Abstract
Important players in triglyceride (TG) metabolism include the liver (production), white adipose tissue (WAT) (storage), heart and skeletal muscle (combustion to generate ATP), and brown adipose tissue (BAT) (combustion toward heat), the collective action of which determine plasma TG levels. Interestingly, recent evidence points to a prominent role of the hypothalamus in TG metabolism through innervating the liver, WAT, and BAT mainly via sympathetic branches of the autonomic nervous system. Here, we review the recent findings in the area of sympathetic control of TG metabolism. Various neuronal populations, such as neuropeptide Y (NPY)-expressing neurons and melanocortin-expressing neurons, as well as peripherally produced hormones (i.e., GLP-1, leptin, and insulin), modulate sympathetic outflow from the hypothalamus toward target organs and thereby influence peripheral TG metabolism. We conclude that sympathetic stimulation in general increases lipolysis in WAT, enhances VLDL-TG production by the liver, and increases the activity of BAT with respect to lipolysis of TG, followed by combustion of fatty acids toward heat. Moreover, the increased knowledge about the involvement of the neuroendocrine system in TG metabolism presented in this review offers new therapeutic options to fight hypertriglyceridemia by specifically modulating sympathetic nervous system outflow toward liver, BAT, or WAT.
Keywords: fatty acids, hypertriglyceridemia, liver, white adipose tissue, brown adipose tissue, hypothalamus
Dyslipidemia is one of the classical risk factors for cardiovascular diseases (CVD) besides hypertension, type 2 diabetes, and smoking (1). Dyslipidemia is defined as an elevation of plasma LDL-cholesterol (LDL-C), triglycerides (TG), or both, with or without a lowering of HDL-cholesterol (HDL-C) (2). Whereas elevated LDL-C is a well-established major predictor of CVD and has been the primary target for lipid-lowering strategies, evidence suggests that an elevated TG level is an independent risk factor for CVD development as well (3, 4). Plasma TG levels are considered elevated when they exceed 150 mg/dl, which is observed in 31% of the adult US population (5). Although hypertriglyceridemia can be caused by rare monogenic disorders, it is mostly caused by a complex interaction between environmental factors and subtle variations in genes involved in lipoprotein metabolism (5). Current treatments for hypertriglyceridemia are aimed at either increasing TG clearance (e.g., fibrates) (6) or at decreasing lipolysis in WAT (e.g., niacin) (7). In addition, reduction of VLDL-TG production lowers plasma TG levels (e.g., exendin-4) (8).
In recent years, the autonomic nervous system, which consists of a sympathetic and a parasympathetic branch, emerged as an important regulator of metabolic homeostasis. Whereas the role of the sympathetic nervous system (SNS) in the regulation of glucose metabolism has been firmly established (for review, see Ref. 9), considerably fewer studies have focused on its role in TG metabolism. This review provides an update specifically on the role of the SNS in the regulation of TG metabolism at the level of production (liver), storage (WAT), and combustion (BAT), and it describes novel therapeutic modalities to diminish hypertriglyceridemia by targeting the SNS.
METABOLISM OF TRIGLYCERIDE-RICH LIPOPROTEINS
The transport of lipids in lipoproteins is highly coordinated, and disturbances in lipid transport can cause dyslipidemia. In the blood, cholesterol is primarily transported via LDL and HDL. In contrast, dietary and endogenously derived TGs are transported to various peripheral tissues via large, TG-rich lipoproteins (TRL) (i.e., chylomicrons and VLDL, respectively) (10). After a meal, dietary fat and cholesterol are taken up by the intestine, assembled within chylomicrons, and subsequently released into the circulation. Hepatocytes assemble lipids derived from chylomicrons, as well as endogenous lipids derived from de novo lipogenesis, to form VLDL (11). Once chylomicrons or VLDL arrive in the circulation, lipoprotein lipase (LPL) present on the capillary beds of adipose tissue and muscle hydrolyze TG into glycerol and FFA. The liberated FFA can subsequently be taken up by white adipocytes to be stored as TG or by muscle cells and brown adipocytes to be combusted toward ATP and heat, respectively (12, 13). Cellular uptake of FFA is mediated by various cell surface receptors, including FA transport proteins and CD36 (14). During lipolysis, the TRL become enriched with apoE. The TRL remnant is either rapidly cleared by the liver via binding of apoE to the LDL receptor or LDL-related protein (LRP) (15), or it is further hydrolyzed to generate LDL. Therefore, there is a continuous flux of FA from the intestine and liver (i.e., TG production) toward WAT (storage), muscle and BAT (combustion), followed by clearance of TRL remnants by the liver and subsequent reinitiation of this cycle. In addition, lipolysis of cellular TG in WAT, for example, under conditions of fasting, contributes to a flux of FA from WAT to liver (e.g., for VLDL-TG synthesis) and to muscle and BAT (i.e., combustion).
ANATOMY OF HYPOTHALAMIC SYMPATHETIC OUTFLOW
The various key target organs involved in TG metabolism (i.e., liver, WAT, and BAT) are densely innervated by the sympathetic branch of the autonomic nervous system and are thus, at least in part, controlled by the brain. The major brain region involved in the regulation of general energy balance is the hypothalamus (9, 16). Within the arcuate nucleus (ARC) of the hypothalamus, two neuronal populations, proopiomelanocortin (POMC)-expressing neurons and neuropeptide Y (NPY)/Agouti-related protein (AgRP)-expressing neurons, oppositely regulate energy metabolism (17). Activation of POMC neurons leads to the production of α-melanocyte-stimulating hormone (α-MSH), which in turn stimulates the melanocortin (MC) receptors within the hypothalamic paraventricular nucleus (PVN) to promote a catabolic state of the body (18). In contrast, activation of NPY/AgRP neurons promotes an anabolic state, partly because AgRP acts as an endogenous antagonist for the melanocortin receptors and hereby directly inhibits the actions of POMC-expressing neurons (19).
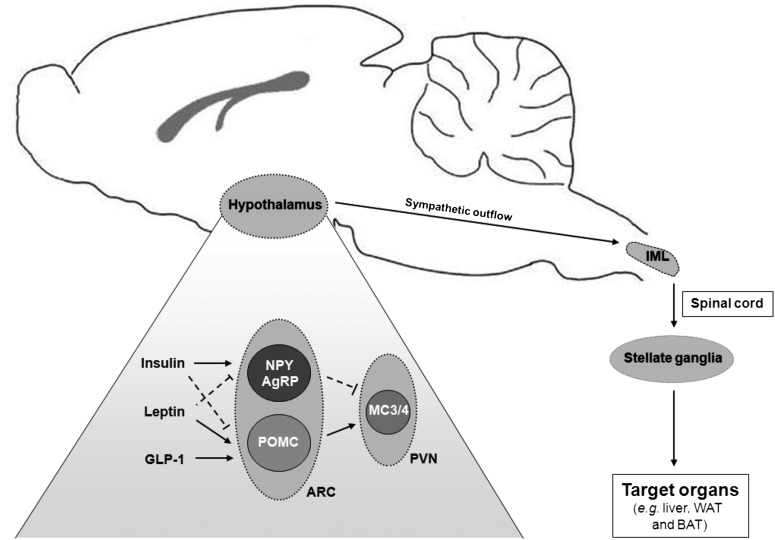
Within the hypothalamus, separate populations of preautonomic nerve fibers reside that project to either parasympathetic or sympathetic nuclei in the brain stem and spinal cord respectively (20). Sympathetic nerve fibers arise from the intermediolateral (IML) column of the thoracic spinal cord and project to stellate ganglia located just outside of the spinal cord. In turn, stellate ganglia give rise to postsynaptic sympathetic nerve fibers, which subsequently innervate the target organ. In general, sympathetic neurons transmit their signal by releasing noradrenalin (i.e., norepinephrin) from their nerve endings (21). Noradrenalin subsequently binds to adrenergic receptors located at the postsynaptic membrane located on the target organ (22). At least nine subtypes of adrenergic receptors, divided into three major classes, have been identified: α1(A/B/D)-adrenergic receptors, α2(A/B/C)-adrenergic receptors, and β(1/2/3)-adrenergic receptors (23). All adrenergic receptors belong to the G-protein-coupled receptor superfamily and couple to Gα proteins. Importantly, each class of adrenergic receptors couples to a different Gα protein, resulting in different intracellular cascades.
SYMPATHETIC CONTROL OF HEPATIC TG METABOLISM
Sympathetic innervation of the liver
The liver is innervated via both sympathetic and parasympathetic nerve fibers that form two separate but intercommunicating plexuses, which enter the liver at its hilus (24). The sympathetic nerve fibers that innervate the liver arise from two major areas within the hypothalamus: the ventromedial hypothalamus (VMH) and the PVN (for review, see Ref. 25). Both nuclei send sympathetic projections toward the liver via the lower brainstem and the IML column of the spinal cord (Fig. 1). Of note, the PVN has many connections with other hypothalamic nuclei involved in energy metabolism (26) and is thereby able to integrate information from other hypothalamic areas with the autonomic control of hepatic energy metabolism (25).
Fig. 1.
Sympathetic innervation of key target organs involved in triglyceride metabolism. Within the ARC of the hypothalamus, both NPY/AgRP-expressing neurons and POMC-expressing neurons are involved in TG metabolism. Their actions are partly mediated via neuronal MC3/4 receptor activation in the hypothalamic PVN. Various peripheral hormones (i.e., insulin, leptin, and GLP-1) can impact on the neuronal populations in the ARC and can thereby influence TG metabolism, a process which is at least in part mediated via the sympathetic nervous system. From the hypothalamus, presympathetic nerve fibers are relayed to the IML column of the thoracic spinal cord. Consecutively, sympathetic nerve fibers from the IML column project to stellate ganglia that are located just outside the spinal cord and give rise to postsynaptic sympathetic nerve fibers that subsequently innervate the target organs including liver, WAT, and BAT.
Evidence for a role of the SNS in regulating hepatic TG metabolism
In vivo studies consistently show that sympathetic activation stimulates VLDL-TG production. In rats, hepatic denervation, which decreases local noradrenalin levels in the liver, as well as adrenalectomy, which results in a generalized decrease of circulating plasma (nor)adrenalin levels, reduced incorporation of exogenously administered fatty acids into the plasma VLDL-TG fraction (27), pointing to decreased VLDL-TG secretion. Accordingly, Bruinstroop et al. (28) recently showed that hepatic sympathetic denervation in rats reduced hepatic VLDL-TG secretion. Of interest, this reduction in VLDL-TG secretion was observed in 19 h-fasted rats but not in postprandial, 4 h-fasted rats. These results indicate that the SNS is involved in regulating hepatic VLDL-TG secretion specifically during fasting, a situation in which lipids become the key substrate for energy metabolism (28).
The regulation of hepatic TG metabolism by the hypothalamus is mediated by both hypothalamic NPY- and MC-expressing neurons. Central NPY administration increased hepatic expression of lipogenic genes and VLDL-TG production in rats (29), an effect found to be largely abolished by hepatic sympathetic denervation (28). Likewise, NPY attenuated the suppression of hepatic VLDL-TG production by insulin in mice, as determined under hyperinsulinemic-euglycemic clamp conditions (30).
In contrast to NPY, central administration of MTII, a synthetic MC3/4 receptor agonist, decreased hepatic expression of lipogenic genes in streptozotocin-induced diabetic mice (31) and decreased hepatic TG content in rats (32), suggestive of decreased hepatic VLDL-TG production. In line with this, administration of SHU9119, a MC3/4 receptor antagonist, markedly increased liver TG content in rats, pointing to increased lipogenesis (33). Importantly, the induction of hepatic lipogenesis by blockade of the central MC system appears to require functional endocrine regulation by the hypothalamic-pituitary-adrenal axis (32). Therefore, the exact contribution of the SNS to the MC effect on hepatic VLDL-TG metabolism needs further attention. In addition, MC receptors have been reported to be expressed in rat liver cells (34), suggesting that melanocortins might also directly interfere with TG metabolism in the liver.
Interestingly, various peripherally produced hormones such as glucagon-like peptide-1 (GLP-1) and leptin likely regulate hepatic TG metabolism via the SNS. GLP-1, an incretin hormone secreted upon food intake by intestinal L-cells and the brain (35), mediates its effects via the GLP-1 receptor, which is widely expressed in various tissues, including the hypothalamus (36). Rats with chronically elevated plasma GLP-1 levels showed decreased expression of lipogenic enzymes and reduced hepatic TG content (37). In line with this, continuous subcutaneous infusion of exendin-4, a GLP-1 receptor agonist, decreased hepatic VLDL-TG production in mice (8). Importantly, also central GLP-1 infusion decreased hepatic TG content and expression of lipogenic enzymes in mice, suggesting that GLP-1 can at least in part affect hepatic TG metabolism via a central mechanism (38, 39). In addition, in vitro activation of GLP-1 receptor signaling in human hepatocytes was shown to directly reduce TG stores (40), thus suggesting that GLP-1 might also directly act on hepatocytes to inhibit lipogenesis. Therefore, the exact role of the SNS in the regulation of hepatic TG metabolism by GLP-1 remains elusive, and experiments combining central and/or peripheral GLP-1 administration with hepatic denervations might prove an effective strategy to resolve this issue.
Leptin, a hormone derived from white adipose tissue (WAT) in case of a positive energy balance, also decreased expression of lipogenic genes and hepatic TG content in mice (41). These effects were dependent on hypothalamic leptin signaling and were, surprisingly, caused by a stimulation of SNS outflow to the liver, as evidenced by an increase in hepatic noradrenalin content (41). In addition, a recent follow-up study showed that leptin administration in 36 h-fasted mice decreased hepatic TG content, lipogenic gene expression, and VLDL-TG production (42). These data, however, should be taken with caution, as 36 h fasting is probably a nonphysiological metabolic condition for mice.
In conclusion, there is clear evidence for a role of the SNS in the regulation of hepatic lipogenesis and VLDL-TG production, in which increased sympathetic outflow to the liver generally leads to an increase in VLDL-TG production, resulting in increased FA availability to be used by peripheral organs (i.e., WAT, BAT, and muscle). This effect is mediated by the NPY system, with increased hypothalamic NPY levels leading to increased sympathetic outflow to the liver and subsequent increased hepatic VLDL-TG output. Furthermore, peripherally produced hormones that are induced upon positive energy balance (e.g., GLP-1 and leptin) reduce hepatic VLDL-TG production.
SYMPATHETIC REGULATION OF TG METABOLISM IN WAT
Sympathetic innervation of WAT
Innervation of WAT by the sympathetic branch of the autonomic nervous system was first evidenced in 1995 by Youngstrom and Bartness by use of anterograde and retrograde fluorescent labeling in Siberian hamsters (43, 44). Of note, they demonstrated that different WAT depots are distinctly innervated by the SNS (44) and that several hypothalamic nuclei involved in regulating peripheral energy metabolism (e.g., the ARC and PVN) are involved in innervation of WAT (45) (Fig. 1).
Evidence for a role of the SNS in regulating TG metabolism in WAT
In general, β-adrenergic SNS stimulation of WAT induces breakdown of TG into glycerol and FFA, a process called lipolysis (46). Stimulation of β1-, β2-, or β3-adrenergic receptors in WAT triggers adenyl cyclase to release cyclic AMP (cAMP) (43). The release of cAMP activates protein kinase A (PKA), which in turn phosphorylates adipose tissue TG lipase (ATGL), hormone-sensitive lipase (HSL), and perilipins, ultimately resulting in lipolysis of stored TG (43).
Just as in liver, the regulation of TG storage in WAT is mediated by hypothalamic NPY- and MC-expressing neurons. Chronic central NPY infusion in rats promoted lipogenesis and thus lipid storage in WAT, independent of its effects on food intake, which is in line with the anabolic actions of NPY (47). Conversely, in rats, knockdown of the NPY gene in the dorsomedial nucleus of the hypothalamus increased expression of genes related to lipolysis and decreased WAT size, suggesting increased lipolysis (48). Taken together, central NPY signaling may decrease sympathetic outflow toward WAT, resulting in decreased lipolysis and thus enhanced storage of energy. However, direct evidence for a role of the SNS in the NPY-induced changes in lipid metabolism in WAT has not been reported.
In contrast, the catabolic MC neurons appear to oppositely regulate TG storage in WAT by stimulating lipolysis. The melanocortin-4 receptor (MC4R) was found to be expressed in SNS outflow neurons to WAT (49). Furthermore, stimulation of the MC4R, by means of chronic central infusion of the MC3/4R agonist MTII, increased the expression of lipolytic genes in WAT of rats (33), thus suggesting an increase in sympathetic outflow toward WAT and enhanced lipolysis. Conversely, inhibition of the MC4R, via chronic central infusion of the MC3/4R antagonist SHU9119, enhanced the expression of lipogenic genes in WAT (33), suggesting decreased sympathetic outflow to WAT and decreased lipolysis. Importantly, these effects were absent in mice lacking the β1,2,3-adrenergic receptors, indicating that the central melanocortin system regulates TG lipolysis by regulating β-adrenergic outflow toward WAT.
Just as in the liver, various peripherally produced hormones likely influence TG metabolism in WAT via modulation of sympathetic outflow toward WAT. GLP-1 generally enhances lipolysis in WAT, which plausibly involves both a central and directly peripheral mechanism. Central infusion of GLP-1 in mice decreased TG content in WAT independent of its anorectic effect and decreased expression of lipogenic genes, shifting TG metabolism toward increased lipolysis (38). These effects were indeed mediated by the SNS, as GLP-1 increased sympathetic nerve activity recorded from nerve endings in WAT and the effect was absent in β1,2,3-adrenoreceptor knockout animals (38). In addition, GLP-1 exerted direct lipolytic actions in isolated rat adipocytes (50).
Within the hypothalamus, insulin and leptin are both inhibitors of NPY/AgRP neurons. However, whereas leptin increased SNS output toward WAT (51, 52), insulin decreased sympathetic outflow, resulting in downregulation of lipolytic genes and upregulation of lipogenic genes in WAT, with net inhibition of lipolysis (53–55). Additionally, we recently showed that circulating insulin stimulates fatty acid retention in WAT in mice, at least in part via the central nervous system (56). Furthermore, insulin directly reduced cellular cAMP levels in WAT, resulting in direct peripheral inhibition of WAT lipolysis (57).
Leptin also regulates TG metabolism in WAT via both central (e.g., via inhibition of NPY/AgRP neurons) and peripheral pathways. First of all, hypothalamic leptin administration in rats decreased lipogenic gene expression (resulting in net lipolysis) in the epididymal WAT depot in rats, an effect abolished after sympathetic denervation of this fat depot (51). Furthermore, leptin administration increased SNS activity in nerves innervating the WAT of rats and this effect was associated with an increase in plasma glycerol and FFA levels (52), indicative of increased WAT lipolysis (51, 52). The differences between insulin and leptin action on WAT lipolysis might be explained by the different signaling cascades evoked by both hormones in AgRP neurons, with leptin inhibiting and insulin stimulating membrane accumulation of the PI3K reporter protein (58). Therefore, Scherer and Buettner (55) proposed a model in which insulin and leptin, by signaling to different populations of second order neurons, activate (i.e., leptin) or inhibit (i.e., insulin) SNS outflow to WAT, consequently leading to either increased or decreased WAT lipolysis, respectively. Peripheral leptin signaling in WAT also appears to be directly involved in TG metabolism in this tissue, as reduced expression of leptin receptors in WAT following partial knockdown increased adiposity and lowered lipolysis in mice (59).
Thus in general, there is convincing evidence for a role of the SNS in modulating lipid storage in WAT, with increased sympathetic outflow to WAT resulting in increased lipolysis and liberation of FAs. These FAs may subsequently be transported to the liver as substrate for VLDL-TG production, as well as to muscle and BAT to be combusted into ATP and heat, respectively. The hypothalamic NPY/MC system mediates this effect, with activation of hypothalamic NPY neurons leading to decreased WAT lipolysis, whereas stimulation of MC3/4 receptors leads to increased WAT lipolysis. Furthermore, the peripherally produced hormones leptin and GLP-1 both increase WAT lipolysis, while the anabolic hormone insulin decreases lipolysis resulting in increased lipid storage.
SYMPATHETIC REGULATION OF TG METABOLISM IN BAT
Sympathetic innervation of BAT
BAT is densely innervated by the SNS, and SNS-mediated BAT thermogenesis and TG clearance are controlled by an area within the preoptic chiasma/anterior hypothalamic nuclei (PO/AH), located in front of the third ventricle (60, 61). Cooling of this area activates BAT (62, 63), whereas warming suppresses its activity (64). The signal is mediated through the VMH and medulla oblongata and then passes through the spinal cord until it reaches the relevant IML neurons (65). From the consequent stellate ganglia (12), thin unmyelinated nerve fibers directly innervate and activate each brown adipocyte, resulting in a dense innervation of the interscapular BAT depot (12, 61) (Fig. 1). Of note, many other brain regions are involved in the innervation of BAT, such as the brain stem, midbrain (central gray and dorsal raphe nucleus), and various regions in the forebrain (i.e., hypothalamic paraventricular nucleus, dorsomedial hypothalamus, suprachiasmatic nucleus, and arcuate nucleus) (66).
Evidence for a role of the SNS in regulating TG metabolism in BAT
In general, increased SNS outflow toward BAT (e.g., following a cold stimulus) results in increased clearance and combustion of TG into heat. Noradrenalin, released by sympathetic nerve fibers, binds to the β adrenergic receptors on brown adipocytes (12). Of the three subtypes of β-adrenergic receptors, the β3 adrenergic receptor is the most significant in mature brown adipocytes from rodents (12). Binding of noradrenalin to the β3-adrenergic receptor results in activation of its coupled stimulatory G protein, after which adenyl cyclase stimulates the formation of cAMP. cAMP activates PKA, resulting in two important downstream effects. First, PKA stimulates phosphorylation of transcription factors that enhance expression and synthesis of uncoupling protein-1 (UCP-1). Furthermore, PKA phosphorylates and actives intracellular HSL, resulting in increased intracellular lipolysis and, consequently, an increased flux of FA toward the mitochondria to be combusted (12). In addition, FAs may bind to a hydrophobic binding pocket on the UCP-1 protein, resulting in its conformational change. This results in an increased proton flux and uncoupling of ATP synthesis so that heat is produced instead of ATP (12).
BAT activity is crucially dependent on SNS input, since mice that lack β-adrenergic receptors are unable to increase thermogenesis by BAT upon cold exposure (67–69). Moreover, animals in which BAT is denervated become rapidly obese and hypertriglyceridemic, underscoring the contribution of BAT to total energy expenditure and TG clearance (70). In addition, Bartelt et al. (71) have shown that housing mice at 4°C for 24 h, a key trigger for sympathetic stimulation of BAT, markedly increased uptake of fatty acids by BAT. This resulted in a rapid normalization of plasma TG levels in hyperlipidemic apoA5 knockout mice (71).
Recently, it became clear that multiple mediators of TG metabolism in liver and WAT also influence thermogenesis in BAT. Since BAT thermogenesis coincides with TG combustion (71), a stimulus that activates BAT is also likely to increase clearance of TRLs toward BAT (71). Unfortunately, most studies that investigated the effect of neuroendocrine factors on BAT thermogenesis have neither focused on lipid metabolism nor performed TG clearance experiments. The effect of these factors on TG clearance therefore remains speculative.
As seen for liver and WAT, the hypothalamic NPY/MC system has also been implied in the regulation of BAT thermogenesis. Knockdown of the NPY gene in the dorsomedial hypothalamus in rats increased the number of brown adipocytes between inguinal WAT and increased thermogenesis in interscapular BAT (48). These data suggest that, in the physiological situation, NPY functions to inhibit BAT activity. Indeed, central infusion of NPY decreased BAT activity and thermogenesis in rats (72) and in mice (M. R. Boon and J. J. Geerling, et al., unpublished data). In addition, NPY signaling in the arcuate nucleus of the hypothalamus decreased sympathetically mediated BAT thermogenesis (73). Though no studies have yet focused on the role of NPY in TG clearance by BAT, the above-mentioned studies suggest that NPY inhibits TG clearance by BAT, which corresponds to its anabolic function.
Similar to NPY-expressing neurons, MC-expressing neurons contribute to the control of SNS-mediated BAT thermogenesis. Since the MC4R colocalizes with SNS outflow neurons to BAT, it might be speculated that the MC4R can affect BAT thermogenesis by modulating the SNS outflow to BAT (74). Indeed, a single injection of MTII, an MC3/4R agonist, into the third ventricle of rats increased BAT thermogenesis and UCP-1 expression (74), which was blocked by surgical sympathetic denervation of BAT (75). On the other hand, three-week ICV infusion of the MC4R antagonist SHU9119 resulted in decreased BAT thermogenesis in rats (76). Thus, activation of the MC4R increases BAT thermogenesis and, as a consequence, likely also increases TG clearance by BAT.
Similar to WAT and liver, peripheral hormones affect BAT thermogenesis and, therefore, likely also affect TG metabolism in BAT. First of all, GLP-1 increases BAT activity, as a recent study in mice showed that central administration of a GLP-1 receptor agonist increased SNS outflow toward BAT, which was accompanied by increased BAT thermogenesis as well as increased expression of LPL (77), pointing to increased TG clearance. Indeed, central administration of the GLP-1 analog exendin-4 specifically increased the uptake of radioactively labeled TG by BAT (E. T. Parlevliet et al., unpublished data). Since GLP-1 may stimulate POMC neurons (78), which results in hypothalamic MC4R activation (79), the effect may have been mediated via this route. Future studies should address the role of the SNS in mediating these effects.
Second, insulin also stimulates BAT thermogenesis. Central injection of insulin into the PO/AH of the hypothalamus induced a dose-dependent increase in BAT thermogenesis and fatty acid oxidation in mice (80). Insulin is known to inhibit NPY/AgRP neurons and to stimulate POMC neurons (80). As these neurons are also involved in mediating BAT activity and thermogenesis (as described above), the effect of insulin on BAT thermogenesis is likely mediated by either increased MC4R activation or decreased NPY activation. On the other hand, insulin may also bind directly to its receptors on brown adipocytes, leading to increased LPL activity and stimulation of TG clearance by BAT (reviewed in Ref. 12). Despite these findings, insulin-resistant mice showed equal if not higher uptake of TRLs by BAT upon cold induction (4°C for 24 h) (71). Furthermore, we recently showed that insulin does not influence TG-derived fatty acid retention in BAT, as assessed under hyperinsulinemic-euglycemic clamp conditions (56). Therefore, insulin is probably not crucially involved in TG clearance by BAT.
Leptin exerts effects on BAT thermogenesis via both central and peripheral pathways. Animals deficient in leptin or its receptor were unable to adapt to acute cold exposure, while activation of central leptin receptors increased SNS output, BAT thermogenesis, and UCP-1 expression (81–84). This is likely mediated via the MC4R of the MC system, as the above-mentioned effects could be blocked with the MC4R antagonist SHU9119 (85). Leptin may also exert a direct peripheral effect on BAT, as intravenous administration of leptin in rats increased glucose utilization and lipolytic activity in BAT, while ICV infusion of leptin did not elicit these effects (86). Thus, though these studies suggest that leptin actives BAT, no studies have focused yet on the effect of (the absence of) central leptin signaling on TG clearance by BAT.
In conclusion, the SNS is an important mediator of BAT activity and increased SNS activity (e.g., as a result of a cold stimulus) results in increased BAT activation and TG clearance. This is mediated by the hypothalamic NPY/MC system, with activation of hypothalamic NPY neurons leading to decreased BAT activation, whereas stimulation of MC3/4 receptors leads to increased BAT activation. Furthermore, the peripheral hormones leptin and GLP-1 both increase BAT thermogenesis. The FAs that are used as a substrate may in part derive from increased WAT lipolysis as is also induced by these hormones, followed by uptake by BAT. The role of insulin in BAT thermogenesis is probably not substantial.
SUMMARY, CLINICAL IMPLICATIONS, AND FUTURE PERSPECTIVES
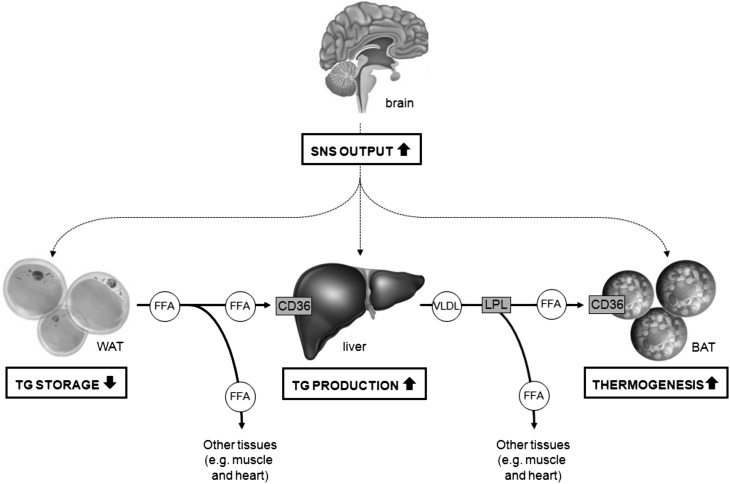
The data summarized in this review indicate that SNS is an important regulator of TG metabolism. In general, increased sympathetic output from the SNS toward WAT and liver increases plasma TG levels by stimulating hepatic VLDL-TG production as well as increasing lipolysis in WAT, the latter resulting in release of FA, which is transported to the liver to fuel the increased synthesis of VLDL-TG. In addition, concomitant activation of BAT results in combustion of excess FA, to generate heat in case of SNS activation by cold exposure or possibly to prevent the occurrence of FA-induced lipotoxicity. Thus, in general, increased sympathetic output increases substrate availability (see Fig. 2). This makes sense physiologically as the SNS is activated by fasting and fight-or-flight responses, both situations in which an organism needs to recruit fuels without being able to eat. In addition, the SNS is not solely involved in innervation of target tissues; it also mediates the effects of various (neuro)peptides and hormones on peripheral TG metabolism (summarized in Table 1).
Fig. 2.
Sympathetic control of TG metabolism. The SNS is upregulated by fasting and fight-or-flight responses, situations in which an organism needs to recruit fuels without being able to eat, and by cold. The increase in SNS output, in general, increases FA release by stimulating hepatic VLDL-TG production as well as increasing lipolysis in WAT, the latter resulting in release of FFA that are transported to the liver to fuel the increased synthesis of VLDL-TG. In addition, BAT is concomitantly activated to combust FFA, to generate heat in case of cold exposure, or possibly to prevent the occurrence of lipotoxicity.
TABLE 1.
Effects of various (neuro)peptides and hormones on peripheral TG metabolism
| Liver | WAT | BAT | |
| Hypothalamic mediators | |||
| NPY | ↑ TG production (28, 29) | ↑ Lipogenesis (47, 48) | ↓ Thermogenesis (48, 72, 73) |
| ∼ TG production (95) | |||
| MC3/4 receptor | ↓ TG content (32, 33) | ↑ Lipolysis (33) | ↑ Thermogenesis (74, 76, 96) |
| ↓ Lipogenesis (31) | |||
| Peripherally produced hormones | |||
| GLP-1 | ↓ TG production (8, 39) | ↑ Lipolysis (50) | ↑ Thermogenesis (77) |
| ↓ Lipogenesis (37, 38) | ↑ TG content (38) | ↑ LPL expression (77) | |
| ↓ Lipogenesis (38) | |||
| Insulin | Not described | ↓ Lipolysis (53–55, 57) | ↑ Thermogenesis (80) |
| ↑ FA retention (56) | |||
| ↑ Lipogenesis (53–55) | |||
| Leptin | ↓ TG content (41, 42) | ↑ Lipolysis (51, 52) | ↑ Thermogenesis (81–84) |
Effects of hypothalamic mediators and peripheral hormones on the major tissues involved in TG metabolism. For some (but not all) effects, the involvement of the SNS has been reported. For more details, refer to the text.
Of note, most insights on the role of the SNS in VLDL-TG metabolism are derived from murine studies. However, accumulating evidence suggests that the SNS is also involved in VLDL-TG metabolism in humans. For instance, in a North European human study cohort, a high level of sympathetic activity was associated with components of the metabolic syndrome, among which were elevated plasma VLDL-TG levels (87). In line with this, the selective β1-adrenergic receptor blocker celiprolol decreased plasma TG levels in patients (88). However, treatment with the nonselective β-adrenergic receptor blocker propranolol increased plasma TG levels (88, 89). As it has been shown that β1-adrenergic receptors are expressed in mature brown adipocytes but are not coupled to any significant extent to intracellular-signaling routes in these cells (64), the decreased plasma TG levels upon celiprolol are presumably caused by a decrease in TG output by both liver and WAT into plasma. In contrast, the β1/2-adrenergic receptor blocker does affect BAT adrenergic receptors, as administration of a low-dose propranolol resulted in a significant reduction of fluorodeoxyglucose (labeled glucose) uptake by BAT in patients undergoing a PET-CT scan, pointing to lowering of BAT activation (90). Therefore, propanolol likely decreases TG combustion in BAT, which can explain the increased TG levels observed in propranolol-treated humans. Since active BAT is present and contributes to energy expenditure in humans (91–94), this might indicate that BAT is crucially involved in TG clearance in humans. We therefore consider BAT as an attractive therapeutic target and propose that future research should be aimed at further delineating the role of this tissue in the regulation of TG metabolism.
Current treatments for hypertriglyceridemia are aimed at increasing fatty acid uptake from plasma and subsequent combustion by, for example, the liver (e.g., fibrates). TG lowering can also be reached by decreasing VLDL-TG production by the liver (e.g., exendin-4) (8) or by decreasing WAT lipolysis (e.g., niacin) (7). However, the increasing knowledge about the involvement of the neuroendocrine system in TG metabolism presented in this review offers new therapeutic options. Compounds that specifically modulate SNS outflow toward liver, BAT, or WAT could be attractive therapeutic agents. Therapeutic agents that decrease sympathetic outflow toward liver and WAT (resulting in decreased TG release) while simultaneously increasing outflow toward BAT (resulting in increased TG uptake) could be very effective. Based on this review, GLP-1 receptor agonists might be a promising new therapeutic strategy, as GLP-1 receptor activation decreases hepatic lipogenesis (37, 38) and VLDL-TG production (8), increases lipolysis (38) and decreases lipogenesis in WAT (50), and increases thermogenesis by BAT (77). However, as all these results were found in a preclinical setting using various animal models, dedicated clinical trials are needed to confirm these results in humans.
In conclusion, TG homeostasis is influenced by various brain circuits that target multiple key organs involved in TG metabolism. As the exact role of the SNS in the regulation of peripheral TG metabolism remains insufficiently studied, it is of importance to further delineate the involvement of the neuroendocrine system, including the SNS. Dedicated studies combining sympathetic denervation of liver, BAT, and WAT with clearance studies using radiolabeled TG could be useful to conclusively determine the role of the SNS in regulating TG clearance by these organs. In addition, experiments studying SNS outflow to target organs under hyperlipidemic circumstances would increase the general knowledge on the role of the SNS in TG metabolism pathologies, which is essential to optimize future therapeutic strategies.
Footnotes
Abbreviations:
- AgRP
- Agouti-related protein
- ARC
- arcuate nucleus
- BAT
- brown adipose tissue
- GLP-1
- glucagon-like peptide-1
- IML
- intermediolateral
- MC
- melanocortin
- NPY
- neuropeptide Y
- PO/AH
- preoptic chiasma/anterior hypothalamic
- POMC
- proopiomelanocortin
- PVN
- paraventricular nucleus
- SNS
- sympathetic nervous system
- TRL
- TG-rich lipoprotein
- UCP-1
- uncoupling protein-1
- VMH
- ventromedial hypothalamus
- WAT
- white adipose tissue
This work is supported by the Dutch Diabetes Research Foundation Grant 2007.00.010 (P.C.N. Rensen). M.R Boon is supported by the board of directors of the Leiden University Medical Center (LUMC), and P.C.N. Rensen is established investigator of the Netherlands Heart Foundation (Grant 2009T038).
REFERENCES
- 1.Lloyd-Jones D. M. 2010. Cardiovascular risk prediction basic concepts, current status, and future directions. Circulation. 121: 1768–1777 [DOI] [PubMed] [Google Scholar]
- 2.Musunuru K. 2010. Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Lipids. 45: 907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langsted A., Freiberg J. J., Tybjaerg-Hansen A., Schnohr P., Jensen G. B., Nordestgaard B. G. 2011. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J. Intern. Med. 270: 65–75 [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard B. G., Benn M., Schnohr P., Tybjaerg-Hansen A. 2007. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 298: 299–308 [DOI] [PubMed] [Google Scholar]
- 5.Miller M., Stone N. J., Ballantyne C., Bittner V., Criqui M. H., Ginsberg H. N., Goldberg A. C., Howard W. J., Jacobson M. S., Kris-Etherton P. M., et al. 2011. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 123: 2292–2333 [DOI] [PubMed] [Google Scholar]
- 6.Staels B., Dallongeville J., Auwerx J., Schoonjans K., Leitersdorf E., Fruchart J. G. 1998. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 98: 2088–2093 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y., Schmidt R. J., Foxworthy P., Emkey R., Oler J. K., Large T. H., Wang H., Su E. W., Mosior M. K., Eacho P. I., et al. 2005. Niacin mediates lipolysis in adipose tissue through its G-protein coupled receptor HM74A. Biochem. Biophys. Res. Commun. 334: 729–732 [DOI] [PubMed] [Google Scholar]
- 8.Parlevliet E. T., Wang Y., Geerling J. J., Schroder-Van der Elst J. P., Picha K., O'Neil K., Stojanovic-Susulic V., Ort T., Havekes L. M., Romijn J. A., et al. 2012. GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice. PLoS ONE. 7: e49152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalsbeek A., Bruinstroop E., Yi C. X., Klieverik L. P., la Fleur S. E., Fliers E. 2010. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann N Y Acad Sci. 1212: 114–12921070249 [Google Scholar]
- 10.Williams K. J. 2008. Molecular processes that handle–and mishandle–dietary lipids. J. Clin. Invest. 118: 3247–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tessari P., Coracina A., Cosma A., Tiengo A. 2009. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 19: 291–302 [DOI] [PubMed] [Google Scholar]
- 12.Cannon B., Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84: 277–359 [DOI] [PubMed] [Google Scholar]
- 13.Bonora M., Patergnani S., Rimessi A., De Marchi E., Suski J. M., Bononi A., Giorgi C., Marchi S., Missiroli S., Poletti F., et al. 2012. ATP synthesis and storage. Purinergic Signal. 8: 343–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glatz J. F. C., Luiken J. J. F. P., Bonen A. 2010. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol. Rev. 90: 367–417 [DOI] [PubMed] [Google Scholar]
- 15.Heeren J., Niemeier A., Merkel M., Beisiegel U. 2002. Endothelial-derived lipoprotein lipase is bound to postprandial triglyceride-rich lipoproteins and mediates their hepatic clearance in vivo. J. Mol. Med. (Berl.). 80: 576–584 [DOI] [PubMed] [Google Scholar]
- 16.Leibowitz S. F., Wortley K. E. 2004. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 25: 473–504 [DOI] [PubMed] [Google Scholar]
- 17.Simpson K. A., Martin N. M., Bloom S. R. 2009. Hypothalamic regulation of food intake and clinical therapeutic applications. Arq. Bras. Endocrinol. Metabol. 53: 120–128 [DOI] [PubMed] [Google Scholar]
- 18.Garfield A. S., Lam D. D., Marston O. J., Przydzial M. J., Heisler L. K. 2009. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol. Metab. 20: 203–215 [DOI] [PubMed] [Google Scholar]
- 19.Chambers A. P., Woods S. C. 2012. The role of neuropeptide Y in energy homeostasis. Handb. Exp. Pharmacol. 2012: 23–45 [DOI] [PubMed] [Google Scholar]
- 20.Buijs R. M., la Fleur S. E., Wortel J., Van H. C., Zuiddam L., Mettenleiter T. C., Kalsbeek A., Nagai K., Niijima A. 2003. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 464: 36–48 [DOI] [PubMed] [Google Scholar]
- 21.Wurtman R. J., Zigmond M. J. 1968. Pharmacologic tools in autonomic nervous system research. Anesthesiology. 29: 714–723 [DOI] [PubMed] [Google Scholar]
- 22.Fluck D. C. 1972. Catecholamines. Br. Heart J. 34: 869–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Insel P. A. 1996. Seminars in medicine of the Beth Israel Hospital, Boston. Adrenergic receptors–evolving concepts and clinical implications. N. Engl. J. Med. 334: 580–585 [DOI] [PubMed] [Google Scholar]
- 24.Tiniakos D. G., Lee J. A., Burt A. D. 1996. Innervation of the liver: morphology and function. Liver. 16: 151–160 [DOI] [PubMed] [Google Scholar]
- 25.Uyama N., Geerts A., Reynaert H. 2004. Neural connections between the hypothalamus and the liver. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 280: 808–820 [DOI] [PubMed] [Google Scholar]
- 26.la Fleur S. E., Kalsbeek A., Wortel J., Buijs R. M. 2000. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 871: 50–56 [DOI] [PubMed] [Google Scholar]
- 27.Carreno F. R., Seelaender M. C. L. 2004. Liver denervation affects hepatocyte mitochondrial fatty acid transport capacity. Cell Biochem. Funct. 22: 9–17 [DOI] [PubMed] [Google Scholar]
- 28.Bruinstroop E., Pei L., Ackermans M. T., Foppen E., Borgers A. J., Kwakkel J., Alkemade A., Fliers E., Kalsbeek A. 2012. Hypothalamic neuropeptide Y (NPY) controls hepatic VLDL-triglyceride secretion in rats via the sympathetic nervous system. Diabetes. 61: 1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stafford J. M., Yu F., Printz R., Hasty A. H., Swift L. L., Niswender K. D. 2008. Central nervous system neuropeptide Y signaling modulates VLDL triglyceride secretion. Diabetes. 57: 1482–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Hoek A. M., Voshol P. J., Karnekamp B. N., Buijs R. M., Romijn J. A., Havekes L. M., Pijl H. 2004. Intracerebroventricular neuropeptide Y infusion precludes inhibition of glucose and VLDL production by insulin. Diabetes. 53: 2529–2534 [DOI] [PubMed] [Google Scholar]
- 31.Leckstrom A., Lew P. S., Poritsanos N. J., Mizuno T. M. 2011. Central melanocortin receptor agonist reduces hepatic lipogenic gene expression in streptozotocin-induced diabetic mice. Life Sci. 88: 664–669 [DOI] [PubMed] [Google Scholar]
- 32.Wiedmer P., Chaudhary N., Rath M., Yi C. X., Ananthakrishnan G., Nogueiras R., Wirth E. K., Kirchner H., Schweizer U., Jonas W., et al. 2012. The HPA axis modulates the CNS melanocortin control of liver triacylglyceride metabolism. Physiol. Behav. 105: 791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nogueiras R., Wiedmer P., Perez-Tilve D., Veyrat-Durebex C., Keogh J. M., Sutton G. M., Pfluger P. T., Castaneda T. R., Neschen S., Hofmann S. M., et al. 2007. The central melanocortin system directly controls peripheral lipid metabolism. J. Clin. Invest. 117: 3475–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik I. A., Triebel J., Posselt J., Khan S., Ramadori P., Raddatz D., Ramadori G. 2012. Melanocortin receptors in rat liver cells: change of gene expression and intracellular localization during acute-phase response. Histochem. Cell Biol. 137: 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips L. K., Prins J. B. 2011. Update on incretin hormones. Ann. N. Y. Acad. Sci. 1243: E55–E74 [DOI] [PubMed] [Google Scholar]
- 36.Willard F. S., Sloop K. W. 2012. Physiology and emerging biochemistry of the glucagon-like peptide-1 receptor. Exp. Diabetes Res. 2012: 470851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Shlomo S., Zvibel I., Shnell M., Shlomai A., Chepurko E., Halpern Z., Barzilai N., Oren R., Fishman S. 2011. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J. Hepatol. 54: 1214–1223 [DOI] [PubMed] [Google Scholar]
- 38.Nogueiras R., Perez-Tilve D., Veyrat-Durebex C., Morgan D. A., Varela L., Haynes W. G., Patterson J. T., Disse E., Pfluger P. T., Lopez M., et al. 2009. Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet-induced obesity. J. Neurosci. 29: 5916–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panjwani N., Mulvihill E. E., Longuet C., Yusta B., Campbell J. E., Brown T. J., Streutker C., Holland D., Cao X. M., Baggio L. L., et al. 2013. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male apoE(−/−) mice. Endocrinology. 154: 127–139 [DOI] [PubMed] [Google Scholar]
- 40.Gupta N. A., Mells J., Dunham R. M., Grakoui A., Handy J., Saxena N. K., Anania F. A. 2010. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 51: 1584–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warne J. P., Alemi F., Reed A. S., Varonin J. M., Chan H., Piper M. L., Mullin M. E., Myers M. G., Corvera C. U., Xu A. W. 2011. Impairment of central leptin-mediated PI3K signaling manifested as hepatic steatosis independent of hyperphagia and obesity. Cell Metab. 14: 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Warne J. P., Varonin J. M., Nielsen S. S., Olofsson L. E., Kaelin C. B., Chua S., Jr, Barsh G. S., Koliwad S. K., Xu A. W. 2013. Coordinated regulation of hepatic energy stores by leptin and hypothalamic agouti-related protein. J. Neurosci. 33: 11972–11985 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Bartness T. J., Song C. K. 2007. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J. Lipid Res. 48: 1655–1672 [DOI] [PubMed] [Google Scholar]
- 44.Youngstrom T. G., Bartness T. J. 1995. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am. J. Physiol. 268: R744–R751 [DOI] [PubMed] [Google Scholar]
- 45.Bamshad M., Aoki V. T., Adkison M. G., Warren W. S., Bartness T. J. 1998. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. 275: R291–R299 [DOI] [PubMed] [Google Scholar]
- 46.Rosell S. 1966. Release of free fatty acids from subcutaneous adipose tissue in dogs following sympathetic nerve stimulation. Acta Physiol. Scand. 67: 343–351 [DOI] [PubMed] [Google Scholar]
- 47.Zarjevski N., Cusin I., Vettor R., Rohnerjeanrenaud F., Jeanrenaud B. 1993. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology. 133: 1753–1758 [DOI] [PubMed] [Google Scholar]
- 48.Chao P. T., Yang L., Aja S., Moran T. H., Bi S. 2011. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 13: 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song C. K., Jackson R. M., Harris R. B. S., Richard D., Bartness T. J. 2005. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289: R1467–R1476 [DOI] [PubMed] [Google Scholar]
- 50.Ruiz-Grande C., Alarcon C., Merida E., Valverde I. 1992. Lipolytic action of glucagon-like peptides in isolated rat adipocytes. Peptides. 13: 13–16 [DOI] [PubMed] [Google Scholar]
- 51.Buettner C., Muse E. D., Cheng A., Chen L., Scherer T., Pocai A., Su K., Cheng B., Li X., Harvey-White J., et al. 2008. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat. Med. 14: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen J., Tanida M., Niijima A., Nagai K. 2007. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci. Lett. 416: 193–197 [DOI] [PubMed] [Google Scholar]
- 53.Koch L., Wunderlich F. T., Seibler J., Konner A. C., Hampel B., Irlenbusch S., Brabant G., Kahn C. R., Schwenk F., Bruning J. C. 2008. Central insulin action regulates peripheral glucose and fat metabolism in mice. J. Clin. Invest. 118: 2132–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scherer T., O'Hare J., Diggs-Andrews K., Schweiger M., Cheng B., Lindtner C., Zielinski E., Vempati P., Su K., Dighe S., et al. 2011. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 13: 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scherer T., Buettner C. 2011. Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism. Rev. Endocr. Metab. Disord. 12: 235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coomans C. P., Geerling J. J., Guigas B., van den Hoek A. M., Parlevliet E. T., Ouwens D. M., Pijl H., Voshol P. J., Rensen P. C. N., Havekes L. M., et al. 2011. Circulating insulin stimulates fatty acid retention in white adipose tissue via K-ATP channel activation in the central nervous system only in insulin-sensitive mice. J. Lipid Res. 52: 1712–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed K., Tunaru S., Tang C., Muller M., Gille A., Sassmann A., Hanson J., Offermanns S. 2010. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 11: 311–319 [DOI] [PubMed] [Google Scholar]
- 58.Xu A. W., Kaelin C. B., Takeda K., Akira S., Schwartz M. W., Barsh G. S. 2005. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Invest. 115: 951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huan J. N., Li J., Han Y. P., Chen K., Wu N., Zhao A. Z. 2003. Adipocyte-selective reduction of the leptin receptors induced by antisense RNA leads to increased adiposity, dyslipidemia, and insulin resistance. J. Biol. Chem. 278: 45638–45650 [DOI] [PubMed] [Google Scholar]
- 60.Boulant J. A. 2000. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin. Infect. Dis. 31: S157–S161 [DOI] [PubMed] [Google Scholar]
- 61.Watanabe J., Mishiro K., Amatsu T., Kanamura S. 1994. Absence of paravascular nerve projection and cross-innervation in interscapular brown adipose tissues of mice. J. Auton. Nerv. Syst. 49: 269–276 [DOI] [PubMed] [Google Scholar]
- 62.Imai-Matsumura K., Matsumura K., Nakayama T. 1984. Involvement of ventromedial hypothalamus in brown adipose tissue thermogenesis induced by preoptic cooling in rats. Jpn. J. Physiol. 34: 939–943 [DOI] [PubMed] [Google Scholar]
- 63.Imai-Matsumura K., Nakayama T. 1987. The central efferent mechanism of brown adipose tissue thermogenesis induced by preoptic cooling. Can. J. Physiol. Pharmacol. 65: 1299–1303 [DOI] [PubMed] [Google Scholar]
- 64.Chen X. M., Hosono T., Yoda T., Fukuda Y., Kanosue K. 1998. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J. Physiol. 512: 883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uno T., Shibata M. 2001. Role of inferior olive and thoracic IML neurons in nonshivering thermogenesis in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280: R536–R546 [DOI] [PubMed] [Google Scholar]
- 66.Bartness T.J., Song C.K. 2005. Innervation of brown adipose tissue and its role in thermogenesis. Canadian Journal of Diabetes. 29(4):420–428 [Google Scholar]
- 67.Bachman E. S., Dhillon H., Zhang C. Y., Cinti S., Bianco A. C., Kobilka B. K., Lowell B. B. 2002. beta AR signaling required for diet-induced thermogenesis and obesity resistance. Science. 297: 843–845 [DOI] [PubMed] [Google Scholar]
- 68.Festuccia W. T., Oztezcan S., Laplante M., Berthiaume M., Michel C., Dohgu S., Denis R. G., Brito M. N., Brito N. A., Miller D. S., et al. 2008. Peroxisome proliferator-activated receptor-gamma-mediated positive energy balance in the rat is associated with reduced sympathetic drive to adipose tissues and thyroid status. Endocrinology. 149: 2121–2130 [DOI] [PubMed] [Google Scholar]
- 69.Sell H., Berger J. P., Samson P., Castriota G., Lalonde J., Deshaies Y., Richard D. 2004. Peroxisome proliferator-activated receptor gamma agonism increases the capacity for sympathetically mediated thermogenesis in lean and ob/ob mice. Endocrinology. 145: 3925–3934 [DOI] [PubMed] [Google Scholar]
- 70.Dulloo A. G., Miller D. S. 1984. Energy balance following sympathetic denervation of brown adipose tissue. Can. J. Physiol. Pharmacol. 62: 235–240 [DOI] [PubMed] [Google Scholar]
- 71.Bartelt A., Bruns O. T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M. G., Tromsdorf U. I., Weller H., Waurisch C., et al. 2011. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17: 200–205 [DOI] [PubMed] [Google Scholar]
- 72.Billington C. J., Briggs J. E., Grace M., Levine A. S. 1991. Effects of intracerebroventricular injection of neuropeptide-Y on energy-metabolism. Am. J. Physiol. 260: R321–R327 [DOI] [PubMed] [Google Scholar]
- 73.Shi Y. C., Lau J., Lin Z., Zhang H., Zhai L., Sperk G., Heilbronn R., Mietzsch M., Weger S., Huang X. F., et al. 2013. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 17: 236–248 [DOI] [PubMed] [Google Scholar]
- 74.Song C. K., Vaughan C. H., Keen-Rhinehart E., Harris R. B., Richard D., Bartness T. J. 2008. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295: R417–R428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams D. L., Bowers R. R., Bartness T. J., Kaplan J. M., Grill H. J. 2003. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology. 144: 4692–4697 [DOI] [PubMed] [Google Scholar]
- 76.Verty A. N. A., Allen A. M., Oldfield B. J. 2010. The endogenous actions of hypothalamic peptides on brown adipose tissue thermogenesis in the rat. Endocrinology. 151: 4236–4246 [DOI] [PubMed] [Google Scholar]
- 77.Lockie S. H., Heppner K. M., Chaudhary N., Chabenne J. R., Morgan D. A., Veyrat-Durebex C., Ananthakrishnan G., Rohner-Jeanrenaud F., Drucker D. J., DiMarchi R., et al. 2012. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like Peptide-1 receptor signaling. Diabetes. 61: 2753–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandoval D. A., Bagnol D., Woods S. C., D'Alessio D. A., Seeley R. J. 2008. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 57: 2046–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hadley M. E., Haskell-Luevano C. 1999. The proopiomelanocortin system. Ann. N. Y. Acad. Sci. 885: 1–21 [DOI] [PubMed] [Google Scholar]
- 80.Sanchez-Alavez M., Tabarean I. V., Osborn O., Mitsukawa K., Schaefer J., Dubins J., Holmberg K. H., Klein I., Klaus J., Gomez L. F., et al. 2010. Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes. 59: 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haynes W. G., Morgan D. A., Djalali A., Sivitz W. I., Mark A. L. 1999. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 33: 542–547 [DOI] [PubMed] [Google Scholar]
- 82.Himms-Hagen J. 1985. Defective brown adipose tissue thermogenesis in obese mice. Int. J. Obes. 9(Suppl. 2): 17–24 [PubMed] [Google Scholar]
- 83.Scarpace P. J., Matheny M. 1998. Leptin induction of UCP1 gene expression is dependent on sympathetic innervation. Am. J. Physiol. 275: E259–E264 [DOI] [PubMed] [Google Scholar]
- 84.Trayhurn P., Thurlby P. L., James W. P. T. 1977. Thermogenic defect in pre-obese ob-ob mice. Nature. 266: 60–62 [DOI] [PubMed] [Google Scholar]
- 85.Satoh N., Ogawa Y., Katsuura G., Numata Y., Masuzaki H., Yoshimasa Y., Nakao K. 1998. Satiety effect and sympathetic activation of leptin are mediated by hypothalamic melanocortin system. Neurosci. Lett. 249: 107–110 [DOI] [PubMed] [Google Scholar]
- 86.Siegrist-Kaiser C. A., Pauli V., Juge-Aubry C. E., Boss O., Pernin A., Chin W. W., Cusin I., Rohner-Jeanrenaud F., Burger A. G., Zapf J., et al. 1997. Direct effects of leptin on brown and white adipose tissue. J. Clin. Invest. 100: 2858–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Licht C. M. M., Vreeburg S. A., Dortland A. K. B. V., Giltay E. J., Hoogendijk W. J. G., Derijk R. H., Vogelzangs N., Zitman F. G., de Geus E. J. C., Penninx B. W. J. H. 2010. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. J. Clin. Endocrinol. Metab. 95: 2458–2466 [DOI] [PubMed] [Google Scholar]
- 88.Fogari R., Zoppi A., Corradi L., Preti P., Mugellini A., Lusardi P. 1999. Beta-blocker effects on plasma lipids during prolonged treatment of hypertensive patients with hypercholesterolemia. J. Cardiovasc. Pharmacol. 33: 534–539 [DOI] [PubMed] [Google Scholar]
- 89.Punda A., Polic S., Rumboldt Z., Bagatin J., Markovic V., Lukin A. 2005. Effects of atenolol and propranolol on platelet aggregation in moderate essential hypertension: randomized crossover trial. Croat. Med. J. 46: 219–224 [PubMed] [Google Scholar]
- 90.Parysow O., Mollerach A. M., Jager V., Racioppi S., San R. J., Gerbaudo V. H. 2007. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin. Nucl. Med. 32: 351–357 [DOI] [PubMed] [Google Scholar]
- 91.Bakker L. E. H., Boon M. R., van der Linden R. A. D., Pereira Arias-Bouda L., van Klinken J. B., Smit F., Verberne H. J., Jukema J. W., Tamsma J. T., Havekes L. M., et al. Brown adipose tissue volume is markedly lower in healthy lean adolescents from South Asian compared to white Caucasian origin. Lancet Diabetes & Endocrinology. Epub ahead of print. November 12, 2013; doi:10.1016/S2213-8587(13)70156-6 [DOI] [PubMed] [Google Scholar]
- 92.Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., et al. 2009. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360: 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., Teule G. J. 2009. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360: 1500–1508 [DOI] [PubMed] [Google Scholar]
- 94.Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerback S., et al. 2009. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360: 1518–1525 [DOI] [PubMed] [Google Scholar]
- 95.Geerling J. J., Wang Y., Havekes L. M., Romijn J. A., Rensen P. C. 2013. Acute central neuropeptide Y administration increases food intake but does not affect hepatic very low-density lipoprotein (VLDL) production in mice. PLoS ONE. 8: e55217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Williams K. W., Margatho L. O., Lee C. E., Choi M., Lee S., Scott M. M., Elias C. F., Elmquist J. K. 2010. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci. 30: 2472–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]