–353 [PubMed] [Google Scholar]
- 93.Korporaal S. J., Meurs I., Hauer A. D., Hildebrand R. B., Hoekstra M., Cate H. T., Pratico D., Akkerman J. W., Van Berkel T. J., Kuiper J., et al. 2011. Deletion of the high-density lipoprotein receptor scavenger receptor BI in mice modulates thrombosis susceptibility and indirectly affects platelet function by elevation of plasma free cholesterol. Arterioscler. Thromb. Vasc. Biol. 31: 34–42 [DOI] [PubMed] [Google Scholar]
- 94.Ma Y., Ashraf M. Z., Podrez E. A. 2010. Scavenger receptor BI modulates platelet reactivity and thrombosis in dyslipidemia. Blood. 116: 1932–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brill A., Yesilaltay A., De Meyer S. F., Kisucka J., Fuchs T. A., Kocher O., Krieger M., Wagner D. D. 2012. Extrahepatic high-density lipoprotein receptor SR-BI and apoA-I protect against deep vein thrombosis in mice. Arterioscler. Thromb. Vasc. Biol. 32: 1841–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eichinger S., Pecheniuk N. M., Hron G., Deguchi H., Schemper M., Kyrle P. A., Griffin J. H. 2007. High-density lipoprotein and the risk of recurrent venous thromboembolism. Circulation. 115: 1609–1614 [DOI] [PubMed] [Google Scholar]
- 97.Deguchi H., Pecheniuk N. M., Elias D. J., Averell P. M., Griffin J. H. 2005. High-density lipoprotein deficiency and dyslipoproteinemia associated with venous thrombosis in men. Circulation. 112: 893–899 [DOI] [PubMed] [Google Scholar]
- 98.Feng Y., Jacobs F., Van Craeyveld E., Brunaud C., Snoeys J., Tjwa M., Van Linthout S., De Geest B. 2008. Human ApoA-I transfer attenuates transplant arteriosclerosis via enhanced incorporation of bone marrow-derived endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 28: 278–283 [DOI] [PubMed] [Google Scholar]
- 99.Spieker L. E., Sudano I., Hurlimann D., Lerch P. G., Lang M. G., Binggeli C., Corti R., Ruschitzka F., Luscher T. F., Noll G. 2002. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 105: 1399–1402 [DOI] [PubMed] [Google Scholar]
- 100.Yuhanna I. S., Zhu Y., Cox B. E., Hahner L. D., Osborne-Lawrence S., Lu P., Marcel Y. L., Anderson R. G., Mendelsohn M. E., Hobbs H. H., et al. 2001. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 7: 853–857 [DOI] [PubMed] [Google Scholar]
- 101.Sorrentino S. A., Besler C., Rohrer L., Meyer M., Heinrich K., Bahlmann F. H., Mueller M., Horvath T., Doerries C., Heinemann M., et al. 2010. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 121: 110–122 [DOI] [PubMed] [Google Scholar]
- 102.Zhang Q. H., Zu X. Y., Cao R. X., Liu J. H., Mo Z. C., Zeng Y., Li Y. B., Xiong S. L., Liu X., Liao D. F., et al. 2012. An involvement of SR-B1 mediated PI3K-Akt-eNOS signaling in HDL-induced cyclooxygenase 2 expression and prostacyclin production in endothelial cells. Biochem. Biophys. Res. Commun. 420: 17–23 [DOI] [PubMed] [Google Scholar]
- 103.Zhang Q., Yin H., Liu P., Zhang H., She M. 2010. Essential role of HDL on endothelial progenitor cell proliferation with PI3K/Akt/cyclin D1 as the signal pathway. Exp. Biol. Med. (Maywood). 235: 1082–1092 [DOI] [PubMed] [Google Scholar]
- 104.Huang C. Y., Lin F. Y., Shih C. M., Au H. K., Chang Y. J., Nakagami H., Morishita R., Chang N. C., Shyu K. G., Chen J. W. 2012. Moderate to high concentrations of high-density lipoprotein from healthy subjects paradoxically impair human endothelial progenitor cells and related angiogenesis by activating Rho-associated kinase pathways. Arterioscler. Thromb. Vasc. Biol. 32: 2405–2417 [DOI] [PubMed] [Google Scholar]
- 105.Miura S., Fujino M., Matsuo Y., Kawamura A., Tanigawa H., Nishikawa H., Saku K. 2003. High density lipoprotein-induced angiogenesis requires the activation of Ras/MAP kinase in human coronary artery endothelial cells. Arterioscler. Thromb. Vasc. Biol. 23: 802–808 [DOI] [PubMed] [Google Scholar]
- 106.Coller B. S. 2005. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler. Thromb. Vasc. Biol. 25: 658–670 [DOI] [PubMed] [Google Scholar]
- 107.Murphy A. J., Akhtari M., Tolani S., Pagler T., Bijl N., Kuo C. L., Wang M., Sanson M., Abramowicz S., Welch C., et al. 2011. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 121: 4138–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barter P. J., Rye K. A., Tardif J. C., Waters D. D., Boekholdt S. M., Breazna A., Kastelein J. J. 2011. Effect of torcetrapib on glucose, insulin, and hemoglobin A1c in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Circulation. 124: 555–562 [DOI] [PubMed] [Google Scholar]
- 109.Sazonov V., Maccubbin D., Sisk C. M., Canner P. L. 2013. Effects of niacin on the incidence of new onset diabetes and cardiovascular events in patients with normoglycaemia and impaired fasting glucose. Int. J. Clin. Pract. 67: 297–302 [DOI] [PubMed] [Google Scholar]
- 110.Fryirs M. A., Barter P. J., Appavoo M., Tuch B. E., Tabet F., Heather A. K., Rye K. A. 2010. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler. Thromb. Vasc. Biol. 30: 1642–1648 [DOI] [PubMed] [Google Scholar]
- 111.Rutti S., Ehses J. A., Sibler R. A., Prazak R., Rohrer L., Georgopoulos S., Meier D. T., Niclauss N., Berney T., Donath M. Y., et al. 2009. Low- and high-density lipoproteins modulate function, apoptosis, and proliferation of primary human and murine pancreatic beta-cells. Endocrinology. 150: 4521–4530 [DOI] [PubMed] [Google Scholar]
- 112.Peterson S. J., Kim D. H., Li M., Positano V., Vanella L., Rodella L. F., Piccolomini F., Puri N., Gastaldelli A., Kusmic C., et al. 2009. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J. Lipid Res. 50: 1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dalla-Riva J., Stenkula K. G., Petrlova J., Lagerstedt J. O. 2013. Discoidal HDL and apoA-I-derived peptides improve glucose uptake in skeletal muscle. J. Lipid Res. 54: 1275–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weng W., Breslow J. L. 1996. Dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity in apolipoprotein A-II knockout mice suggest a complex role for apolipoprotein A-II in atherosclerosis susceptibility. Proc. Natl. Acad. Sci. USA. 93: 14788–14794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ruan X., Li Z., Zhang Y., Yang L., Pan Y., Wang Z., Feng G. S., Chen Y. 2011. Apolipoprotein A-I possesses an anti-obesity effect associated with increase of energy expenditure and up-regulation of UCP1 in brown fat. J. Cell. Mol. Med. 15: 763–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Terasaka N., Wang N., Yvan-Charvet L., Tall A. R. 2007. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA. 104: 15093–15098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Suc I., Escargueil-Blanc I., Troly M., Salvayre R., Negre-Salvayre A. 1997. HDL and ApoA prevent cell death of endothelial cells induced by oxidized LDL. Arterioscler. Thromb. Vasc. Biol. 17: 2158–2166 [DOI] [PubMed] [Google Scholar]
- 118.de Souza J. A., Vindis C., Negre-Salvayre A., Rye K. A., Couturier M., Therond P., Chantepie S., Salvayre R., Chapman M. J., Kontush A. 2010. Small, dense HDL 3 particles attenuate apoptosis in endothelial cells: pivotal role of apolipoprotein A-I. J. Cell. Mol. Med. 14: 608–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Riwanto M., Rohrer L., Roschitzki B., Besler C., Mocharla P., Mueller M., Perisa D., Heinrich K., Altwegg L., von Eckardstein A., et al. 2013. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 127: 891–904 [DOI] [PubMed] [Google Scholar]
- 120.Roehrich M. E., Mooser V., Lenain V., Herz J., Nimpf J., Azhar S., Bideau M., Capponi A., Nicod P., Haefliger J. A., et al. 2003. Insulin-secreting beta-cell dysfunction induced by human lipoproteins. J. Biol. Chem. 278: 18368–18375 [DOI] [PubMed] [Google Scholar]
- 121.Xia P., Wang L., Gamble J. R., Vadas M. A. 1999. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J. Biol. Chem. 274: 34499–34505 [DOI] [PubMed] [Google Scholar]
- 122.Kimura T., Sato K., Malchinkhuu E., Tomura H., Tamama K., Kuwabara A., Murakami M., Okajima F. 2003. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler. Thromb. Vasc. Biol. 23: 1283–1288 [DOI] [PubMed] [Google Scholar]
- 123.Theilmeier G., Schmidt C., Herrmann J., Keul P., Schafers M., Herrgott I., Mersmann J., Larmann J., Hermann S., Stypmann J., et al. 2006. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 114: 1403–1409 [DOI] [PubMed] [Google Scholar]
- 124.Kontush A., Therond P., Zerrad A., Couturier M., Negre-Salvayre A., de Souza J. A., Chantepie S., Chapman M. J. 2007. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler. Thromb. Vasc. Biol. 27: 1843–1849 [DOI] [PubMed] [Google Scholar]
- 125.Nofer J. R., Levkau B., Wolinska I., Junker R., Fobker M., von Eckardstein A., Seedorf U., Assmann G. 2001. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J. Biol. Chem. 276: 34480–34485 [DOI] [PubMed] [Google Scholar]
- 126.Riwanto M., Landmesser U. 2013. High density lipoproteins and endothelial functions: mechanistic insights and alterations in cardiovascular disease. J. Lipid Res. 54: 3227–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]